Abstract
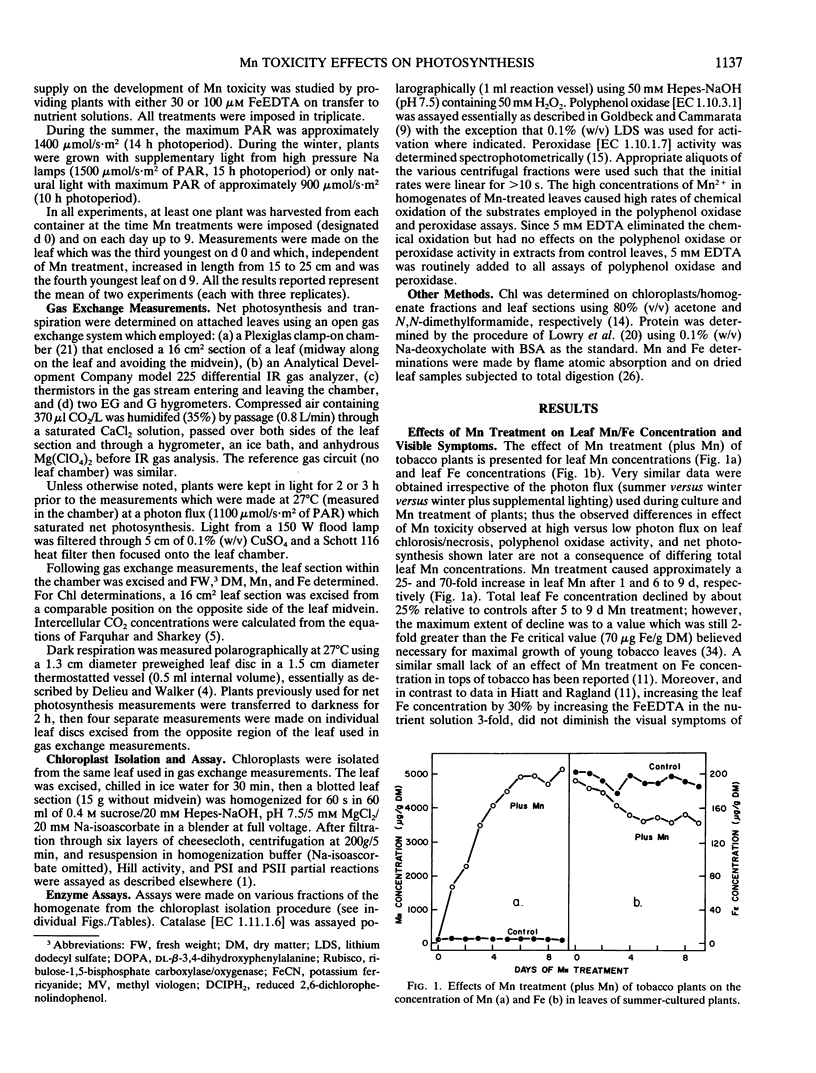
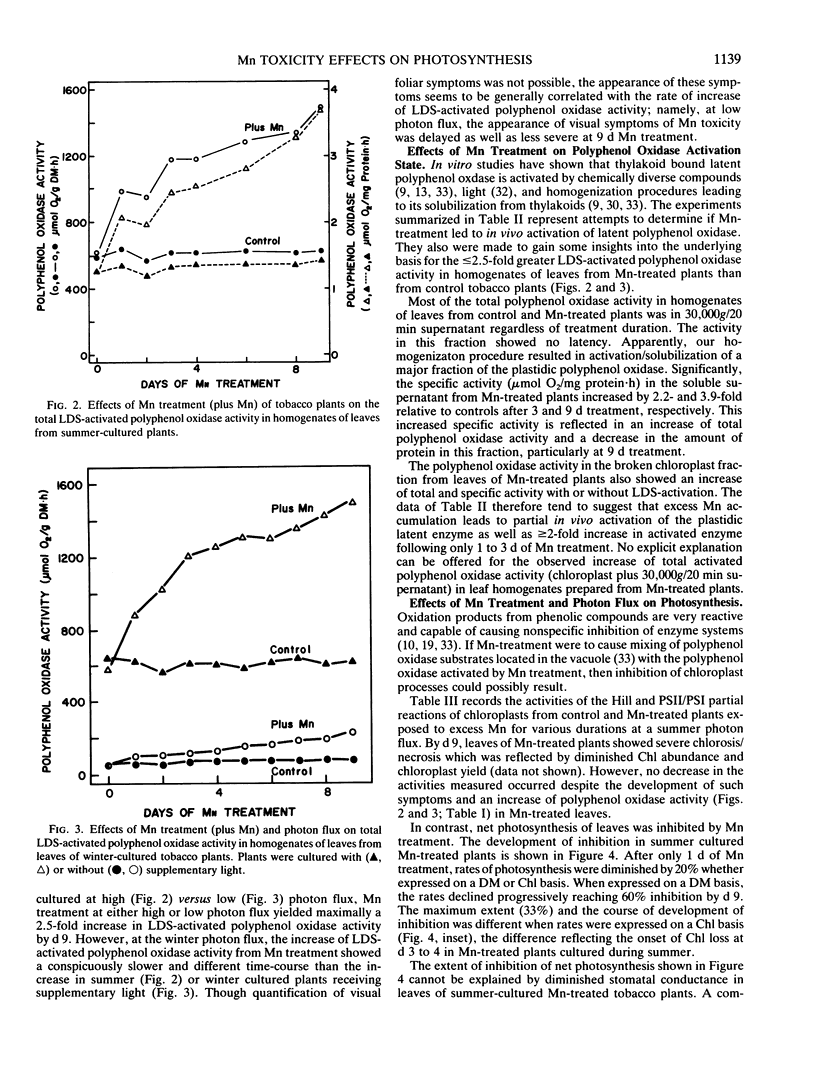
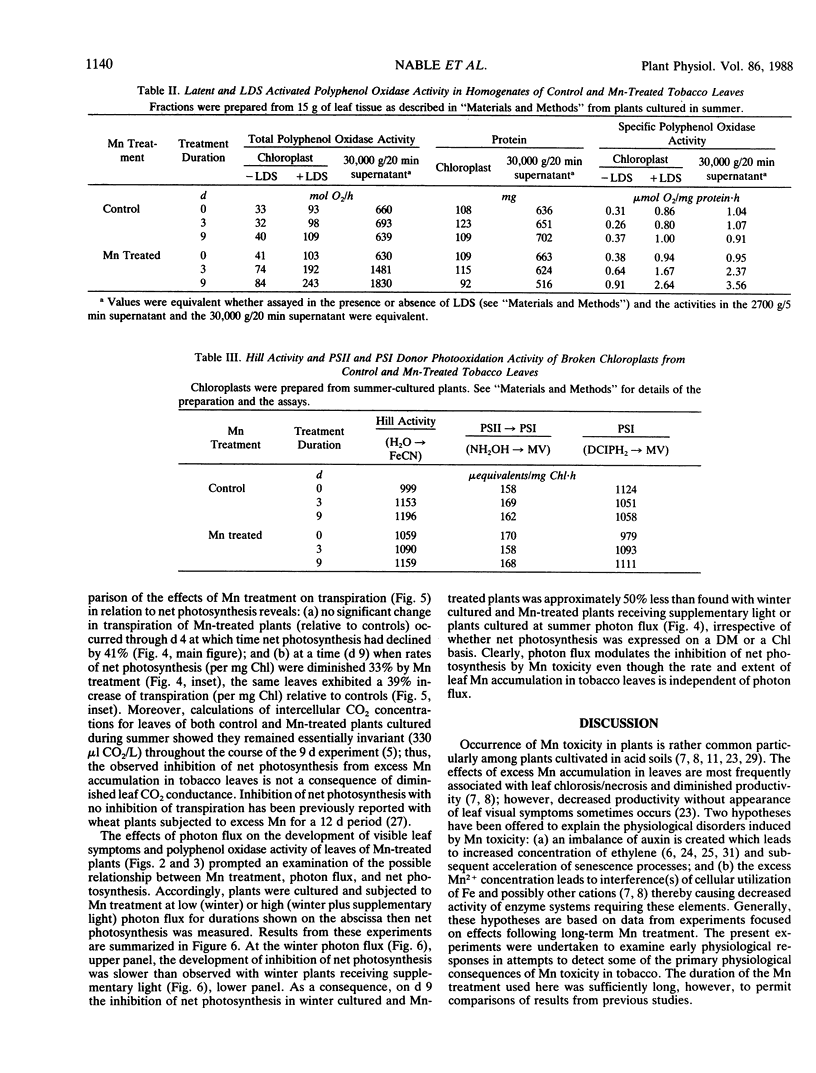
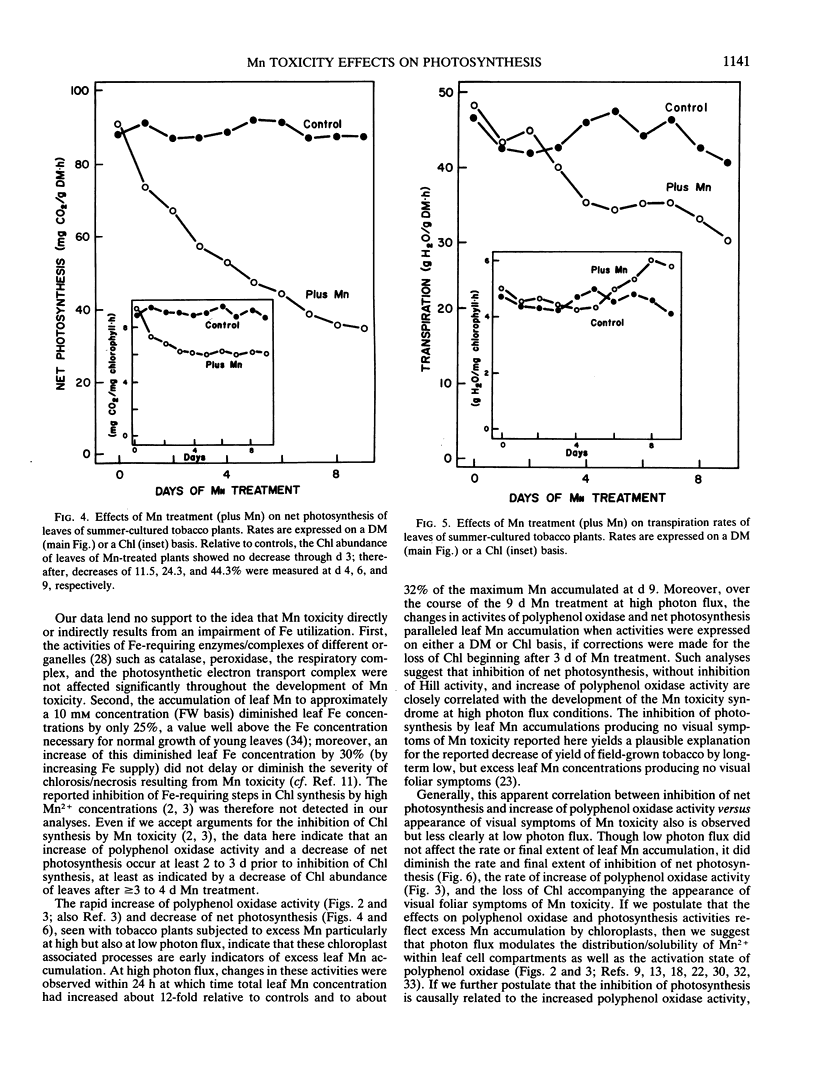
Early physiological effects of developing Mn toxicity in young leaves of burley tobacco (Nicotiana tabacum L. cv KY 14) were examined in glass-house/water cultured plants grown at high (summer) and low (winter) photon flux. Following transfer of plants to solutions containing 1 millimolar Mn2+, sequential samplings were made at various times for the following 9 days, during which Mn accumulation by leaves increased rapidly from ∼70 on day 0 to ∼1700 and ∼5000 microgram per gram dry matter after 1 and 9 days, respectively. In plants grown at high photon flux, net photosynthesis declined by ∼20 and ∼60% after 1 and 9 days, respectively, and the onset of this decline preceded appearance (after 3 to 4 days) of visible foliar symptoms of Mn toxicity. Intercellular CO2 concentrations and rates of transpiration were not significantly affected; moreover, the activity of the Hill and photosystem I and II partial reactions of chloroplasts remained constant despite ultimate development of severe necrosis. Though the activity of latent or activated polyphenol oxidase increased in parallel with Mn accumulation, neither leaf respiration nor the activity of catalase [EC 1.11.1.6] and peroxidase [EC 1.10.1.7] were greatly affected. These effects from Mn toxicity could not be explained by any changes in protein or chlorophyll abundance. Additionally, they were not a consequence of Mn induced Fe deficiency. Therefore, inhibition of net photosynthesis and enhancement of polyphenol oxidase activity are early indicators of excess Mn accumulation in tobacco leaves. These changes, as well as leaf visual symptoms of Mn toxicity, were less severe in plants cultured and treated at low photon flux even though the rates of leaf Mn accumulation at high and low photon flux were essentially equivalent.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Callahan F. E., Cheniae G. M. Studies on the photoactivation of the water-oxidizing enzyme : I. Processes limiting photoactivation in hydroxylamine-extracted leaf segments. Plant Physiol. 1985 Nov;79(3):777–786. doi: 10.1104/pp.79.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clairmont K. B., Hagar W. G., Davis E. A. Manganese toxicity to chlorophyll synthesis in tobacco callus. Plant Physiol. 1986 Jan;80(1):291–293. doi: 10.1104/pp.80.1.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csatorday K., Gombos Z., Szalontai B. Mn and Co toxicity in chlorophyll biosynthesis. Proc Natl Acad Sci U S A. 1984 Jan;81(2):476–478. doi: 10.1073/pnas.81.2.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler J. L., Morgan P. W. The relationship of the peroxidative indoleacetic Acid oxidase system to in vivo ethylene synthesis in cotton. Plant Physiol. 1972 Apr;49(4):555–559. doi: 10.1104/pp.49.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golbeck J. H., Cammarata K. V. Spinach Thylakoid Polyphenol Oxidase : ISOLATION, ACTIVATION, AND PROPERTIES OF THE NATIVE CHLOROPLAST ENZYME. Plant Physiol. 1981 May;67(5):977–984. doi: 10.1104/pp.67.5.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J. C., Kung S. D., Wildman S. G. Polypeptide chains of the large and small subunits of fraction I protein from tobacco. Arch Biochem Biophys. 1978 Jan 15;185(1):272–281. doi: 10.1016/0003-9861(78)90167-4. [DOI] [PubMed] [Google Scholar]
- Hutcheson S. W., Buchanan B. B. Polyphenol Oxidation by Vicia faba Chloroplast Membranes: STUDIES ON THE LATENT MEMBRANE-BOUND POLYPHENOL OXIDASE AND ON THE MECHANISM OF PHOTOCHEMICAL POLYPHENOL OXIDATION. Plant Physiol. 1980 Dec;66(6):1150–1154. doi: 10.1104/pp.66.6.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inskeep W. P., Bloom P. R. Extinction coefficients of chlorophyll a and B in n,n-dimethylformamide and 80% acetone. Plant Physiol. 1985 Feb;77(2):483–485. doi: 10.1104/pp.77.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar M., Mishra D. Catalase, Peroxidase, and Polyphenoloxidase Activities during Rice Leaf Senescence. Plant Physiol. 1976 Feb;57(2):315–319. doi: 10.1104/pp.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Loomis W. D. Overcoming problems of phenolics and quinones in the isolation of plant enzymes and organelles. Methods Enzymol. 1974;31:528–544. doi: 10.1016/0076-6879(74)31057-9. [DOI] [PubMed] [Google Scholar]
- Morgan P. W., Joham H. E., Amin J. V. Effect of manganese toxicity on the indoleacetic Acid oxidase system of cotton. Plant Physiol. 1966 Apr;41(4):718–724. doi: 10.1104/pp.41.4.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirkar S., Amin J. V. The manganese toxicity of cotton. Plant Physiol. 1974 Oct;54(4):539–543. doi: 10.1104/pp.54.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert N. E. Activation of polyphenol oxidase of chloroplasts. Plant Physiol. 1973 Feb;51(2):234–244. doi: 10.1104/pp.51.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]


