Abstract
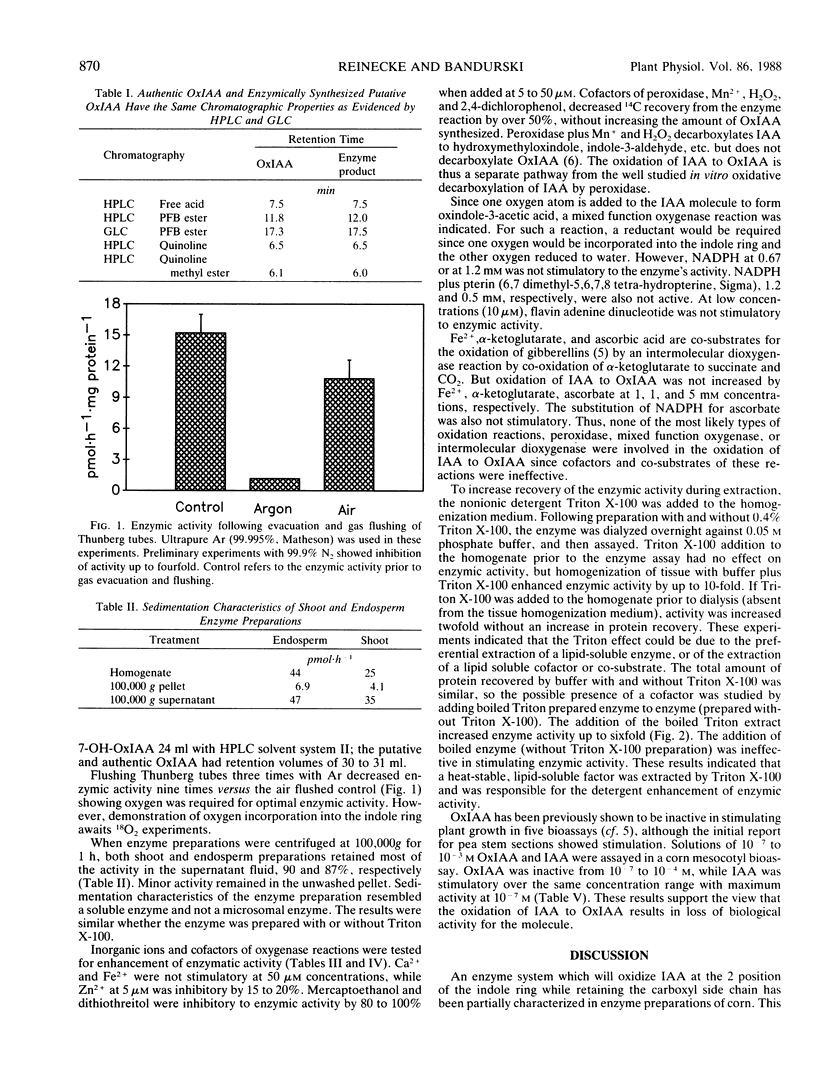
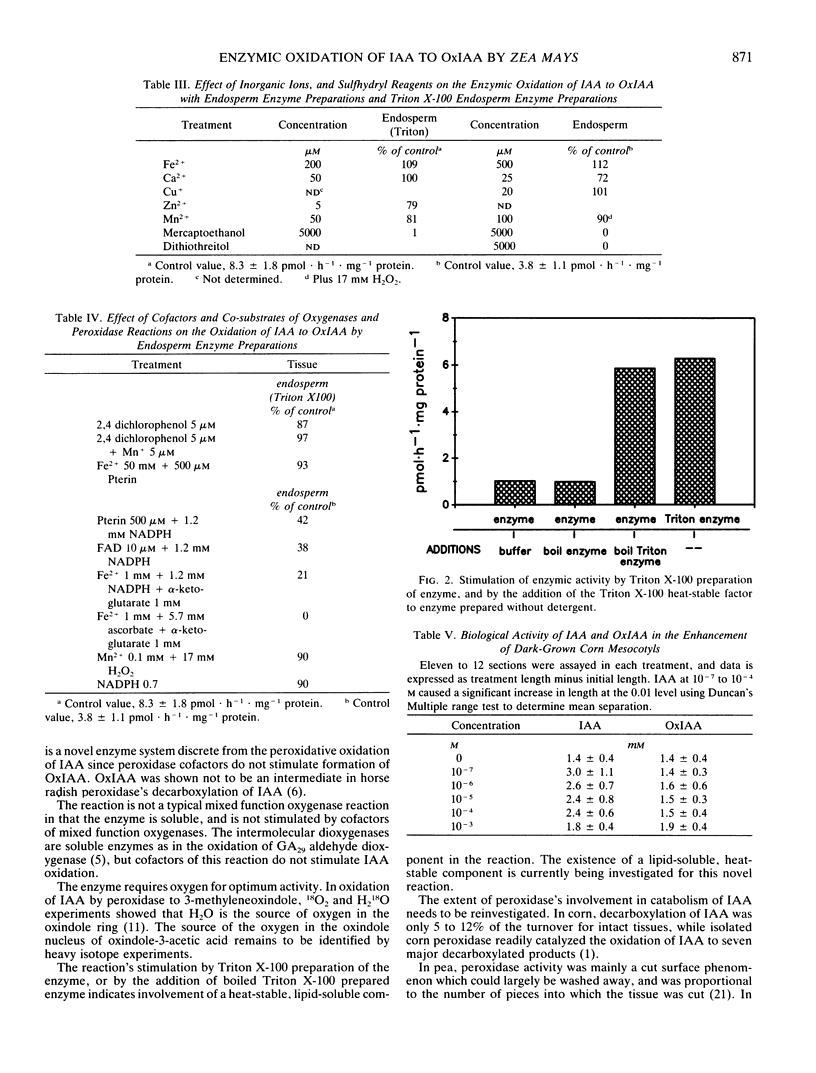
Indole-3-acetic acid is oxidized to oxindole-3-acetic acid by Zea mays tissue extracts. Shoot, root, and endosperm tissues have enzyme activities of 1 to 10 picomoles per hour per milligram protein. The enzyme is heat labile, is soluble, and requires oxygen for activity. Cofactors of mixed function oxygenase, peroxidase, and intermolecular dioxygenase are not stimulatory to enzymic activity. A heat-stable, detergent-extractable component from corn enhances enzyme activity 6- to 10-fold. This is the first demonstration of the in vitro enzymic oxidation of indole-3-acetic acid to oxindole-3-acetic acid in higher plants.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Epstein E., Cohen J. D., Bandurski R. S. Concentration and Metabolic Turnover of Indoles in Germinating Kernels of Zea mays L. Plant Physiol. 1980 Mar;65(3):415–421. doi: 10.1104/pp.65.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HINMAN R. L., LANG J. PEROXIDASE-CATALYZED OXIDATION OF INDOLE-3-ACETIC ACID. Biochemistry. 1965 Jan;4:144–158. doi: 10.1021/bi00877a023. [DOI] [PubMed] [Google Scholar]
- Hall P. L., Bandurski R. S. Movement of Indole-3-acetic Acid and Tryptophan-derived Indole-3-acetic Acid from the Endosperm to the Shoot of Zea mays L. Plant Physiol. 1978 Mar;61(3):425–429. doi: 10.1104/pp.61.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewer P., Bandurski R. S. Occurrence and metabolism of 7-hydroxy-2-indolinone-3-acetic acid in Zea mays. Phytochemistry. 1987;26(5):1247–1250. doi: 10.1016/s0031-9422(00)81790-2. [DOI] [PubMed] [Google Scholar]
- Nitsch J. P., Nitsch C. Studies on the Growth of Coleoptile and First Internode Sections. A New, Sensitive, Straight-Growth Test for Auxins. Plant Physiol. 1956 Mar;31(2):94–111. doi: 10.1104/pp.31.2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonhebel H. M., Bandurski R. S. Oxidation of indole-3-acetic acid and oxindole-3-acetic acid to 2,3-dihydro-7-hydroxy-2-oxo-1H indole-3-acetic acid-7'-O-beta-D-glucopyranoside in Zea mays seedlings. Plant Physiol. 1984;76:979–983. doi: 10.1104/pp.76.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinecke D. M., Bandurski R. S. Metabolic conversion of 14C-indole-3-acetic acid to 14C-oxindole-3-acetic acid. Biochem Biophys Res Commun. 1981 Nov 30;103(2):429–433. doi: 10.1016/0006-291x(81)90470-8. [DOI] [PubMed] [Google Scholar]
- Reinecke D. M., Bandurski R. S. Oxindole-3-acetic Acid, an Indole-3-acetic Acid Catabolite in Zea mays. Plant Physiol. 1983 Jan;71(1):211–213. doi: 10.1104/pp.71.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurumi S., Wada S. Identification of 3-(O-beta-Glucosyl)-2-Indolone-3-Acetylaspartic Acid as a New Indole-3-Acetic Acid Metabolite in Vicia Seedlings. Plant Physiol. 1985 Nov;79(3):667–671. doi: 10.1104/pp.79.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldrum J. D., Davies E. Subcellular Localization of IAA Oxidase in Peas. Plant Physiol. 1981 Dec;68(6):1303–1307. doi: 10.1104/pp.68.6.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]


