Abstract
Cupping therapy is a common intervention for the management of musculoskeletal impairment. Previous studies have demonstrated that cupping therapy can improve muscle hemodynamic responses using single-channel near-infrared spectroscopy (NIRS). However, the effects of cupping therapy on spatial hemodynamic responses as well as the correlation between oxyhemoglobin and deoxy-hemoglobin are largely unknown. The cross-correlation function (CCF) algorithm was used to determine the correlation between time-series NIRS signals from inside and outside the cup as well as time-series oxyhemoglobin and deoxy-hemoglobin under 4 cupping intensities, including −225 and −300 mmHg for 5 and 10 min. The main finding was that the maximum CCF values of oxyhemoglobin was significantly higher than those in deoxy-hemoglobin (p < 0.05). Furthermore, it was found that there was a correlation between deoxy-hemoglobin with a longer duration and a larger magnitude of negative pressure. This is the first study investigating time-series hemodynamic responses after cupping therapy using cross-correlation function analysis of multi-channel NIRS signals.
1. Introduction
Chronic musculoskeletal impairment, such as muscle fatigue and pain, is a common problem and significantly impacts the quality of life in people of industrialized countries (1). Sports performance can be affected by musculoskeletal impairment caused by intense training and sports competition. Various interventions have been proposed to reduce muscle fatigue for the prevention of musculoskeletal injury and improvement of sports performance, including sports massage, spa treatments and cupping therapy [2]. To date, there is no sufficient evidence on the effectiveness of these interventions on reducing muscle fatigue and risk for musculoskeletal impairment [3]. Cupping therapy is an intervention by creating vacuum to induce a vasodilatory response to local tissues (the skin and muscles), and has been gradually becoming a popular intervention to reduce muscle fatigue recently [4,5]. Traditional cupping therapy has been widely in the Middle East and Asia, and is not widely accepted in the Europe and United States for the lack of evidence. It is imperative to investigate the mechanism of cupping therapy for improving clinical effectiveness of using cupping therapy to improve musculoskeletal impairment [6,7].
The literature indicates that cupping therapy may improve local blood circulation and relieve muscle pain [8–10]. Li et al. (2023) demonstrated that different combinations of cupping pressures and durations could induce various hemodynamic responses [8]. Lauche et al. demonstrated that cupping therapy increases microcirculation and repairs capillary endothelial cells, which may reduce muscle fatigue and heal muscle impairment [11,12]. Additionally, research studies have established an association between exercise tolerance, muscle fatigue recovery speed, and autonomic nerve regulation after exercise [13,14].
Li et al. (2017) used NIRS to assess hemodynamic changes before and after cupping therapy, and found that cupping therapy could reduce deoxy-hemoglobin and increase oxyhemoglobin at an area outside the cupping cup after cupping therapy [15]. Additionally, cupping therapy has been shown to maintain blood microcirculation and hemodynamic function by inducing a significant increase in deoxy-hemoglobin levels and a significant decrease in oxyhemoglobin levels at an area inside the cupping cup during cupping therapy [16]. Oxygen saturation and deoxy-hemoglobin were found to be more closely related to muscle fatigue by Scano and colleagues, while the correlation between total hemoglobin and the median frequency of the electromyographic signal was low [17]. The median frequency of electromyographic signal is a common index to assess muscle fatigue [4]. In addition, Cage et al. also found that an increase in cupping time would increase the surface temperature (baseline = 89.37 ± 2.09 °F, after 5 minutes = 90.49 ± 2.08 °F, after 10 minutes = 91.65 ± 2.18 °F, and after 15 minutes = 91.62 ± 2.26°F, p < 0.001) [18]. Li and colleagues (2023) demonstrated that muscle hemodynamic response to cupping therapy is affected by the pressure and duration of cupping therapy [8]. In their study, only four popular combinations of cupping therapy were tested, and many common settings of pressure and duration of cupping therapy remain to be investigated. These studies indicate a need to assess the effect of various combinations of pressure and duration of cupping therapy on muscle hemodynamic responses.
Previous studies demonstrate that near-infrared spectroscopy (NIRS) can be used to assess muscle hemodynamic responses under various conditions [19–21]. Thus, it is reasonable to postulate that NIRS can be used to assess the effect of various doses of cupping therapy on local hemodynamic responses after cupping therapy. According to Jöbsis et al., near-infrared light penetrates deeply into biological tissues, and can be used to measure blood flow and oxygen levels in biological tissues [22]. By analyzing the sample's near-infrared absorption spectrum, near-infrared spectroscopy can infer the concentration of its components without destroying the sample [23]. As a result, the concentration in oxyhemoglobin [HbO2] and deoxy-hemoglobin [Hb] can be measured in human tissue non-invasively [24].
The cross-correlation function (CCF) method has been used to estimate the correlation to assess the relationship levels of the two signals [25]. CCF estimation's advantage is to identify the hidden information and quantify the temporal similarity [25]. Furthermore, studies have shown that CCF estimation could examine the sense of balance between the autonomic system and baroreflex control [26,27]. For example, cross-correlation baroreflex sensitivity was adopted to analyze the database of EUROBAVAR, including patients with heart transplant and autonomic neuropathy. CCF estimation pointed out that a delay in baroreflex occurred in patients with heart transplant [26]. Although there is no previous studies using CCF to assess physiological mechanisms of cupping therapy, it appears that CCF could be a useful tool to explore hidden interactions in the hemodynamic response after cupping therapy, especially when multi-channel NIRS is used to assess the spatial hemodynamic response.
To date, it is unclear whether different sites of hemodynamic responses could affect each other after cupping therapy. Also, it is largely unknown about the correlation between oxyhemoglobin and deoxy-hemoglobin in response to cupping therapy. Previous studies usually adopted a single channel NIRS or reporting the average of the hemodynamic response from multi-channel NIRS to assess the hemodynamic response after cupping therapy [8]. The use of a multi-channel NIRS device may provide additional information about the spatial hemodynamic response and the use of CCF may shed light on the interactions of these hemodynamic responses. Our group has been conducting a series of studies on assessing physiological benefits of cupping therapy using various biomedical devices, such as laser Doppler flowmetry, elastographic ultrasound, and near-infrared spectroscopy [5,8,9,28,29]. The use of multi-channel NIRS can complete the understanding of physiological benefits of cupping therapy.
Therefore, we intended to demonstrate the feasibility of using the cross-correlation function analysis of multi-channel NIRS to assess the effect of cupping therapy on spatial hemodynamic responses. The primary aim of this study was to analyze the effects of cupping therapy on spatial hemodynamic response to cupping therapy assessed by using a multi-channel NIRS with the CCF algorithm. This study may help shed light on the effect of cupping therapy on the spatial hemodynamic regulations, including areas inside and outside the cupping cup. The secondary aim was to assess the correlation between oxyhemoglobin and deoxy-hemoglobin inside and outside the cupping cup using CCF. The findings from this study could help understand the effects of cupping therapy on the spatial hemodynamic response to cupping therapy for improving clinical effectiveness of cupping therapy.
2. Materials and methods
This study was approved by the University Institutional Review Board of the University of Illinois at Urbana-Champaign (IRB #22900). This is part of a bigger project investigating various combinations of pressure and duration of cupping therapy on muscle hemodynamic responses. The results reported in this study are not presented elsewhere.
2.1. Participants
Eighteen healthy individuals (12 women, 6 men) volunteered to participate in the present study and their characteristics were: age 25.0 ± 4.6 years, systolic blood pressure 106.2 ± 9.7 mmHg, diastolic blood pressure 62.7 ± 8.0 mmHg, arm length 32.9 ± 2.2 cm, and arm circumference 27.4 ± 3.6 cm. All research participants were right handed. The inclusion criteria were healthy individuals aged between 18 and 40 years. The exclusion criteria were as follows: non-blanchable response of the red skin areas over the biceps and triceps of the dominant side, open wounds, scar or tattoo over the tested area, diagnosed ischemic cardiovascular, hypertension (SBP > 140mmHg or DBP > 90mmHg), diabetes and smoking history. Subjects were briefed on the experimental procedures before signing the informed consent form.
2.2. Instrumentation
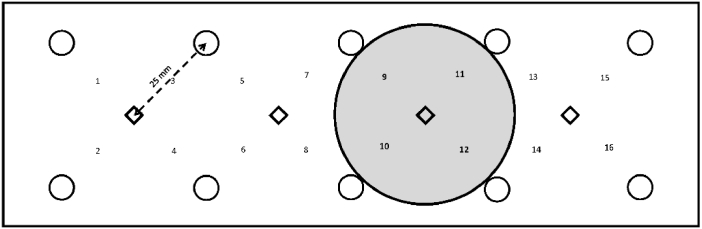
A functional NIRS sensor band (fNIR Imager 1000, fNIR Devices, LLC, Potomac, MD) was used to measure changes in hemodynamic responses of the biceps muscle, including oxyhemoglobin (Δ[HbO2], µM) and deoxy-hemoglobin (Δ[Hb], µM) (Fig. 1]. The fNIRS sensor band consisted of 10 photo detectors and 4 light emitters for a combination of 16 channels. The oxyhemoglobin shows an absorption peak at around 850-900 nm and deoxy-hemoglobin has an absorption peak at around 730-750 nm. The source-detector distance is 2.5 cm, which allows about 1.25 cm measurement depth. This penetration depth is sufficient for capturing major hemodynamic activity of the biceps muscle. The sampling rate was 2 Hz. In this study, the area measured by 4 channels (9, 10, 11 and 12] was used to measure hemodynamic responses inside the cupping cup rim and 4 channels [7,8,13 and 14] were used to measure hemodynamic responses outside the cupping cup rim. The raw fNIRS signals were low-pass filtered within a finite impulse response filter of cut-off frequency at 0.14 Hz to eliminate possible respiration and heart rate signals and unwanted high frequency noise. The NIRS signal of 5-min pre-cupping period was used to calculate the relative change of fNIRS signals with the concentration change of oxyhemoglobin and deoxy-hemoglobin.
Fig. 1.
Schematic drawing of the sensor pad of the near infrared spectroscopy and the relation of the channels inside the cupping cup (shaded area under channels 9, 10, 11, and 12) and outside the cupping cup (channels 7, 8, 13 and 14).
This study used an automatic suction device (P1000-PCS, California Medical Device Manufacturing Facility, CA) to create a negative pressure inside the cup by using an electrical suction pump. The device consists of a vacuum gauge, a vacuum regulator, a power switch, and a vacuum cup. This device can produce negative pressure ranging from 0 to −760 mmHg by automatic suction. The cup with the inner diameter of 45 mm and the outer diameter of 53 mm (round shape with a range of 53-55 mm) was used in this study. The selection of this cup size was based on our preliminary research results showing that the 45-mm cup was more effective in reducing the stiffness of the triceps muscle compared with the 35-mm cup [4,5]. Our previous research showed that both −300 mmHg and −225 mmHg of cupping therapy are safe and effective for increasing skin blood flow [9]. Therefore, the cupping pressure intensities chosen in this study were at −225 mmHg and −300 mmHg.
2.3. Experimental protocol
Four protocols of cupping therapy included: A: 5-min duration at-225mmHg pressure; B: 10-min duration at-225mmHg pressure; C: 5-min duration at −300mmHg pressure; and D: 10-min duration at −300 mmHg pressure (Table 1].
Table 1. Characteristics of 4 cupping protocols conducted at 4 different days.
| Cupping Protocols | Pre-Cupping | Cupping | Post-Cupping | |
|---|---|---|---|---|
| Pressure (mmHg) | Duration (min) | |||
| A | 5 min | −225 | 5 | 10 min |
| B | 5 min | −225 | 10 | 10 min |
| C | 5 min | −300 | 5 | 10 min |
| D | 5 min | −300 | 10 | 10 min |
All experiments were performed in the Rehabilitation Engineering Laboratory at the University of Illinois at Urbana-Champaign. The room temperature was maintained at 24-26 °C throughout the experiment. Participants were asked to remain supine for at least 30 minutes to stabilize the baseline muscle hemodynamic activities and acclimate to the room temperature. The participant fully extended the dominant-side elbow with the palm facing upward. The selection of the right dominant side was to reduce the potential influence of muscle metabolism between the dominant and non-dominant arms on the hemodynamic response to cupping therapy. The angle between the arm and the body was 45 degrees (Fig. 1]. The participant was instructed to relax. A mark was drawn on the biceps muscle (cubital fossa to one-third of acromion) to identify the location for cupping therapy. Before the NIRS sensor was used to monitor the 5-min baseline of hemodynamic status, three marks on the four corners of the NIRS sensor were drawn to mark the location of the NIRS band, as shown in Fig. 1. To secure the NIRS sensor and minimize the influence of environmental light, the elastic bandage was used to wrap the NIRS sensor to the arm. Then, the NIRS sensor was removed, and one cupping cup (the inner diameter as 45 mm) was applied on the biceps muscle for either 5-min or 10-min at either −225mmHg or −300 mmHg based on the predetermined random order of cupping protocols. Then, the same procedures were applied to test another three protocols after 2-4 days. All 4 cupping protocols were conducted at 4 different days to reduce potential influence of carry over effect from cupping therapy. The order of all 4 cupping protocols for each participant was different to minimize the influence of testing order effect on hemodynamic responses.
2.4. Cross-correlation function estimation for similarity
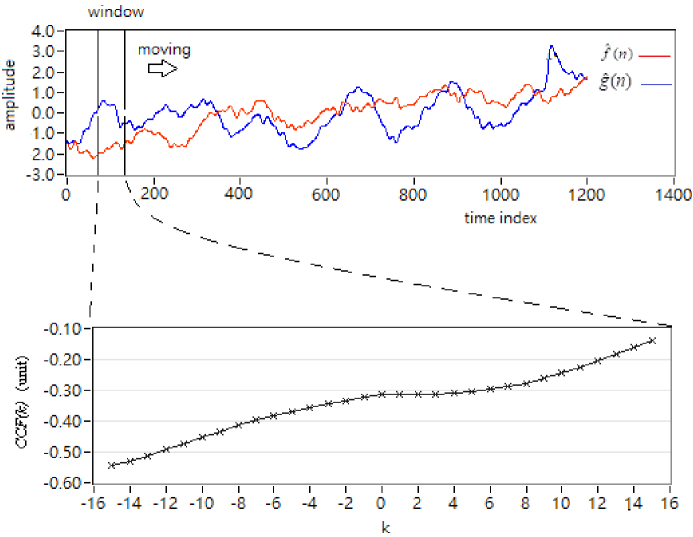
The cross-correlation function analysis is a method to assess the degree of similarity between two data sets [25]. The cross-correlation function could be used to determine the association between two signals. In this study, CCF was used to determine the spatial association of hemodynamic response to cupping therapy, specifically, 1) areas inside and outside the cup and 2) oxyhemoglobin and deoxy-hemoglobin. Taking the average hemodynamic signal as the input data for estimation, the relation and phase between the two channels of the signals can be determined by using CCF. The correlation between two signals is used to examine the cupping therapy effect under various cupping intensities on the microvascular system. Let the cross-correlation function be expressed as CCF(k), W is the window length, k is the number of peak-to-peak displacement points, and N is the total signal length. In this study, assume two signals by each channel are and , respectively (Fig. 2]. f^(n) and g^(n) are either a time-series signal from a single channel or the averaged time-series signal from the four channels (9, 10, 11, and 12) inside the cup and outside the cup for the channels (7, 8, 13, and 14). Then, the CCF value can be calculated as follows:
where is an estimate of the cross-covariance in the ith time window and defined as
Fig. 2.
Presentation of the CCF estimation. The upper plot indicates the input with two signals with a moving window. The bottom plot shows the CCF value estimated from the range of the window.
Also , and .
3. Results
Table 2 shows the maximum CCF and SD values inside and outside cupping rim with oxyhemoglobin. It can be found that outside the cupping rim (channel 7 and 14) time and pressure induced SD changed significantly (p < 0.05, A&C vs. B&D; C&D vs. A&D). Other CCF and SD values inside and outside the cupping rim remain stable for each channel with oxyhemoglobin.
Table 2. Maximum CCF and SD values of oxyhemoglobin inside and outside cup rim.
| Inside the cup rim | |||||||||
| Ch 9 | Ch 10 | Ch 11 | Ch 12 | ||||||
| Oxyhemoglobin | Cupping protocol | CCF | SD | CCF | SD | CCF | SD | CCF | SD |
| A&B | −0.04 | 0.56 | 0.14 | 0.50 | 0.07 | 0.54 | 0.20 | 0.53 | |
| A&C | 0.48 | 0.58 | 0.20 | 0.53 | 0.18 | 0.57 | 0.16 | 0.53 | |
| C&D | 0.05 | 0.56 | 0.08 | 0.55 | 0.17 | 0.59 | 0.32 | 0.48 | |
| B&D | 0.17 | 0.58 | 0.15 | 0.52 | 0.12 | 0.54 | 0.15 | 0.55 | |
| A&D | 0.05 | 0.55 | 0.21 | 0.54 | 0.23 | 0.57 | 0.29 | 0.51 | |
| Outside the cup rim | |||||||||
| Ch 7 | Ch 8 | Ch 13 | Ch 14 | ||||||
| Oxyhemoglobin | Cupping protocol | CCF | SD | CCF | SD | CCF | SD | CCF | SD |
| A&B | 0.12 | 0.56 | 0.22 | 0.52 | 0.20 | 0.56 | 0.20 | 0.53 | |
| A&C | 0.10 | 0.61 a | 0.04 | 0.56 | 0.12 | 0.60 | 0.14 | 0.56 | |
| C&D | 0.13 | 0.56 | 0.15 | 0.55 | 0.13 | 0.62 | 0.27 | 0.49 b | |
| B&D | 0.22 | 0.55 | 0.21 | 0.52 | 0.18 | 0.56 | 0.33 | 0.55 | |
| A&D | 0.00 | 0.55 | 0.12 | 0.58 | 0.17 | 0.60 | 0.24 | 0.60 | |
p < 0.05: A&C vs. B&D;
p < 0.05: C&D vs. A&D
Table 3 shows the maximum CCF and SD values inside and outside cupping rim with deoxy-hemoglobin. It can be found that inside the cupping rim (channel 9, 10 and 11) would be influenced by the protocols with time and pressure factors induced CCF and SD values changed significantly (p < 0.05). However, outside the cupping rim showed fewer change of CCF and SD values significantly. Therefore, cupping protocols would affect deoxy-hemoglobin more inside the cupping rim.
Table 3. Maximum CCF and SD values of deoxy-hemoglobin inside and outside cup rim.
| Inside the cup rim | |||||||||
| Ch 9 | Ch 10 | Ch 11 | Ch 12 | ||||||
| Deoxy-hemoglobin | Cupping protocol | CCF | SD | CCF | SD | CCF | SD | CCF | SD |
| A&B | 0.60 a a | 0.46 | 0.67 | 0.42 | 0.45 | 0.46 d e | 0.60 | 0.50 | |
| A&C | 0.51 | 0.52 | 0.52 b c | 0.55 b c | 0.37 | 0.57 | 0.51 | 0.56 | |
| C&D | 0.53 | 0.52 | 0.64 | 0.46 | 0.38 | 0.60 f g | 0.58 | 0.50 | |
| B&D | 0.59 | 0.44 | 0.72 | 0.42 | 0.45 | 0.48 | 0.62 | 0.47 | |
| A&D | 0.58 | 0.48 | 0.71 | 0.40 | 0.44 | 0.52 | 0.61 | 0.46 | |
| Outside the cup rim | |||||||||
| Ch 7 | Ch 8 | Ch 13 | Ch 14 | ||||||
| Deoxy-hemoglobin | Cupping protocol | CCF | SD | CCF | SD | CCF | SD | CCF | SD |
| A&B | 0.50 | 0.57 | 0.59 | 0.51 | 0.42 | 0.48 j k | 0.59 | 0.49 | |
| A&C | 0.44 | 0.58 | 0.45 h | 0.61 i | 0.29 | 0.60 | 0.41 | 0.57 | |
| C&D | 0.43 | 0.57 | 0.55 | 0.52 | 0.28 | 0.59 | 0.42 | 0.54 | |
| B&D | 0.46 | 0.54 | 0.63 | 0.51 | 0.23 | 0.55 | 0.54 | 0.55 | |
| A&D | 0.46 | 0.60 | 0.63 | 0.46 | 0.36 | 0.54 | 0.59 | 0.51 | |
p < 0.05: A&B vs A&C;
p < 0.05: A&C vs B&D;
p < 0.05: A&C vs A&D;
p < 0.05 A&B vs. A&C;
p < 0.05: A &B vs. A&D;
p < 0.05: C&D vs. B&D;
p < 0.05: C&D vs. A&D;
p < 0.05: A&C vs. B D;
p < 0.05: A&C vs. A&D;
p < 0.05: A&B vs. A&C;
p < 0.05: A&B vs. C&D.
According to the results shown in Tables 2 and 3, it can be found that cupping protocol effect of different pressures and durations could induce disturbance on oxyhemoglobin outside the cup rim. On the other hand, it causes a more obvious effect on deoxy-hemoglobin by the change of CCF and SD inside the cup rim rather than those of outside cup rim.
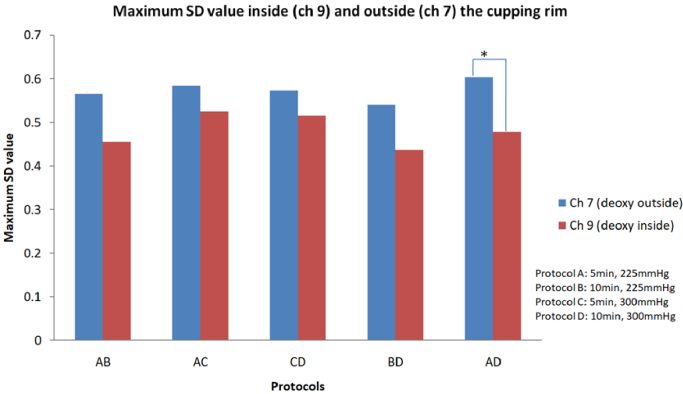
Figure 3 shows that maximum SD values in channel 7 and 9 with deoxy-hemoglobin (A&D) changed significantly (p < 0.05).
Fig. 3.
The plot shows maximum SD values in channels7 and 9 with deoxy-hemoglobin (*p < 0.05).
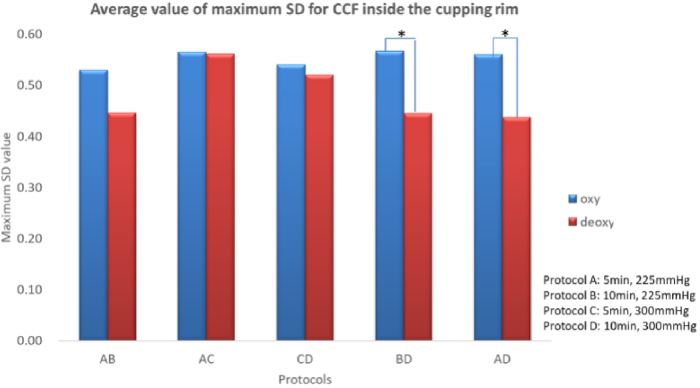
Figure 4 shows the average maximum SD of CCF values of oxyhemoglobin and deoxy-hemoglobin inside the cupping rim. In deoxy-hemoglobin, longer cupping durations (10 min) and higher magnitude of negative pressure (−300 mmHg) would result in a lower maximum SD of CCF values (Protocols B&D and A&D, p < 0.05).
Fig. 4.
The average maximum SD of CCF values of oxyhemoglobin and deoxy-hemoglobin inside the cupping rim (*p < 0.05, cupping therapy would lead to a significant maximum SD difference of CCF value between oxyhemoglobin and deoxy-hemoglobin blood in protocol B&D and A&D.)
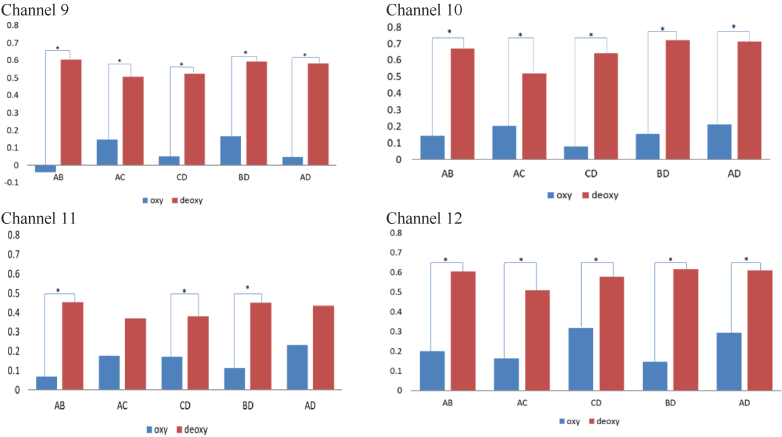
Figure 5 shows the maximum CCF values for different cupping durations and pressures for each channel inside the cupping rim. The results showed that maximum CCF values of deoxy-hemoglobin were higher than those in oxyhemoglobin significantly (p < 0.05) in all comparisons. In addition, the results indicate that a longer cupping duration and higher magnitude of negative pressure would increase correlation in deoxy-hemoglobin.
Fig. 5.
Comparisons of maximum CCF values between oxyhemoglobin and deoxy-hemoglobin under different cupping protocols (Protocol A: 5 min, 225 mmHg; Protocol B: 10 min, 225 mmHg; Protocol C: 5 min, 300 mmHg; Protocol D: 10 min, 300 mmHg) for each channel inside the cupping rim (*p < 0.05).
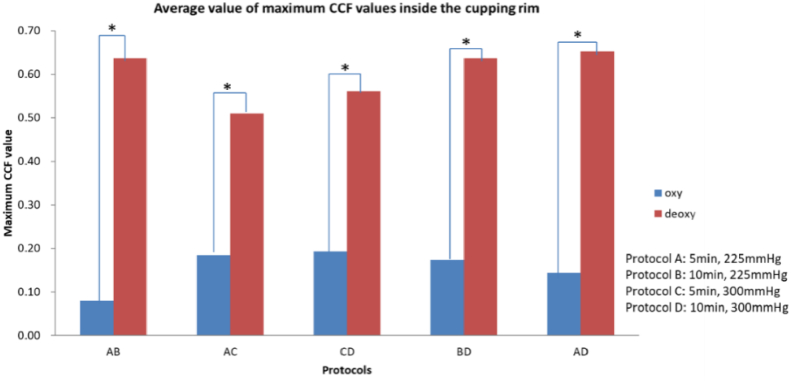
The results of the average maximum CCF values for different cupping durations and negative pressures are shown in Fig. 6. The results showed that the maximum CCF values in oxyhemoglobin were significantly higher than those in deoxy-hemoglobin (p < 0.05). Furthermore, it was found that there was a correlation between deoxy-hemoglobin and a longer duration and a higher magnitude of negative pressure of cupping.
Fig. 6.
The maximum CCF values of average oxyhemoglobin and deoxy-hemoglobin in different cupping durations and pressures (*p < 0.05).
4. Discussion
Our results demonstrate that CCF can be used to quantify the correlation of time-series changes of NIRS signals assessed after cupping therapy. To the best of our knowledge, this is the first study investigating the dynamic correlation of spatial hemodynamic responses after cupping therapy. The use of CCF can complement the current approach on reporting the average of NIRS signals after cupping therapy. The correlation between time-series changes between spatial hemodynamic responses as well as time-series changes between oxyhemoglobin and deoxy-hemoglobin can shed light on the complex mechanism of cupping therapy on reducing musculoskeletal impairment.
Deoxy-hemoglobin inside the cup showed lower variations in maximum SD of CCF under different cupping intensities inside the cup, especially for a longer cupping duration and a higher magnitude of negative pressure. Interestingly, the maximum SD of CCF values of different cupping intensities shown in Fig. 4 are significantly higher (p < 0.05) with a longer cupping duration and a higher magnitude of negative pressure in oxyhemoglobin. According to this finding, cupping therapy could produce a more significant change in oxyhemoglobin inside the cupping rim.
Based on the maximum CCF value between oxyhemoglobin and deoxy-hemoglobin, Fig. 5 shows an analysis of CCF values for oxyhemoglobin and deoxy-hemoglobin. In Fig. 6, CCF values for oxyhemoglobin and deoxy-hemoglobin between the areas inside and outside the cup are shown within the CCF analysis. There might be a correlation between two time-series represented by the maximum CCF value. Our study observed the maximum CCF values for oxyhemoglobin and deoxy-hemoglobin during two cupping intensities. In Fig. 5 (each channel inside the cup rim) and Fig. 6 (averaged by 4 channels inside the rim), the maximum CCF value were significantly different (p < 0.05) between two cupping intensities. This may indicate that oxyhemoglobin would be changed more with a longer cupping period and a higher magnitude of negative pressures because all of the maximum CCF values of oxyhemoglobin were significantly lower than those of deoxy-hemoglobin (p < 0.05). Based on our results, a longer duration of cupping therapy and a higher magnitude of negative pressure should be applied to induce an increase in oxyhemoglobin into muscle microcirculation. A previous study reported that the phase of NIRS signals could be used to explore the relationship between oxyhemoglobin and deoxy-hemoglobin [30]. Duo to the CCF analysis could explore the relation between two time-series dynamically, CCF analysis was adopted to investigate multi-channel NIRS time-series to observe the interaction between oxyhemoglobin and deoxy-hemoglobin in this study. Moreover, in Fig. 6, the difference between oxyhemoglobin and deoxy-hemoglobin is observed under protocols A (−225 mmHg for 5 min) and B (−225 mmHg for 10 min), which might indicate that the duration factor is relatively more dominant under −225 mmHg.
A multi-channel NIRS device was used in this study to investigate spatial hemodynamic responses to cupping therapy. The use of NIRS can help to understand the benefits of cupping therapy on managing musculoskeletal impairment. Previous studies used either one channel or reporting the average of multiple channels of NIRS to assess cupping therapy effects, which may limit the understanding of the spatial hemodynamic responses. To the best of our knowledge, this is the first study to assess the relationship from different channels of NIRS for assessing cupping effects on the hemodynamic responses.
The use of CCF allows us to quantify the correlation of time-series changes of oxyhemoglobin and deoxy-hemoglobin after cupping therapy. This approach provides a new window to examine the hemodynamics rather than the reported average of time-series NIRS signals. In the previous study [8], the average value of oxyhemoglobin and deoxy-hemoglobin inside the cupping rim was reported and demonstrated that the factors of pressure and duration of cupping therapy may affect hemodynamic responses. In this study, the use of CCF to analyze oxyhemoglobin and deoxy-hemoglobin inside and both the cupping cup further reveals that deoxy-hemoglobin was correlated with a longer duration and a larger magnitude of negative pressure. Both this study and Li et al. (2023) demonstrate that oxyhemoglobin is more sensitive to the cupping intensity [8]. The use of NIRS to assess muscle hemodynamic response to mechanical stimulus is reported in the literature [31]. Using moving cupping therapy, Liu et al. demonstrated that cupping therapy could alleviate some chemotherapy-related side effects in patients with colorectal cancer for improving their quality of life [32]. Arce-Esquivel et al. examined the effect of cupping negative pressure on blood flow [33]. Based on our results, the results from these studies may benefit from using CCF to explore the spatial hemodynamic response, especially under moving cupping therapy.
More than 600 medical studies have been conducted on cupping negative pressure therapy. According to the research, the main physiological effects include promoting local blood circulation, improving nervous system activity, increasing joint mobility, reducing fatigue, and managing pain. However, the use of near-infrared spectroscopy to explore negative pressure therapy research is still limited, especially the analysis of the response from different channels of NIRS for assessing the spatial hemodynamic response. The application advantages of near-infrared spectroscopy have considerable advantages in the field of biomedicine, primarily because of the advantage of detecting a variety of samples and the convenient use of detection equipment [34]. It can indeed be used as a non-invasive tool for observing human muscle tissue [35], and it is being used in health care, sports science, and medicine to advance research [24,36]. For example, observing muscle microcirculation (oxygenated hemoglobin, deoxygenated hemoglobin, blood volume) [37–39], the literature points out that when the nerve stops activation (deactivation), deoxy-hemoglobin will begin to rise, while oxyhemoglobin will decrease [40]. However, the interaction from different areas are largely unknown that prevents researchers to take the advantage of using multi-channel NIRS to assess physiological benefits of cupping therapy [41,42]. Our study using the CCF analysis provides a foundation for exploring hidden interactions from the areas inside and outside the cup on the hemodynamic regulations after cupping therapy.
According to Stephens, oxygenated hemoglobin and total hemoglobin levels increase after 8 minutes of cupping therapy, thereby relieving patients with non-specific neck pain [43]. Liu et al. (2022) also found that the −0.03 MPa group experienced a maximum increase in body surface temperature of 0.92 °C while the −0.04 MPa group experienced a maximum increase of 1.42 °C. Wang et al. (2020) indicated that a higher magnitude of negative pressure (−300 mmHg) and shorter the action time (5 min) can result in higher skin blood flow. This study confirms that the negative pressure between −225 and −300 mmHg are effective intensity on inducing muscle hemodynamic responses [44].
There are limitations of this study. First, we have more female participants than male participants. It is unclear whether this gender effect would significantly affect our findings. Future research may need to assess the gender effect on the efficacy of cupping therapy. Second, the NIRS sensor pad was applied to measure pre-cupping hemodynamic response as the baseline. When cupping therapy was applied, the NIRS sensor pad was removed. After cupping therapy, the sensor pad was replaced back to the arm. This may affect the calibration result. Future research may need to develop embedded sensor pad with cupping cup; thus, the NIRS sensor could be applied before and after cupping therapy.
5. Conclusion
This study demonstrates that the cross-correlation function (CCF) can be used to analyze multi-channel NIRS signals for assessing the correlation of spatial hemodynamic responses (inside and outside of the cup) as well as the correlation between oxyhemoglobin and deoxy-hemoglobin after cupping therapy. To the best of our knowledge, this is the first study demonstrating the correlation between oxyhemoglobin and deoxy-hemoglobin in response to cupping therapy at the sites inside and outside the cupping cup using CCF. The analysis of time-series NIRS signals can complement current approaches on reporting the average of NIRS signals that ignores the time-series information as well as the interaction between time-series signals. Our findings indicate that based on the comparison of CCF values between oxyhemoglobin and deoxy-hemoglobin, oxyhemoglobin is more sensitive to changes in a longer cupping duration and a higher magnitude of negative pressures.
Funding
National Science and Technology Council10.13039/100020595 (MOST 110-2637-E-241-002; MOST 111-2221-E-468-002).
Disclosures
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Data availability
Data underlying the results presented in this paper are not publicly available at this time but maybe obtained from the authors upon reasonable request.
References
- 1.Hou X., Liu J., Weng K., Griffin L., Rice L. A., Jan Y. K., “Effects of various physical interventions on reducing neuromuscular fatigue assessed by electromyography: a systematic review and meta-analysis,” Front. Bioeng. Biotechnol. 9, 659138 (2021). 10.3389/fbioe.2021.659138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnett A., “Using Recovery Modalities between Training Sessions in Elite Athletes,” Sports Med. 36(9), 781–796 (2006). 10.2165/00007256-200636090-00005 [DOI] [PubMed] [Google Scholar]
- 3.Mohamed A. A., Zhang X., Jan Y. K., “Evidence-based and adverse-effects analyses of cupping therapy in musculoskeletal and sports rehabilitation: A systematic and evidence-based review,” J. Back Musculoskelet Rehabil 36(1), 3–19 (2023). 10.3233/BMR-210242 [DOI] [PubMed] [Google Scholar]
- 4.Hou X., Wang X., Griffin L., Liao F., Peters J., Jan Y. K., “Immediate and delayed effects of cupping therapy on reducing neuromuscular fatigue,” Front. Bioeng. Biotechnol. 9, 678153 (2021). 10.3389/fbioe.2021.678153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jan Y. K., Hou X., He X., Guo C., Jain S., Bleakney A., “Using elastographic ultrasound to assess the effect of cupping size of cupping therapy on stiffness of triceps muscle,” Am. J. Phys. Med. Rehabil. 100(7), 694–699 (2021). 10.1097/PHM.0000000000001625 [DOI] [PubMed] [Google Scholar]
- 6.Al-Bedah A. M., Elsubai I. S., Qureshi N. A., Aboushanab T. S., Ali G. I., El-Olemy A. T., Khalil A. A., Khalil M. K., Alqaed M. S., “The medical perspective of cupping therapy: Effects and mechanisms of action,” J. Tradit. Med. Complement. 9(2), 90–97 (2019). 10.1016/j.jtcme.2018.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen W. C., Jan Y. K., Liau B. Y., Lin Q., Wang S., Tai C. C., Lung C. W., “Effectiveness of self-management of dry and wet cupping therapy for low back pain: A systematic review and meta-analysis,” Medicine 101(51), e32325 (2022). 10.1097/MD.0000000000032325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y., Mo P. C., Lin C. F., Pauly S., Kundal N., Hernandez M. E., Jan Y. K., “Using near-infrared spectroscopy to investigate the effects of pressures and durations of cupping therapy on muscle blood volume and oxygenation,” J. Biophotonics. 16(7), e202200342 (2023). 10.1002/jbio.202200342 [DOI] [PubMed] [Google Scholar]
- 9.Wang X., Zhang X., Elliott J., Liao F., Tao J., Jan Y. K., “Effect of Pressures and Durations of Cupping Therapy on Skin Blood Flow Responses,” Front. Bioeng. Biotechnol. 8, 608509 (2020). 10.3389/fbioe.2020.608509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehtaa P., Dhapteb V., “Cupping therapy: A prudent remedy for a plethora of medical ailments,” J. Tradit. Complement. Med. 5(3), 127–134 (2015). 10.1016/j.jtcme.2014.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lauche R., Dhapteb V., Cramer H., Haller H., Stange R., Dobos G., Rampp T., “Effectiveness of Home-Based Cupping Massage Compared to Progressive Muscle Relaxation in Patients with Chronic Neck Pain—A Randomized Controlled Tria,” PLoS ONE 8(6), e65378 (2013). 10.1371/journal.pone.0065378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanan S., Eman S., “Cupping therapy (al-hijama): it’s impact on persistent non-specific lower back pain and client disability,” Life Science Journal 10, 4 (2013). [Google Scholar]
- 13.Lewis M. J., Kingsley M., Short A. L., Simpson K., “Rate of reduction of heart rate variability during exercise as an index of physical work capacity,” Scand. J. Med. Sci. 17(6), 696–702 (2007). 10.1111/j.1600-0838.2006.00616.x [DOI] [PubMed] [Google Scholar]
- 14.Micklewright D. P., Beneke R., Gladwell V., Sellens M. H., “Blood lactate removal using combined massage and active recovery,” Medicine & Science in Sports & Exercise 35, 4 (2006). [Google Scholar]
- 15.Li T., Li Y., Lin Y., Li K., “Significant and sustaining elevation of blood oxygen induced by Chinese cupping therapy as assessed by near-infrared spectroscopy,” Biomed. Opt. Express 8(1), 223–229 (2017). 10.1364/BOE.8.000223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao C., Wang M., He L., He Y., Li T., “Alternations of hemodynamic parameters during Chinese cupping therapy assessed by an embedded near-infrared spectroscopy monitor,” Biomed. Opt. Express 10(1), 196–203 (2019). 10.1364/BOE.10.000196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scano A., Pirovano I., Manunza M. E., Spinelli L., Contini D., Torricelli A., Re R., “Sustained fatigue assessment during isometric exercises with time-domain near infrared spectroscopy and surface electromyography signals,” Biomed. Opt. Express 11(12), 7357–7375 (2020). 10.1364/BOE.403976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cage S., Warner B., Gallegos D. M., “Effect of Cupping Therapy on Skin Surface Temperature in Healthy Individuals’,” Journal of Sports Medicine and Allied Health Sciences 5, 3 (2020). 10.25035/jsmahs.05.03.02 [DOI] [Google Scholar]
- 19.Boushel R., Piantadosi C. A., “Near-infrared spectroscopy for monitoring muscle oxygenation,” Acta Phys. Scand. 168(4), 615–622 (2000). 10.1046/j.1365-201x.2000.00713.x [DOI] [PubMed] [Google Scholar]
- 20.Boushel R., Langberg H., Olesen J., Gonzales-Alonzo J., Bulow J., Kjaer M., “Monitoring tissue oxygen availability with near infrared spectroscopy (NIRS) in health and disease,” Scand J. Med. Sci. Sports. 11(4), 213–222 (2001). 10.1034/j.1600-0838.2001.110404.x [DOI] [PubMed] [Google Scholar]
- 21.Jan Y. K., Crane B. A., Liao F. Y., Woods J. A., Ennis W. J., “Comparison of Muscle and Skin Perfusion Over the Ischial Tuberosities in Response to Wheelchair Tilt-in-Space and Recline Angles in People With Spinal Cord Injury,” Arch. Phys. Med. Rehabil. 94(10), 1990–1996 (2013). 10.1016/j.apmr.2013.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jöbsis F. F., “Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters,” Science 198(4323), 1264–1267 (1977). 10.1126/science.929199 [DOI] [PubMed] [Google Scholar]
- 23.Chen C.-F. L. C.-W., Huang T.-L., “Development and application of near infrared spectrometer,” Instruments Today 29, 2 (2007). [Google Scholar]
- 24.Hamaoka T., McCully K. K., Niwayama M., Chance B., “The use of muscle near-infrared spectroscopy in sport, health and medical sciences: recent developments,” Phil. Trans. R. Soc. A. 369(1955), 4591–4604 (2011). 10.1098/rsta.2011.0298 [DOI] [PubMed] [Google Scholar]
- 25.Derrick T., Thomas J., “Time series analysis: the crosscorrelation function,” in Innovative Analyses of Human Movement (Human Kinetics Publishers, 2004). [Google Scholar]
- 26.Westerhof B. E., Gisolf J., Stok W. J., Wesseling K. H., Karemaker J. M., “Time-domain cross-correlation baroreflex sensitivity: performance on the EUROBAVAR data set,” J. Hypertens. 22(7), 1371–1380 (2004). 10.1097/01.hjh.0000125439.28861.ed [DOI] [PubMed] [Google Scholar]
- 27.Silvani A., Magosso E., Bastianini S., Lenzi P., Ursino M., “Mathematical modeling of cardiovascular coupling: central autonomic commands and baroreflex control,” Auton Neurosci Basic Clin. 162(1-2), 66–71 (2011). 10.1016/j.autneu.2011.04.003 [DOI] [PubMed] [Google Scholar]
- 28.Hou X., He X., Zhang X., Liao F., Hung Y. J., Jan Y. K., “Using laser Doppler flowmetry with wavelet analysis to study skin blood flow regulations after cupping therapy,” Skin Res. Technol. 27(3), 393–399 (2021). 10.1111/srt.12970 [DOI] [PubMed] [Google Scholar]
- 29.He X., Zhang X., Liao F., He L., Xu X., Jan Y. K., “Using reactive hyperemia to investigate the effect of cupping sizes of cupping therapy on skin blood flow responses,” J. Back Musculoskelet Rehabil. 34(2), 327–333 (2021). 10.3233/BMR-200120 [DOI] [PubMed] [Google Scholar]
- 30.Hakim U., Pinti P., Noah A. J., Zhang X., Burgess P., Hamilton A., Hirsch J., Tachtsidis I., “Investigation of functional near-infrared spectroscopy signal quality and development of the hemodynamic phase correlation signal,” Neurophotonics. 9(2), 025001 (2022). 10.1117/1.NPh.9.2.025001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu Tiantian S. J., Ping Y., Qinlao Y., Jun W., “Influence of active and passive stretching on blood oxygen in ligament and muscle assessed using near-infrared spectroscopy,” Chinese Journal of Tissue Engineering Research 23, 20 (2019). [Google Scholar]
- 32.Liu Y. W., Su Y. L., Chang C. L., Tsai M. Y., “Cupping Therapy as an Adjunctive Therapy for Side Effects of Colorectal Cancer Treatment: A Prospective Observational Study,” J. Chiropr. Med. 21(4), 280–287 (2022). 10.1016/j.jcm.2022.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arce-Esquivel A. A., Warner B. J., Gallegos D. M., Cage S. A., “Effect of Dry Cupping on Vascular Function among Young Individuals,” Int. J. Health Sci. 5(3), 10–15 (2017). 10.15640/ijhs.v5n3a2 [DOI] [Google Scholar]
- 34.Bec K. B., Grabska J., Huck C. W., “Near-Infrared Spectroscopy in Bio-Applications,” Molecules. 25(12), 2948 (2020). 10.3390/molecules25122948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Curra A., Gasbarrone R., Cardillo A., Trompetto C., Fattapposta F., Pierelli F., Missori P., Bonifazi G., Serranti S., “Near-infrared spectroscopy as a tool for in vivo analysis of human muscles,” Sci. Rep. 9(1), 8623 (2019). 10.1038/s41598-019-44896-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu T., Pelowski M., Pang C., Zhou Y., Cai J., “Near-infrared spectroscopy as a tool for driving research,” Ergonomics. 59(3), 368–379 (2016). 10.1080/00140139.2015.1076057 [DOI] [PubMed] [Google Scholar]
- 37.De Backer D., Donadello K., Cortes D. O., “Monitoring the microcirculation,” J. Clin. Monit. Comput. 26(5), 361–366 (2012). 10.1007/s10877-012-9383-8 [DOI] [PubMed] [Google Scholar]
- 38.Tripodaki E. S., Tasoulis A., Koliopoulou A., Vasileiadis I., Vastardis L., Giannis G., Argiriou M., Charitos C., Nanas S., “Microcirculation and macrocirculation in cardiac surgical patients,” Crit. Care Res. Pract. 2012, 1–9 (2012). 10.1155/2012/654381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buono M. J., Miller P. W., Hom C., Pozos R. S., Kolkhorst F. W., “Skin blood flow affects in vivo near-infrared spectroscopy measurements in human skeletal muscle,” Jpn. J. Physiol. 55(4), 241–244 (2005). 10.2170/jjphysiol.T649 [DOI] [PubMed] [Google Scholar]
- 40.Ferrari M., Muthalib M., Quaresima V., “The use of near-infrared spectroscopy in understanding skeletal muscle physiology: recent developments,” Phil. Trans. R. Soc. A. 369(1955), 4577–4590 (2011). 10.1098/rsta.2011.0230. [DOI] [PubMed] [Google Scholar]
- 41.McCully K. K., “Near infrared spectroscopy: what can it tell us,” Exercise and Sport Sciences Reviews 28, 3 (2000). [PubMed] [Google Scholar]
- 42.Barstow T. J., “Understanding near infrared spectroscopy and its application to skeletal muscle research,” J. Appl. Phys. (1985) 126(5), 1360–1376 (2019). 10.1152/japplphysiol.00166.2018 [DOI] [PubMed] [Google Scholar]
- 43.Stephens S. L., Selkow N. M., Hoffman N. L., “Dry Cupping Therapy for Improving Nonspecific Neck Pain and Subcutaneous Hemodynamics,” J Athl Train. 55(7), 682–690 (2020). 10.4085/1062-6050-236-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu X., Wang Y., Wu Z., “Infrared thermal imaging-based skin temperature response during cupping at two different negative pressures,” Sci. Rep. 12(1), 15506 (2022). 10.1038/s41598-022-19781-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data underlying the results presented in this paper are not publicly available at this time but maybe obtained from the authors upon reasonable request.