Abstract
Background
The undetermined efficacy of the current standard-of-care neoadjuvant treatment, anthracycline/platinum-based chemotherapy, in patients with early-stage triple-negative breast cancer (TNBC) and germline BRCA mutations emphasizes the need for biomarker-targeted treatment, such as poly(ADP-ribose) polymerase inhibitors, in this setting. This phase II, single-arm, open-label study evaluated the efficacy and safety of neoadjuvant talazoparib in patients with germline BRCA1/2-mutated early-stage TNBC.
Patients and Methods
Patients with germline BRCA1/2-mutated early-stage TNBC received talazoparib 1 mg once daily for 24 weeks (0.75 mg for moderate renal impairment) followed by surgery. The primary endpoint was pathologic complete response (pCR) by independent central review (ICR). Secondary endpoints included residual cancer burden (RCB) by ICR. Safety and tolerability of talazoparib and patient-reported outcomes were assessed.
Results
Of 61 patients, 48 received ≥80% talazoparib doses, underwent surgery, and were assessed for pCR or progressed before pCR assessment and considered nonresponders. pCR rate was 45.8% (95% confidence interval [CI], 32.0%-60.6%) and 49.2% (95% CI, 36.7%-61.6%) in the evaluable and intent-to-treat (ITT) population, respectively. RCB 0/I rate was 45.8% (95% CI, 29.4%-63.2%) and 50.8% (95% CI, 35.5%-66.0%) in the evaluable and ITT population, respectively. Treatment-related adverse events (TRAE) were reported in 58 (95.1%) patients. Most common grade 3 and 4 TRAEs were anemia (39.3%) and neutropenia (9.8%). There was no clinically meaningful detriment in quality of life. No deaths occurred during the reporting period; 2 deaths due to progressive disease occurred during long-term follow-up (>400 days after first dose).
Conclusions
Neoadjuvant talazoparib monotherapy was active despite pCR rates not meeting the prespecified threshold; these rates were comparable to those observed with combination anthracycline- and taxane-based chemotherapy regimens. Talazoparib was generally well tolerated.
ClinicalTrials.gov identifier
Keywords: poly(ADP-ribose) polymerase inhibitors, triple-negative breast neoplasms, neoadjuvant therapy, antineoplastic agents
The undetermined efficacy of the current standard-of-care neoadjuvant treatment of early-stage triple-negative breast cancer (TNBC) with germline BRCA mutations emphasizes the need for biomarker-targeted treatment in this setting. This article evaluates the efficacy and safety of neoadjuvant talazoparib in patients with germline BRCA1/2-mutated early-stage TNBC.
Implications for Practice.
This phase II study showed that talazoparib monotherapy was active with an observed pathologic complete response (pCR) rate of 45.8% in patients with early-stage triple-negative breast cancer and germline BRCA1/2 mutations. Neoadjuvant talazoparib monotherapy was comparable to anthracycline- and taxane-based chemotherapy combination regimens and was generally well tolerated. Side effects were consistent with the established safety profile of talazoparib with no clinically meaningful detriment in global health status or quality of life. Multiple ongoing neoadjuvant studies may further clarify the optimal use of poly(ADP-ribose) polymerase (PARP) inhibitors in this setting.
Introduction
Despite the overall progress in breast cancer treatments, some patients continue to have a high risk of recurrence and death after systemic therapy.1 Mutations in BRCA1/2 account for approximately 30% of hereditary breast cancer and approximately 5% of all breast cancers.2-4 Germline mutations in BRCA1/2 increase the probability of developing breast cancer during a lifetime to >70%.4,5BRCA1/2 are tumor suppressor genes that mediate the repair of DNA double-strand breaks via homologous recombination repair (HRR).6 Patients with germline BRCA1 mutations have a predisposition for triple-negative breast cancer (TNBC). In one analysis, approximately 70% of patients with BRCA1 mutations and 16% of patients with BRCA2 mutations had TNBC.7
Cancer cells with germline BRCA1/2 mutations rely on poly(ADP-ribose) polymerase (PARP) enzymes 1 and 2 for DNA repair.8 PARP inhibitors inhibit PARP1/2 catalytic activity and trap PARP to single-strand breaks inducing cell death via synthetic lethality.6 This supports the rationale for the use of PARP inhibitors in the neoadjuvant setting for patients with germline BRCA1/2 mutations.
Neoadjuvant chemotherapy has become a standard approach for most patients with early-stage TNBC,9,10 including patients with BRCA1/2 mutations.11 The efficacy of neoadjuvant platinum chemotherapy for patients with germline BRCA mutations remains unclear,12 despite several studies demonstrating the efficacy of platinum-containing regimens for TNBC in the neoadjuvant setting,13,14 including the KEYNOTE-522 study, which evaluated paclitaxel-, carboplatin-, and anthracycline-based chemotherapy with either pembrolizumab or placebo.10 Lack of clear benefit has been demonstrated in both the INFORM study, in which 70% of patients had TNBC, and the GeparSixto study, in which 54% of patients had TNBC and showed that patients with germline BRCA mutations did not receive further benefit from the addition of platinum.15,16 These results highlight the need to investigate targeted therapies for patients with TNBC and germline BRCA mutations.
In the phase III BrighTNess study, the PARP inhibitor veliparib plus carboplatin in addition to weekly neoadjuvant paclitaxel followed by doxorubicin and cyclophosphamide in patients with TNBC demonstrated a clinically significant improvement of pathologic complete response (pCR) vs. paclitaxel alone followed by doxorubicin and cyclophosphamide (P < .0001).9 However, the addition of veliparib to carboplatin/paclitaxel combination did not appear to add significant benefit compared with carboplatin/paclitaxel alone.9 This study failed to demonstrate an improvement in pCR from adding PARP inhibition to standard of care therapy but did not evaluate the use of single-agent PARP inhibition as a neoadjuvant strategy by itself.9
Talazoparib is a PARP inhibitor approved in the United States, the European Union, and multiple other countries as monotherapy for the treatment of patients with deleterious or suspected deleterious germline BRCA-mutated, human epidermal growth factor receptor 2 (HER2)-negative, locally advanced or metastatic breast cancer.17,18 Talazoparib is the first single-agent targeted therapy to achieve pCR in germline BRCA-positive, HER2-negative patients with early breast cancer, including TNBC.19 Results from a prior investigator-initiated study (NCT02282345) showed that of 19 patients enrolled with early-stage I to III breast cancer and germline BRCA mutations with pathologic response data, 53% (95% confidence interval [CI], 32%-73%) achieved pCR and 63% (95% CI, 41%-81%) achieved residual cancer burden (RCB) 0/I with neoadjuvant talazoparib monotherapy for 24 weeks followed by surgery.19 The most common adverse events (AEs) observed in this treatment-naive patient population were anemia and nausea, which were consistent with those previously reported with PARP inhibitors in the metastatic breast cancer setting.19-21 Moreover, these early results provided the rationale for the current confirmatory phase II study in a larger patient population (NEOTALA; NCT03499353).
Methods
Study Design and Patients
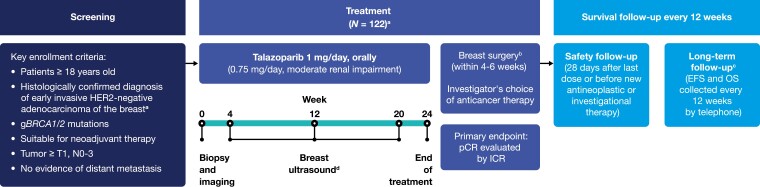
NEOTALA was an open-label, multicenter, single-arm, phase II study initially designed to enroll 122 patients (Fig. 1). However, due to lower-than-expected recruitment rate, target sample size was reduced to 60 adult patients with a histologically confirmed diagnosis of early breast cancer with hormone receptor (HR)-negative, HER2-negative disease and germline BRCA1/2 mutations (Fig. 1). Although inclusion criteria changed during the course of the study to allow HR-positive patients (Amendment 4; August 14, 2019), no HR-positive patients were enrolled by the time the study closed to new patients in March 2020. Further key inclusion criteria included patients ≥18 years of age, adenocarcinoma of the breast, germline BRCA1/2 mutations, tumors ≥T1, N0-3, no evidence of distant metastasis, and those suitable for neoadjuvant therapy (Fig. 1). All patients had BRCA mutation status determined via screening (BRACAnalysis CDx® [Myriad Genetics, Salt Lake City, Utah]) or historical evidence of a BRCA1/2 mutation by Myriad BRACAnalysis CDx® post 2016. Key exclusion criteria included any previous antitumor therapies for the current breast cancer event, previous or concomitant systemic anticancer therapies used for the treatment of cancer in the last 3 years, prior treatment with a PARP inhibitor in any disease setting, and patients with myelodysplastic syndrome (MDS).
Figure 1.
Study design. Abbreviations: BC, breast cancer; EFS, event-free survival; gBRCA1/2, germline breast cancer susceptibility genes 1 and 2; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; ICR, independent central review; N, regional lymph nodes; OS, overall survival; pCR, pathologic complete response; T, primary tumor. aStudy design was amended to include HR-positive, HER2-negative patients with BC and the patient numbers were reduced from 122 to 60 to address lower than expected enrollment. bBreast/axillary tumor tissue must be removed by either lumpectomy or mastectomy with clinically appropriate axillary surgery. Patients who had disease progression were to discontinue treatment on study and switch to alternative systemic therapy or go straight to surgery. cLong-term follow-up was planned to be at least 3 years, starting from the date of surgery for EFS and after the first dose of drug for OS. However, Pfizer decided to make a strategic change in the development program for talazoparib in neoadjuvant BC and decided not to pursue further development in this setting. The study was closed after all patients completed safety follow-up, and EFS/OS was not reached. dBreast ultrasound scans at week 12 were used to assist in clinical response assessment during treatment and to provide investigator reassurance that the patient was not progressing. Breast ultrasound was not used to assess response.
In keeping with the initial pilot study, the prescribed treatment phase was 24 weeks (6 cycles of 4 weeks). Patients were administered a starting dose of oral talazoparib 1 mg/day, or 0.75 mg/day for moderate renal impairment. Treatment was followed by breast surgery within 6 weeks of completing neoadjuvant treatment, and pCR was assessed by both independent central review (ICR) and investigator. Standard-of-care treatment could be initiated following progressive disease (PD) with investigator’s choice of therapy. Investigator’s choice of systemic therapy could also be initiated post-surgery. The safety follow-up period was 28 days after the last dose of talazoparib or initiation of a new anticancer therapy. Long-term follow-up of event-free survival (EFS), defined as the time from surgery date to first documentation of local or distant recurrence, death, or initiation of antineoplastic therapy before documentation of first relapse, assessed starting after surgery, and overall survival (OS), assessed from first dose, was planned for 3 years, and collected every 12 weeks by telephone.
Because of a strategic change in the development program by the sponsor, not related to safety or efficacy concerns for talazoparib in the neoadjuvant breast cancer setting, the study was closed on September 23, 2020, after all patients completed the safety follow-up, and therefore, the long-term EFS and OS endpoints were not met or evaluated.
The study protocol was reviewed and approved by an institutional review board and ethics committee and research ethics board, and conducted under the International Ethical Guidelines for Biomedical Research Involving Human Patients, the International Council for Harmonisation Guideline for Good Clinical Practice, the Declaration of Helsinki, and other applicable national and local regulations. Written evidence of informed consent was required before patients could be enrolled into the study.
Endpoints
The primary endpoint was pCR in the evaluable population assessed by ICR, and defined as the absence of invasive cancer in the breast and axillary lymph nodes on H&E staining of the complete resected breast specimen, and all sampled regional lymph nodes following completion of neoadjuvant systemic therapy (ie, ypT0/Tis ypN0 in the current American Joint Committee on Cancer staging system).22 The evaluable population was the primary analysis population and consisted of patients who received ≥80% of the planned talazoparib doses prescribed at treatment initiation, underwent breast/axillary surgery, and had a pCR assessment. A reduction in relative dose intensity (RDI) below 80% is considered a clinically significant reduction from planned therapy and maintaining RDI has been associated with improved survival, hence in this study a cut-off of 80% was chosen.23 In addition, patients who progressed before pCR could be assessed were included in the evaluable population (and considered as nonresponders).
Isolated tumor cells (ITCs) were not specifically collected, therefore, no data on ITCs are available. Key secondary endpoints included pCR rate in the intent-to-treat (ITT) population assessed by ICR, and pCR by investigator assessment in both ITT and evaluable populations as well as RCB by ICR in both populations. All patients who took ≥1 dose of talazoparib were included in the ITT population and evaluated for efficacy and safety.
RCB is a 4-category index derived from primary tumor dimensions, cellularity of the tumor bed, and axillary nodal burden. RCB 0 refers to no residual invasive cancer, or pCR. RCB I refers to minimal RCB, RCB II to moderate RCB, and RCB III to extensive RCB (PD).24 Only patients who received surgery and histologically assessed by ICR were evaluated for RCB. Safety and tolerability of talazoparib were also assessed. EFS and OS were to be assessed at 3 years.
Patient-Reported Outcome Endpoints
Patient-reported outcomes (PROs) were measured at baseline, and every 4 weeks for 24 weeks or at disease progression via the European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire (QLQ-C30), the EORTC breast cancer module (QLQ-BR23), and the European Quality of Life 5-Domain 5-Level Scale (EQ-5D-5L) health questionnaire. Missed expected menstrual periods were assessed via the Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) questionnaire.
The EORTC QLQ-C30 is a standardized instrument that assesses cancer-specific patient-reported global quality of life, functioning, and disease/treatment-related symptoms. Patients self-rate their self-care, activity level, pain/discomfort, and mental health during the past week by choosing responses from a 4-point Likert scale. On all EORTC scales, responses to all items are converted to a 0-to-100 scale with a standard scoring algorithm. For functional and Global Health Status/Quality of Life (GHS/QoL) scales, higher scores indicate a better level of functioning and quality of life. For symptom scales, a higher score indicates greater symptom severity.
The EQ-5D-5L consists of 5 dimensions (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression), each of which have 5 possible responses indicating the level of the problem (1, no problem; 2, slight problem; 3, moderate problem; 4, severe problem; 5, extreme problem). Results of the 5 dimensions are combined to derive an EQ-5D index score that ranges from −0.594 to 1.0 corresponding to worst and best health status (using a UK valuation based on the EQ-5D-3L).25
The PRO-CTCAE item library questionnaire is a PRO measure developed to evaluate symptomatic toxicity in patients on cancer clinical trials.
Statistical Analysis
The study was to be considered a success if the posterior probability that the true pCR rate exceeds 45% was ≥0.8. An interim analysis was to be conducted once 28 evaluable patients were assessed for pCR, and if ≥12 responses were observed, the study was to continue enrolling up to 60 patients.
The pCR rate was defined as the percentage of patients achieving pCR after talazoparib treatment in both the evaluable and ITT populations by ICR and investigator, and related exact 2-sided 80% and 95% CIs were based on the Blaker’s method. RCB was calculated by ICR in both the evaluable and ITT populations, along with the corresponding exact 2-sided 95% CIs based on the Goodman’s method. Safety and tolerability of talazoparib were evaluated in the ITT population.
Prespecified PRO analyses included the overall mean change from baseline (estimated using the longitudinal mixed-effects model) and the time to clinically meaningful deterioration (summarized using Kaplan-Meier methods). The time to clinically meaningful deterioration according to the GHS/QoL scale was defined as the first observation with a decrease of ≥10 points and no subsequent observations with a decrease of <10 points from baseline based on previously established thresholds.26 Similarly, time to deterioration according to the nausea/vomiting symptoms scale was defined as the first observation with an increase of ≥10 points and no subsequent observations with an increase of <10 points from baseline. Missed expected menstrual periods are reported descriptively as the proportion of patients having ≥1 missing menstrual period over the 6 cycles.
Results
Patient Disposition
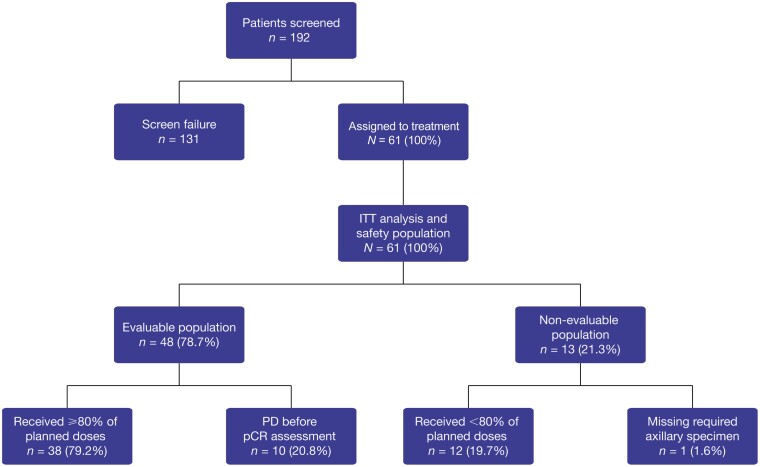
Between August 27, 2018, and February 5, 2020, a total of 192 patients in the United States were screened (including for germline BRCA1/2 mutations) and 61 patients were treated (ITT population); 48 patients were included in the evaluable population (Fig. 2), and 13 patients were considered non-evaluable, 12 of whom received <80% of planned doses, and 1 patient did not have surgery at the investigator site. Of the 12 patients who received <80% of planned doses, 10 had AEs leading to dose interruption or dose reduction, one was not compliant with treatment administration and decided to discontinue treatment, and one withdrew from the study. The study results met the threshold for the continuation of enrollment (13 pCR out of the first 28 evaluable patients). Forty-five patients completed the neoadjuvant treatment phase with talazoparib, and 49 patients completed the surgical safety follow-up phase (Supplementary Table S1). Fifty-eight patients entered the long-term follow-up phase, of whom 55 (90.2%) patients discontinued due to study closure, 2 patients died due to PD (>400 days after first dose), and 1 patient withdrew consent (Supplementary Table S1).
Figure 2.
Patient populations. Abbreviations: ITT, intent-to-treat; pCR, pathologic complete response; PD, progressive disease.
Baseline Patient Characteristics
All patients were female with a median age of 42 years and an average duration since onset of breast cancer of 4.5 weeks (Table 1). Thirty-six (59%) patients were premenopausal and 25 (41%) patients were postmenopausal, with the majority of patients being White (77.0%), Black/African American (11.5%), or Asian (4.9%). All women were diagnosed with TNBC with 78.7% germline BRCA1-positive and 21.3% germline BRCA2-positive (Table 1). Of the 61 patients in the ITT population, 60 patients had adenocarcinoma and 1 patient had squamous carcinoma with spindle cell features.
Table 1.
Baseline patient characteristics (ITT population).
| Patient baseline characteristic | Talazoparib (N = 61) |
|---|---|
| Age (years) | |
| Median (min-max) | 42 (26-75) |
| Sex, n (%) | |
| Female | 61 (100.0) |
| Menopausal status, n (%) | |
| Premenopausal | 36 (59.0) |
| Postmenopausal | 25 (41.0) |
| Race, n (%) | |
| White | 47 (77.0) |
| Black or African American | 7 (11.5) |
| Asian | 3 (4.9) |
| Not reported | 4 (6.6) |
| Ethnicity, n (%) | |
| African American | 7 (11.5) |
| Ashkenazi Jewish | 1 (1.6) |
| Chinese | 1 (1.6) |
| Othera | 52 (85.2) |
| Hispanic or Latino | 3 (4.9) |
| Not Hispanic or Latino | 42 (68.9) |
| Not reported | 7 (11.5) |
| Breast cancer | |
| Duration since onset (weeks) | |
| Mean (min-max) | 4.54 (0.4-21.1) |
| Adenocarcinoma, n (%) | 60 (98.4) |
| Squamous carcinoma with spindle cell, n (%) | 1 (1.6) |
| TNBC, n (%) | 61 (100.0) |
| BRCA1, n (%) | 48 (78.7) |
| BRCA2, n (%) | 13 (21.3) |
| Staging, n (%) | |
| Stage I | 20 (32.8) |
| Stage II | 27 (44.3) |
| Stage III | 14 (23.0) |
Abbreviations: BRCA1/2, breast cancer susceptibility genes 1 and 2; ITT, intent-to-treat; max, maximum; min, minimum; TNBC, triple-negative breast cancer.
a“Other” includes 3 patients who were not recorded in the “Other” category (designation left blank) but reported their ethnicity as Hispanic or Latino (n = 1), Not Hispanic or Latino (n = 1) and Not Reported (n = 1)
Pathologic Complete Response
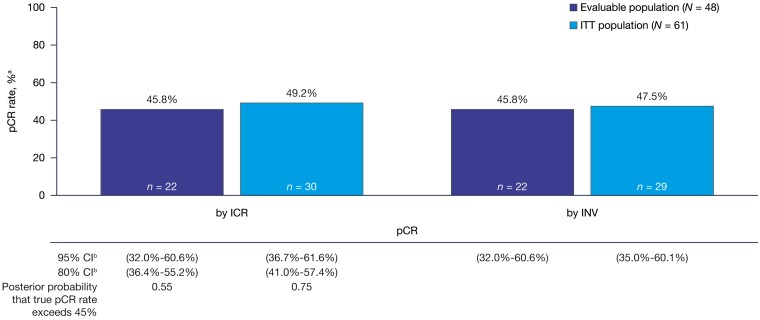
Twenty-two (45.8%) of 48 patients in the evaluable population achieved pCR (95% CI, 32.0%-60.6%) by both ICR and investigator assessment. Among patients in the ITT population, the pCR rate was 49.2% (30/61) (95% CI, 36.7%-61.6%) and 47.5% (29/61) (95% CI, 35.0%-60.1%) by ICR and investigator assessments, respectively (Fig. 3). Nine patients in the ITT population who achieved pCR had breast cancer stage I at initial diagnosis, 11 patients had stage II, and 10 patients had stage III/other (Supplementary Table S2). Of the 12 patients who received <80% of the planned treatment, 8 achieved pCR. The posterior probability that true pCR rate exceeds 45% was 0.55 in the evaluable population and 0.75 in the ITT population. The prespecified threshold was not passed (corresponding to 25 pCR in 48 evaluable patients and 31 in 61 ITT patients).
Figure 3.
Pathologic complete response. Abbreviations: CI, confidence interval; ICR, independent central review; INV, investigator; ITT, intent-to-treat; pCR, pathologic complete response. aThe denominator is N, the number of patients in the evaluable/ITT analysis set as per ICR/INV. bThe exact CI was calculated using the Blaker’s method.
Residual Cancer Burden
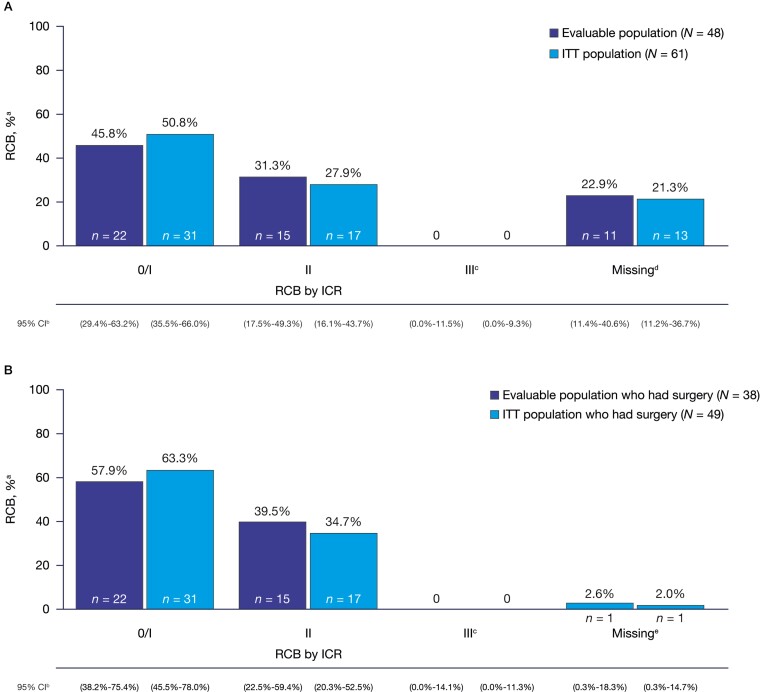
Among 48 patients in the evaluable population, 45.8% (n = 22; 95% CI, 29.4%-63.2%) achieved RCB 0/I by ICR compared with 50.8% (n = 31/61; 95% CI, 35.5%-66.0%) in the ITT population (Fig. 4A). Fifteen patients (31.3%; 95% CI, 17.5%-49.3%) in the evaluable population and 17 patients (27.9%; 95% CI, 16.1%-43.7%) in the ITT population had RCB II. A summary of breast cancer stage at initial diagnosis by RCB in the ITT population is presented in Supplementary Table S2. Ten patients had PD among the evaluable and ITT population, of whom 2 patients had stage I, 6 patients had stage II, and 2 patients had stage III breast cancer at initial diagnosis. Patients who had progression during study treatment and switched to chemotherapy were not assessed by ICR for RCB and are counted in the “Missing” category. However, per Symmans et al. (2017), these 10 patients would have been considered RCB III (Fig. 4A).24
Figure 4.
(A) Residual cancer burden (all patients); (B) Residual cancer burden among patients who had surgery. Abbreviations: CI, confidence interval; ICR, independent central review; ITT, intent-to-treat; PD, progressive disease; RCB, residual cancer burden. aThe denominator is N, the number of patients in the evaluable/ITT analysis set as per ICR. bThe simultaneous exact CI was calculated using Goodman’s method. cNone of the patients who had pathology submitted are classified as RCB III. d10 patients in the evaluable and ITT population had PD and are counted in the “Missing” category. Two patients did not have surgery for other reasons (early discontinuation and consent withdrawal; ITT population), and 1 patient was unable to be assessed because of missing a required axillary specimen. eOne patient was unable to be assessed because of missing a required axillary specimen.
Among patients who had surgery (n = 38 evaluable population; n = 49 ITT population), 57.9% (n = 22; 95% CI, 38.2%-75.4%) of the evaluable population compared with 63.3% (n = 31; 95% CI, 45.5%-78.0%) of the ITT population achieved RCB 0/I (Fig. 4B). RCB II was found in 15 (39.5%; 95% CI, 22.5%-59.4%) patients in the evaluable population and in 17 (34.7%; 95% CI, 20.3%-52.5%) patients in the ITT population.
Drug Exposure
Mean talazoparib exposure was 23.3 weeks, and 90.2% (n = 55) of patients received talazoparib for ≥20 weeks (Supplementary Table S3). Patients with dose interruptions due to toxicities could make up missed doses per investigator discretion extending the patient’s treatment longer than 24 weeks. Forty-five (73.8%) patients received talazoparib for ≥24 weeks. The mean overall RDI was 84.5% (Supplementary Table S3).
Safety
In total, 58 (95.1%) patients experienced treatment-related AEs (TRAEs) (Table 2). The most common TRAEs ≥10% were fatigue (n = 47; 77%), nausea (n = 39; 63.9%), and alopecia (n = 35; 57.4%). Most TRAEs were grade 1 or 2. Most common grade 3 and 4 TRAEs were anemia (n = 24; 39.3%) and neutropenia (n = 6; 9.8%). No cases of MDS were reported.
Table 2.
The most common treatment-related adverse events experienced by ≥10% of patients (ITT population).
| Number (%) of patients by preferred terma | Talazoparib (N = 61) | ||||
|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Total | |
| Any adverse event | 22 (36.1) | 9 (14.8) | 26 (42.6) | 1 (1.6) | 58 (95.1) |
| Fatigue | 34 (55.7) | 12 (19.7) | 1 (1.6) | 0 | 47 (77.0) |
| Nausea | 31 (50.8) | 7 (11.5) | 1 (1.6) | 0 | 39 (63.9) |
| Alopecia | 33 (54.1) | 2 (3.3) | 0 | 0 | 35 (57.4) |
| Anemia | 4 (6.6) | 1 (1.6) | 24 (39.3) | 0 | 29 (47.5) |
| Headache | 16 (26.2) | 2 (3.3) | 1 (1.6) | 0 | 19 (31.1) |
| Dizziness | 11 (18.0) | 1 (1.6) | 0 | 0 | 12 (19.7) |
| Constipation | 9 (14.8) | 2 (3.3) | 0 | 0 | 11 (18.0) |
| Neutrophil count decreased | 1 (1.6) | 2 (3.3) | 5 (8.2) | 1 (1.6) | 9 (14.8) |
| White blood cell count decreased | 5 (8.2) | 3 (4.9) | 1 (1.6) | 0 | 9 (14.8) |
| Decreased appetite | 7 (11.5) | 1 (1.6) | 0 | 0 | 8 (13.1) |
| Diarrhea | 6 (9.8) | 2 (3.3) | 0 | 0 | 8 (13.1) |
Abbreviations: CTCAE, Common Terminology Criteria for Adverse Events; ITT, intent-to-treat; MedDRA, Medical Dictionary for Regulatory Activities; NCI-CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Events.
aIncludes all data collected since the first dose of study drug. If the same patient had more than 1 occurrence in the same preferred term event category, only the occurrence with maximum CTCAE grade is counted. Patients are counted only once per event. MedDRA v23.1 coding dictionary applied. NCI-CTCAE version 4.03.
Patient-Reported Outcomes
EORTC QLQ-C30
Based on the repeated measures mixed-effect model, there was no clinically meaningful overall change from baseline in GHS/QoL (estimated mean −9.44; 95% CI, −13.11 to −5.77). The median time to definitive deterioration (TTD) was not estimable, and the 6-month probability of not experiencing a definitive deterioration in GHS/QoL was 59.4%. Based on the repeated measures mixed-effect model, there was no clinically meaningful overall change from baseline in nausea/vomiting (estimated mean 7.36; 95% CI, 4.67-10.05). The median TTD was not estimable, and the 6-month probability of not experiencing a definitive deterioration in nausea/vomiting was 81.8%.
EQ-5D-5L
Based on the repeated measures mixed-effect model, there was no clinically meaningful change from baseline27 in EQ-5D index score (estimated mean −0.02; 95% CI, −0.04 to 0.00).
PRO-CTCAE Missed Menstrual Period
Of the 32 premenopausal patients, 6 (18.8%) patients reported missing at least 1 menstrual period in the last 7 days across the 6 cycles.
Dose Modifications and Supportive Care
In the event of a grade 3 or greater toxicity, daily dosing was interrupted. Upon resuming treatment, talazoparib was administered at the next lower dose level (reduced increments of 0.25 mg/day). Twenty (32.8%) patients had AEs leading to dose interruptions and 24 (39.3%) had AEs leading to dose reductions (Table 3).
Table 3.
Dose modifications and permanent drug discontinuation (ITT population).
| Dose interruptions, reductions, transfusions, and discontinuations | Talazoparib (N = 61) |
|---|---|
| Number (%) of patients | n (%) |
| Dose interruptions due to AEs | 20 (32.8) |
| Dose reductions due to AEs | 24 (39.3) |
| Packed red blood cell transfusion | 13 (21.3) |
| Transfusion | 7 (11.5) |
| Permanent drug discontinuation | |
| Adverse event | 3 (4.9) |
| Death | 0 |
| Progressive disease | 10 (16.4) |
| Withdrawal by patienta | 2 (3.3) |
| Otherb | 1 (1.6) |
Abbreviations: AE, adverse event; ITT, intent-to-treat.
aOne patient completed only 4 months of treatment and decided to have surgery early and 1 patient withdrew consent from the treatment and also permanently discontinued from the study.
bPatient had surgery early in another country and specimen was not provided to central laboratory.
Anemia is a known AE for talazoparib. In this study, 24 patients (39.3%) experienced treatment-related grade 3 anemia (Table 2). Median time from the first dose of talazoparib to the onset of the first grade 3 anemia event was 85 days (range: 41-141). The earliest onset of grade 3 anemia was at the end of cycle 2; however, most grade 3 anemia events started in either cycle 3 (n = 9) or cycle 4 (n = 11). Nine patients had recurrent grade 3 anemia during the treatment period. Twenty (32.8%) patients received packed red blood cell (PRBC)/blood transfusions due to grade 3 anemia. Eight (13.1%) patients received 1 unit of PRBCs; 2 (3.3%) patients received 2 units; 3 (4.9%) patients received 3 units; 4 (6.6%) patients received 4 units; 1 (1.6%) patient received 5 units; and 2 (3.3%) patients received 8 units. Of the patients with grade 3 anemia (n = 24), the majority received PRBC/blood transfusions within one day of onset and all patients had initial dosing interruptions/reductions as defined by the protocol dose-modification guidance. Four patients had more than 1 dose interruption, 7 patients had dose reductions, and 2 patients discontinued talazoparib because of recurrent grade 3 anemia.
Discontinuation and Long-term Follow-up
Sixteen (26.2%) patients discontinued study treatment before completion of the 24-week period: 3 patients discontinued due to AEs, 10 patients discontinued due to PD, 2 patients withdrew consent, and 1 patient discontinued early to have surgery (Table 3).
In the long-term follow-up phase, 4/30 (13.3%) patients with pCR, 10/16 (62.5%) patients with pathologic partial response (pPR), and 2/2 (100%) patients with no response in the ITT population received a combination of adjuvant cyclophosphamide, doxorubicin, platinum, or taxane-based therapy. In addition, of the 10/16 patients with pPR who received adjuvant therapy, 2 patients received capecitabine and 1 patient each received fluorouracil and zoledronic acid.
Of the 10 patients who experienced PD, 5 patients received additional neoadjuvant chemotherapy within 1-8 days from the date of PD. Of the 5 patients who received neoadjuvant chemotherapy, all received platinum therapy, 4 patients received paclitaxel, and 4 patients received cyclophosphamide and doxorubicin. Among the 4 patients with PD who received adjuvant chemotherapy following surgery, patients received taxane, cyclophosphamide, pertuzumab, trastuzumab, atezolizumab, antimetabolite, and/or platinum therapy. One patient received both additional neoadjuvant and adjuvant chemotherapy.
Discussion
The results from the NEOTALA study showed that talazoparib monotherapy was active, despite not meeting the predefined threshold, and was well tolerated in the neoadjuvant setting. The pCR rate by ICR was 45.8% (95% CI, 32.0%-60.6%) and 49.2% (95% CI, 36.7%-61.6%) in the evaluable and ITT analysis population, respectively, and comparable with pCR rates previously observed with neoadjuvant chemotherapy regimens with combination anthracycline- and taxane-based chemotherapy regimens.9 Furthermore, the pCR rates were comparable with a phase I pilot study of neoadjuvant niraparib monotherapy where 40% (n = 6/15) of patients with BRCA mutations and TNBC achieved pCR.28 While efficacy is typically higher in the evaluable population compared to the ITT population, the pCR rate in the NEOTALA study was greater in the ITT population since 8 patients were considered non-evaluable as they received <80% of the planned talazoparib dose but still achieved pCR. Thus, despite the fact a decrease in RDI below 80% is considered a clinically significant reduction from standard therapy,23 the results of the NEOTALA study potentially suggest a shorter neoadjuvant treatment duration in future studies, although further investigation in larger patient populations is warranted.
In this study, 10 (16.4%) patients experienced PD with talazoparib, which may reflect PARP inhibitor resistance in these patients. In the phase II PETREMAC study, which evaluated neoadjuvant olaparib monotherapy, no patients with primary TNBC and BRCA mutations experienced PD.29 However, the lower PD rate achieved with neoadjuvant olaparib may be explained by the small patient population with gBRCA1/2 mutations (n = 4) enrolled in the study. PARP inhibitor resistance can occur through various mechanisms, including reversion of mutated BRCA genes,30 HRR restoration, and DNA replication fork protection, which reduce PARP inhibitor sensitivity and thus negatively impact response.31,32 Nonetheless, further research is required to understand how these mechanisms translate to PARP inhibitor resistance observed in the clinic.31-33
TRAEs were consistent with the established safety profile of talazoparib20,21 with no clinically meaningful detriment in GHS/QoL or nausea/vomiting. Twenty (32.8%) patients had dose interruptions for AEs. Most common TRAEs included fatigue, nausea, alopecia, and anemia. Twenty-four patients (39.3%) experienced treatment-related grade 3 anemia with a median time to onset of 85 days. Twenty (32.8%) patients received transfusions to support anemia, which is comparable with previous talazoparib safety studies in the advanced/metastatic setting including the ABRAZO and EMBRACA studies in which PRBC transfusions were administered to 28% and 38% of patients, respectively.20,21
NEOTALA was limited by its non-randomized, single-arm design, small sample size, and early termination due to sponsor decision. In addition, response assessment per breast ultrasound scan was non-standardized so could not be determined.
Other clinical studies have demonstrated the utility of PARP inhibitors in this patient population. The phase III OlympiA study assessed olaparib in patients with high-risk HER2-negative germline BRCA1/2-mutated breast cancer randomized to 1-year adjuvant olaparib vs. placebo.34 The 3-year invasive, disease-free survival was greater in patients treated with olaparib vs. patients administered placebo (85.9% vs. 77.1%; hazard ratio for invasive disease or death, 0.58; 99.5% CI, 0.41-0.82; P < .001).34 Results from the OlympiA study suggest that PARP inhibitors have a substantial role in the treatment of patients with a germline BRCA mutation and early breast cancer. Similarly, in the GeparOLA study, paclitaxel was combined with either olaparib or carboplatin for the initial segment of neoadjuvant therapy in patients with HER2-negative breast cancer followed by epirubicin and cyclophosphamide.35 The primary endpoint of pCR rate for patients who received paclitaxel-olaparib was 55.1% (90% CI, 44.5%-65.3%) compared to 48.6% (90% CI, 34.4%-63.2%) for patients who received paclitaxel-carboplatin, and paclitaxel-olaparib was better tolerated.35
Multiple ongoing neoadjuvant studies may further clarify the optimal use of PARP inhibitors in germline BRCA-mutated early breast cancers. Notably, the PARTNER study (NCT03150576) is a randomized phase II/III study evaluating the addition of olaparib to platinum-based neoadjuvant chemotherapy in patients with TNBC and/or germline BRCA-mutated breast cancer (N = 756) and pCR as the primary endpoint.36 In addition, following FDA approval of pembrolizumab plus platinum-containing neoadjuvant chemotherapy as a standard of care for patients with TNBC,37 further investigation of PARP inhibitors with immunotherapy is in progress for patients with TNBC (talazoparib plus avelumab, NCT03330405; olaparib plus durvalumab, NCT03544125 and NCT03740893; niraparib plus dostarlimab, NCT04837209).
Conclusion
In summary, despite not reaching the predefined threshold for the primary endpoint, in this phase II study that investigated neoadjuvant talazoparib monotherapy in patients with early-stage TNBC and germline BRCA mutations, the pCR rate was 45.8% in the evaluable population and 49.2% in the ITT population, and was comparable to observed pCR rates with anthracycline- and taxane-based chemotherapy combination regimens. The TRAEs in this study were consistent with the established safety profile of talazoparib.20,21
Supplementary Material
Acknowledgments
The authors acknowledge the contribution of Helen Bhattacharyya, of Pfizer, for the acquisition and analysis of the patient-reported outcomes data. Medical writing support was provided by Maddie Higgins, MBiolSci, of CMC AFFINITY, a division of IPG Health Medical Communications, and was funded by Pfizer.
Contributor Information
Jennifer K Litton, Department of Breast Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
J Thaddeus Beck, Department of Medical Oncology and Hematology, Highlands Oncology, Springdale, AR, USA.
Jason M Jones, Avera Medical Group Oncology & Hematology, Avera Cancer Institute, Sioux Falls, SD, USA.
Jay Andersen, Medical Oncology, Compass Oncology, West Cancer Center, US Oncology Network, Tigard, OR, USA.
Joanne L Blum, Department of Oncology, Texas Oncology-Baylor Charles A. Sammons Cancer Center, US Oncology Network, Dallas, TX, USA.
Lida A Mina, Hematology Oncology Department, Banner MD Anderson Cancer Center, Gilbert, AZ, USA.
Raymond Brig, Medical Oncology, Brig Center for Cancer Care and Survivorship, Knoxville, TN, USA.
Michael Danso, Medical Oncology, Virginia Oncology Associates, Norfolk, VA, USA.
Yuan Yuan, Department of Medical Oncology & Therapeutics Research, Cedars-Sinai Cancer Center, West Hollywood, CA, USA.
Antonello Abbattista, Clinical Statistics, Pfizer Oncology, Milan, Italy.
Kay Noonan, Clinical Oncology, Pfizer Inc., Groton, CT, USA.
Alexander Niyazov, Oncology Value & Evidence, Pfizer Inc., New York, NY, USA.
Jayeta Chakrabarti, Medical Oncology, Pfizer Ltd., Walton Oaks, Surrey, UK.
Akos Czibere, Oncology Drug Development, Pfizer Inc., Cambridge, MA, USA.
William F Symmans, Department of Pathology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Melinda L Telli, Department of Medicine, Stanford University School of Medicine, Stanford, CA, USA.
Funding
This study was sponsored by Pfizer.
Conflict of Interest
Jennifer K. Litton reports research support (to her institution) from AstraZeneca, EMD Serono, Genentech, GlaxoSmithKline, Medivation/Pfizer, Merck, Novartis, Pfizer, and Zenith Epigenetics; fees for participation on the speakers’ bureaus for Clinical Care Options, Med Learning Group, Medpage, Medscape, Physicians’ Education Resource, PrIME Oncology, and UpToDate; honoraria and patent/royalty payments from UpToDate; travel fees from Clinical Care Options, Med Learning Group, Medscape, and Physicians’ Education Resource; and consulting/advisory fees from AstraZeneca, Ayala Pharmaceuticals, Medivation/Pfizer, and Pfizer – all uncompensated. J. Thaddeus Beck reports research support (to his institution) from AbbVie, Amgen, Ascentage Pharma Group, AstraZeneca, Bayer, Boston Biomedical/Bristol Myers Squibb, Celgene, Daiichi Sankyo, EMD Serono, Evelo Biosciences, Genentech/Roche, GlaxoSmithKline, Hutchison, Immunomedics, Laekna Therapeutics, Lilly, Mirati Therapeutics, Nektar, Novartis, Pfizer, Polynoma, and Seattle Genetics. Jason M. Jones and Raymond Brig have nothing to disclose. Jay Andersen reports fees for participation on the speakers’ bureaus for AstraZeneca/Daiichi Sankyo, Genentech, Genomic Health, Immunomedics, Novartis, Puma Biotechnology, and Seattle Genetics; and consulting/advisory fees from AstraZeneca/Daiichi Sankyo, Athenex, Biotheranostics, Myriad, Novartis, Pfizer, Puma Biotechnology, and Seattle Genetics.
Joanne L. Blum was a physician at Texas Oncology-Baylor Charles A. Sammons Cancer Center, US Oncology Network at the time these analyses were performed and reports consulting/advisory fees and honoraria from AstraZeneca, Athenex, Inc., Biotheranostics, Immunomedics, OncLive, Pfizer, Puma Biotechnology, Research to Practice, Sanofi, Tempus, and TD2 and participation on the speakers’ bureaus for Pfizer, and Tempus. Lida A. Mina was an employee at Banner MD Anderson Cancer Center at the time these analyses were performed and has nothing to disclose. Michael Danso reports consulting/advisory fees from Immunomedics, Novartis, Pfizer, and Seattle Genetics; and honoraria from Amgen. Yuan Yuan was an employee of the City of Hope Comprehensive Cancer Center and Beckman Research Institute at the time of these analyses and reports research support from Eisai, Genentech, and Merck; consulting/advisory fees from BCI Pharma, Daiichi Sankyo, Eisai, Genentech, Gilead, Novartis, Pfizer, and Puma Biotechnology; fees for participation on the speakers’ bureaus for AstraZeneca, Daiichi Sankyo/Lilly, Eisai, Genentech, Gilead, Merck, and Pfizer; and fees for expert testimony for Novartis. Antonello Abbattista, Alexander Niyazov, Jayeta Chakrabarti, and Akos Czibere are employees of Pfizer and hold stock. Kay Noonan was an employee at Pfizer at the time of this study. William F. Symmans reports consulting/advisory fees from Almac Diagnostics and Merck; support for travel from Luminex and Merck; stock ownership in Delphi Diagnostics, Eiger BioPharmaceuticals, ISIS Pharmaceuticals, and Nuvera Biosciences; and an uncompensated relationship with Delphi Diagnostics. Melinda L. Telli reports research support (to her institution) from AbbVie, Bayer, Biothera, Calithera Biosciences, EMD Serono, Genentech, Medivation, Merck, Novartis, OncoSec, Pfizer, PharmaMar, Tesaro, and Vertex; and consulting/advisory fees from AbbVie, Aduro Biotech, AstraZeneca, Celgene, Daiichi Sankyo, G1 Therapeutics, Genentech/Roche, Guardant, Immunomedics, Lilly, Merck, Natera, Novartis, OncoSec, Pfizer, and Sanofi.
Author Contributions
Conception/design: J.K.L., L.A.M., M.D., Y.Y., A.A., A.N., W.F.S., M.L.T. Provision of study material or patients: J.T.B., J.M.J., L.A.M., M.D., Y.Y., K.N. Collection and/or assembly of data: J.K.L., J.T.B., J.A., J.L.B., L.A.M., M.D., Y.Y., W.F.S., M.L.T. Data analysis and interpretation: J.K.L., J.T.B., Y.Y., A.A., A.N., A.C., M.L.T., R.B. Manuscript writing and final approval of manuscript: All authors.
Data Availability
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
References
- 1. Wang H, Yee D.. I-SPY 2: a neoadjuvant adaptive clinical trial designed to improve outcomes in high-risk breast cancer. Curr Breast Cancer Rep. 2019;11(4):303-310. 10.1007/s12609-019-00334-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Campeau PM, Foulkes WD, Tischkowitz MD.. Hereditary breast cancer: new genetic developments, new therapeutic avenues. Hum Genet. 2008;124(1):31-42. 10.1007/s00439-008-0529-1. [DOI] [PubMed] [Google Scholar]
- 3. Economopoulou P, Dimitriadis G, Psyrri A.. Beyond BRCA: new hereditary breast cancer susceptibility genes. Cancer Treat Rev. 2015;41(1):1-8. 10.1016/j.ctrv.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 4. Gonçalves A, Bertucci A, Bertucci F.. PARP inhibitors in the treatment of early breast cancer: the step beyond? Cancers (Basel). 2020;12(6):1378. 10.3390/cancers12061378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kuchenbaecker KB, Hopper JL, Barnes DR, et al. ; BRCA1 and BRCA2 Cohort Consortium. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017;317(23):2402-2416. 10.1001/jama.2017.7112. [DOI] [PubMed] [Google Scholar]
- 6. Lord CJ, Ashworth A.. PARP inhibitors: synthetic lethality in the clinic. Science. 2017;355(6330):1152-1158. 10.1126/science.aam7344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mavaddat N, Barrowdale D, Andrulis IL, et al. ; HEBON. Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA). Cancer Epidemiol Biomarkers Prev. 2012;21(1):134-147. 10.1158/1055-9965.EPI-11-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Javle M, Curtin NJ.. The potential for poly (ADP-ribose) polymerase inhibitors in cancer therapy. Ther Adv Med Oncol. 2011;3(6):257-267. 10.1177/1758834011417039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Loibl S, O’Shaughnessy J, Untch M, et al. Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): a randomised, phase 3 trial. Lancet Oncol. 2018;19(4):497-509. 10.1016/S1470-2045(18)30111-6. [DOI] [PubMed] [Google Scholar]
- 10. Schmid P, Cortes J, Pusztai L, et al. ; KEYNOTE-522 Investigators. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382(9):810-821. 10.1056/NEJMoa1910549. [DOI] [PubMed] [Google Scholar]
- 11. Lee JS, Yost SE, Yuan Y.. Neoadjuvant treatment for triple negative breast cancer: recent progresses and challenges. Cancers (Basel). 2020;12(6):1404. 10.3390/cancers12061404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang C-J, Xu Y, Lin Y, et al. Platinum-based neoadjuvant chemotherapy for breast cancer with BRCA mutations: a meta-analysis. Front Oncol. 2020;10(2410):592998. 10.3389/fonc.2020.592998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. von Minckwitz G, Schneeweiss A, Loibl S, et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol. 2014;15(7):747-756. 10.1016/s1470-2045(14)70160-3. [DOI] [PubMed] [Google Scholar]
- 14. Sikov WM, Berry DA, Perou CM, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol. 2015;33(1):13-21. 10.1200/JCO.2014.57.0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tung N, Arun B, Hacker MR, et al. TBCRC 031: randomized phase II study of neoadjuvant cisplatin versus doxorubicin-cyclophosphamide in germline BRCA carriers with HER2-negative breast cancer (the INFORM trial). J Clin Oncol. 2020;38(14):1539-1548. 10.1200/JCO.19.03292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Loibl S, Weber KE, Timms KM, et al. Survival analysis of carboplatin added to an anthracycline/taxane-based neoadjuvant chemotherapy and HRD score as predictor of response-final results from GeparSixto. Ann Oncol. 2018;29(12):2341-2347. 10.1093/annonc/mdy460. [DOI] [PubMed] [Google Scholar]
- 17. European Medicines Agency. TALZENNA® (talazoparib) Summary of Product Characteristics. November 2020. https://www.ema.europa.eu/en/documents/product-information/talzenna-epar-product-information_en.pdf (accessed March 1, 2022).
- 18. U.S. Food and Drug Administration. TALZENNA® (talazoparib) prescribing information. September 2021. Accessed March 2, 2023. http://labeling.pfizer.com/ShowLabeling.aspx?id=11046.
- 19. Litton JK, Scoggins ME, Hess KR, et al. Neoadjuvant talazoparib for patients with operable breast cancer with a germline BRCA pathogenic variant. J Clin Oncol. 2020;38(5):388-394. 10.1200/JCO.19.01304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hurvitz SA, Gonçalves A, Rugo HS, et al. Talazoparib in patients with a germline BRCA-mutated advanced breast cancer: detailed safety analyses from the Phase III EMBRACA trial. Oncologist. 2020;25(3):e439-e450. 10.1634/theoncologist.2019-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Turner NC, Telli ML, Rugo HS, et al. ; ABRAZO Study Group. A phase II study of talazoparib after platinum or cytotoxic nonplatinum regimens in patients with advanced breast cancer and germline BRCA1/2 mutations (ABRAZO). Clin Cancer Res. 2019;25(9):2717-2724. 10.1158/1078-0432.CCR-18-1891. [DOI] [PubMed] [Google Scholar]
- 22. Amin MB, Edge SB, Greene FL, et al. , eds. AJCC Cancer Staging Manual. 8th ed.New York: Springer; 2017. [Google Scholar]
- 23. Nielson CM, Bylsma LC, Fryzek JP, Saad HA, Crawford J.. Relative dose intensity of chemotherapy and survival in patients with advanced stage solid tumor cancer: a systematic review and meta-analysis. Oncologist. 2021;26(9):e1609-e1618. 10.1002/onco.13822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Symmans WF, Wei C, Gould R, et al. Long-term prognostic risk after neoadjuvant chemotherapy associated with residual cancer burden and breast cancer subtype. J Clin Oncol. 2017;35(10):1049-1060. 10.1200/JCO.2015.63.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35(11):1095-1108. 10.1097/00005650-199711000-00002. [DOI] [PubMed] [Google Scholar]
- 26. Snyder CF, Blackford AL, Sussman J, et al. Identifying changes in scores on the EORTC-QLQ-C30 representing a change in patients’ supportive care needs. Qual Life Res. 2015;24(5):1207-1216. 10.1007/s11136-014-0853-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pickard AS, Neary MP, Cella D.. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes. 2007;5:70. 10.1186/1477-7525-5-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Spring LM, Han H, Liu MC, et al. Neoadjuvant study of niraparib in patients with HER2-negative, BRCA-mutated, resectable breast cancer. Nat Cancer. 2022;3(8):927-931. 10.1038/s43018-022-00400-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eikesdal HP, Yndestad S, Elzawahry A, et al. Olaparib monotherapy as primary treatment in unselected triple negative breast cancer. Ann Oncol. 2021;32(2):240-249. 10.1016/j.annonc.2020.11.009. [DOI] [PubMed] [Google Scholar]
- 30. Barber LJ, Sandhu S, Chen L, et al. Secondary mutations in BRCA2 associated with clinical resistance to a PARP inhibitor. J Pathol. 2013;229(3):422-429. 10.1002/path.4140. [DOI] [PubMed] [Google Scholar]
- 31. Lee EK, Matulonis UA.. PARP inhibitor resistance mechanisms and implications for post-progression combination therapies. Cancers (Basel). 2020;12(8):2054. 10.3390/cancers12082054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li H, Liu ZY, Wu N, et al. PARP inhibitor resistance: the underlying mechanisms and clinical implications. Mol Cancer. 2020;19(1):107. 10.1186/s12943-020-01227-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Telli ML, Litton JK, Beck JT, et al. Neoadjuvant talazoparib (TALA) in patients (pts) with germline BRCA1/2 (gBRCA1/2) mutation-positive, early HER2-negative breast cancer (BC): exploration of tumor BRCA mutational status and zygosity and overall mutational landscape in a phase 2 study. Presented at: ASCO; June 4-8, 2021. Virtual. [Google Scholar]
- 34. Tutt ANJ, Garber JE, Kaufman B, et al. ; OlympiA Clinical Trial Steering Committee and Investigators. Adjuvant olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. N Engl J Med. 2021;384(25):2394-2405. 10.1056/NEJMoa2105215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fasching PA, Link T, Hauke J, et al. ; German Breast Group and Arbeitsgemeinschaft Gynäkologische Onkologie Breast. Neoadjuvant paclitaxel/olaparib in comparison to paclitaxel/carboplatinum in patients with HER2-negative breast cancer and homologous recombination deficiency (GeparOLA study). Ann Oncol. 2021;32(1):49-57. 10.1016/j.annonc.2020.10.471. [DOI] [PubMed] [Google Scholar]
- 36. Drewett L, Lucey R, Pinilla KA, et al. PARTNER: a randomized, phase II/III trial to evaluate the safety and efficacy of the addition of olaparib to platinum-based neoadjuvant chemotherapy in patients with triple-negative and/or germline BRCA-mutated breast cancer. J Clin Oncol. 2022;40(16_suppl):TPS619-TPS619. 10.1200/jco.2022.40.16_suppl.tps619. https://meetings.asco.org/abstracts-presentations/213442). ASCO abstract TPS619. [DOI] [Google Scholar]
- 37. U.S. Food and Drug Administration. FDA approves pembrolizumab for high-risk early-stage triple-negative breast cancer. 2021. Accessed March 1, 2022https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-high-risk-early-stage-triple-negative-breast-cancer.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.