Abstract
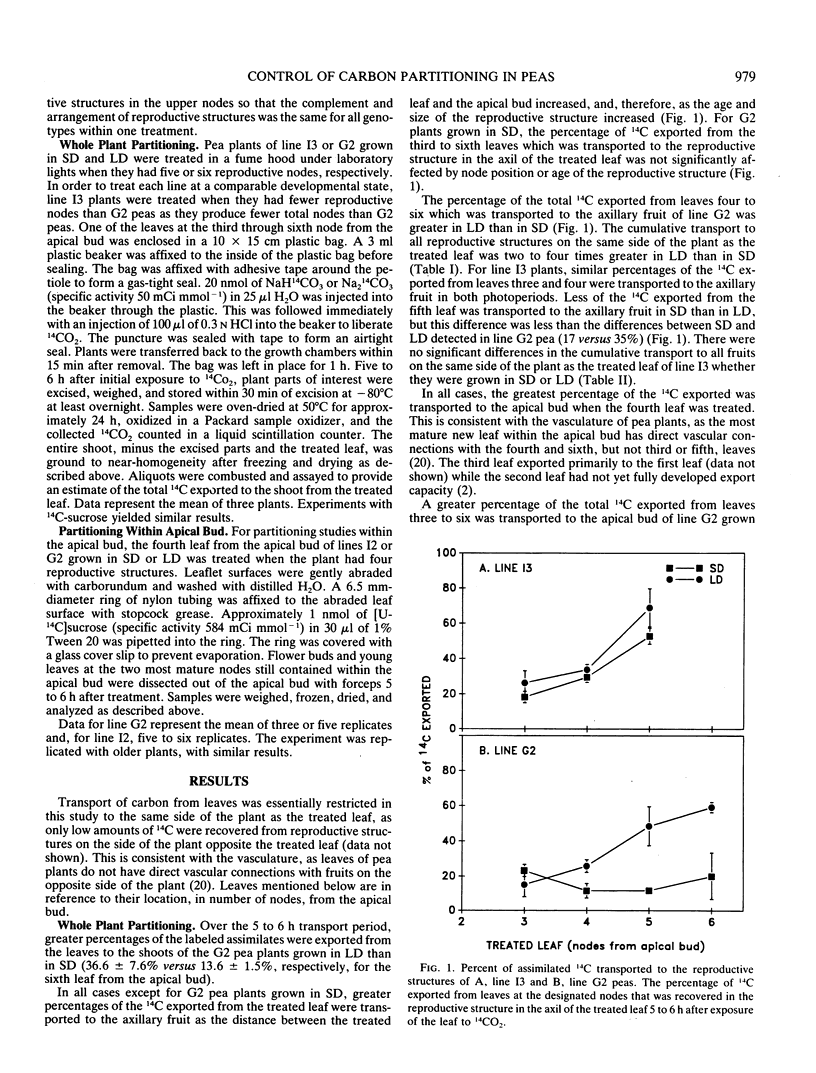
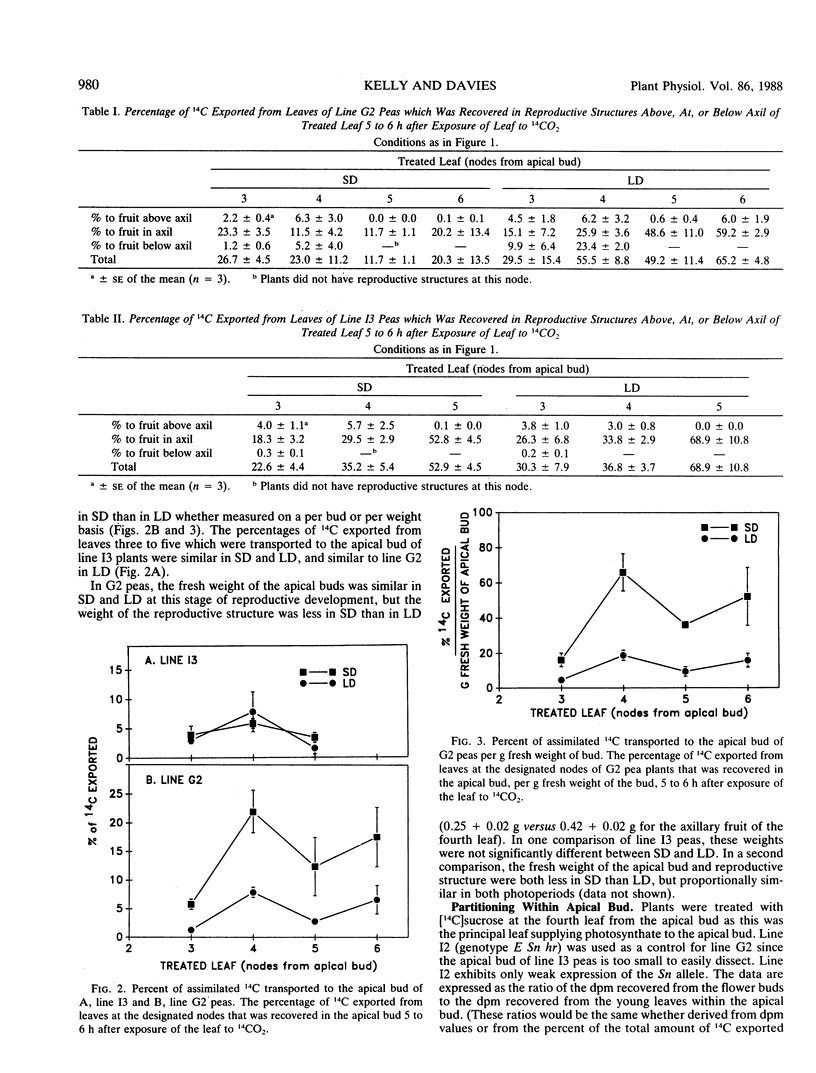
Apical senescence but not flower initiation is delayed by short days (SD) compared to long days (LD) in pea plants (Pisum sativum L.) of genotype E Sn Hr. We recently reported that delay of senescence correlated with slower reproductive development, suggesting that fruits are weaker sinks for assimilates under delayed senescence conditions. Thus, we have examined assimilate partitioning in peas to determine if genotype and photoperiod regulate relative sink strength. Assimilate diversion by developing fruit has been implicated in senescence induction. A greater percentage of leaf-exported 14C was transported to fruits and a smaller percentage to the apical bud of G2 peas (genotype E Sn Hr) in LD than in SD. Relatively more of the 14C delivered to the apical bud of G2 peas was transported to flower buds than to young leaves in LD as compared to SD. There was no striking photoperiodic difference in carbon partitioning in genetic lines without the Sn Hr allele combination. The Sn Hr allele combination and photoperiod may regulate the relative strength of reproductive and vegetative sinks. Photoperiodic differences in sink strength early in reproduction suggest that these genes regulate sink strength by affecting the physiology of the whole plant. High vegetative sink strength in SD may maintain assimilate supply to the apical bud, delaying senescence.
Full text
PDF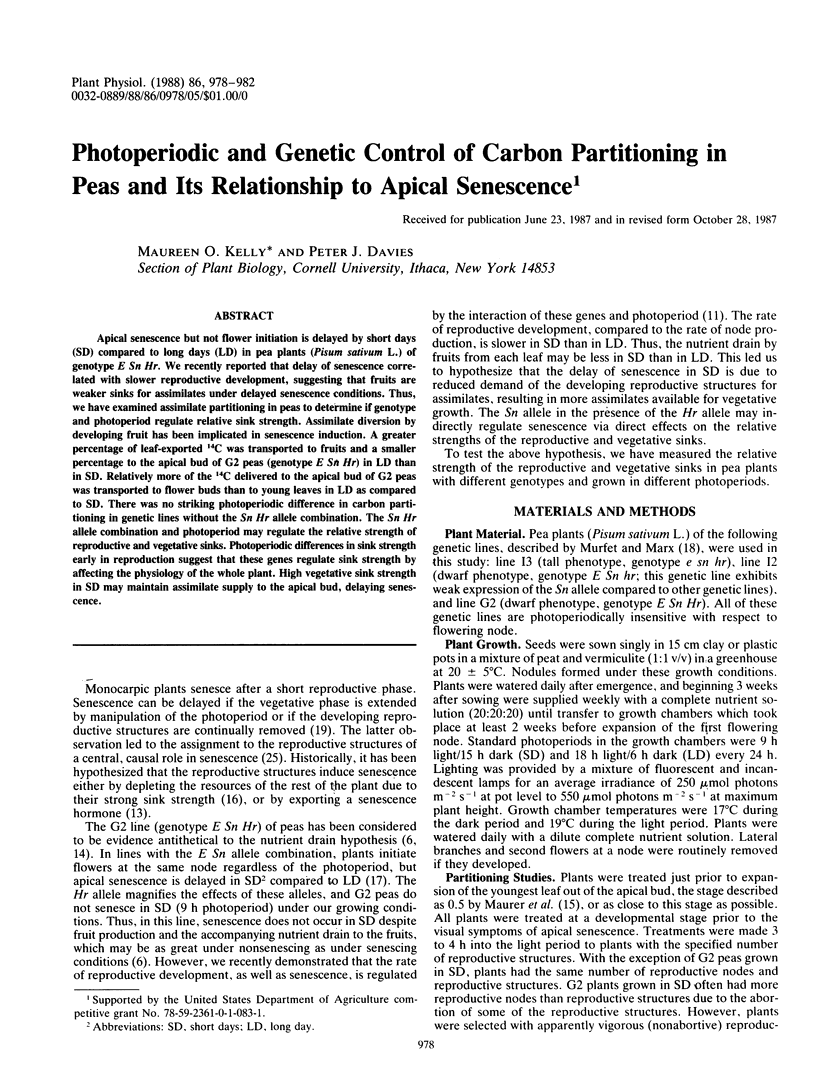


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burke J. J., Kalt-Torres W., Swafford J. R., Burton J. W., Wilson R. F. Studies on Genetic Male-Sterile Soybeans : III. The Initiation of Monocarpic Senescence. Plant Physiol. 1984 Aug;75(4):1058–1063. doi: 10.1104/pp.75.4.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr D. J., Pate J. S. Ageing in the whole plant. Symp Soc Exp Biol. 1967;21:559–599. [PubMed] [Google Scholar]
- Chatterton N. J., Silvius J. E. Photosynthate Partitioning into Starch in Soybean Leaves: I. Effects of Photoperiod versus Photosynthetic Period Duration. Plant Physiol. 1979 Nov;64(5):749–753. doi: 10.1104/pp.64.5.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crafts-Brandner S. J., Below F. E., Harper J. E., Hageman R. H. Effects of pod removal on metabolism and senescence of nodulating and nonnodulating soybean isolines: I. Metabolic constituents. Plant Physiol. 1984 Jun;75(2):311–317. doi: 10.1104/pp.75.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P. J., Birnberg P. R., Maki S. L., Brenner M. L. Photoperiod modification of [C]gibberellin a(12) aldehyde metabolism in shoots of pea, line g2. Plant Physiol. 1986 Aug;81(4):991–996. doi: 10.1104/pp.81.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold A. C., Niedergang-Kamien E., Janick J. Experimental Modification of Plant Senescence. Plant Physiol. 1959 Sep;34(5):570–573. doi: 10.1104/pp.34.5.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proebsting W. M., Davies P. J., Marx G. A. Photoperiodic control of apical senescence in a genetic line of peas. Plant Physiol. 1976 Dec;58(6):800–802. doi: 10.1104/pp.58.6.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wareing P. F., Seth A. K. Ageing and senescence in the whole plant. Symp Soc Exp Biol. 1967;21:543–558. [PubMed] [Google Scholar]


