Abstract
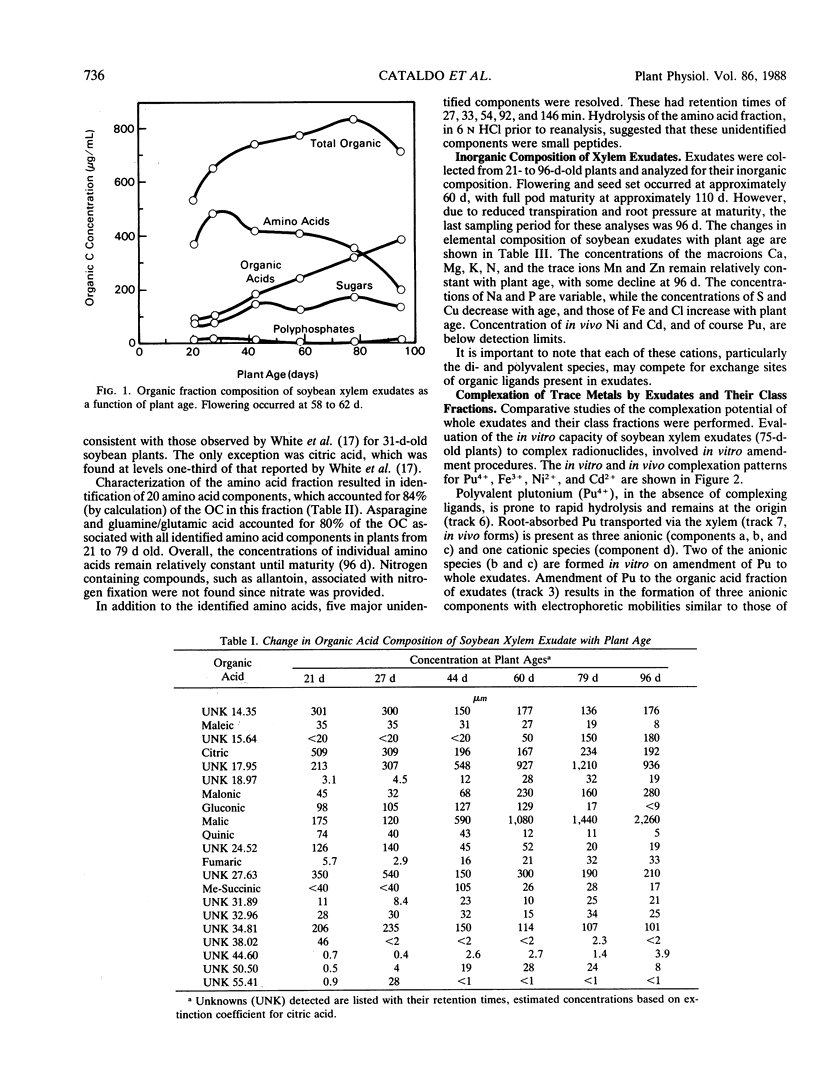
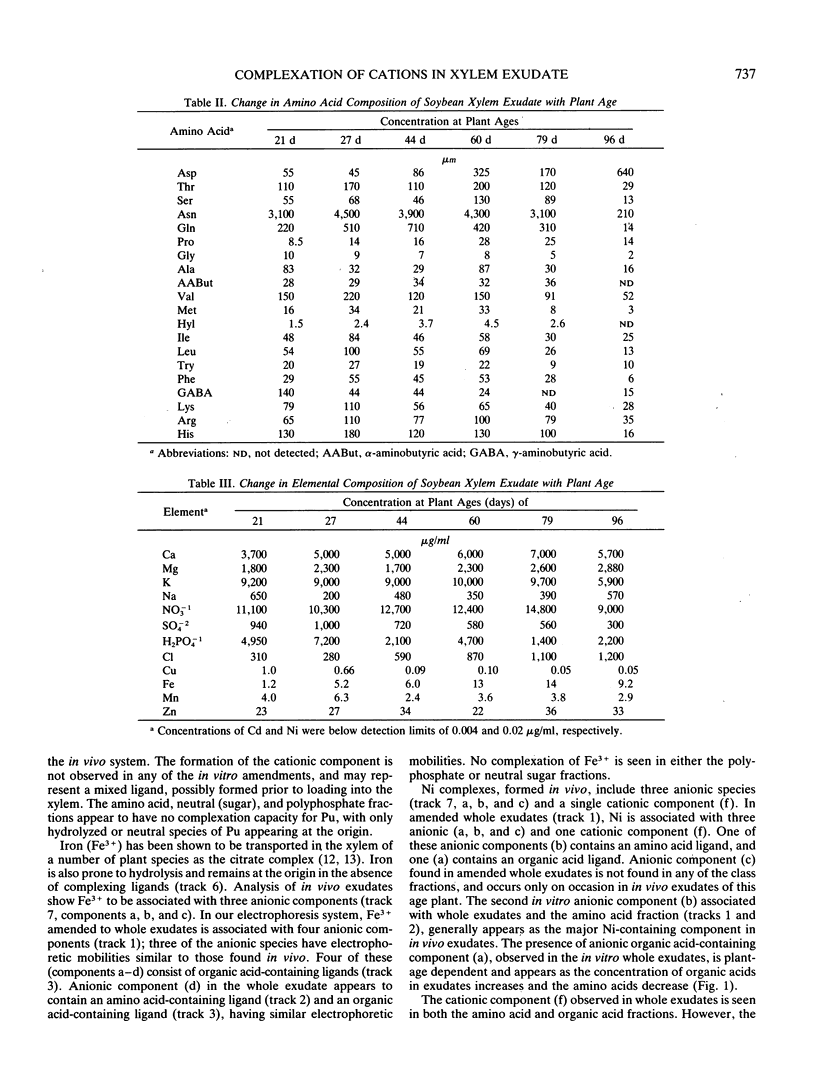
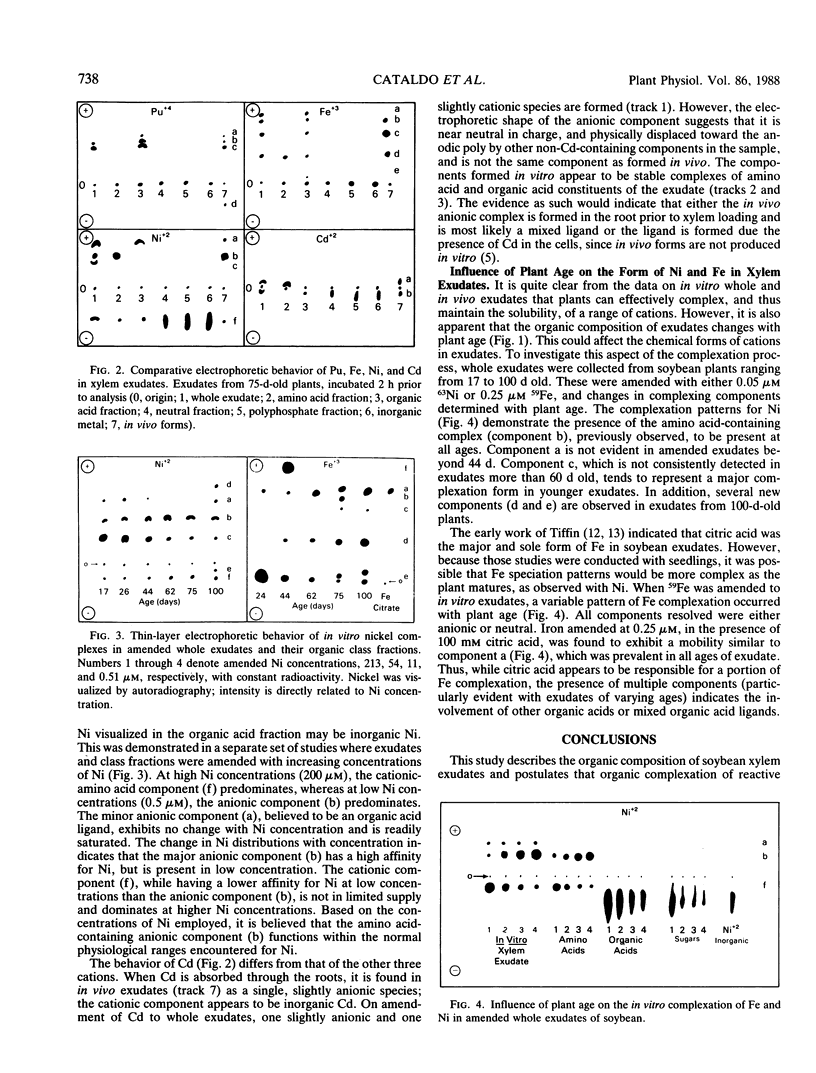
The xylem exudates of soybean (Glycine max cv Williams), provided with fixed N, were characterized as to their organic constituents and in vivo and in vitro complexation of plutonium, iron, cadmium, and nickel. Ion exchange fractionation of whole exudates into their compound classes (organic acid, neutral, amino acid, and polyphosphate), followed by thinlayer electrophoresis, permitted evaluation of the types of ligands which stabilize each element. The polyvalent elements plutonium(IV) and iron(III) are found primarily as organic acid complexes, while the divalent elements nickel(II) and cadmium(II) are associated primarily with components of the amino acid/peptide fraction. For plutonium and cadmium, it was not possible to fully duplicate complexes formed in vivo by back reaction with whole exudates or individual class fractions, indicating the possible importance of plant induction processes, reaction kinetics, and/or the formation of mixed ligand complexes. The number and distribution of specific iron- and nickel-containing complexes varies with plant age and appears to be related to the relative concentration of organic acids and amino acids/peptides being produced and transported in the xylem as the plant matures.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bremner I., Knight A. H. The complexes of zinc, copper and manganese present in ryegrass. Br J Nutr. 1970 Mar;24(1):279–289. doi: 10.1079/bjn19700027. [DOI] [PubMed] [Google Scholar]
- Cataldo D. A., Garland T. R., Wildung R. E. Cadmium distribution and chemical fate in soybean plants. Plant Physiol. 1981 Oct;68(4):835–839. doi: 10.1104/pp.68.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataldo D. A., Garland T. R., Wildung R. E. Cadmium uptake kinetics in intact soybean plants. Plant Physiol. 1983 Nov;73(3):844–848. doi: 10.1104/pp.73.3.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataldo D. A., Garland T. R., Wildung R. E. Nickel in Plants: II. Distribution and Chemical Form in Soybean Plants. Plant Physiol. 1978 Oct;62(4):566–570. doi: 10.1104/pp.62.4.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataldo D. A., Wildung R. E. The role of soil and plant metabolic processes in controlling trace element behavior and bioavailability to animals. Sci Total Environ. 1983 Jun;28:159–168. doi: 10.1016/s0048-9697(83)80015-1. [DOI] [PubMed] [Google Scholar]
- Garland T. R., Cataldo D. A., Wildung R. E. Absorption, transport, and chemical fate of plutonium in soybean plants. J Agric Food Chem. 1981 Sep-Oct;29(5):915–920. doi: 10.1021/jf00107a007. [DOI] [PubMed] [Google Scholar]
- Tiffin L. O. Translocation of iron citrate and phosphorus in xylem exudate of soybean. Plant Physiol. 1970 Mar;45(3):280–283. doi: 10.1104/pp.45.3.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M. C. Metal Complexation in Xylem Fluid : I. CHEMICAL COMPOSITION OF TOMATO AND SOYBEAN STEM EXUDATE. Plant Physiol. 1981 Feb;67(2):292–300. doi: 10.1104/pp.67.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M. C. Metal Complexation in Xylem Fluid : II. THEORETICAL EQUILIBRIUM MODEL AND COMPUTATIONAL COMPUTER PROGRAM. Plant Physiol. 1981 Feb;67(2):301–310. doi: 10.1104/pp.67.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M. C. Metal Complexation in Xylem Fluid : III. ELECTROPHORETIC EVIDENCE. Plant Physiol. 1981 Feb;67(2):311–315. doi: 10.1104/pp.67.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]


