Abstract
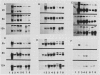
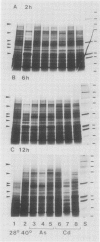
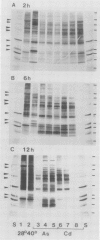
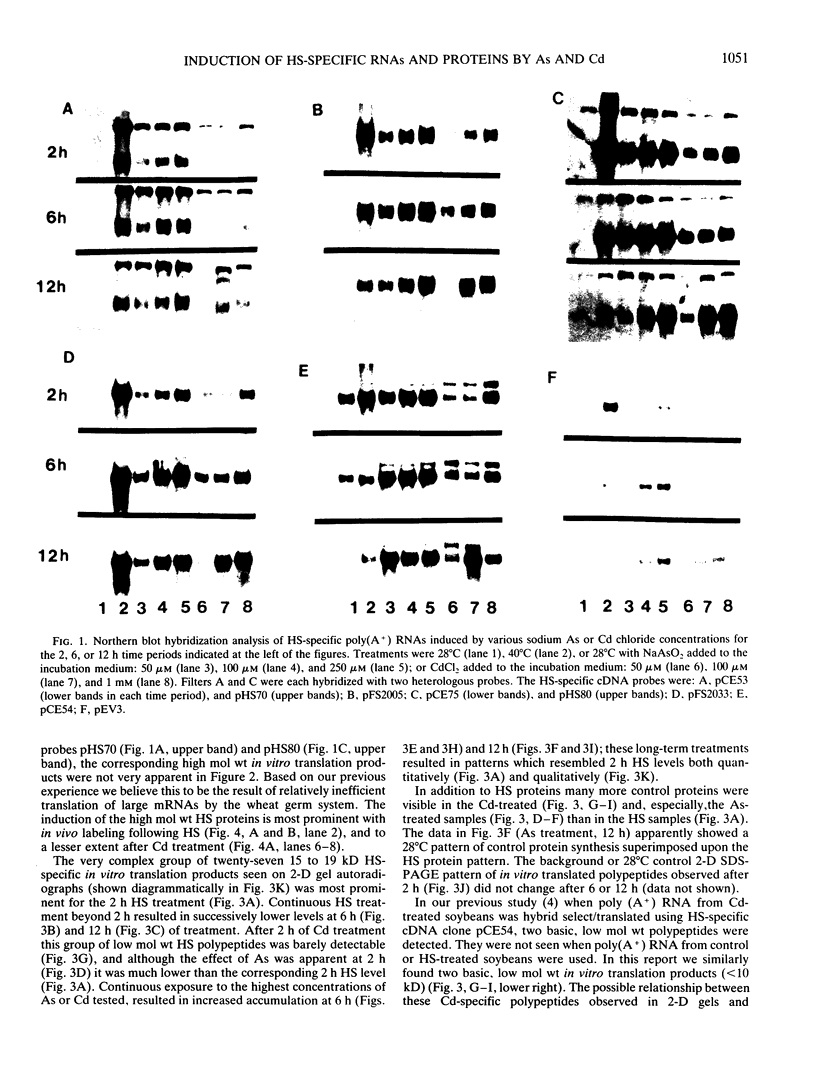
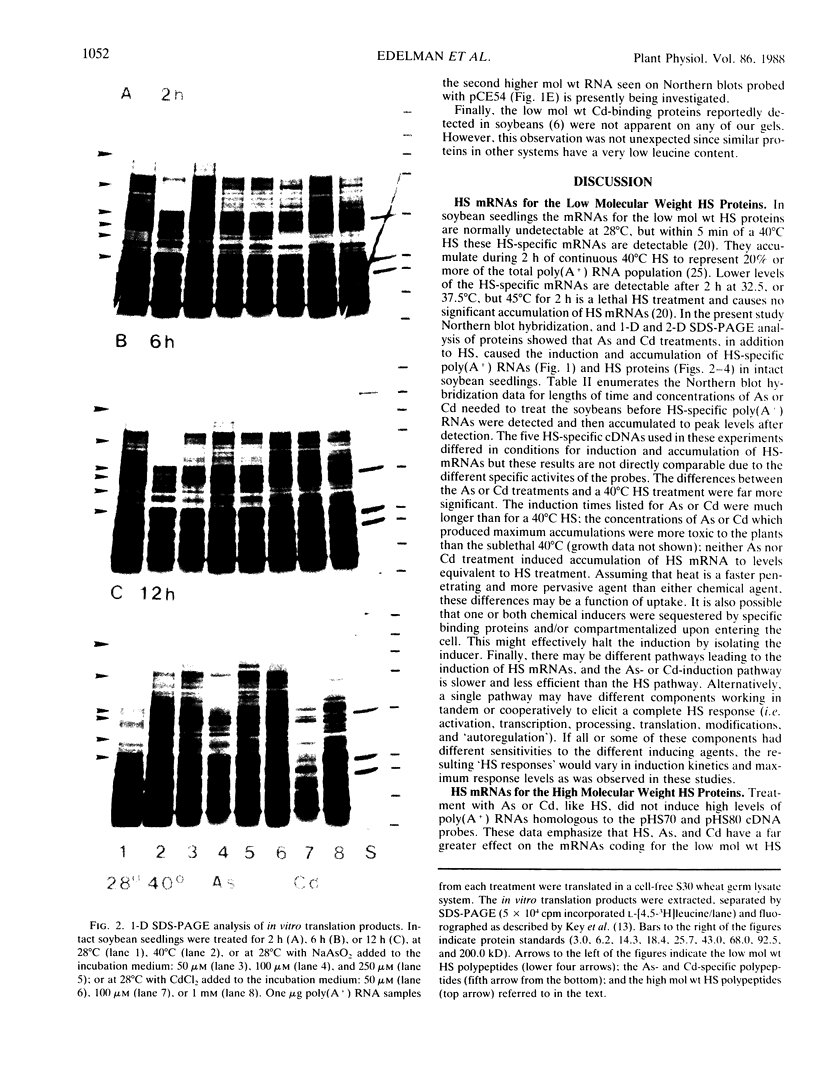
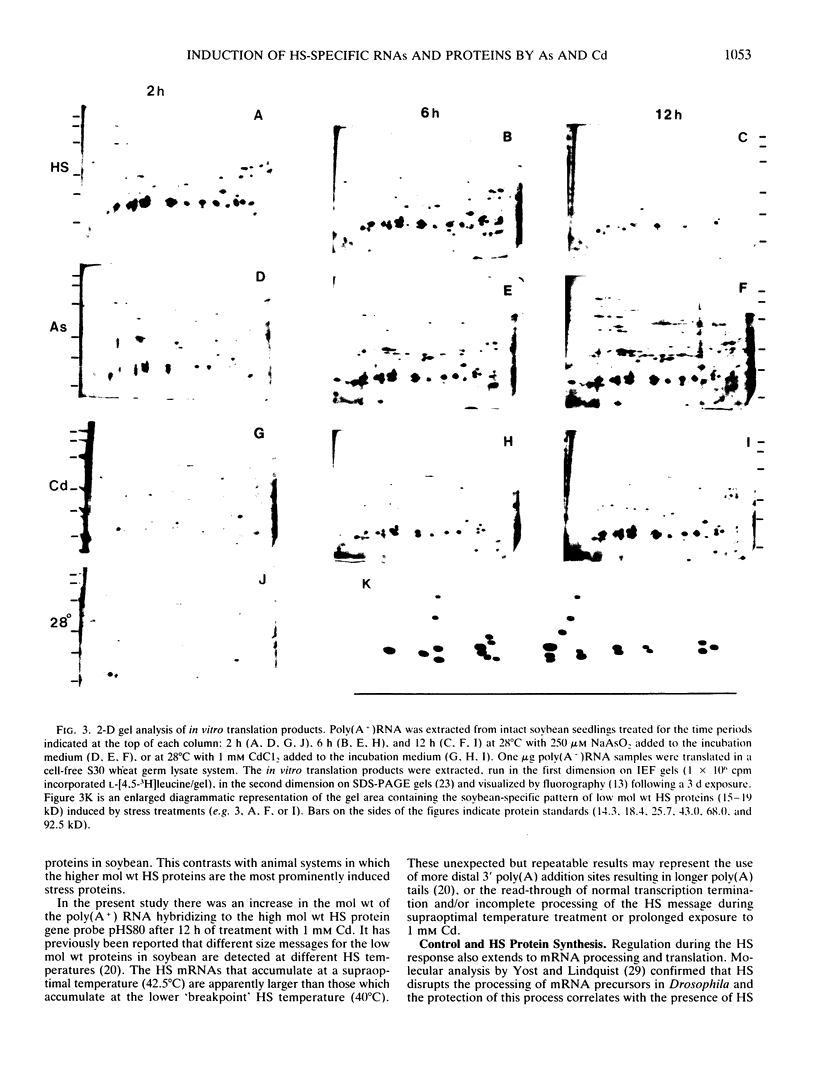
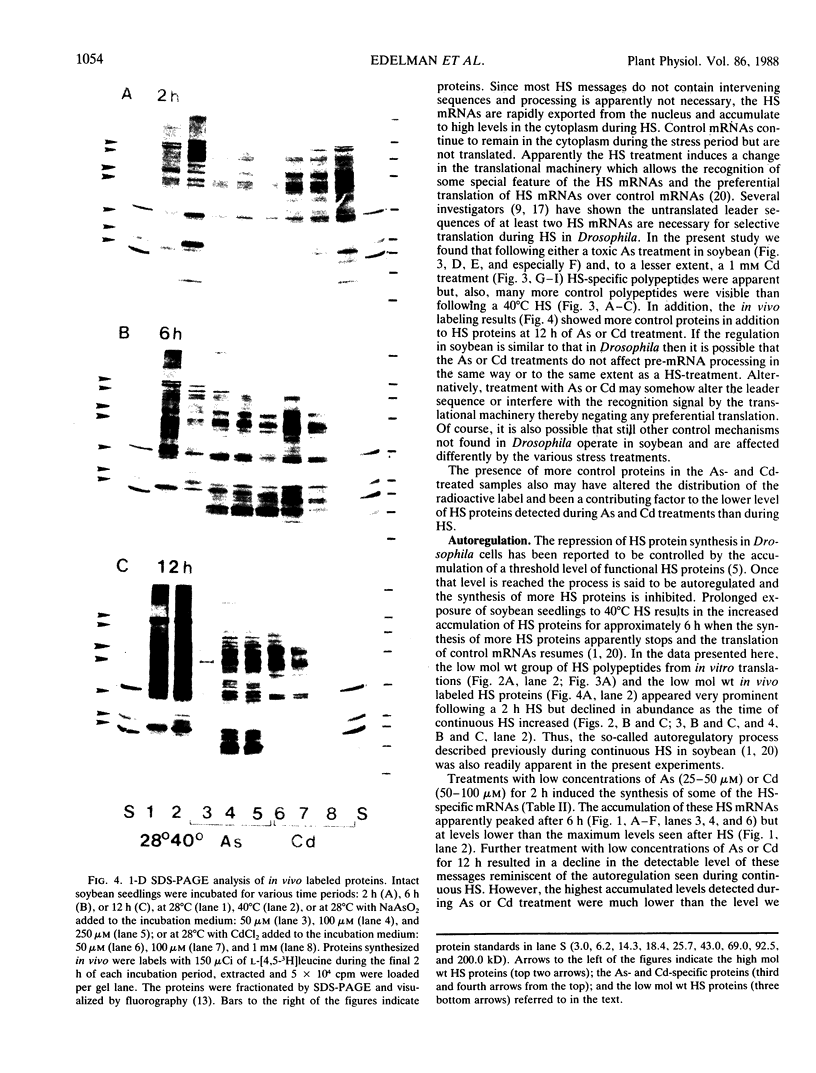
Northern blot hybridization analyzes revealed that poly(A+) RNAs homologous to eight heat shock (HS)-specific cDNA clones were induced by arsenite (As) or Cd treatments. The mRNAs accumulated slower, and maximum accumulations were consistently lower than HS-induced levels. Prolonged treatment with low concentrations (50-100 micromolar) of As for 6 hours, or Cd for 12 hours, resulted in decreased accumulations of HS-specific mRNAs. This response resembled the `autoregulation' observed during continuous 40°C HS. However, no autoregulation was evident when soybean seedlings were exposed to high concentrations of As (250 micromolar) or Cd (1 millimolar) for 12 hours. The cDNA probe pCE54 detected a second higher molecular weight poly(A+) RNA following As or Cd treatments which accumulated concomitantly with the lower molecular weight HS-specific poly(A+) RNA. The patterns of low molecular weight HS polypeptides from in vitro translations induced by HS, As, and Cd, and analyzed by one-dimensional and two-dimensional SDS-PAGE, were similar but temporal differences were apparent. In addition to HS proteins, many control proteins were also detected in both in vitro and in vivo labeling patterns from As and, to a lesser extent, Cd treatments. The chemical agents used in this study apparently induced the accumulation and translation of HS messages in vivo but not in the selective manner as observed during HS treatment.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ananthan J., Goldberg A. L., Voellmy R. Abnormal proteins serve as eukaryotic stress signals and trigger the activation of heat shock genes. Science. 1986 Apr 25;232(4749):522–524. doi: 10.1126/science.3083508. [DOI] [PubMed] [Google Scholar]
- Bishop J. O., Rosbash M. Polynucleotide sequences in eukaryotic DNA and RNA that form ribonuclease-resistant complexes with polyuridylic acid. J Mol Biol. 1974 May 5;85(1):75–86. doi: 10.1016/0022-2836(74)90130-2. [DOI] [PubMed] [Google Scholar]
- Czarnecka E., Nagao R. T., Key J. L., Gurley W. B. Characterization of Gmhsp26-A, a stress gene encoding a divergent heat shock protein of soybean: heavy-metal-induced inhibition of intron processing. Mol Cell Biol. 1988 Mar;8(3):1113–1122. doi: 10.1128/mcb.8.3.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiDomenico B. J., Bugaisky G. E., Lindquist S. The heat shock response is self-regulated at both the transcriptional and posttranscriptional levels. Cell. 1982 Dec;31(3 Pt 2):593–603. doi: 10.1016/0092-8674(82)90315-4. [DOI] [PubMed] [Google Scholar]
- Heikkila J. J., Schultz G. A., Iatrou K., Gedamu L. Expression of a set of fish genes following heat or metal ion exposure. J Biol Chem. 1982 Oct 25;257(20):12000–12005. [PubMed] [Google Scholar]
- Huang C. Y., Bazzaz F. A., Vanderhoef L. N. The inhibition of soybean metabolism by cadmium and lead. Plant Physiol. 1974 Jul;54(1):122–124. doi: 10.1104/pp.54.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultmark D., Klemenz R., Gehring W. J. Translational and transcriptional control elements in the untranslated leader of the heat-shock gene hsp22. Cell. 1986 Feb 14;44(3):429–438. doi: 10.1016/0092-8674(86)90464-2. [DOI] [PubMed] [Google Scholar]
- Jackson M., Ingle J. The interpretation of studies on rapidly labeled ribonucleic Acid in higher plants. Plant Physiol. 1973 Feb;51(2):412–414. doi: 10.1104/pp.51.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston D., Oppermann H., Jackson J., Levinson W. Induction of four proteins in chick embryo cells by sodium arsenite. J Biol Chem. 1980 Jul 25;255(14):6975–6980. [PubMed] [Google Scholar]
- Key J. L., Lin C. Y., Chen Y. M. Heat shock proteins of higher plants. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3526–3530. doi: 10.1073/pnas.78.6.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levinson W., Oppermann H., Jackson J. Transition series metals and sulfhydryl reagents induce the synthesis of four proteins in eukaryotic cells. Biochim Biophys Acta. 1980;606(1):170–180. doi: 10.1016/0005-2787(80)90108-2. [DOI] [PubMed] [Google Scholar]
- Lin C. Y., Roberts J. K., Key J. L. Acquisition of Thermotolerance in Soybean Seedlings : Synthesis and Accumulation of Heat Shock Proteins and their Cellular Localization. Plant Physiol. 1984 Jan;74(1):152–160. doi: 10.1104/pp.74.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry T. J., Lindquist S. The preferential translation of Drosophila hsp70 mRNA requires sequences in the untranslated leader. Cell. 1985 Oct;42(3):903–911. doi: 10.1016/0092-8674(85)90286-7. [DOI] [PubMed] [Google Scholar]
- Munro S., Pelham H. What turns on heat shock genes? Nature. 1985 Oct 10;317(6037):477–478. doi: 10.1038/317477a0. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C. What the bacteriologists have learned about heat shock. Genes Dev. 1987 Apr;1(2):109–110. doi: 10.1101/gad.1.2.109. [DOI] [PubMed] [Google Scholar]
- Parker C. S., Topol J. A Drosophila RNA polymerase II transcription factor binds to the regulatory site of an hsp 70 gene. Cell. 1984 May;37(1):273–283. doi: 10.1016/0092-8674(84)90323-4. [DOI] [PubMed] [Google Scholar]
- Vierling E., Mishkind M. L., Schmidt G. W., Key J. L. Specific heat shock proteins are transported into chloroplasts. Proc Natl Acad Sci U S A. 1986 Jan;83(2):361–365. doi: 10.1073/pnas.83.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson D. G., Nemer M. Metallothionein genes MTa and MTb expressed under distinct quantitative and tissue-specific regulation in sea urchin embryos. Mol Cell Biol. 1987 Jan;7(1):48–58. doi: 10.1128/mcb.7.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]