This systematic review and network meta-analysis examines evidence about the use of probiotics, prebiotics, lactoferrin, and their combination to gauge the comparative effectiveness of alternative prophylactic strategies for morbidity and mortality in premature infants.
Key Points
Question
In premature infants, what association do probiotics, prebiotics, lactoferrin, and their combination have with major morbidity, mortality, and intervention-related adverse effects?
Findings
This systematic review and network meta-analysis including 106 trials involving 25 840 preterm infants found that multiple-strain probiotics were associated with reductions in all-cause mortality, necrotizing enterocolitis, feeding intolerance, and hospitalization. When combined with oligosaccharides, multiple-strain probiotics were associated with reductions in NEC and feeding intolerance and the best effectiveness for these outcomes but did not have high-certainty evidence for other outcomes.
Meaning
Moderate- to high-certainty evidence shows multiple-strain probiotics alone or possibly in combination with oligosaccharides to be superior to alternative prophylactic interventions.
Abstract
Importance
Modulation of intestinal microbiome by administering probiotics, prebiotics, or both may prevent morbidity and mortality in premature infants.
Objective
To assess the comparative effectiveness of alternative prophylactic strategies through a network meta-analysis (NMA) of randomized clinical trials.
Data Sources
MEDLINE, EMBASE, Science Citation Index Expanded, CINAHL, Scopus, Cochrane CENTRAL, and Google Scholar from inception until May 10, 2023.
Study Selection
Eligible trials tested probiotics, prebiotics, lactoferrin, and combination products for prevention of morbidity or mortality in preterm infants.
Data Extraction and Synthesis
A frequentist random-effects model was used for the NMA, and the certainty of evidence and inferences regarding relative effectiveness were assessed using the GRADE approach.
Main Outcomes and Measures
All-cause mortality, severe necrotizing enterocolitis, culture-proven sepsis, feeding intolerance, time to reach full enteral feeding, and duration of hospitalization.
Results
A total of 106 trials involving 25 840 preterm infants were included. Only multiple-strain probiotics were associated with reduced all-cause mortality compared with placebo (risk ratio [RR], 0.69; 95% CI, 0.56 to 0.86; risk difference [RD], −1.7%; 95% CI, −2.4% to −0.8%). Multiple-strain probiotics alone (vs placebo: RR, 0.38; 95% CI, 0.30 to 0.50; RD, −3.7%; 95% CI, −4.1% to −2.9%) or in combination with oligosaccharides (vs placebo: RR, 0.13; 95% CI, 0.05 to 0.37; RD, −5.1%; 95% CI, −5.6% to −3.7%) were among the most effective interventions reducing severe necrotizing enterocolitis. Single-strain probiotics in combination with lactoferrin (vs placebo RR, 0.33; 95% CI, 0.14 to 0.78; RD, −10.7%; 95% CI, −13.7% to −3.5%) were the most effective intervention for reducing sepsis. Multiple-strain probiotics alone (RR, 0.61; 95% CI, 0.46 to 0.80; RD, −10.0%; 95% CI, −13.9% to −5.1%) or in combination with oligosaccharides (RR, 0.45; 95% CI, 0.29 to 0.67; RD, −14.1%; 95% CI, −18.3% to −8.5%) and single-strain probiotics (RR, 0.61; 95% CI, 0.51 to 0.72; RD, −10.0%; 95% CI, −12.6% to −7.2%) proved of best effectiveness in reduction of feeding intolerance vs placebo. Single-strain probiotics (MD, −1.94 days; 95% CI, −2.96 to −0.92) and multistrain probiotics (MD, −2.03 days; 95% CI, −3.04 to −1.02) proved the most effective in reducing the time to reach full enteral feeding compared with placebo. Only single-strain and multistrain probiotics were associated with greater effectiveness compared with placebo in reducing duration of hospitalization (MD, −3.31 days; 95% CI, −5.05 to −1.58; and MD, −2.20 days; 95% CI, −4.08 to −0.31, respectively).
Conclusions and Relevance
In this systematic review and NMA, moderate- to high-certainty evidence demonstrated an association between multistrain probiotics and reduction in all-cause mortality; these interventions were also associated with the best effectiveness for other key outcomes. Combination products, including single- and multiple-strain probiotics combined with prebiotics or lactoferrin, were associated with the largest reduction in morbidity and mortality.
Introduction
The human gastrointestinal tract is a complex ecosystem that becomes rapidly colonized by microorganisms that facilitate digestion and modulate the immune system.1 Administration of interventions that involve probiotics, prebiotics, lactoferrin, or synbiotics (products that contain both probiotics and prebiotics) results in alteration of the gut microbial flora that may prevent or treat a number of diseases.1,2
Necrotizing enterocolitis (NEC) is a devastating inflammatory disorder of the intestine and among the leading causes of mortality and morbidity in neonatal intensive care units.3,4 With an incidence ranging from 2% to 10% in infants born before 32 weeks’ gestation and 5% to 22% among those with a birthweight of less than 1000 g, NEC has no effective treatments, and surgical management is associated with high rates of mortality.4,5,6 Late-onset sepsis (LOS) also has a significant burden worldwide, and despite preventive strategies such as antimicrobial stewardship, limited corticosteroid use, early enteral feeding, and hand hygiene, the incidence has, in recent years, remained stable in preterm infants.7,8
Modulation of the intestinal microbiome has long been suggested as a potentially effective preventive strategy for NEC and LOS.9,10,11,12 A wide range of probiotics and synbiotics with different formulations and including different probiotic species have been studied, and although numerous systematic reviews and meta-analyses of randomized clinical trials (RCTs)7,8,13,14,15,16,17,18,19 and observational studies2,20,21 have addressed the use of probiotics, prebiotics, or lactoferrin to prevent NEC and LOS, no review has simultaneously addressed the comparative effectiveness of all these agents. Therefore, we conducted a systematic review and network meta-analysis (NMA) of RCTs addressing the effectiveness of these interventions in reducing mortality and morbidity in preterm infants.
Methods
Search Strategy and Selection Criteria
We registered our protocol with PROSPERO (CRD42016043873) and previously published a detailed protocol.22 Using the strategies reported in our published protocol and without language restrictions,22 we searched MEDLINE, EMBASE, Science Citation Index Expanded and Social Sciences Citation Index, Cumulative Index of Nursing and Allied Health (CINAHL), Scopus, Cochrane Central Register of Controlled Trials (CENTRAL), and Google Scholar from inception until May 10, 2023, for published RCTs (eMethods 1 in Supplement 1). We reviewed reference lists from eligible trials and related reviews for additional eligible RCTs. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) extension statement for reporting of systematic reviews incorporating NMA.23
Pairs of reviewers (Y.W., I.D.F., R.L.M., F.F., Y.C., H.N.C., D.Z., M.M.B., R.Q.M., B.T., S.S., X.W.) independently screened the titles and abstracts of all identified studies and subsequently assessed eligibility of the full-text articles. Discrepancies were resolved through discussion or, if needed, by adjudication from a third reviewer (Y.W. or B.S.). Eligible trials used probiotics, prebiotics (including inulin, galacto-oligosaccharides, fructo-oligosaccharides, and human milk oligosaccharides), lactoferrin, and combination products for prevention of morbidity or mortality in preterm infants (gestational age <37 weeks) and/or those with low birth weight (birth weight <2500 g). eMethods 2 in Supplement 1 provides further details regarding eligibility criteria.
Data Abstraction and Risk-of-Bias Assessment
Pairs of reviewers extracted data and assessed risk of bias independently and in duplicate using a modified Cochrane risk-of-bias 1.0 instrument24,25 (eMethods 2 in Supplement 1). Outcomes included severe NEC (stage 2 or 3) based on criteria from Bell et al,26,27 all-cause and NEC-related mortality, culture-proven LOS, feeding intolerance, duration of hospitalization, time to full enteral feeding (days), weight at discharge or at 37 weeks’ postnatal age, and associated adverse events.
Data Synthesis and Statistical Analysis
For each direct paired comparison, we calculated the risk ratio (RR) and associated 95% CIs for dichotomous outcomes. For continuous outcomes, we calculated weighted mean differences (MDs) with corresponding 95% CIs. For each outcome, risk differences (RDs) were calculated by applying relative risk estimates from the NMA to baseline risk calculated using the median risk in the placebo arms of included trials.
Initially, we performed conventional pairwise meta-analysis using DerSimonian-Laird random effects in Stata version 17.0 (StataCorp). We then performed frequentist NMA using the methodology of multivariate meta-analysis and assuming a common heterogeneity parameter.28,29
We evaluated the presence of incoherence (inconsistency) by comparing direct evidence with indirect evidence using the side-splitting method.30,31 We confirmed the coherence assumption in the entire network using a design-by-treatment model (global test) as described by Higgins et al.32 We performed network meta-regression adjusting effect estimates for a priori defined covariates (gestational age, birth weight, percentage of infants exclusively fed by mothers’ or donors’ milk, percentage born by cesarean delivery, and risk of bias) at the study level assuming a common fixed coefficient across comparisons. We estimated ranking probabilities using surface under the cumulative ranking curve (familiarly known as SUCRA), mean ranks, and rankograms.33,34
Assessing Certainty of the Evidence
We rated the certainty (quality) of evidence for each network estimate using theGrading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach, using the null as the target of our certainty rating.35,36,37,38 We rated the certainty of the indirect evidence focusing on the dominant lowest-order loop,36 rating the certainty of indirect evidence as the lowest certainty of the contributing direct comparisons.
We determined network estimate certainty considering the relative contribution of direct and indirect evidence to the network estimate. We considered rating down the certainty in the network estimate if there was incoherence between the indirect and direct estimates or if there was imprecision (wide CIs) around the treatment effect.36,37 eMethods 2 in Supplement 1 provides details about the certainty assessments.
Summary of Results Using the GRADE Approach
To optimize NMA results for interpretation, we applied a novel minimally contextualized GRADE approach, in which we categorized the interventions from most effective to least effective based on the effect estimates obtained from the NMA and their associated certainty of evidence.39,40,41 For each outcome, we created categories of groups of interventions as follows: (1) the reference intervention (placebo) and interventions with no evidence of difference compared with placebo (ie, 95% CI includes null value), termed among the worst; (2) interventions superior to placebo but not superior to any other of the interventions superior to placebo, termed among the intermediate; and (3) interventions that proved superior to at least 1 category 2 intervention, termed among the best. We then divided all 3 categories into 2 groups: those with moderate- or high-certainty evidence relative to placebo and those with low- or very low-certainty evidence relative to placebo.
Results
Description of the Evidence
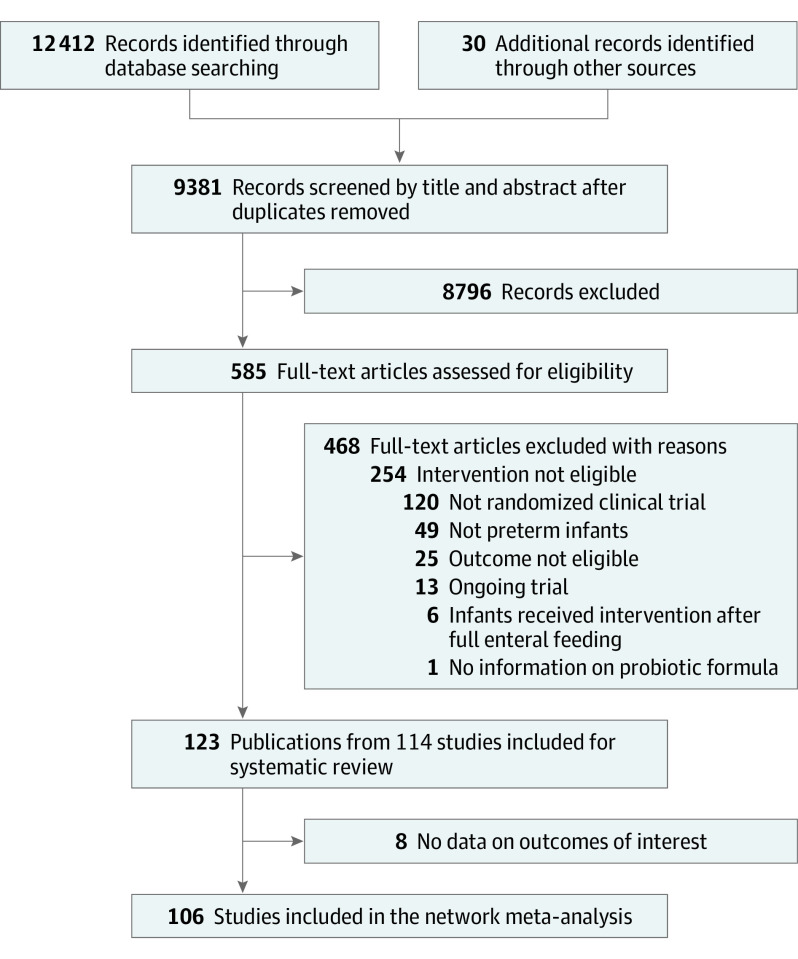
Our literature search identified 12 442 records, of which we included 123 publications from 114 RCTs. Eight trials failed to report any of our target outcomes42,43,44,45,46,47,48,49; thus, 106 RCTs involving 25 840 infants were used for the NMA (eMethods 3 in Supplement 1). Figure 1 presents the details of the study selection process.
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Flow Diagram for Study Selection.
Across the eligible trials, the median (IQR) of the mean birth weight was 1237.1 g (1130.1-1470.6 g), and the median (IQR) of the mean gestational age was 30.0 weeks (28.9-31.5 weeks). eTable 1 in Supplement 1 summarizes the characteristics of the participants.
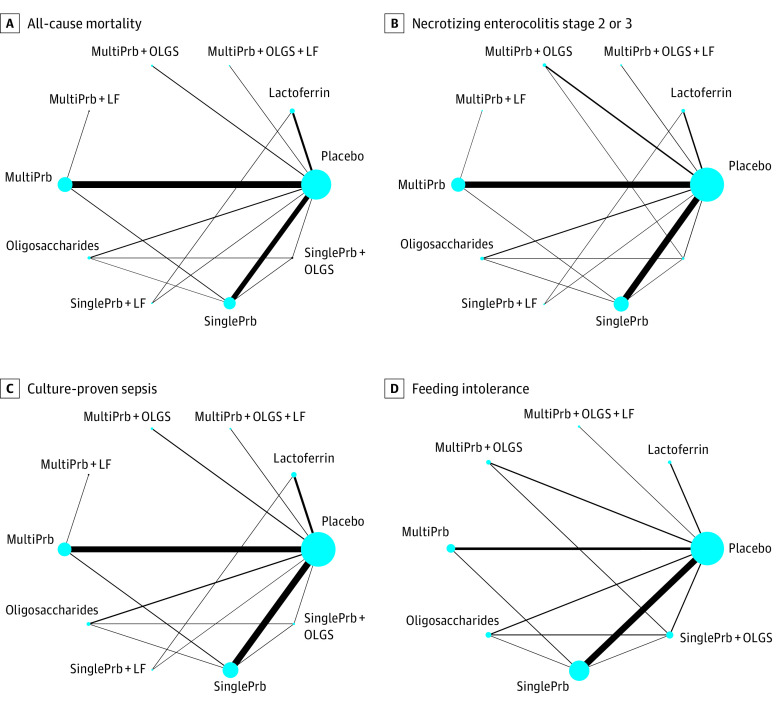
Of the 106 RCTs, 41 studied single-strain and 44 studied multiple-strain probiotics, 13 combination products, 10 lactoferrin, and 6 oligosaccharides (human milk oligosaccharides, fructo-oligosaccharides, and/or galacto-oligosaccharides). A total of 16 different probiotic strains were used among the studies assessing multistrain probiotics and combination products, most of which included both Bifidobacterium and Lactobacillus species (38 of 44 and 9 of 13 studies, respectively). eTable 2 in Supplement 1 presents the characteristics of the interventions and outcomes. Figure 2 and eFigures 1-4 in Supplement 1 present the networks of eligible comparisons for each outcome.
Figure 2. Network of Eligible Comparisons.
The size of the node corresponds to the number of infants randomized to that intervention. The interventions directly compared are linked with a line; the thickness of the line corresponds to the number of trials that assessed the comparison. LF indicates lactoferrin; MultiPrb, multiple-strain probiotics; OLGS, oligosaccharides (human milk oligosaccharides, fructo-oligosaccharides, and/or galacto-oligosaccharides); SinglePrb, single-strain probiotics.
Of the 106 RCTs, 72 had a low risk of bias in terms of allocation concealment and 88 in terms of missing participant outcome data; 25 studies had a high risk of bias for blinding of infants’ parents/caregivers and study personnel, while 48 studies had a high risk of bias for masking of outcome assessments. eTable 3 in Supplement 1 provides details of risk-of-bias assessments.
Figure 3 summarizes the key findings for each outcome, providing network estimates and their 95% CIs for each intervention compared with placebo, the associated certainty of evidence as high or moderate vs low or very low, and the classification of interventions as among the best, among the intermediate, and among the worst.
Figure 3. Network Meta-Analysis Results Sorted Based on Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) Certainty of Evidence and Effect Estimates for the Comparisons of Active Interventions vs Placebo.
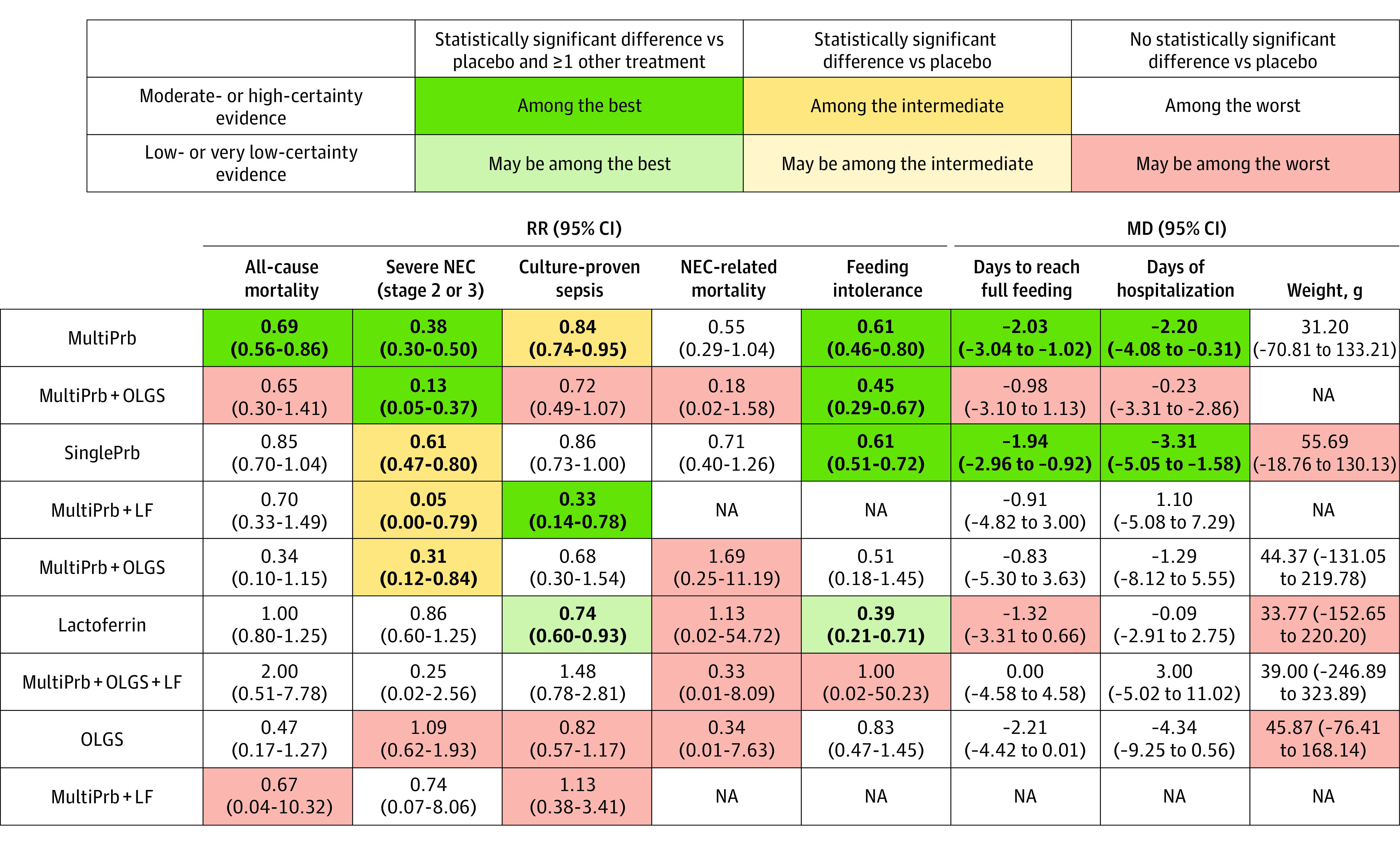
Numbers in bold indicate statistically significant results. LF indicates lactoferrin; MD, mean difference; MultiPrb, multiple-strain probiotics; NA, not available; NEC, necrotizing enterocolitis; OLGS, oligosaccharides (human milk oligosaccharides, fructo-oligosaccharides, and/or galacto-oligosaccharides); RR, risk ratio; SinglePrb, single-strain probiotics.
All-Cause Mortality
The 80 studies reporting all-cause mortality enrolled 21 793 infants and addressed 9 preventive interventions (Figure 2A). No statistically significant incoherence was observed in any of the network loops (eFigure 5 in Supplement 1). Of the 14 available direct comparisons, in 6 comparisons, 2 or more studies were available for conventional pairwise meta-analysis in which the I2 was zero in 2 comparisons, less than 50% in 3 comparisons, and more than 50% in 1 comparison (eTable 4 in Supplement 1).
Compared with placebo, only multiple-strain probiotics were associated with reduced mortality (RR, 0.69; 95% CI, 0.56 to 0.86; high certainty; RD, −1.7%; 95% CI, −2.4% to −0.8%) (Table, Figure 3, and Figure 4). No other interventions showed statistically significant benefits. Figure 4 shows the comparative effectiveness and certainty of evidence for all pairwise comparisons.
Table. Network Meta-Analysis Results for the Comparisons of Interventions vs Placebo Ranked Using the GRADE Approacha.
| Certainty (GRADE) | Classification | Intervention | RR (95% CI) | SUCRAb | RD per 1000 (95% CI)c |
|---|---|---|---|---|---|
| All-cause mortality | |||||
| High (moderate to high) | The most effective | Multistrain probiotics | 0.69 (0.56 to 0.86)d | 62.7 | -16.71 (−23.72 to −7.55)d |
| Among the least effective | Single-strain probiotics | 0.85 (0.70 to 1.04) | 44.1 | −8.09 (−16.17 to 2.16) | |
| Oligosaccharides | 0.47 (0.17 to 1.27) | 76.0 | −28.57 (−44.74 to 14.55) | ||
| Single-strain probiotics + OLGS | 0.34 (0.10 to 1.15) | 85.0 | −35.57 (−48.51 to 8.09) | ||
| Single-strain probiotics + lactoferrin | 0.70 (0.33 to 1.49) | 56.7 | −16.17 (−36.11 to 26.41) | ||
| Lactoferrin | 1.00 (0.80 to 1.25) | 25.6 | 0.01 (−10.78 to 13.48) | ||
| Multistrain probiotics + OLGS + lactoferrin | 2.00 (0.51 to 7.78) | 11.3 | 53.90 (−26.41 to 365.44) | ||
| Low (low to very low) | May be among the least effective | Multistrain probiotics + OLGS | 0.65 (0.30 to 1.41) | 61.0 | −18.87 (−37.73 to 22.10) |
| Multistrain probiotics + lactoferrin | 0.67 (0.04 to 10.32) | 53.0 | −17.79 (−51.74 to 502.35) | ||
| Severe NEC (stage 2 or 3) | |||||
| High (moderate to high) | Among the most effective | Multistrain probiotics + OLGS | 0.13 (0.05 to 0.37)d | 85.5 | -51.24 (−55.96 to −37.12)d |
| Multistrain probiotics | 0.38 (0.30 to 0.50)d | 61.3 | -36.52 (−41.23 to −29.45)d | ||
| Inferior to the most effective/superior to the least effective | Single-strain probiotics + lactoferrin | 0.05 (0.00 to 0.79)d | 91.3 | -55.96 (−58.90 to −12.37)d | |
| Single-strain probiotics + OLGS | 0.31 (0.12 to 0.84)d | 65.3 | -40.64 (−51.83 to −9.42)d | ||
| Single-strain probiotics | 0.61 (0.47 to 0.80)d | 42.7 | -22.97 (−31.22 to −11.78)d | ||
| Among the least effective | Multistrain probiotics + OLGS + lactoferrin | 0.25 (0.02 to 2.56) | 65.1 | −44.18 (−57.72 to 91.88) | |
| Multistrain probiotics + lactoferrin | 0.74 (0.07 to 8.06) | 35.3 | −15.31 (−54.78 to 415.83) | ||
| Lactoferrin | 0.86 (0.60 to 1.25) | 24.8 | −8.25 (−23.56 to 14.73) | ||
| Low (low to very low) | May be the least effective | Oligosaccharides | 1.09 (0.62 to 1.93) | 13.4 | 5.30 (−22.38 to 54.78) |
| Culture-proven sepsis | |||||
| High (moderate to high) | The most effective | Single-strain probiotics + lactoferrin | 0.33 (0.14 to 0.78)d | 96.5 | -106.73 (−137.00 to −35.05)d |
| Inferior to the most effective/superior to the least effective | Multistrain probiotics | 0.84 (0.74 to 0.95)d | 49.7 | -25.49 (−41.42 to −7.97)d | |
| Among the least effective | Single-strain probiotics + OLGS | 0.68 (0.30 to 1.54) | 64.0 | −50.98 (−111.51 to 86.02) | |
| Single-strain probiotics | 0.86 (0.73 to 1.00) | 45.9 | −22.30 (−43.01 to 0.00) | ||
| Multistrain probiotics + OLGS + lactoferrin | 1.48 (0.78 to 2.81) | 8.1 | 76.46 (−35.05 to 288.33) | ||
| Low (low to very low) | May be the most effective | Lactoferrin | 0.74 (0.60 to 0.93)d | 66.6 | -41.42 (−63.72 to −11.15)d |
| May be among the least effective | Multistrain probiotics + OLGS | 0.72 (0.49 to 1.07) | 66.0 | −44.60 (−81.24 to 11.15) | |
| Oligosaccharides | 0.82 (0.57 to 1.17) | 52.3 | −28.67 (−68.50 to 27.08) | ||
| Multistrain probiotics + lactoferrin | 1.13 (0.38 to 3.41) | 29.8 | 20.71 (−98.76 to 383.91) | ||
| Feeding intolerance | |||||
| High (moderate to high) | Among the most effective | Multistrain probiotics + OLGS | 0.45 (0.29 to 0.67)d | 76.4 | -141.52 (−182.68 to −84.91)d |
| Multistrain probiotics | 0.61 (0.46 to 0.80)d | 50.1 | -100.35 (−138.94 to −51.46)d | ||
| Single-strain probiotics | 0.61 (0.51 to 0.72)d | 49.3 | -100.35 (−126.07 to −72.04)d | ||
| Among the least effective | Single-strain probiotics + OLGS | 0.51 (0.18 to 1.45) | 61.8 | −126.08 (−210.99 to 115.79) | |
| Oligosaccharides | 0.83 (0.47 to 1.45) | 27.1 | −43.74 (−136.37 to 115.79) | ||
| Low (low to very low) | May be the most effective | Lactoferrin | 0.39 (0.21 to 0.71)d | 82.6 | -156.95 (−203.27 to −74.62)d |
| May be among the least effective | Multistrain probiotics + OLGS + lactoferrin | 1.00 (0.02 to 50.23) | 40.7 | 0.01 (−252.15 to 12666.88) | |
Abbreviations: GRADE, Grading of Recommendations, Assessment, Development, and Evaluation; NEC, necrotizing enterocolitis; OLGS, oligosaccharides (human milk, fructo- and/or galacto-oligosaccharides); RD, risk difference; RR, risk ratio; SUCRA, surface under the cumulative ranking curve.
Results are RR and associated 95% CIs between the intervention and placebo from the network meta-analysis. An RR <1 indicates the intervention is superior to placebo.
The larger the SUCRA value, the better the rank of the treatment.
RDs were calculated using baseline risk acquired from the placebo/control arm of included trials. Baseline risk for all-cause mortality, 5.39%; severe NEC (stage 2 or 3), 5.89%; culture-proven sepsis, 15.93%; and feeding intolerance, 25.73%.
Results were statistically significant.
Figure 4. Network Meta-Analysis Results for All-Cause Mortality (Bottom-Left Half) and Necrotizing Enterocolitis Stage 2 or 3 (Top-Right Half).
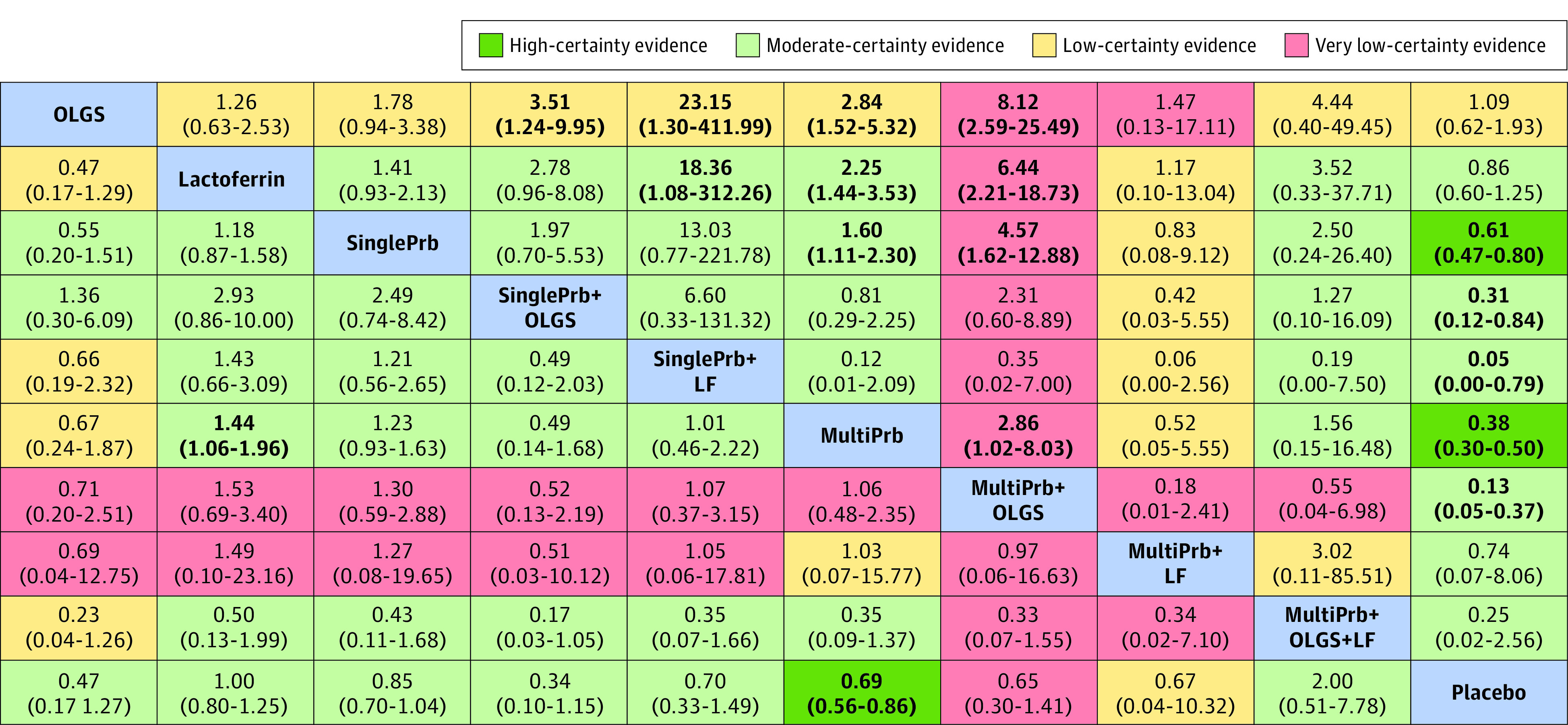
Results are risk ratio (95% CI) from the network meta-analysis. For each column compared with the row, a risk ratio less than 1 means the intervention in the top-left blue cell is better (a risk ratio >1 favors the intervention in the column). Numbers in bold indicate statistically significant results. Each result cell’s color represents the certainty of the evidence for the pairwise comparison. LF indicates lactoferrin; MultiPrb, multiple-strain probiotics; OLGS, oligosaccharides (human milk oligosaccharides, fructo-oligosaccharides, and/or galacto-oligosaccharides); SinglePrb, single-strain probiotics.
Severe NEC (Stage 2 or 3)
Of the 106 RCTs, 90 with 22 709 infants involving 9 preventive interventions addressed the outcome severe NEC (Figure 2B). We observed incoherence in 1 closed loop of evidence involving placebo, single-strain probiotics, and oligosaccharides (eFigure 6 in Supplement 1). Of the 15 direct comparisons, 8 involved 2 studies or more, none of which showed substantial heterogeneity (I2 > 50%). The observed incoherence did not result in biased estimates because the pooled estimates for all direct comparisons proved to be similar to the NMA estimates (Table and eTable 5 in Supplement 1).
Multiple-strain probiotics alone (vs placebo: RR, 0.38; 95% CI, 0.30 to 0.50; high certainty; RD, 3.7%; 95% CI, −4.1% to −2.9%) or in combination with oligosaccharides (vs placebo: RR, 0.13; 95% CI, 0.05 to 0.37; moderate certainty; RD, −5.1%; 95% CI, −5.6% to −3.7%) were among the most effective interventions in reducing severe necrotizing enterocolitis (Figure 3). Single-strain probiotics alone (vs placebo: RR, 0.61; 95% CI, 0.47 to 0.80; high certainty; RD, −2.3%; 95% CI, −3.1% to −1.2%) or in combination with lactoferrin (vs placebo: RR, 0.05; 95% CI, 0.01 to 0.79; moderate certainty; RD, −5.6%; 95% CI, −5.9% to −1.2%) or oligosaccharides (vs placebo: RR, 0.31; 95% CI, 0.12 to 0.84; moderate certainty; RD, −4.1%; 95% CI, −5.2% to −0.9%) were associated with intermediate effectiveness (Table, Figure 3, and Figure 4). Figure 4 shows the comparative effectiveness and certainty for all pairwise comparisons.
Culture-Proven Late-Onset Sepsis
Culture-proven LOS was reported in 81 RCTs involving 21 808 infants comparing 9 preventive interventions with 14 direct comparisons (Figure 2C). Our analysis showed no evidence of incoherence (eFigure 7 in Supplement 1). Heterogeneity in 2 of 8 direct comparisons with placebo was substantial (I2 = 52.3% for multiple-strain probiotics and 55.9% for lactoferrin vs placebo) (eTable 6 in Supplement 1).
The combination of single-strain probiotics with lactoferrin was the most effective intervention in reduction of LOS (vs placebo: RR, 0.33; 95% CI, 0.14 to 0.78; moderate certainty; RD, −10.7%; 95% CI, −13.7% to −3.5%) (Figure 3). Multiple-strain probiotics (vs placebo: RR, 0.84; 95% CI, 0.74 to 0.95; moderate certainty; RD, −2.5%; 95% CI, −4.1% to −0.8%) proved of intermediate effectiveness (Figure 3 and Table). Low-certainty evidence suggests lactoferrin may be associated with a reduction in culture-proven LOS (vs placebo: RR, 0.74; 95% CI, 0.60 to 0.93; RD, −4.1%; 95% CI, −6.4% to −1.1%) (Figure 3 and Table). No other intervention showed statistically significant benefit. eTable 7 in Supplement 1 shows the comparative effectiveness and certainty for all pairwise comparisons.
Other Outcomes
eTables 8-11 in Supplement 1 provide the results of NMA for other outcomes, and Figure 3 summarizes these results. eTables 12-17 in Supplement 1 present the results of direct comparisons. The magnitude and direction of the effects for all NMA estimates across secondary outcomes were comparable with those of direct estimates.
NEC-Related Mortality
NEC-related mortality was reported in 34 studies involving 9553 infants and 7 interventions (eFigure 1 in Supplement 1). When compared with placebo, no intervention showed statistically significant benefit (Figure 3 and eTable 8 in Supplement 1). We found no evidence of incoherence (eFigure 8 in Supplement 1).
Feeding Intolerance
The 25 studies that reported feeding intolerance enrolled 5074 infants and involved 7 interventions (Figure 2D). Of the 12 direct comparisons, 6 were informed by 2 or more studies with low statistical heterogeneity (I2 > 50%, eTable 13 in Supplement 1). We found no statistical evidence of incoherence (eFigure 9 in Supplement 1). Multiple-strain probiotics alone (RR, 0.61; 95% CI, 0.46 to 0.80; high certainty; RD, −10.0%; 95% CI, −13.9% to −5.1%) or in combination with oligosaccharides (RR, 0.45; 95% CI, 0.29 to 0.67; high certainty; RD, −14.1%; 95% CI, −18.3% to −8.5%) and single-strain probiotics (vs placebo: RR, 0.61; 95% CI, 0.51 to 0.72; high certainty; RD, −10.0%; 95% CI, −12.6% to −7.2%) proved of best effectiveness in reduction of feeding intolerance vs placebo (Figure 3 and Table). Low-certainty evidence suggests lactoferrin may be associated with a reduction in feeding intolerance (vs placebo, RR, 0.39; 95% CI, 0.21 to 0.71; RD, −15.7%; 95% CI, −20.3% to −7.5%) (Figure 3 and Table). No other interventions showed statistically significant benefit (eTable 8 in Supplement 1).
Time to Reach Full Enteral Feeding
We identified 64 studies (13 634 infants) that reported time to reach full enteral feeding involving 8 interventions. Of the 6 direct comparisons with 2 or more studies, 5 comparisons had substantial heterogeneity (I2 > 50%) (eFigure 2 and eTable 14 in Supplement 1). We observed incoherence in 1 study with closed loops of evidence involving placebo, oligosaccharides, and single-strain probiotics plus oligosaccharides (eFigure 10 in Supplement 1). Single-strain probiotics (MD, −1.94 days; 95% CI, −2.96 to −0.92; moderate certainty) and multistrain probiotics (MD, −2.03 days; 95% CI, −3.04 to −1.02; moderate certainty) proved the most effective in reducing the number of days to reach full enteral feeding compared with placebo (Figure 3 and eTables 9 and 10 in Supplement 1).
Duration of Hospital Stay
The 55 studies (15 174 infants) that reported duration of hospital stay involved 8 interventions in 5 direct comparisons with 2 or more studies, of which 3 had substantial heterogeneity (I2 > 50%) (eFigure 3 and eTable 15 in Supplement 1). We found no statistical evidence of incoherence (eFigure 11 in Supplement 1). Only single-strain and multistrain probiotics were associated with greater effectiveness compared with placebo in reducing the duration of hospitalization (MD, −3.31 days; 95% CI, −5.05 to −1.58; moderate certainty; and MD, −2.20 days; 95% CI, −4.08 to −0.31; moderate certainty, respectively) (Figure 3 and eTables 9 and 11 in Supplement 1).
Weight Change
The 17 studies that reported weight at discharge or 37 weeks’ postnatal age involved 3393 infants and 6 interventions in 3 direct comparisons with 2 or more studies (eFigure 4 and eTable 16 in Supplement 1). When compared with placebo, no intervention was associated with statistically significant change in weight (eTable 11 in Supplement 1). We found no evidence of incoherence (eFigure 12 in Supplement 1).
Additional Analysis
eFigures 13-20 in Supplement 1 provide probability ranking curves for all the outcomes. Comparison-adjusted funnel plots across outcomes showed no evidence of small-study effect (eFigures 21-28 in Supplement 1). We performed network meta-regression on all-cause mortality, severe NEC, LOS, and feeding intolerance but did not find any important evidence of effect modifications for any of the variables we tested. While only a few trials investigated adverse events, no study found any intervention-related adverse effects.
Discussion
In this systematic review and NMA comparing the benefits of probiotics, prebiotics, lactoferrin, and combination products for prevention of mortality and morbidity in preterm infants, we found moderate- to high-certainty evidence that multiple-strain probiotics were associated with reduced mortality (unlike any other intervention) and were among the best interventions associated with important reductions in all-cause mortality, severe NEC, LOS, feeding intolerance, time to reach full feeding, and duration of hospital stay (Figure 3). Prebiotics alone likely have little to no benefit. Combination products, including single- and multiple-strain probiotics combined with prebiotics or lactoferrin, were associated with the largest reduction in morbidity and mortality (Figure 3). We did not observe any effect modification for risk of bias, birth weight, gestational age, feeding with breast milk, or delivery type.
Strengths of our review include a comprehensive comparative assessment of all relevant interventions to reduce morbidity and mortality in low-birth-weight infants; a comprehensive search for such studies; duplicate assessment of eligibility, risk of bias, and data abstraction; and use of the GRADE approach for assessing the certainty in the NMA effect estimates as well as in providing a simple and user-friendly presentation of the relative performance of each intervention on each outcome (Figure 3).
There are 3 previously published NMAs and 2 strain-specific meta-analyses investigating the associations of different probiotic strains with morbidity and mortality in preterm infants.15,18,19,50,51 Two strain-specific meta-analyses of RCTs and non-RCTs involving preterm infants showed no significant benefits for B breve (M-16V)50 associated with severe NEC, late-onset sepsis, all-cause mortality, and time to reach full enteral feedings, while L reuteri (DSM 17938)15 was associated with significantly reduced LOS, time to reach full feedings, and duration of hospitalization and nonsignificant reductions in severe NEC and all-cause mortality. Two of these NMAs provided evidence that a combination of strains (multiple-strain probiotics) is usually better than any single-strain probiotics, but the paucity of studies addressing particular strains or combinations of strains limited inferences regarding comparative benefits.19,51 Beghetti et al18 included fewer trials but performed stratified NMA by feeding type and found a minority of single-strain probiotics may be associated with reduction of NEC. However, their conclusions were based on ranking probabilities and limited by the small number of trials included in the analysis.
The enhanced preventive effects of multiple-strain probiotics compared with single-stain formulations might be owing to the combined effects of multiple strains, each with specific biological properties (eg, anti-inflammatory, immune modulation, intestinal motility, epithelial barrier function) or simply the consequence of a higher probiotic dose.13 Though multistrain probiotics are the only intervention with strong evidence for reductions in mortality and morbidity in preterm infants, limited evidence on combination products suggest considerable uncertainty around their comparative effectiveness. The underlying mechanisms of action for the beneficial effects of synbiotics and combination products remain uncertain but might be based on an additive or synergistic interaction when combining lactoferrin or prebiotics and probiotics.52 One possible explanation is that prebiotics and lactoferrin can act as a food source for probiotic colonization. In healthy term infants, the capacity of bifidobacteria strains to consume human milk oligosaccharides present in human breast milk as substrate is considered a primary mechanism promoting bacterial colonization.53,54
The most severe harm associated with the use of probiotics for prevention of morbidity and mortality in preterm infants is sepsis caused by administered probiotics due to translocation across the intestinal epithelial barrier.53,55 This is reported to be extremely rare13,55 with none of our included studies reporting cases of probiotic-associated sepsis, which is consistent with what other recent systematic reviews have reported.8,13,15,17,20,50,51
Limitations
The limitations of the review are largely those of the primary evidence: for many interventions, only low-quality or very low-quality evidence was available (Figure 3). Variability in probiotic composition (ie, diverse strains, species, and doses) makes it difficult to identify with certainty the most effective probiotic species or synbiotics products for clinical use. In a separate study, we performed an NMA to investigate the effectiveness of different probiotic species19; despite limited evidence and variability in probiotic strains used across trials, we found combinations of 1 or more Lactobacillus species and 1 or more Bifidobacterium species were superior to single- and other multiple-strain probiotic products.
Conclusions
In this systematic review and NMA, moderate- to high-certainty evidence demonstrated the superiority of multistrain probiotics over alternative prophylactic interventions. Combination products, including single- and multiple-strain probiotics combined with prebiotics or lactoferrin, were associated with the largest reduction in morbidity and mortality, but these benefits were only supported by a small number of trials directly comparing combination products with single- or multiple-strain probiotics. While certain probiotics with evidence of efficacy and safety are now used in clinical practice, the findings of this study suggest that multistrain probiotics alone or combination products are associated with greater benefit in preventing morbidity and mortality in preterm infants and should be prioritized in future trials.
eMethods 1. Search strategies
eMethods 2. Additional methods
eMethods 3. References of trials included in the network meta-analysis
eTable 1. Characteristics of participants of trials included in the network meta-analysis
eTable 2. Intervention characteristics of trials and outcomes included in the network meta-analysis
eTable 3. Summary of risk of bias assessments for included trials
eTable 4. Results of direct pairwise comparisons with number of trials and events for each trial arm and certainty of evidence (CoE) for all-cause mortality
eTable 5. Results of direct pairwise comparisons with number of trials and events for each trial arm and certainty of evidence (CoE) for severe NEC (Bell’s criteria stage II or more)
eTable 6. Results of direct pairwise comparisons with number of trials and events for each trial arm and certainty of evidence (CoE) for culture proven late-onset sepsis
eTable 7. Network meta-analysis results for culture-proven late-onset sepsis
eTable 8. Network meta-analysis results for NEC-related mortality (bottom half) and feed intolerance (top half)
eTable 9. Network meta-analysis results (mean difference and the 95% CIs) sorted based on GRADE certainty of evidence (CoE) for the comparisons of active interventions vs. placebo (PLC) for time to reach full enteral feed (days) and duration of hospitalization (days)
eTable 10. Network meta-analysis results time to reach full enteral feed (days – top half)
eTable 11. Network meta-analysis results for duration of hospitalization (days – top half) and weight at discharge or 37 weeks’ postnatal age (grams–bottom half)
eTable 12. Results of direct pairwise comparisons with number of trials and events for each trial arm and certainty of evidence (CoE) NEC-related mortality
eTable 13. Results of direct pairwise comparisons with number of trials and events for each trial arm and certainty of evidence (CoE) feed intolerance
eTable 14. Results of direct pairwise comparisons with number of trials and participants for each trial arm and certainty of evidence (CoE) for time to reach full enteral feed (days)
eTable 15. Results of direct pairwise comparisons with number of trials and participants for each trial arm and certainty of evidence (CoE) for duration of hospitalization (days)
eTable 16. Results of direct pairwise comparisons with number of trials and participants for each trial arm and certainty of evidence (CoE) for weight at discharge or 37 weeks’ postnatal age (grams)
eTable 17. Results of sensitive analysis for severe NEC (Bell’s criteria stage II or more) with data excluded from Hay 2018 trial arm
eFigure 1. Network of eligible comparisons for NEC-related mortality
eFigure 2. Network of eligible comparisons for time to reach full enteral feed
eFigure 3. Network of eligible comparisons for duration of hospitalization
eFigure 4. Network of eligible comparisons for weight at discharge or 37 weeks’ postnatal age
eFigure 5. Incoherence plot for all-cause mortality
eFigure 6. Incoherence plot for severe NEC (Bell’s criteria stage II or more)
eFigure 7. Incoherence plot for culture proven late-onset sepsis
eFigure 8. Incoherence plot for NEC-related mortality
eFigure 9. Incoherence plot for feed intolerance
eFigure 10. Incoherence plot for time to reach full enteral feed
eFigure 11. Incoherence plot for duration of hospitalization
eFigure 12. Incoherence plot for weight at discharge or 37 weeks’ postnatal age
eFigure 13. Plots of the surface under the cumulative ranking curves for all interventions for the ‘all-cause mortality’ outcome
eFigure 14. Plots of the surface under the cumulative ranking curves for all interventions for the ‘severe NEC (Bell’s criteria stage II or more)’ outcome
eFigure 15. Plots of the surface under the cumulative ranking curves for all interventions for the ‘culture proven late-onset sepsis’ outcome
eFigure 16. Plots of the surface under the cumulative ranking curves for all interventions for the ‘NEC-related mortality’ outcome
eFigure 17. Plots of the surface under the cumulative ranking curves for all interventions for the ‘feed intolerance’ outcome
eFigure 18. Plots of the surface under the cumulative ranking curves for all interventions for the ‘time to reach full enteral feed’ outcome
eFigure 19. Plots of the surface under the cumulative ranking curves for all interventions for the ‘duration of hospitalization’ outcome
eFigure 20. Plots of the surface under the cumulative ranking curves for all interventions for the ‘weight at discharge or at 37 weeks postnatal age’ outcome
eFigure 21. Comparison-adjusted funnel plot with pseudo 95% confidence limits including all the comparisons of the active intervention vs. placebo for all-cause mortality
eFigure 22. Comparison-adjusted funnel plot with pseudo 95% confidence limits including all the comparisons of the active intervention vs. placebo for severe NEC (Bell’s criteria stage II or more)
eFigure 23. Comparison-adjusted funnel plot with pseudo 95% confidence limits including all the comparisons of the active intervention vs. placebo for culture proven late-onset sepsis
eFigure 24. Comparison-adjusted funnel plot with pseudo 95% confidence limits including all the comparisons of the active intervention vs. placebo for NEC-related mortality
eFigure 25. Comparison-adjusted funnel plot with pseudo 95% confidence limits including all the comparisons of the active intervention vs. placebo for feed intolerance
eFigure 26. Comparison-adjusted funnel plot with pseudo 95% confidence limits including all the comparisons of the active intervention vs. placebo for time to reach full enteral feed
eFigure 27. Comparison-adjusted funnel plot with pseudo 95% confidence limits including all the comparisons of the active intervention vs. placebo for duration of hospitalization
eFigure 28. Comparison-adjusted funnel plot with pseudo 95% confidence limits including all the comparisons of the active intervention vs. placebo for ‘weight at discharge or at 37 weeks postnatal age
eReferences
Data sharing statement
References
- 1.Patel R, DuPont HL. New approaches for bacteriotherapy: prebiotics, new-generation probiotics, and synbiotics. Clin Infect Dis. 2015;60(suppl 2):S108-S121. doi: 10.1093/cid/civ177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dermyshi E, Wang Y, Yan C, et al. The “golden age” of probiotics: a systematic review and meta-analysis of randomized and observational studies in preterm infants. Neonatology. 2017;112(1):9-23. doi: 10.1159/000454668 [DOI] [PubMed] [Google Scholar]
- 3.Eaton S, Rees CM, Hall NJ. Current research on the epidemiology, pathogenesis, and management of necrotizing enterocolitis. Neonatology. 2017;111(4):423-430. doi: 10.1159/000458462 [DOI] [PubMed] [Google Scholar]
- 4.Battersby C, Santhalingam T, Costeloe K, Modi N. Incidence of neonatal necrotising enterocolitis in high-income countries: a systematic review. Arch Dis Child Fetal Neonatal Ed. 2018;103(2):F182-F189. doi: 10.1136/archdischild-2017-313880 [DOI] [PubMed] [Google Scholar]
- 5.Yee WH, Soraisham AS, Shah VS, Aziz K, Yoon W, Lee SK; Canadian Neonatal Network . Incidence and timing of presentation of necrotizing enterocolitis in preterm infants. Pediatrics. 2012;129(2):e298-e304. doi: 10.1542/peds.2011-2022 [DOI] [PubMed] [Google Scholar]
- 6.Lin PW, Stoll BJ. Necrotising enterocolitis. Lancet. 2006;368(9543):1271-1283. doi: 10.1016/S0140-6736(06)69525-1 [DOI] [PubMed] [Google Scholar]
- 7.Sharma D, Shastri S. Lactoferrin and neonatology: role in neonatal sepsis and necrotizing enterocolitis: present, past and future. J Matern Fetal Neonatal Med. 2016;29(5):763-770. doi: 10.3109/14767058.2015.1017463 [DOI] [PubMed] [Google Scholar]
- 8.Rao SC, Athalye-Jape GK, Deshpande GC, Simmer KN, Patole SK. Probiotic supplementation and late-onset sepsis in preterm infants: a meta-analysis. Pediatrics. 2016;137(3):e20153684. doi: 10.1542/peds.2015-3684 [DOI] [PubMed] [Google Scholar]
- 9.Caplan MS, Jilling T. New concepts in necrotizing enterocolitis. Curr Opin Pediatr. 2001;13(2):111-115. doi: 10.1097/00008480-200104000-00004 [DOI] [PubMed] [Google Scholar]
- 10.Deshpande G, Rao S, Patole S. Probiotics for prevention of necrotising enterocolitis in preterm neonates with very low birthweight: a systematic review of randomised controlled trials. Lancet. 2007;369(9573):1614-1620. doi: 10.1016/S0140-6736(07)60748-X [DOI] [PubMed] [Google Scholar]
- 11.Warner BB, Tarr PI. Necrotizing enterocolitis and preterm infant gut bacteria. Semin Fetal Neonatal Med. 2016;21(6):394-399. doi: 10.1016/j.siny.2016.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neu J, Pammi M. Pathogenesis of NEC: impact of an altered intestinal microbiome. Semin Perinatol. 2017;41(1):29-35. doi: 10.1053/j.semperi.2016.09.015 [DOI] [PubMed] [Google Scholar]
- 13.Chang HY, Chen JH, Chang JH, Lin HC, Lin CY, Peng CC. Multiple strains probiotics appear to be the most effective probiotics in the prevention of necrotizing enterocolitis and mortality: an updated meta-analysis. PLoS One. 2017;12(2):e0171579. doi: 10.1371/journal.pone.0171579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sawh SC, Deshpande S, Jansen S, Reynaert CJ, Jones PM. Prevention of necrotizing enterocolitis with probiotics: a systematic review and meta-analysis. PeerJ. 2016;4:e2429. doi: 10.7717/peerj.2429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Athalye-Jape G, Rao S, Patole S. Lactobacillus reuteri DSM 17938 as a probiotic for preterm neonates: a strain-specific systematic review. JPEN J Parenter Enteral Nutr. 2016;40(6):783-794. doi: 10.1177/0148607115588113 [DOI] [PubMed] [Google Scholar]
- 16.Pammi M, Abrams SA. Oral lactoferrin for the prevention of sepsis and necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev. 2015;(2):CD007137. doi: 10.1002/14651858.CD007137.pub4 [DOI] [PubMed] [Google Scholar]
- 17.AlFaleh K, Anabrees J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Evid Based Child Health. 2014;9(3):584-671. doi: 10.1002/ebch.1976 [DOI] [PubMed] [Google Scholar]
- 18.Beghetti I, Panizza D, Lenzi J, et al. Probiotics for preventing necrotizing enterocolitis in preterm infants: a network meta-analysis. Nutrients. 2021;13(1):192. doi: 10.3390/nu13010192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morgan RL, Preidis GA, Kashyap PC, Weizman AV, Sadeghirad B; McMaster Probiotic, Prebiotic, and Synbiotic Work Group . Probiotics reduce mortality and morbidity in preterm, low-birth-weight infants: a systematic review and network meta-analysis of randomized trials. Gastroenterology. 2020;159(2):467-480. doi: 10.1053/j.gastro.2020.05.096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olsen R, Greisen G, Schrøder M, Brok J. Prophylactic probiotics for preterm infants: a systematic review and meta-analysis of observational studies. Neonatology. 2016;109(2):105-112. doi: 10.1159/000441274 [DOI] [PubMed] [Google Scholar]
- 21.Schulzke SM, Deshpande GC, Patole SK. Neurodevelopmental outcomes of very low-birth-weight infants with necrotizing enterocolitis: a systematic review of observational studies. Arch Pediatr Adolesc Med. 2007;161(6):583-590. doi: 10.1001/archpedi.161.6.583 [DOI] [PubMed] [Google Scholar]
- 22.Sadeghirad B, Florez ID, Chang Y, et al. Comparative effectiveness of prophylactic therapies for necrotizing enterocolitis in preterm infants: protocol for a network meta-analysis of randomized trials. Int J Prev Med. 2018;9:83. doi: 10.4103/ijpvm.IJPVM_328_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777-784. doi: 10.7326/M14-2385 [DOI] [PubMed] [Google Scholar]
- 24.Higgins JPT, Altman DG, Gøtzsche PC, et al. ; Cochrane Bias Methods Group; Cochrane Statistical Methods Group . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akl EA, Sun X, Busse JW, et al. Specific instructions for estimating unclearly reported blinding status in randomized trials were reliable and valid. J Clin Epidemiol. 2012;65(3):262-267. doi: 10.1016/j.jclinepi.2011.04.015 [DOI] [PubMed] [Google Scholar]
- 26.Bell MJ, Ternberg JL, Feigin RD, et al. Neonatal necrotizing enterocolitis: therapeutic decisions based upon clinical staging. Ann Surg. 1978;187(1):1-7. doi: 10.1097/00000658-197801000-00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. 1986;33(1):179-201. doi: 10.1016/S0031-3955(16)34975-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sadeghirad B, Foroutan F, Zoratti MJ, et al. Theory and practice of Bayesian and frequentist frameworks for network meta-analysis. BMJ Evid Based Med. 2023;28(3):204-209. doi: 10.1136/bmjebm-2022-111928 [DOI] [PubMed] [Google Scholar]
- 29.White IR, Barrett JK, Jackson D, Higgins JP. Consistency and inconsistency in network meta-analysis: model estimation using multivariate meta-regression. Res Synth Methods. 2012;3(2):111-125. doi: 10.1002/jrsm.1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu G, Ades AE. Assessing evidence inconsistency in mixed treatment comparisons. J Am Stat Assoc. 2006;101(474):447-459. doi: 10.1198/016214505000001302 [DOI] [Google Scholar]
- 32.Higgins JP, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods. 2012;3(2):98-110. doi: 10.1002/jrsm.1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in Stata. PLoS One. 2013;8(10):e76654. doi: 10.1371/journal.pone.0076654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chaimani A, Salanti G. Visualizing assumptions and results in network meta-analysis: the network graphs package. Stata J. 2015;15(4):905-950. doi: 10.1177/1536867X1501500402 [DOI] [Google Scholar]
- 35.Guyatt GH, Oxman AD, Vist GE, et al. ; GRADE Working Group . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924-926. doi: 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Puhan MA, Schünemann HJ, Murad MH, et al. ; GRADE Working Group . A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ. 2014;349:g5630. doi: 10.1136/bmj.g5630 [DOI] [PubMed] [Google Scholar]
- 37.Brignardello-Petersen R, Bonner A, Alexander PE, et al. ; GRADE Working Group . Advances in the GRADE approach to rate the certainty in estimates from a network meta-analysis. J Clin Epidemiol. 2018;93:36-44. doi: 10.1016/j.jclinepi.2017.10.005 [DOI] [PubMed] [Google Scholar]
- 38.Zeng L, Brignardello-Petersen R, Hultcrantz M, et al. GRADE guidelines 32: GRADE offers guidance on choosing targets of GRADE certainty of evidence ratings. J Clin Epidemiol. 2021;137:163-175. doi: 10.1016/j.jclinepi.2021.03.026 [DOI] [PubMed] [Google Scholar]
- 39.Busse JW, Sadeghirad B, Oparin Y, et al. Management of acute pain from non-low back, musculoskeletal injuries: a systematic review and network meta-analysis of randomized trials. Ann Intern Med. 2020;173(9):730-738. doi: 10.7326/M19-3601 [DOI] [PubMed] [Google Scholar]
- 40.Phillips MR, Sadeghirad B, Busse JW, et al. Development and design validation of a novel network meta-analysis presentation tool for multiple outcomes: a qualitative descriptive study. BMJ Open. 2022;12(6):e056400. doi: 10.1136/bmjopen-2021-056400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brignardello-Petersen R, Florez ID, Izcovich A, et al. ; GRADE working group . GRADE approach to drawing conclusions from a network meta-analysis using a minimally contextualised framework. BMJ. 2020;371:m3900. doi: 10.1136/bmj.m3900 [DOI] [PubMed] [Google Scholar]
- 42.Li Y, Shimizu T, Hosaka A, Kaneko N, Ohtsuka Y, Yamashiro Y. Effects of bifidobacterium breve supplementation on intestinal flora of low birth weight infants. Pediatr Int. 2004;46(5):509-515. doi: 10.1111/j.1442-200x.2004.01953.x [DOI] [PubMed] [Google Scholar]
- 43.Coleta E, Gheonea M, Sarbu M. Oral supplementation with probiotics in premature infants-a randomised clinical trial. Presented at: 24th Annual Meeting of the European Society of Paediatric and Neonatal Intensive Care; June 12, 2013; Rotterdam, Netherlands. [Google Scholar]
- 44.Koksal N, Varal I, Ozkan H, Bagci O, Dotan P. Effect of probiotic support on feeding intolerance and mortality at preterm infants. Presented at: 12th World Congress of Perinatal Medicine. November 3, 2015; Madrid, Spain. [Google Scholar]
- 45.Kapiki A, Costalos C, Oikonomidou C, Triantafyllidou A, Loukatou E, Pertrohilou V. The effect of a fructo-oligosaccharide supplemented formula on gut flora of preterm infants. Early Hum Dev. 2007;83(5):335-339. doi: 10.1016/j.earlhumdev.2006.07.003 [DOI] [PubMed] [Google Scholar]
- 46.Wang C, Shoji H, Sato H, et al. Effects of oral administration of bifidobacterium breve on fecal lactic acid and short-chain fatty acids in low birth weight infants. J Pediatr Gastroenterol Nutr. 2007;44(2):252-257. doi: 10.1097/01.mpg.0000252184.89922.5f [DOI] [PubMed] [Google Scholar]
- 47.Zeber-Lubecka N, Kulecka M, Ambrozkiewicz F, et al. Effect of Saccharomyces boulardii and mode of delivery on the early development of the gut microbial community in preterm infants. PLoS One. 2016;11(2):e0150306. doi: 10.1371/journal.pone.0150306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Indrio F, Riezzo G, Raimondi F, Bisceglia M, Cavallo L, Francavilla R. The effects of probiotics on feeding tolerance, bowel habits, and gastrointestinal motility in preterm newborns. J Pediatr. 2008;152(6):801-806. doi: 10.1016/j.jpeds.2007.11.005 [DOI] [PubMed] [Google Scholar]
- 49.Pärtty A, Luoto R, Kalliomaki M, Salminen S, Isolauri E. Effects of early prebiotic and probiotic supplementation on development of gut microbiota and fussing and crying in preterm infants: a randomized, double-blind, placebo-controlled trial. J Pediatr. 2013;163(5):1271-1272. doi: 10.1016/j.jpeds.2013.05.035 [DOI] [PubMed] [Google Scholar]
- 50.Athalye-Jape G, Rao S, Simmer K, Patole S. Bifidobacterium breve M-16V as a probiotic for preterm infants: a strain-specific systematic review. JPEN J Parenter Enteral Nutr. 2018;42(4):677-688. [DOI] [PubMed] [Google Scholar]
- 51.van den Akker CHP, van Goudoever JB, Szajewska H, et al. ; ESPGHAN Working Group for Probiotics, Prebiotics and Committee on Nutrition . Probiotics for preterm infants: a strain-specific systematic review and network meta-analysis. J Pediatr Gastroenterol Nutr. 2018;67(1):103-122. doi: 10.1097/MPG.0000000000001897 [DOI] [PubMed] [Google Scholar]
- 52.Johnson-Henry KC, Abrahamsson TR, Wu RY, Sherman PM. Probiotics, prebiotics, and synbiotics for the prevention of necrotizing enterocolitis. Adv Nutr. 2016;7(5):928-937. doi: 10.3945/an.116.012237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Underwood MA. Impact of probiotics on necrotizing enterocolitis. Semin Perinatol. 2017;41(1):41-51. doi: 10.1053/j.semperi.2016.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu ZT, Chen C, Newburg DS. Utilization of major fucosylated and sialylated human milk oligosaccharides by isolated human gut microbes. Glycobiology. 2013;23(11):1281-1292. doi: 10.1093/glycob/cwt065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Doron S, Snydman DR. Risk and safety of probiotics. Clin Infect Dis. 2015;60(suppl 2):S129-S134. doi: 10.1093/cid/civ085 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Search strategies
eMethods 2. Additional methods
eMethods 3. References of trials included in the network meta-analysis
eTable 1. Characteristics of participants of trials included in the network meta-analysis
eTable 2. Intervention characteristics of trials and outcomes included in the network meta-analysis
eTable 3. Summary of risk of bias assessments for included trials
eTable 4. Results of direct pairwise comparisons with number of trials and events for each trial arm and certainty of evidence (CoE) for all-cause mortality
eTable 5. Results of direct pairwise comparisons with number of trials and events for each trial arm and certainty of evidence (CoE) for severe NEC (Bell’s criteria stage II or more)
eTable 6. Results of direct pairwise comparisons with number of trials and events for each trial arm and certainty of evidence (CoE) for culture proven late-onset sepsis
eTable 7. Network meta-analysis results for culture-proven late-onset sepsis
eTable 8. Network meta-analysis results for NEC-related mortality (bottom half) and feed intolerance (top half)
eTable 9. Network meta-analysis results (mean difference and the 95% CIs) sorted based on GRADE certainty of evidence (CoE) for the comparisons of active interventions vs. placebo (PLC) for time to reach full enteral feed (days) and duration of hospitalization (days)
eTable 10. Network meta-analysis results time to reach full enteral feed (days – top half)
eTable 11. Network meta-analysis results for duration of hospitalization (days – top half) and weight at discharge or 37 weeks’ postnatal age (grams–bottom half)
eTable 12. Results of direct pairwise comparisons with number of trials and events for each trial arm and certainty of evidence (CoE) NEC-related mortality
eTable 13. Results of direct pairwise comparisons with number of trials and events for each trial arm and certainty of evidence (CoE) feed intolerance
eTable 14. Results of direct pairwise comparisons with number of trials and participants for each trial arm and certainty of evidence (CoE) for time to reach full enteral feed (days)
eTable 15. Results of direct pairwise comparisons with number of trials and participants for each trial arm and certainty of evidence (CoE) for duration of hospitalization (days)
eTable 16. Results of direct pairwise comparisons with number of trials and participants for each trial arm and certainty of evidence (CoE) for weight at discharge or 37 weeks’ postnatal age (grams)
eTable 17. Results of sensitive analysis for severe NEC (Bell’s criteria stage II or more) with data excluded from Hay 2018 trial arm
eFigure 1. Network of eligible comparisons for NEC-related mortality
eFigure 2. Network of eligible comparisons for time to reach full enteral feed
eFigure 3. Network of eligible comparisons for duration of hospitalization
eFigure 4. Network of eligible comparisons for weight at discharge or 37 weeks’ postnatal age
eFigure 5. Incoherence plot for all-cause mortality
eFigure 6. Incoherence plot for severe NEC (Bell’s criteria stage II or more)
eFigure 7. Incoherence plot for culture proven late-onset sepsis
eFigure 8. Incoherence plot for NEC-related mortality
eFigure 9. Incoherence plot for feed intolerance
eFigure 10. Incoherence plot for time to reach full enteral feed
eFigure 11. Incoherence plot for duration of hospitalization
eFigure 12. Incoherence plot for weight at discharge or 37 weeks’ postnatal age
eFigure 13. Plots of the surface under the cumulative ranking curves for all interventions for the ‘all-cause mortality’ outcome
eFigure 14. Plots of the surface under the cumulative ranking curves for all interventions for the ‘severe NEC (Bell’s criteria stage II or more)’ outcome
eFigure 15. Plots of the surface under the cumulative ranking curves for all interventions for the ‘culture proven late-onset sepsis’ outcome
eFigure 16. Plots of the surface under the cumulative ranking curves for all interventions for the ‘NEC-related mortality’ outcome
eFigure 17. Plots of the surface under the cumulative ranking curves for all interventions for the ‘feed intolerance’ outcome
eFigure 18. Plots of the surface under the cumulative ranking curves for all interventions for the ‘time to reach full enteral feed’ outcome
eFigure 19. Plots of the surface under the cumulative ranking curves for all interventions for the ‘duration of hospitalization’ outcome
eFigure 20. Plots of the surface under the cumulative ranking curves for all interventions for the ‘weight at discharge or at 37 weeks postnatal age’ outcome
eFigure 21. Comparison-adjusted funnel plot with pseudo 95% confidence limits including all the comparisons of the active intervention vs. placebo for all-cause mortality
eFigure 22. Comparison-adjusted funnel plot with pseudo 95% confidence limits including all the comparisons of the active intervention vs. placebo for severe NEC (Bell’s criteria stage II or more)
eFigure 23. Comparison-adjusted funnel plot with pseudo 95% confidence limits including all the comparisons of the active intervention vs. placebo for culture proven late-onset sepsis
eFigure 24. Comparison-adjusted funnel plot with pseudo 95% confidence limits including all the comparisons of the active intervention vs. placebo for NEC-related mortality
eFigure 25. Comparison-adjusted funnel plot with pseudo 95% confidence limits including all the comparisons of the active intervention vs. placebo for feed intolerance
eFigure 26. Comparison-adjusted funnel plot with pseudo 95% confidence limits including all the comparisons of the active intervention vs. placebo for time to reach full enteral feed
eFigure 27. Comparison-adjusted funnel plot with pseudo 95% confidence limits including all the comparisons of the active intervention vs. placebo for duration of hospitalization
eFigure 28. Comparison-adjusted funnel plot with pseudo 95% confidence limits including all the comparisons of the active intervention vs. placebo for ‘weight at discharge or at 37 weeks postnatal age
eReferences
Data sharing statement