Abstract
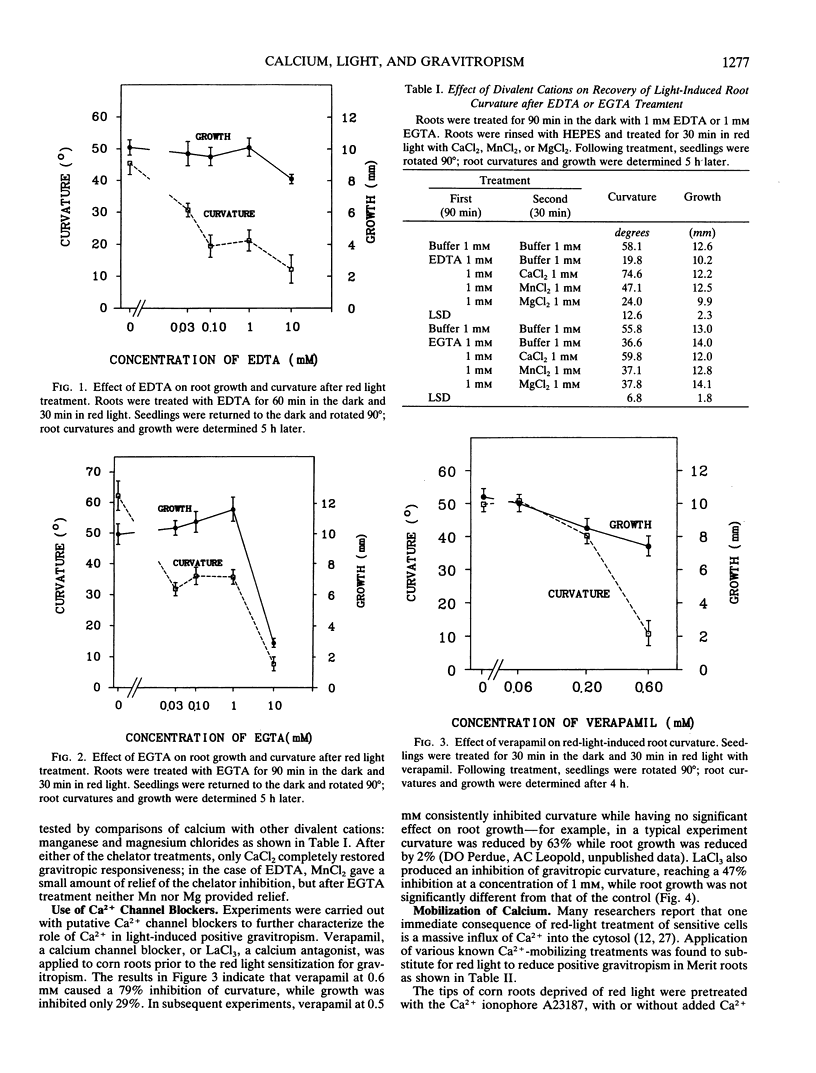
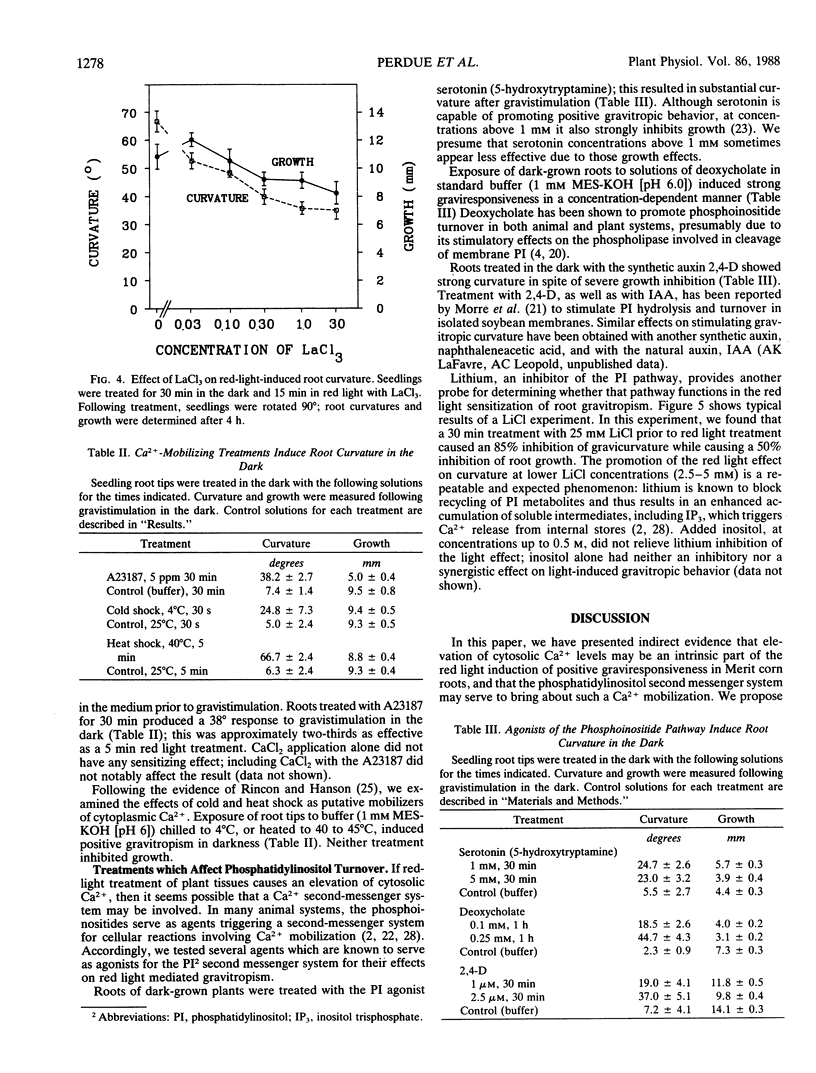
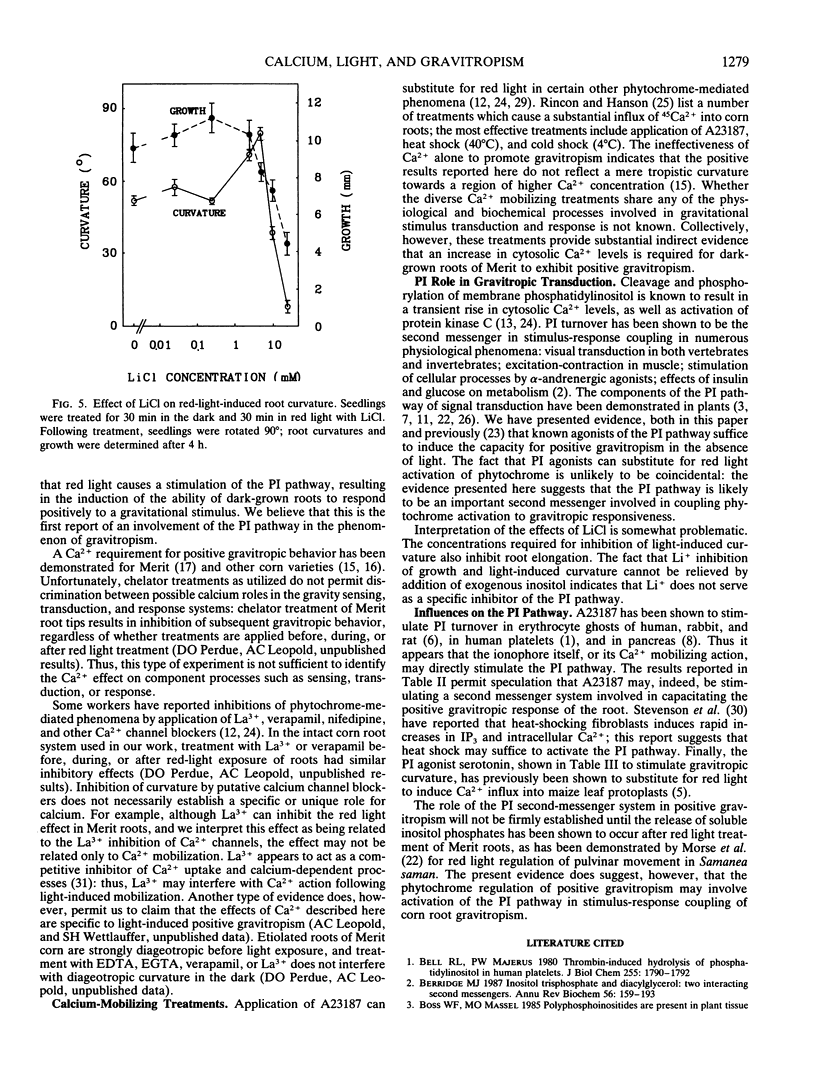
The red light requirement for positive gravitropism in roots of corn (Zea mays cv “Merit”) provides an entry for examining the participation of calcium in gravitropism. Applications of calcium chelators inhibit the light response. Calcium channel blockers (verapamil, lanthanum) can also inhibit the light response, and a calcium ionophore, A23187, can substitute for light. One can substitute for red light by treatments which have elsewhere been shown to trigger Ca2+ influx into the cytosol, e.g. heat or cold shock. Agents which are known to be agonists of the phosphatidylinositol second messenger system (serotonin, 2,4-dichlorophenoxyacetic acid, deoxycholate) can each partially substitute for the red light, and Li+ can inhibit the light effect. These experiments suggest that the induction of positive gravitropism by red light involves a rise in cytoplasmic Ca2+ concentration, and that a contribution to this end may be made by the phosphatidylinositol second messenger system.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell R. L., Majerus P. W. Thrombin-induced hydrolysis of phosphatidylinositol in human platelets. J Biol Chem. 1980 Mar 10;255(5):1790–1792. [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Das R., Sopory S. K. Evidence of regulation of calcium uptake by phytochrome in maize protoplasts. Biochem Biophys Res Commun. 1985 May 16;128(3):1455–1460. doi: 10.1016/0006-291x(85)91103-9. [DOI] [PubMed] [Google Scholar]
- Downes C. P., Michell R. H. The polyphosphoinositide phosphodiesterase of erythrocyte membranes. Biochem J. 1981 Jul 15;198(1):133–140. doi: 10.1042/bj1980133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drøbak B. K., Ferguson I. B. Release of Ca2+ from plant hypocotyl microsomes by inositol-1,4,5-trisphosphate. Biochem Biophys Res Commun. 1985 Aug 15;130(3):1241–1246. doi: 10.1016/0006-291x(85)91747-4. [DOI] [PubMed] [Google Scholar]
- Farese R. V., Larson R. E., Sabir M. A. Effects of Ca2+ ionophore A23187 and Ca2+ deficiency on pancreatic phospholipids and amylase release in vitro. Biochim Biophys Acta. 1980 Dec 15;633(3):479–484. doi: 10.1016/0304-4165(80)90205-6. [DOI] [PubMed] [Google Scholar]
- Feldman L. J., Briggs W. R. Light-regulated gravitropism in seedling roots of maize. Plant Physiol. 1987;83:241–243. doi: 10.1104/pp.83.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman L. J. Root gravitropism. Physiol Plant. 1985;65:341–344. doi: 10.1111/j.1399-3054.1985.tb02405.x. [DOI] [PubMed] [Google Scholar]
- Heim S., Wagner K. G. Evidence of phosphorylated phosphatidylinositols in the growth cycle of suspension cultured plant cells. Biochem Biophys Res Commun. 1986 Feb 13;134(3):1175–1181. doi: 10.1016/0006-291x(86)90374-8. [DOI] [PubMed] [Google Scholar]
- Kikkawa U., Nishizuka Y. The role of protein kinase C in transmembrane signalling. Annu Rev Cell Biol. 1986;2:149–178. doi: 10.1146/annurev.cb.02.110186.001053. [DOI] [PubMed] [Google Scholar]
- Lee J. S., Evans M. L. Polar transport of 45Ca2+ across the elongation zone of gravistimulated roots. Plant Cell Physiol. 1985;26(8):1587–1595. [PubMed] [Google Scholar]
- Lee J. S., Mulkey T. J., Evans M. L. Gravity-Induced Polar Transport of Calcium across Root Tips of Maize. Plant Physiol. 1983 Dec;73(4):874–876. doi: 10.1104/pp.73.4.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. S., Mulkey T. J., Evans M. L. Reversible loss of gravitropic sensitivity in maize roots after tip application of calcium chelators. Science. 1983 Jun 24;220(4604):1375–1376. doi: 10.1126/science.220.4604.1375. [DOI] [PubMed] [Google Scholar]
- Mandoli D. F., Tepperman J., Huala E., Briggs W. R. Photobiology of diagravitropic maize roots. Plant Physiol. 1984 Jun;75(2):359–363. doi: 10.1104/pp.75.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morré D. J., Gripshover B., Monroe A., Morré J. T. Phosphatidylinositol turnover in isolated soybean membranes stimulated by the synthetic growth hormone 2,4-dichlorophenoxyacetic acid. J Biol Chem. 1984 Dec 25;259(24):15364–15368. [PubMed] [Google Scholar]
- Morse M. J., Crain R. C., Satter R. L. Light-stimulated inositolphospholipid turnover in Samanea saman leaf pulvini. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7075–7078. doi: 10.1073/pnas.84.20.7075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poovaiah B. W., Reddy A. S. Calcium messenger system in plants. CRC Crit Rev Plant Sci. 1987;6(1):47–103. doi: 10.1080/07352688709382247. [DOI] [PubMed] [Google Scholar]
- Rincon M., Boss W. F. myo-Inositol Trisphosphate Mobilizes Calcium from Fusogenic Carrot (Daucus carota L.) Protoplasts. Plant Physiol. 1987 Feb;83(2):395–398. doi: 10.1104/pp.83.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux S. J., Serlin B. S. Cellular mechanisms controlling light-stimulated gravitropism: role of calcium. CRC Crit Rev Plant Sci. 1987;5(3):205–236. doi: 10.1080/07352688709382240. [DOI] [PubMed] [Google Scholar]
- Sekar M. C., Hokin L. E. The role of phosphoinositides in signal transduction. J Membr Biol. 1986;89(3):193–210. doi: 10.1007/BF01870664. [DOI] [PubMed] [Google Scholar]
- Serlin B. S., Roux S. J. Modulation of chloroplast movement in the green alga Mougeotia by the Ca2+ ionophore A23187 and by calmodulin antagonists. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6368–6372. doi: 10.1073/pnas.81.20.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson M. A., Calderwood S. K., Hahn G. M. Rapid increases in inositol trisphosphate and intracellular Ca++ after heat shock. Biochem Biophys Res Commun. 1986 Jun 13;137(2):826–833. doi: 10.1016/0006-291x(86)91154-x. [DOI] [PubMed] [Google Scholar]
- Van Breemen C., Farinas B. R., Gerba P., McNaughton E. D. Excitation-contraction coupling in rabbit aorta studied by the lanthanum method for measuring cellular calcium influx. Circ Res. 1972 Jan;30(1):44–54. doi: 10.1161/01.res.30.1.44. [DOI] [PubMed] [Google Scholar]


