Abstract
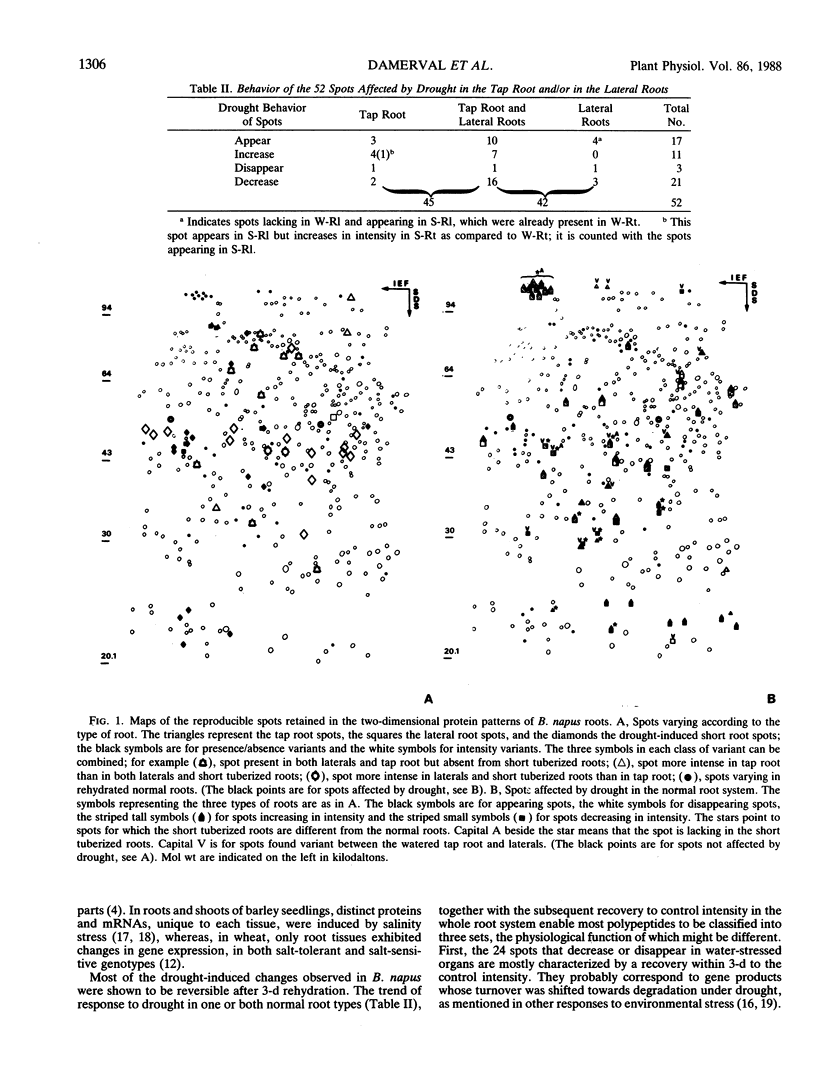
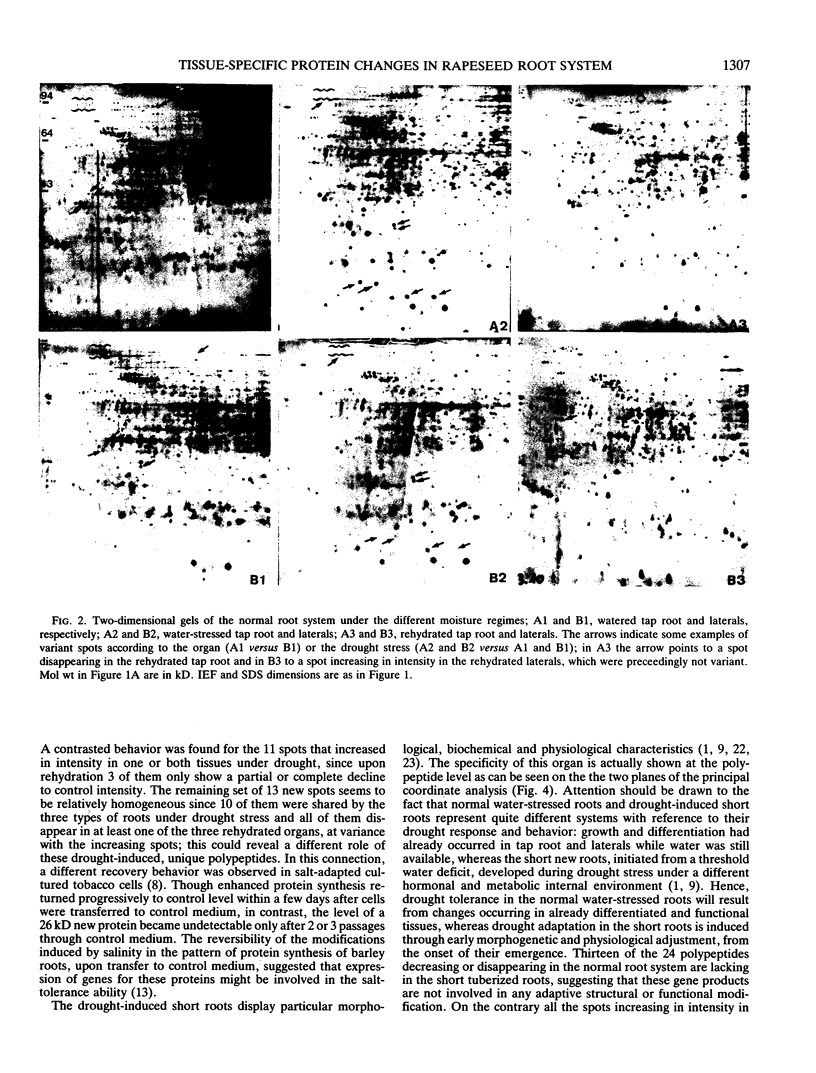
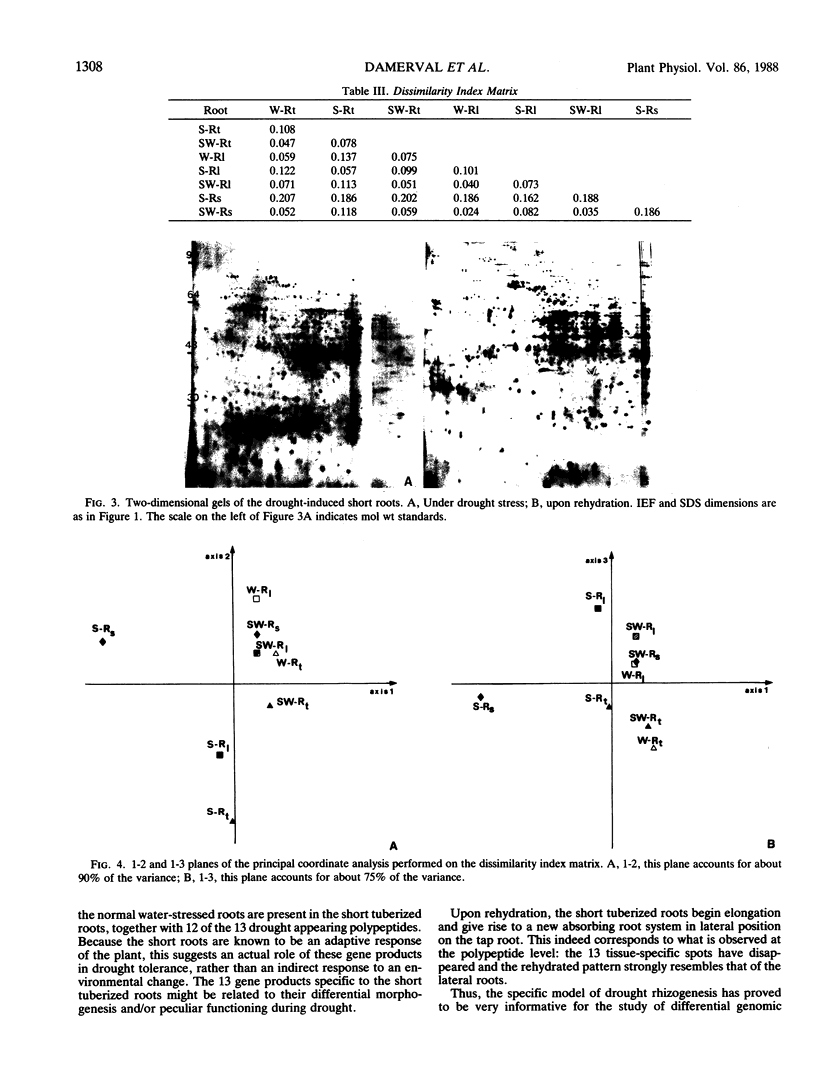
Differential two-dimensional protein patterns as related to tissue specificity and water conditions were investigated within Brassica napus var oleifera root system. The different parts of the root system (tap root, lateral roots, and drought-induced short roots) were analyzed under various moisture regimes (regular watering at field capacity, progressive drought stress, and rewatering). Tissue specificity was evident from 25 differences in protein patterns (qualitative and quantitative) between well-watered lateral and tap roots. Twice as many polypeptides (52) were drought-affected and the response to the water stress was shown to be similar in both root types. In addition, more than half of the polypeptides detected as organ-specific were affected by drought. Based upon the trend of variation observed under drought and rehydration, three categories of polypeptides could be defined that might be differently involved in drought susceptibility or tolerance. A highly differentiated protein pattern characterized the drought-induced short roots. This pattern appeared as far from the watered as from the water-stressed normal roots. In particular, 13 unique polypeptides were detected which could be relevant to their adaptive morphogenesis and/or their specific drought tolerance induction. Upon rehydration, their polypeptide pattern and their specific morphology returned to a normal well-watered lateral root type.
Full text
PDF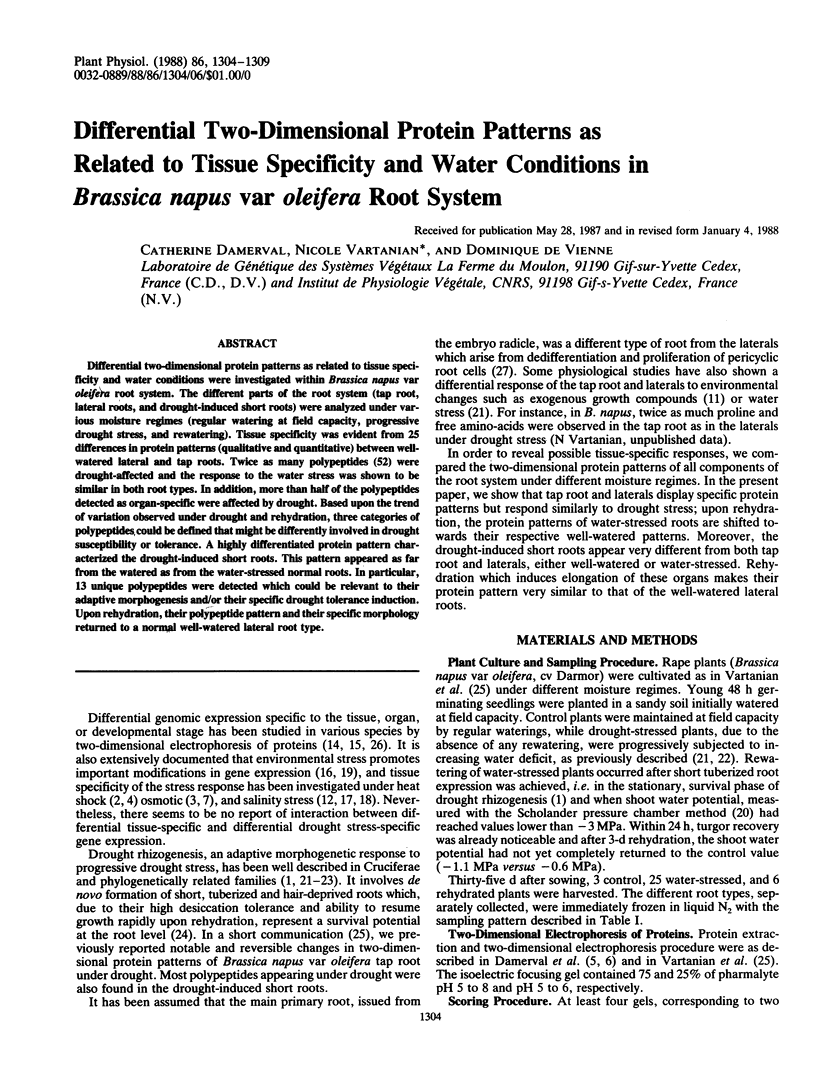


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baszczynski C. L., Walden D. B., Atkinson B. G. Regulation of gene expression in corn (Zea Mays L.) by heat shock. Can J Biochem. 1982 May;60(5):569–579. doi: 10.1139/o82-070. [DOI] [PubMed] [Google Scholar]
- Cooper P., Ho T. H., Hauptmann R. M. Tissue specificity of the heat-shock response in maize. Plant Physiol. 1984 Jun;75(2):431–441. doi: 10.1104/pp.75.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson M. C., Alfinito S. H. Proteins Produced during Salt Stress in Tobacco Cell Culture. Plant Physiol. 1984 Mar;74(3):506–509. doi: 10.1104/pp.74.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulick P., Dvorák J. Gene induction and repression by salt treatment in roots of the salinity-sensitive Chinese Spring wheat and the salinity-tolerant Chinese Spring x Elytrigia elongata amphiploid. Proc Natl Acad Sci U S A. 1987 Jan;84(1):99–103. doi: 10.1073/pnas.84.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurkman W. J., Tanaka C. K. The effects of salt on the pattern of protein synthesis in barley roots. Plant Physiol. 1987 Mar;83(3):517–524. doi: 10.1104/pp.83.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klose J. Genetic variability of soluble proteins studied by two-dimensional electrophoresis on different inbred mouse strains and on different mouse organs. J Mol Evol. 1982;18(5):315–328. doi: 10.1007/BF01733898. [DOI] [PubMed] [Google Scholar]
- Matters G. L., Scandalios J. G. Changes in plant gene expression during stress. Dev Genet. 1986;7(4):167–175. doi: 10.1002/dvg.1020070402. [DOI] [PubMed] [Google Scholar]
- Ramagopal S. Differential mRNA transcription during salinity stress in barley. Proc Natl Acad Sci U S A. 1987 Jan;84(1):94–98. doi: 10.1073/pnas.84.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramagopal S. Salinity stress induced tissue-specific proteins in barley seedlings. Plant Physiol. 1987 Jun;84(2):324–331. doi: 10.1104/pp.84.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholander P. F., Bradstreet E. D., Hemmingsen E. A., Hammel H. T. Sap Pressure in Vascular Plants: Negative hydrostatic pressure can be measured in plants. Science. 1965 Apr 16;148(3668):339–346. doi: 10.1126/science.148.3668.339. [DOI] [PubMed] [Google Scholar]
- Vartanian N., Damerval C., de Vienne D. Drought-Induced Changes in Protein Patterns of Brassica napus var. oleifera Roots. Plant Physiol. 1987 Aug;84(4):989–992. doi: 10.1104/pp.84.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]




