Abstract
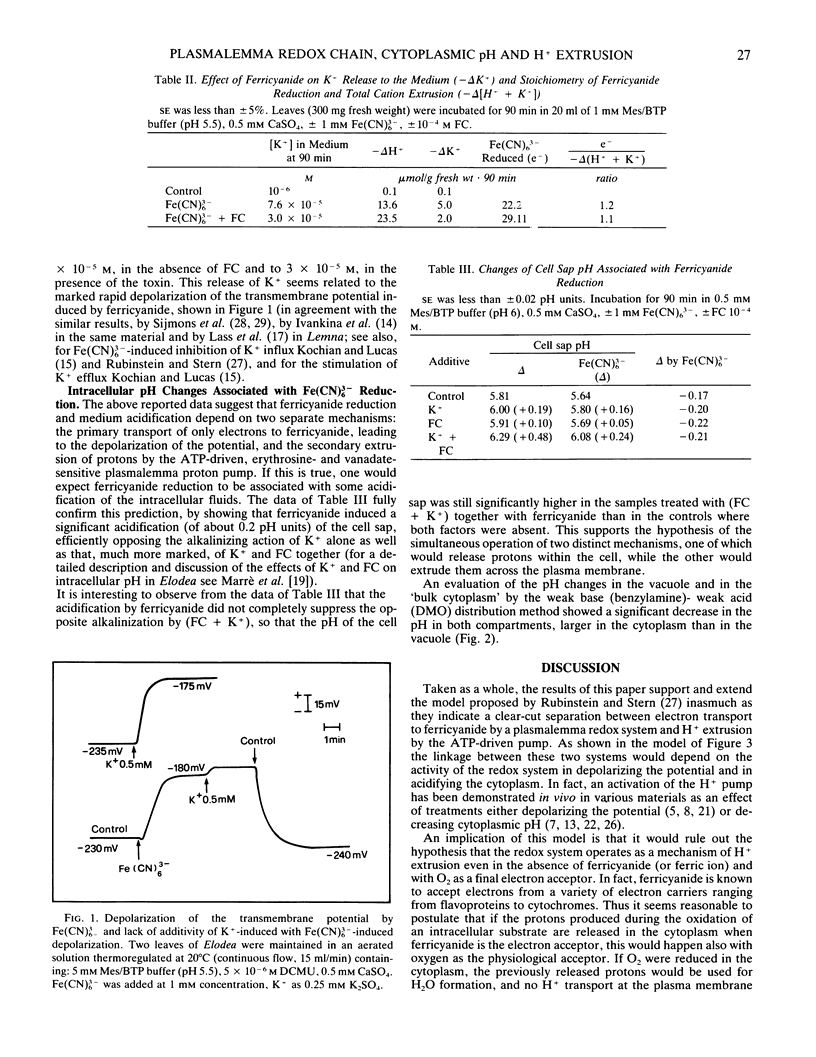
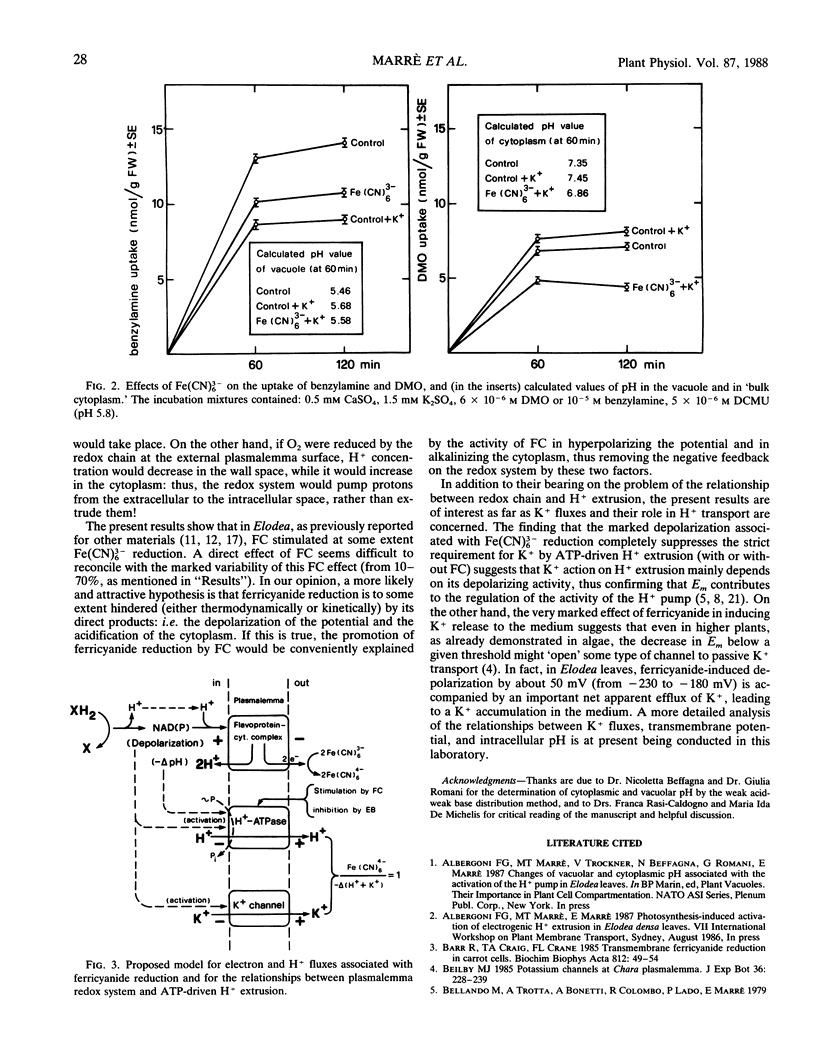
Ferricyanide reduction by Elodea densa leaves, in the dark, is associated with: (a) acidification of the medium; (b) decrease (about 0.2-0.3 units) of intracellular pH (measured in cell sap, cytoplasm, and vacuole); (c) depolarization of the transmembrane potential; (d) net efflux of K+ to the medium. Ferricyanide-induced acid secretion is markedly increased by the presence of fusicoccin (FC), and this effect is severely inhibited by the proton pump inhibitors erythrosine B and vanadate. In the presence of ferricyanide FC-induced H+ extrusion no longer requires the presence of K+ in the medium. The (ferricyanide reduced)/(H+ extruded) ratio varies from about 2, in the absence of FC, to about 1 when the toxin is present, and to more than 4, when ATP-driven H+ extrusion is inhibited by erythrosine B or by vanadate. Fusicoccin markedly reduces K+ release to the medium. The ratio (ferricyanide reduced)/(H+ extruded + K+ released) approaches unity under all of the three conditions considered. These results indicate that ferricyanide reduction depends on a plasmalemma system transporting only electrons to the extracellular acceptor, with consequent potential depolarization and cytoplasm acidification. Most of the protons released in the cytoplasm would be secondarily extruded by the ATP-driven pump, stimulated by both intracellular acidification and depolarization. K+ efflux would depend on potential depolarization.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barr R., Craig T. A., Crane F. L. Transmembrane ferricyanide reduction in carrot cells. Biochim Biophys Acta. 1985 Jan 10;812(1):49–54. doi: 10.1016/0005-2736(85)90520-6. [DOI] [PubMed] [Google Scholar]
- Federico R., Giartosio C. E. A transplasmamembrane electron transport system in maize roots. Plant Physiol. 1983 Sep;73(1):182–184. doi: 10.1104/pp.73.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochian L. V., Lucas W. J. Potassium Transport in Corn Roots : III. Perturbation by Exogenous NADH and Ferricyanide. Plant Physiol. 1985 Feb;77(2):429–436. doi: 10.1104/pp.77.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W. Further Characterization on the Transport Property of Plasmalemma NADH Oxidation System in Isolated Corn Root Protoplasts. Plant Physiol. 1984 Feb;74(2):219–222. doi: 10.1104/pp.74.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld E., Bown A. W. A plasmamembrane redox system and proton transport in isolated mesophyll cells. Plant Physiol. 1987 Apr;83(4):895–899. doi: 10.1104/pp.83.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasi-Caldogno F., De Michelis M. I., Pugliarello M. C., Marrè E. H-pumping driven by the plasma membrane ATPase in membrane vesicles from radish: stimulation by fusicoccin. Plant Physiol. 1986 Sep;82(1):121–125. doi: 10.1104/pp.82.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasi-Caldogno F., Pugliarello M. C. Fusicoccin stimulates the H+-ATPase of plasmalemma in isolated membrane vesicles from radish. Biochem Biophys Res Commun. 1985 Nov 27;133(1):280–285. doi: 10.1016/0006-291x(85)91872-8. [DOI] [PubMed] [Google Scholar]
- Romani G., Marrè M. T., Bellando M., Alloatti G., Marrè E. H extrusion and potassium uptake associated with potential hyperpolarization in maize and wheat root segments treated with permeant weak acids. Plant Physiol. 1985 Nov;79(3):734–739. doi: 10.1104/pp.79.3.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein B., Stern A. I. Relationship of Transplasmalemma Redox Activity to Proton and Solute Transport by Roots of Zea mays. Plant Physiol. 1986 Apr;80(4):805–811. doi: 10.1104/pp.80.4.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijmons P. C., Lanfermeijer F. C., de Boer A. H., Prins H. B., Bienfait H. F. Depolarization of Cell Membrane Potential during Trans-Plasma Membrane Electron Transfer to Extracellular Electron Acceptors in Iron-Deficient Roots of Phaseolus vulgaris L. Plant Physiol. 1984 Dec;76(4):943–946. doi: 10.1104/pp.76.4.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijmons P. C., van den Briel W., Bienfait H. F. Cytosolic NADPH is the electron donor for extracellular fe reduction in iron-deficient bean roots. Plant Physiol. 1984 May;75(1):219–221. doi: 10.1104/pp.75.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]


