Abstract
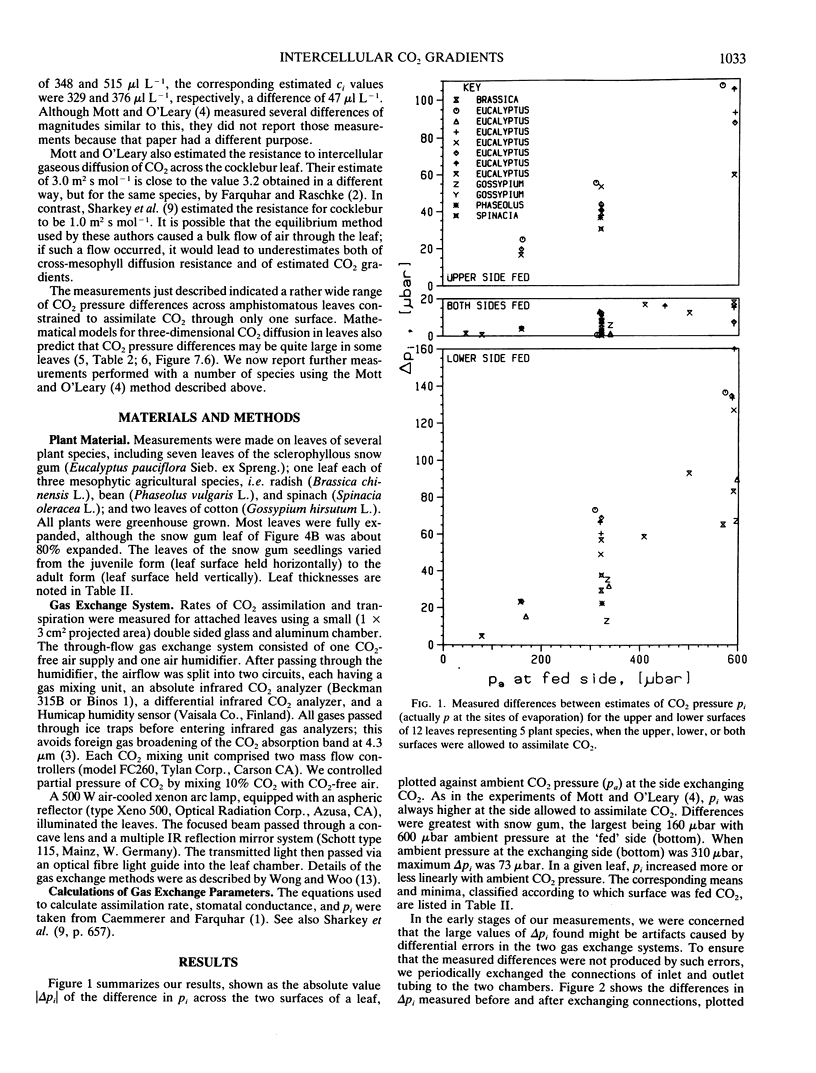
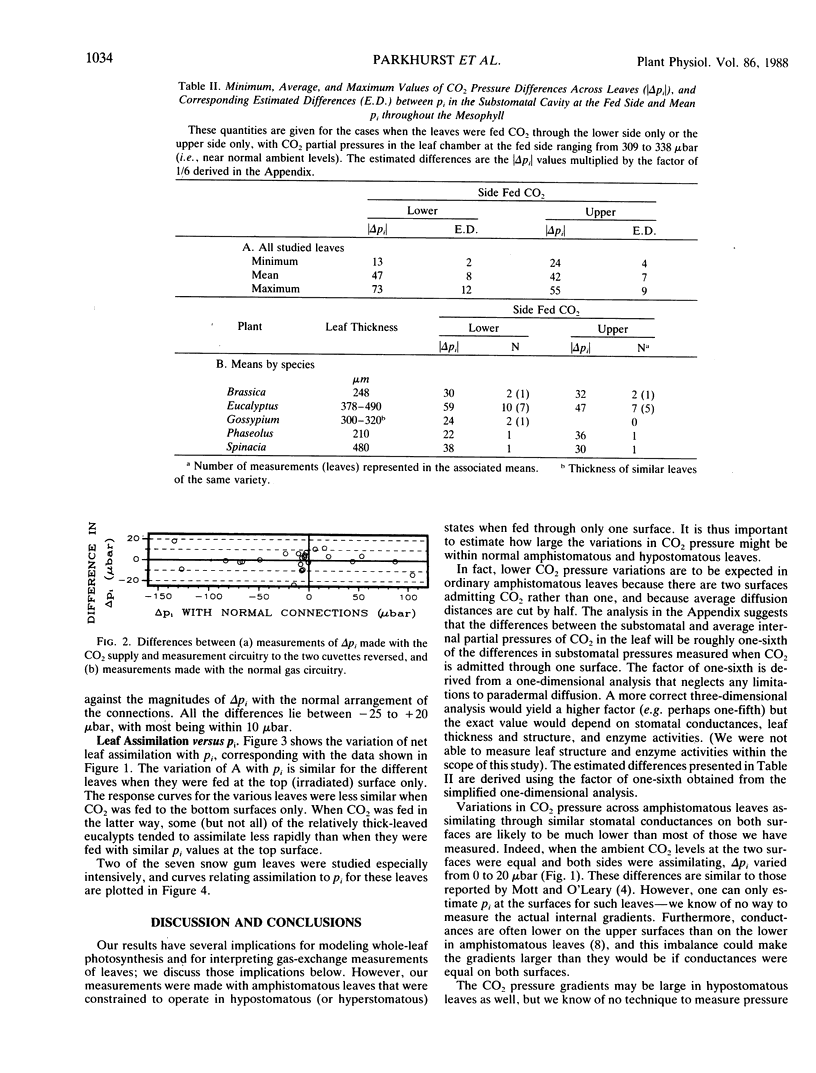
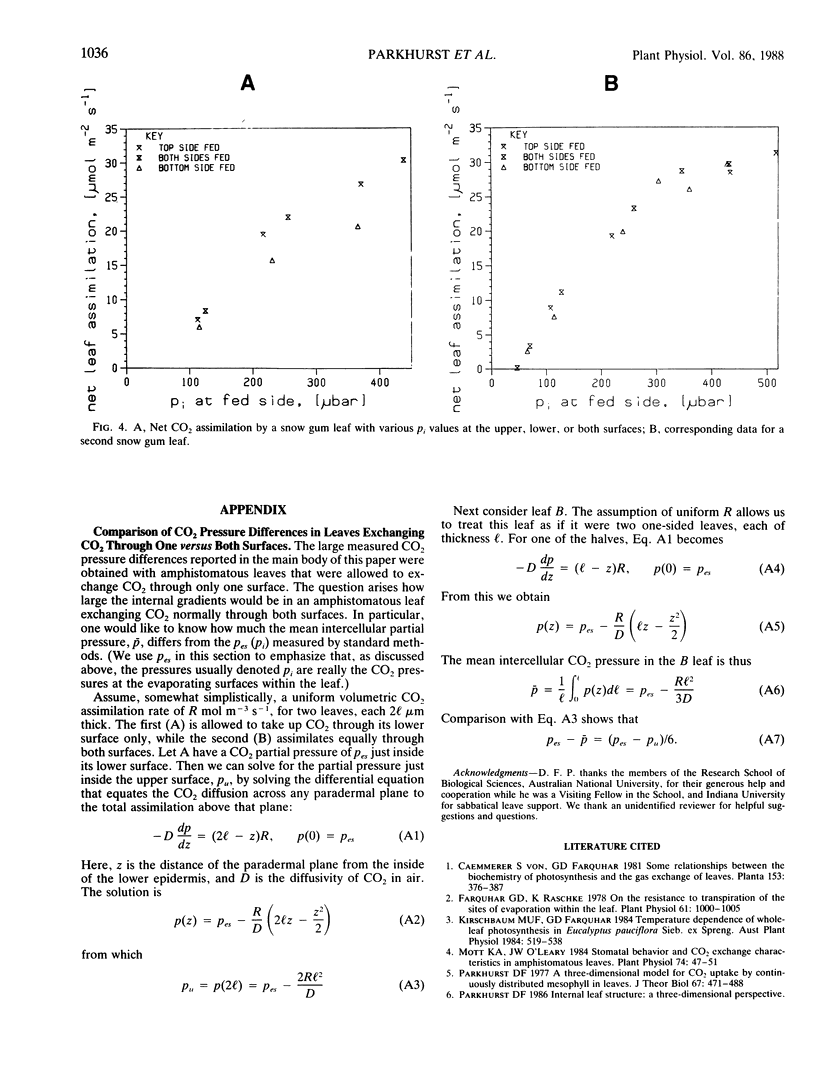
Most current photosynthesis models, and interpretations of many wholeleaf CO2 gas exchange measurements, are based on the often unstated assumption that the partial pressure of CO2 is nearly uniform throughout the airspaces of the leaf mesophyll. Here we present measurements of CO2 gradients across amphistomatous leaves allowed to assimilate CO2 through only one surface, thus simulating hypostomatous leaves. We studied five species: Eucalyptus pauciflora Sieb. ex Spreng., Brassica chinensis L., Gossypium hirsutum L., Phaseolus vulgaris L., and Spinacia oleracea L. For Eucalyptus, maximum CO2 pressure differences across the leaf mesophyll were 73 and 160 microbar when the pressures outside the lower leaf surface were 310 and 590 microbar, respectively. Using an approximate theoretical calculation, we infer that if the CO2 had been supplied equally at both surfaces then the respective mean intercellular CO2 pressures would have been roughly 12 and 27 microbar less than the pressures in the substomatal cavities in these cases. For ambient CO2 pressures near 320 microbar, the average and minimum pressure differences across the mesophyll were 45 and 13 microbar. The corresponding mean intercellular CO2 pressures would then be roughly 8 and 2 microbar less than those in the substomatal cavities. Pressure differences were generally smaller for the four agricultural species than for Eucalyptus, but they were nevertheless larger than previously reported values.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Farquhar G. D., Raschke K. On the Resistance to Transpiration of the Sites of Evaporation within the Leaf. Plant Physiol. 1978 Jun;61(6):1000–1005. doi: 10.1104/pp.61.6.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott K. A., O'leary J. W. Stomatal Behavior and CO(2) Exchange Characteristics in Amphistomatous Leaves. Plant Physiol. 1984 Jan;74(1):47–51. doi: 10.1104/pp.74.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhurst D. F. A three-dimensional model for CO2 uptake by continuously distributed mesophyll in leaves. J Theor Biol. 1977 Aug 7;67(3):471–488. doi: 10.1016/0022-5193(77)90050-9. [DOI] [PubMed] [Google Scholar]
- Sharkey T. D., Imai K., Farquhar G. D., Cowan I. R. A Direct Confirmation of the Standard Method of Estimating Intercellular Partial Pressure of CO(2). Plant Physiol. 1982 Mar;69(3):657–659. doi: 10.1104/pp.69.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S. C., Woo K. C. Simultaneous Measurements of Steady State Chlorophyll a Fluorescence and CO(2) Assimilation in Leaves: The Relationship between Fluorescence and Photosynthesis in C(3) and C(4) Plants. Plant Physiol. 1986 Apr;80(4):877–883. doi: 10.1104/pp.80.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]


