Abstract
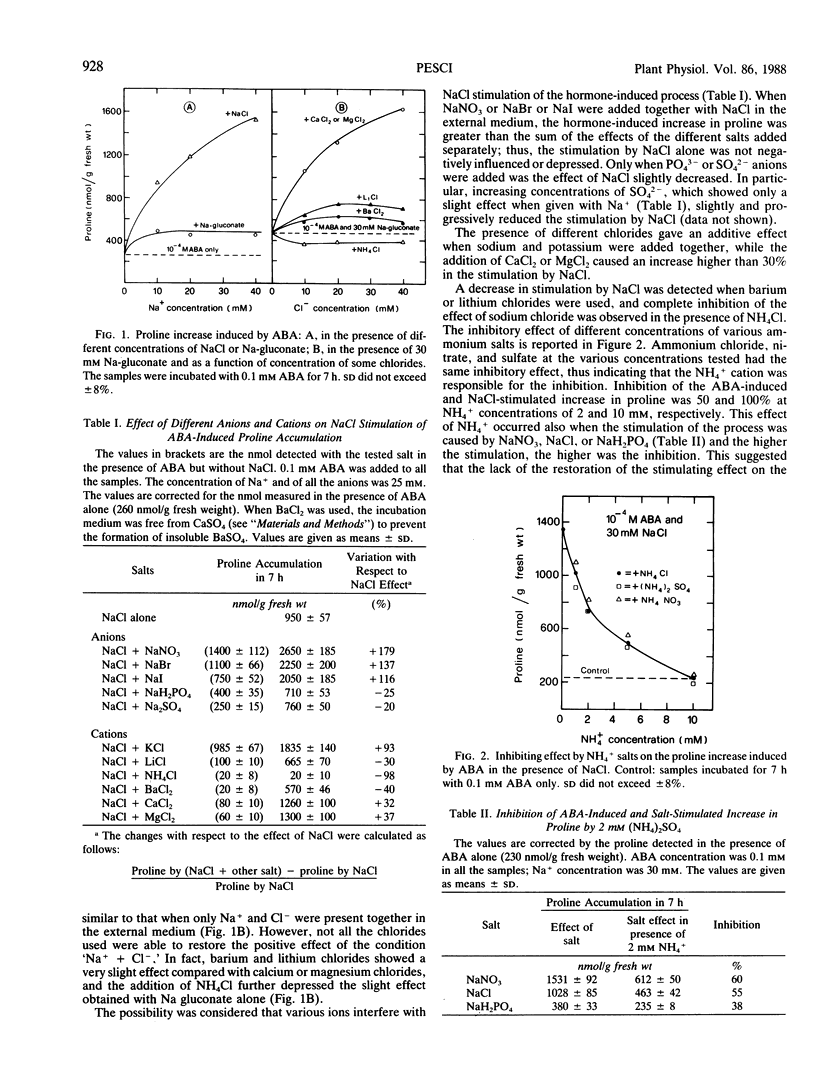
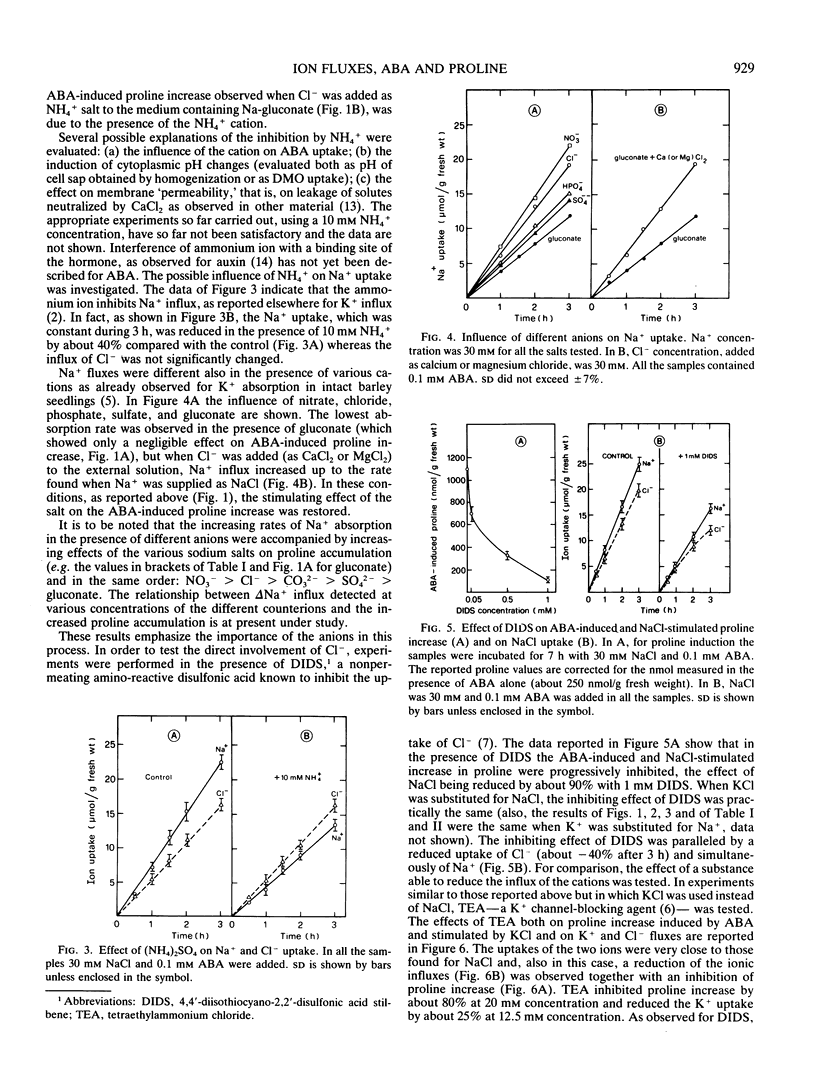
The increase in proline induced by ABA, a process stimulated by NaCl or KCl in barley leaves, did not occur when Na+ (or K+) was present in the external medium as the gluconate salt, namely with an anion unable to permeate the plasma membrane. However, proline increase was restored, to different extents, by the addition of various chloride salts but not by ammonium chloride. Moreover, it was shown that the stimulation of the process by NaCl (or KCl) was variously affected by the presence of different salts; all the ammonium salts (10 millimolar NH4+ concentration) inhibited this stimulation almost completely. Inhibition by NH4+ was accompanied by a decreased Na+ influx (−40%). Also, in the case of Na-gluconate, Na+ uptake was reduced and the addition of Cl− as the calcium or magnesium salt (but not as ammonium salt) restored both the ion influxes and the increase in proline typical of NaCl treatments. Both 4,4′-diisothiocyano-2,2′-disulfonic acid stilbene (DIDS), an anion transport inhibitor, and tetraethylammonium chloride (TEA), a K+ channels-blocking agent, caused, as well as with a reduction of ion influxes, an inhibition of the proline accumulation. The inhibition was practically total with 1 millimolar DIDS and about 80% with 20 millimolar TEA. A possible role of ion influxes in the process leading to the increase in proline induced by ABA is proposed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumwald E., Poole R. J. Na/H Antiport in Isolated Tonoplast Vesicles from Storage Tissue of Beta vulgaris. Plant Physiol. 1985 May;78(1):163–167. doi: 10.1104/pp.78.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane-Drummond C. E., Glass A. D. Short Term Studies of Nitrate Uptake into Barley Plants Using Ion-Specific Electrodes and ClO(3): II. Regulation of NO(3) Efflux by NH(4). Plant Physiol. 1983 Sep;73(1):105–110. doi: 10.1104/pp.73.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ephritikhine G., Barbier-Brygoo H., Muller J. F., Guern J. Auxin effect on the transmembrane potential difference of wild-type and mutant tobacco protoplasts exhibiting a differential sensitiity to auxin. Plant Physiol. 1987 Apr;83(4):801–804. doi: 10.1104/pp.83.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochian L. V., Xin-Zhi J., Lucas W. J. Potassium Transport in Corn Roots : IV. Characterization of the Linear Component. Plant Physiol. 1985 Nov;79(3):771–776. doi: 10.1104/pp.79.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W. Inhibition of anion transport in corn root protoplasts. Plant Physiol. 1981 Aug;68(2):435–438. doi: 10.1104/pp.68.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poovaiah B. W., Leopold A. C. Effects of inorganic salts on tissue permeability. Plant Physiol. 1976 Aug;58(2):182–185. doi: 10.1104/pp.58.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poovaiah B. W., Leopold A. C. Effects of inorganic solutes on the binding of auxin. Plant Physiol. 1976 Dec;58(6):783–785. doi: 10.1104/pp.58.6.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauf C. L., Wilson K. J. Effects of abscisic acid on K+ channels in Vicia faba guard cell protoplasts. Biochem Biophys Res Commun. 1987 May 29;145(1):284–290. doi: 10.1016/0006-291x(87)91318-0. [DOI] [PubMed] [Google Scholar]


