Abstract
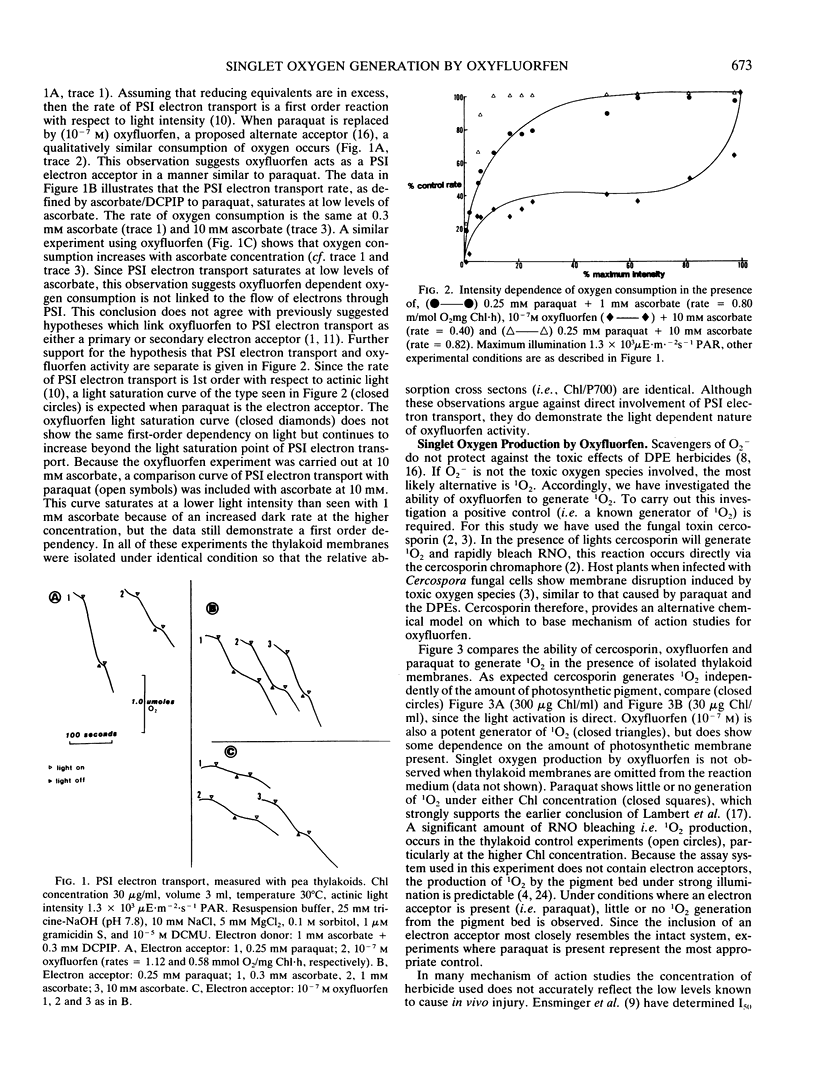
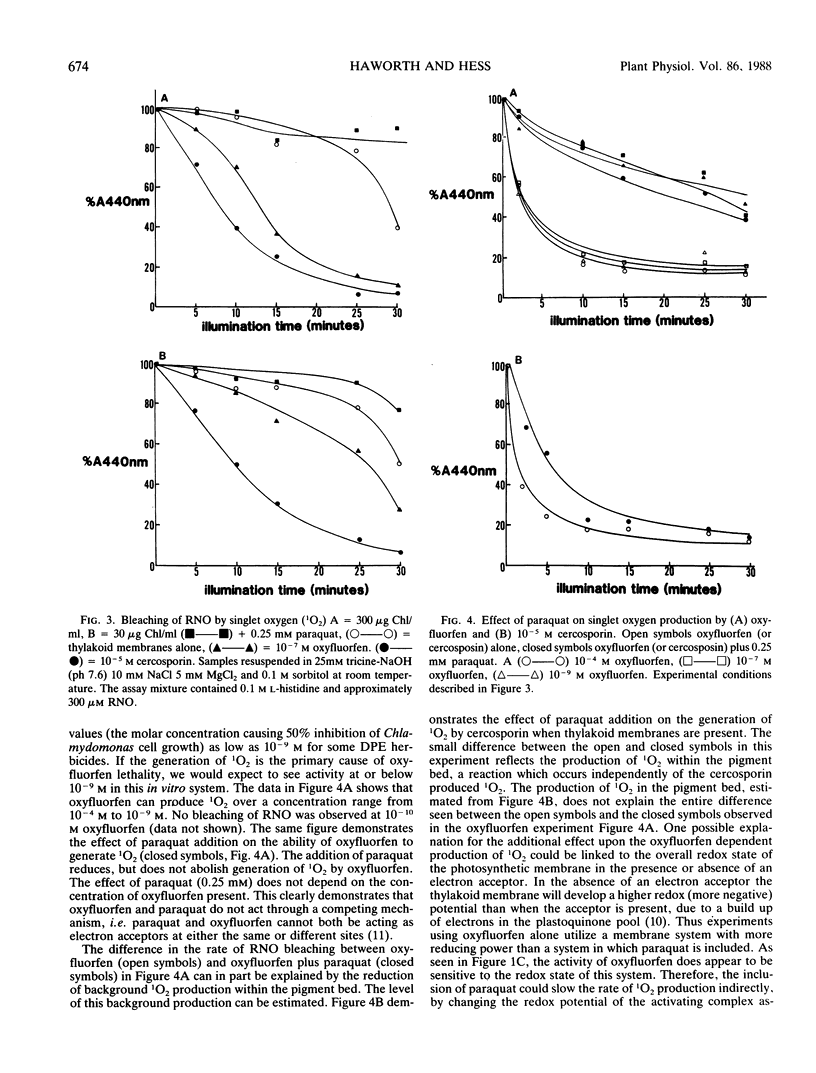
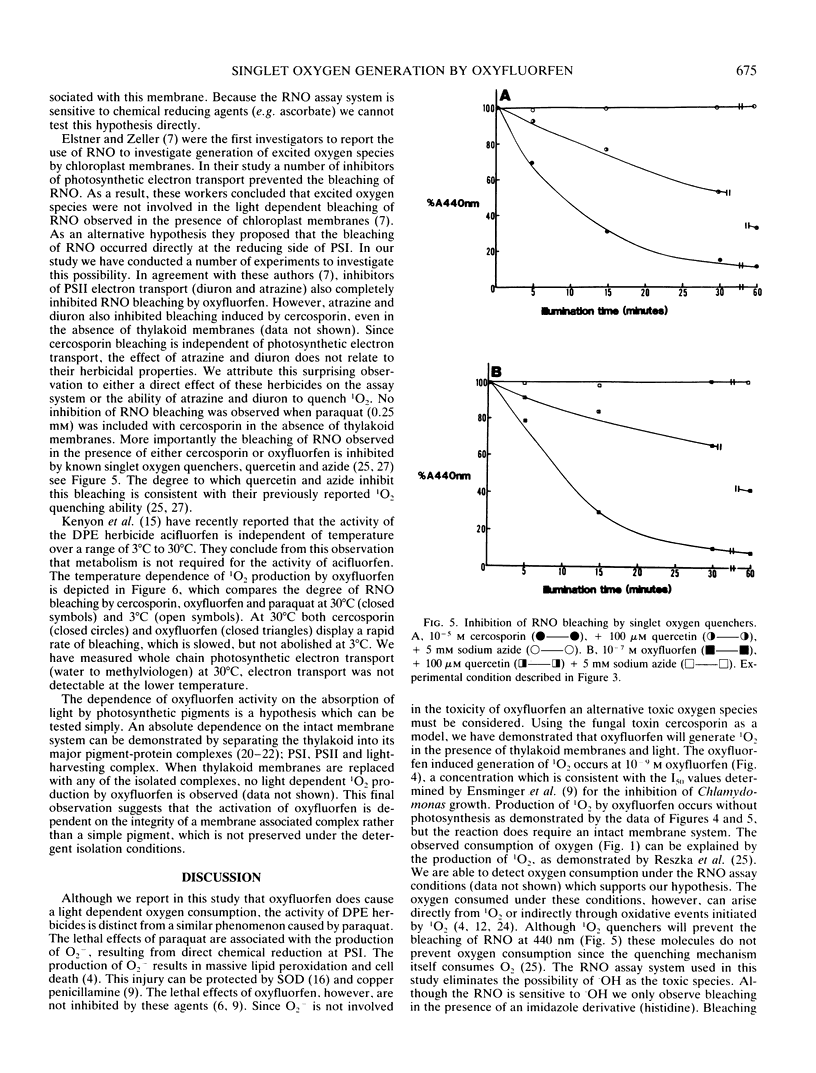
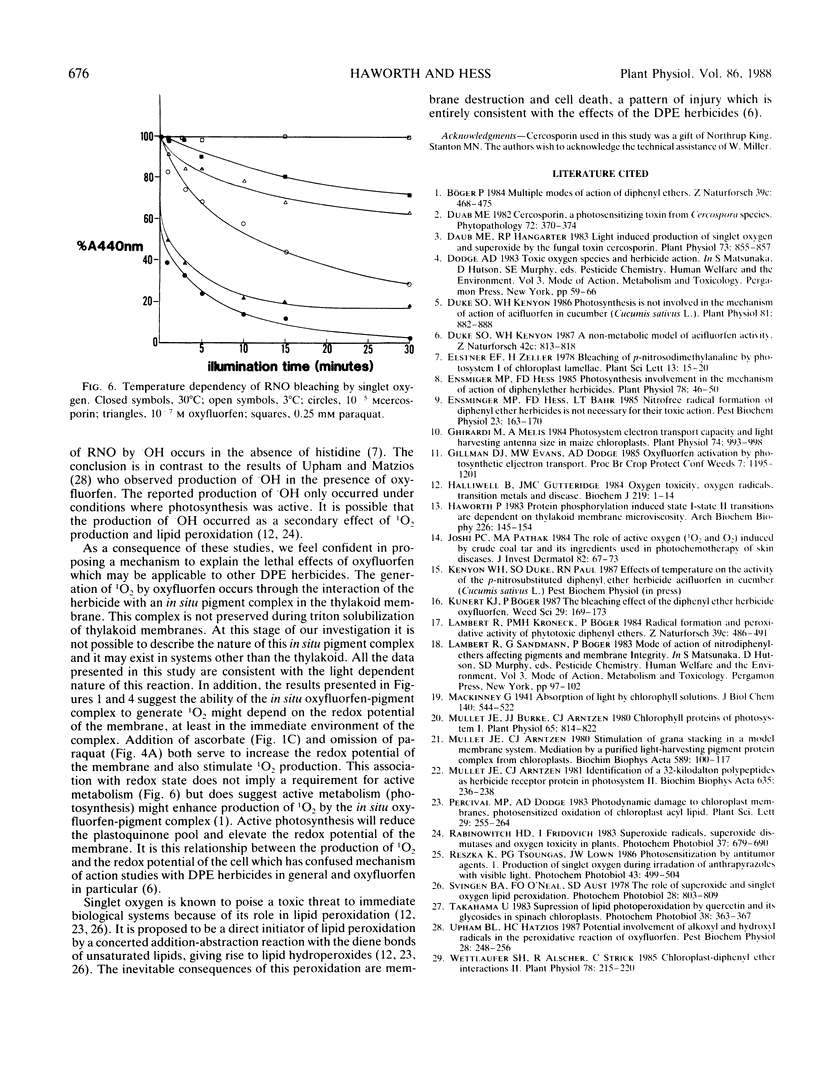
The mechanism of action of the p-nitrodiphenyl ether herbicides has remained ambiguous because of conflicting reports in the literature. The diphenyl ether herbicide oxyfluorfen causes a light induced consumption of oxygen which resembles the electron acceptor reaction of paraquat. However, this reaction is not linked to the transport of electrons through photosystem I. This conclusion is based on the observation that the rate of oxygen consumption, in the presence of oxyfluorfen, does not demonstrate a first order rate dependence on light intensity. Using the bleaching of N,N-dimethyl p-nitrosoaniline as a specific detector of singlet oxygen, we demonstrate that oxyfluorfen is a potent generator of this toxic radical. The production of singlet oxygen occurs in the presence of inhibitors of photosynthetic electron transport (oxyfluorfen at 10−4 molar and paraquat) and also under temperature conditions (3°C) which prevent electron transport. This light induced reaction results in oxygen consumption and is the primary cause of lethality for oxyfluorfen. The production of singlet oxygen occurs rapidly and at low herbicide concentrations (10−9 molar). The reaction occurs without photosynthetic electron transport but does require an intact thylakoid membrane.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Daub M. E., Hangarter R. P. Light-induced production of singlet oxygen and superoxide by the fungal toxin, cercosporin. Plant Physiol. 1983 Nov;73(3):855–857. doi: 10.1104/pp.73.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke S. O., Kenyon W. H. Photosynthesis Is Not Involved in the Mechanism of Action of Acifluorfen in Cucumber (Cucumis sativus L.). Plant Physiol. 1986 Jul;81(3):882–888. doi: 10.1104/pp.81.3.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensminger M. P., Hess F. D. Photosynthesis involvement in the mechanism of action of diphenyl ether herbicides. Plant Physiol. 1985 May;78(1):46–50. doi: 10.1104/pp.78.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghirardi M. L., Melis A. Photosystem electron-transport capacity and light-harvesting antenna size in maize chloroplasts. Plant Physiol. 1984 Apr;74(4):993–998. doi: 10.1104/pp.74.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984 Apr 1;219(1):1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi P. C., Pathak M. A. The role of active oxygen (1O2 and O(2)) induced by crude coal tar and its ingredients used in photochemotherapy of skin diseases. J Invest Dermatol. 1984 Jan;82(1):67–73. doi: 10.1111/1523-1747.ep12259146. [DOI] [PubMed] [Google Scholar]
- Mullet J. E., Arntzen C. J. Identification of a 32-34-kilodalton polypeptide as a herbicide receptor protein in photosystem II. Biochim Biophys Acta. 1981 Apr 13;635(2):236–248. doi: 10.1016/0005-2728(81)90023-2. [DOI] [PubMed] [Google Scholar]
- Mullet J. E., Burke J. J., Arntzen C. J. Chlorophyll proteins of photosystem I. Plant Physiol. 1980 May;65(5):814–822. doi: 10.1104/pp.65.5.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reszka K., Tsoungas P. G., Lown J. W. Photosensitization by antitumor agents--1. Production of singlet oxygen during irradiation of anthrapyrazoles with visible light. Photochem Photobiol. 1986 May;43(5):499–504. doi: 10.1111/j.1751-1097.1986.tb09526.x. [DOI] [PubMed] [Google Scholar]
- Svingen B. A., O'Neal F. O., Aust S. D. The role of superoxide and singlet oxygen in lipid peroxidation. Photochem Photobiol. 1978 Oct-Nov;28(4-5):803–809. doi: 10.1111/j.1751-1097.1978.tb07022.x. [DOI] [PubMed] [Google Scholar]
- Wettlaufer S. H., Alscher R., Strick C. Chloroplast-Diphenyl Ether Interactions II. Plant Physiol. 1985 Jun;78(2):215–220. doi: 10.1104/pp.78.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]


