Abstract
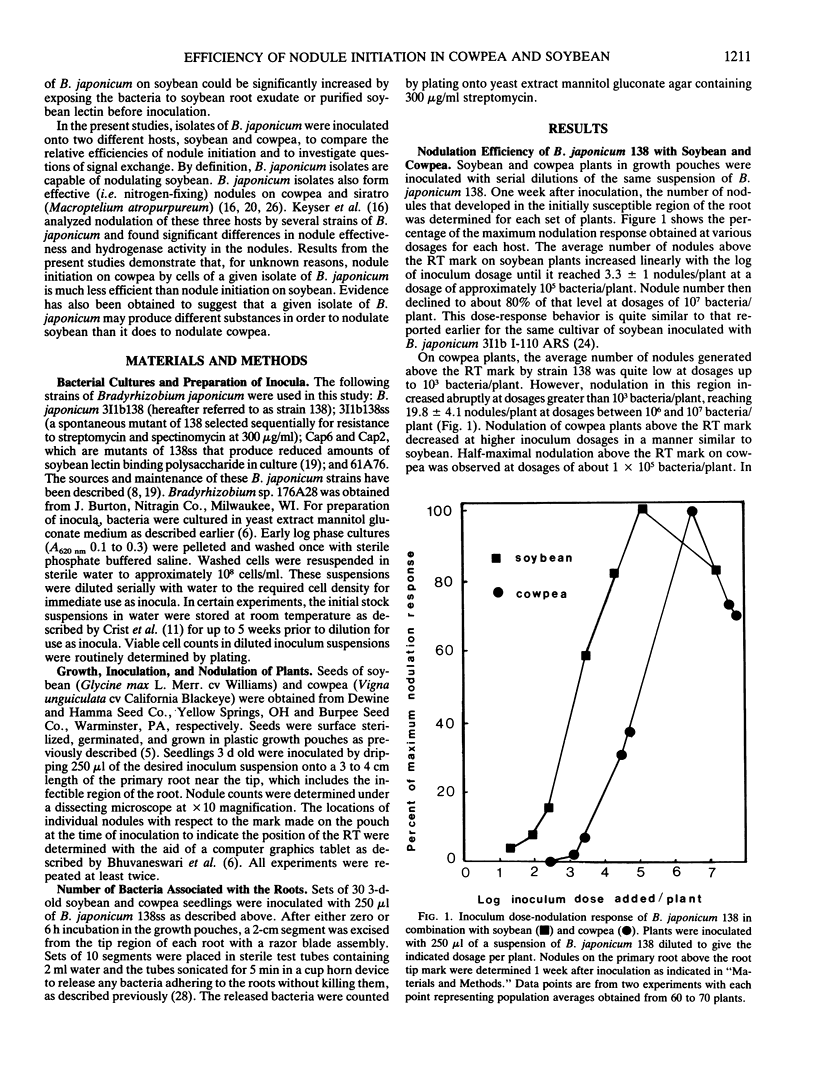
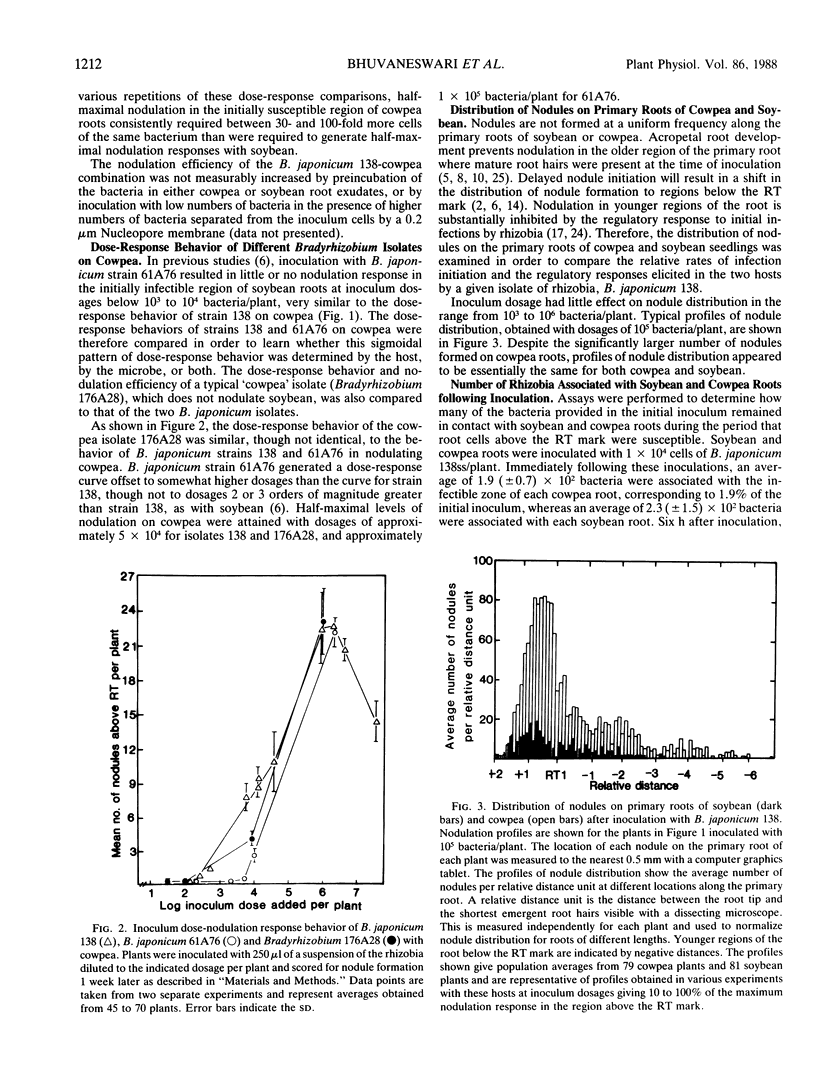
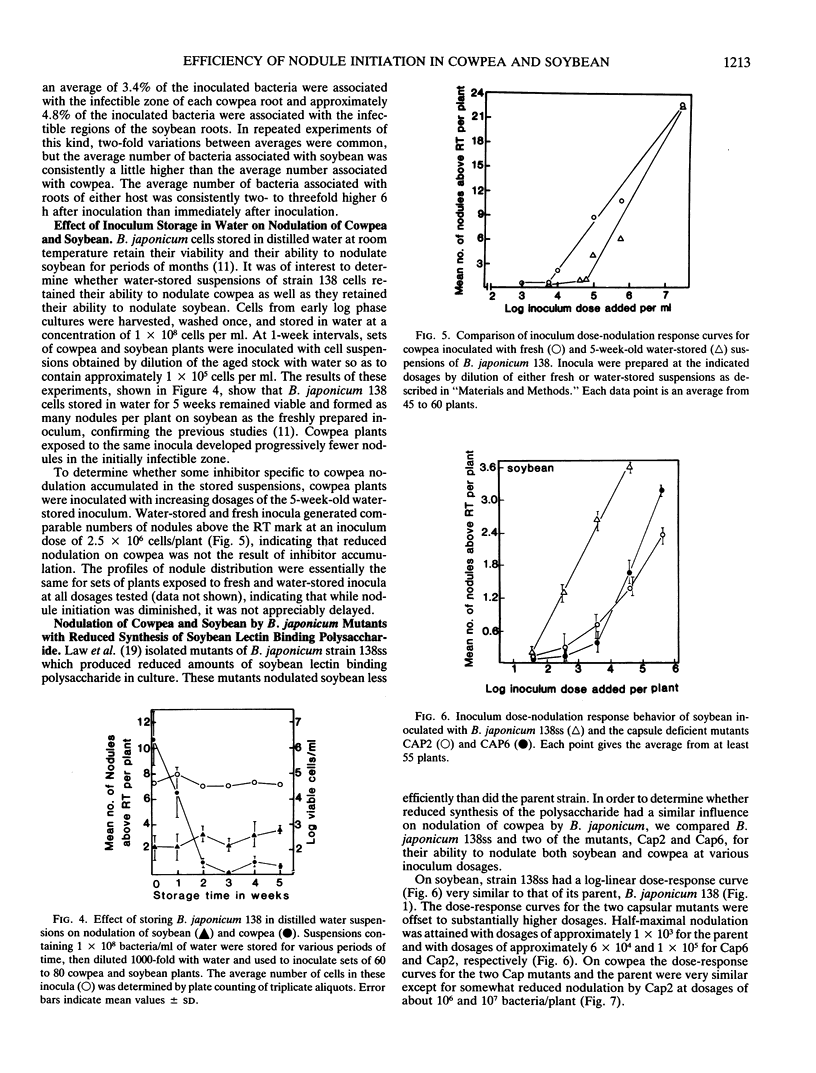
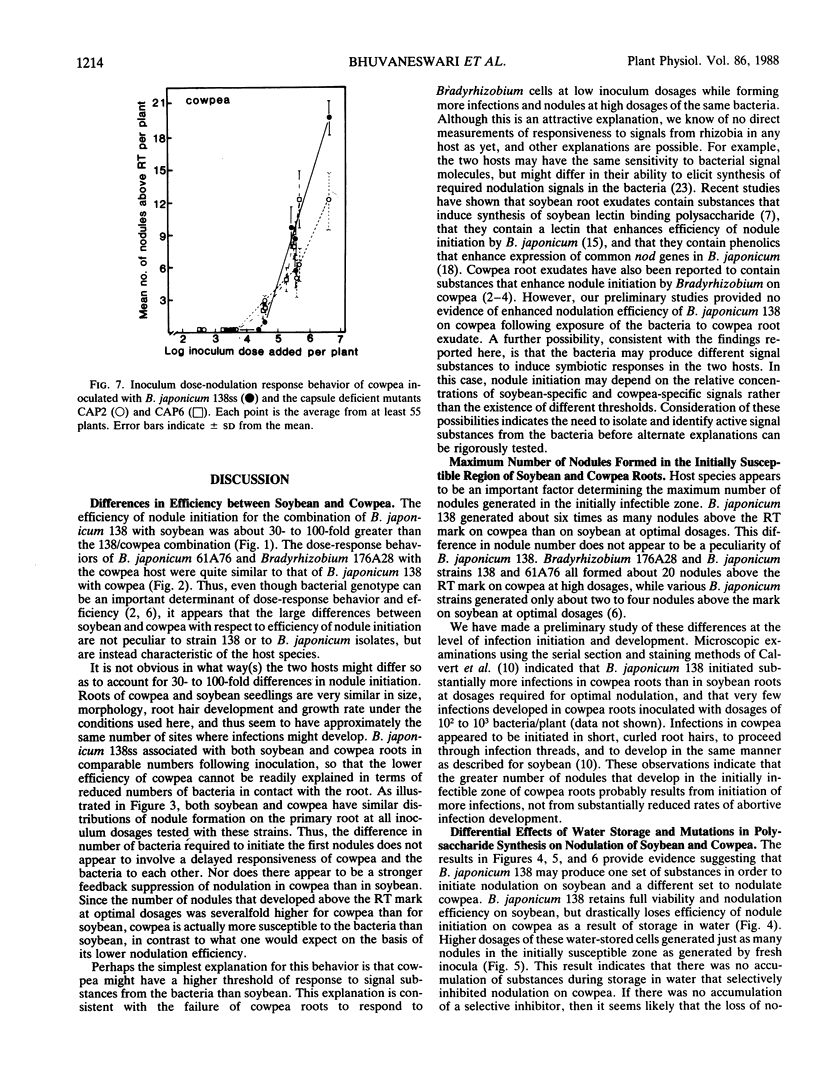
When serial dilutions of a suspension of Bradyrhizobium japonicum strain 138 were inoculated onto both soybean and cowpea roots, the formation of nodules in the initially susceptible region of the roots of both hosts was found to be linearly dependent on the log of the inoculum dosage until an optimum dosage was reached. Approximately 30- to 100-fold higher dosages were required to elicit half-maximal nodulation on cowpea than on soybean in the initially susceptible zone of the root. However, at optimal dosages, about six times as many nodules formed in this region on cowpea roots than on soybean roots. There was no appreciable difference in the apparent rate of nodule initiation on these two hosts nor in the number of inoculum bacteria in contact with the root. These results are consistent with the possibility that cowpea roots have a substantially higher threshold of response to symbiotic signals from the bacteria than do soybean roots. Storage of B. japonicum cells in distilled water for several weeks did not affect their viability or efficiency of nodule initiation on soybean. However, the nodulation efficiency of these same cells on cowpea diminished markedly over a 2 week period. These differential effects of water storage indicate that at least some aspects of signal production by the bacteria during nodule initiation are different on the two hosts. Mutants of B. japonicum 138 defective in synthesis of soybean lectin binding polysaccharide were defective in their efficiency of nodule initiation on soybean but not on cowpea. These results also suggest that B. japonicum may produce different substances to initiate nodules on these two hosts.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhagwat A. A., Thomas J. Legume-Rhizobium interactions: cowpea root exudate elicits faster nodulation response by Rhizobium species. Appl Environ Microbiol. 1982 Apr;43(4):800–805. doi: 10.1128/aem.43.4.800-805.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuvaneswari T. V., Bauer W. D. Role of Lectins in Plant-Microorganism Interactions: III. Influence of Rhizosphere/Rhizoplane Culture Conditions on the Soybean Lectin-binding Properties of Rhizobia. Plant Physiol. 1978 Jul;62(1):71–74. doi: 10.1104/pp.62.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuvaneswari T. V., Bhagwat A. A., Bauer W. D. Transient susceptibility of root cells in four common legumes to nodulation by rhizobia. Plant Physiol. 1981 Nov;68(5):1144–1149. doi: 10.1104/pp.68.5.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuvaneswari T. V., Mills K. K., Crist D. K., Evans W. R., Bauer W. D. Effects of culture age on symbiotic infectivity of Rhizobium japonicum. J Bacteriol. 1983 Jan;153(1):443–451. doi: 10.1128/jb.153.1.443-451.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuvaneswari T. V., Turgeon B. G., Bauer W. D. Early Events in the Infection of Soybean (Glycine max L. Merr) by Rhizobium japonicum: I. LOCALIZATION OF INFECTIBLE ROOT CELLS. Plant Physiol. 1980 Dec;66(6):1027–1031. doi: 10.1104/pp.66.6.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlool B. B., Schmidt E. L. Lectins: a possible basis for specificity in the Rhizobium--legume root nodule symbiosis. Science. 1974 Jul 19;185(4147):269–271. doi: 10.1126/science.185.4147.269. [DOI] [PubMed] [Google Scholar]
- Crist D. K., Wyza R. E., Mills K. K., Bauer W. D., Evans W. R. Preservation of Rhizobium viability and symbiotic infectivity by suspension in water. Appl Environ Microbiol. 1984 May;47(5):895–900. doi: 10.1128/aem.47.5.895-900.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halverson L. J., Stacey G. Effect of lectin on nodulation by wild-type Bradyrhizobium japonicum and a nodulation-defective mutant. Appl Environ Microbiol. 1986 Apr;51(4):753–760. doi: 10.1128/aem.51.4.753-760.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halverson L. J., Stacey G. Host recognition in the Rhizobium-soybean symbiosis: detection of a protein factor in soybean root exudate which is involved in the nodulation process. Plant Physiol. 1984 Jan;74(1):84–89. doi: 10.1104/pp.74.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyser H. H., van Berkum P., Weber D. F. A Comparative Study of the Physiology of Symbioses Formed by Rhizobium japonicum with Glycine max, Vigna unguiculata, and Macroptilium atropurpurem. Plant Physiol. 1982 Dec;70(6):1626–1630. doi: 10.1104/pp.70.6.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosslak R. M., Bohlool B. B. Suppression of nodule development of one side of a split-root system of soybeans caused by prior inoculation of the other side. Plant Physiol. 1984 May;75(1):125–130. doi: 10.1104/pp.75.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutman P. S. Genetics of symbiosis and nitrogen fixation in legumes. Proc R Soc Lond B Biol Sci. 1969 Apr 1;172(1029):417–437. doi: 10.1098/rspb.1969.0030. [DOI] [PubMed] [Google Scholar]
- Peters N. K., Frost J. W., Long S. R. A plant flavone, luteolin, induces expression of Rhizobium meliloti nodulation genes. Science. 1986 Aug 29;233(4767):977–980. doi: 10.1126/science.3738520. [DOI] [PubMed] [Google Scholar]
- Pierce M., Bauer W. D. A rapid regulatory response governing nodulation in soybean. Plant Physiol. 1983 Oct;73(2):286–290. doi: 10.1104/pp.73.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey G., Paau A. S., Brill W. J. Host recognition in the Rhizobium-soybean symbiosis. Plant Physiol. 1980 Oct;66(4):609–614. doi: 10.1104/pp.66.4.609. [DOI] [PMC free article] [PubMed] [Google Scholar]


