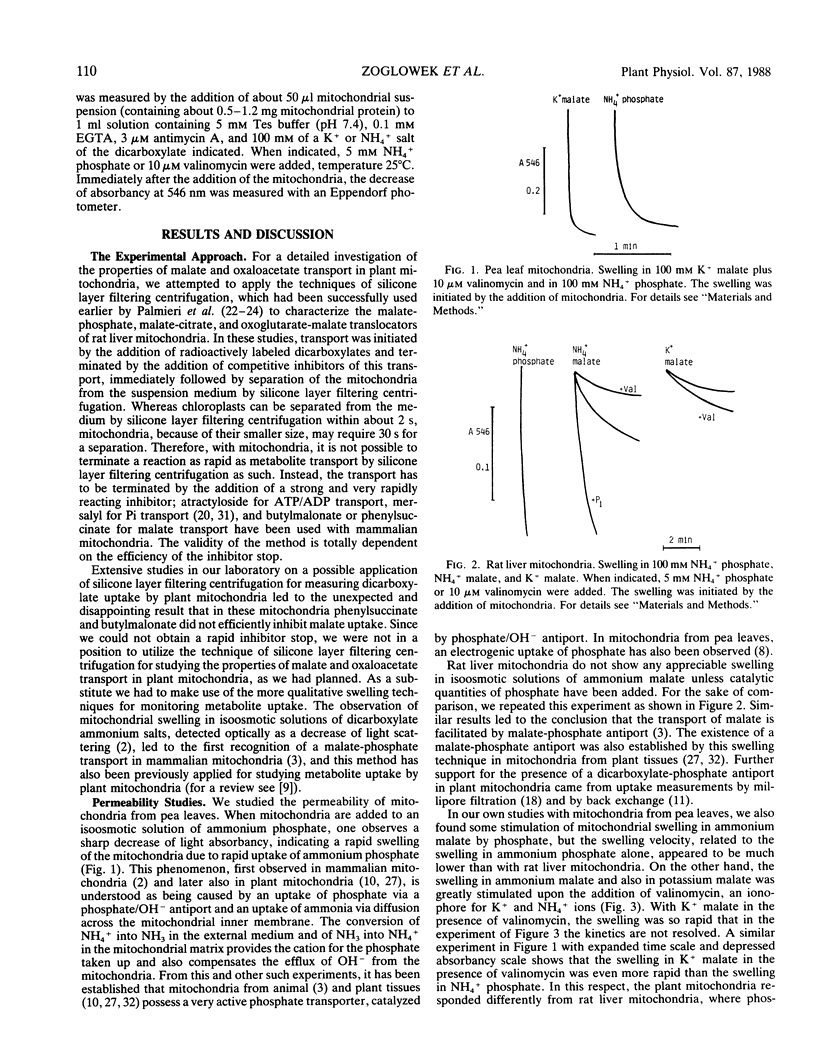
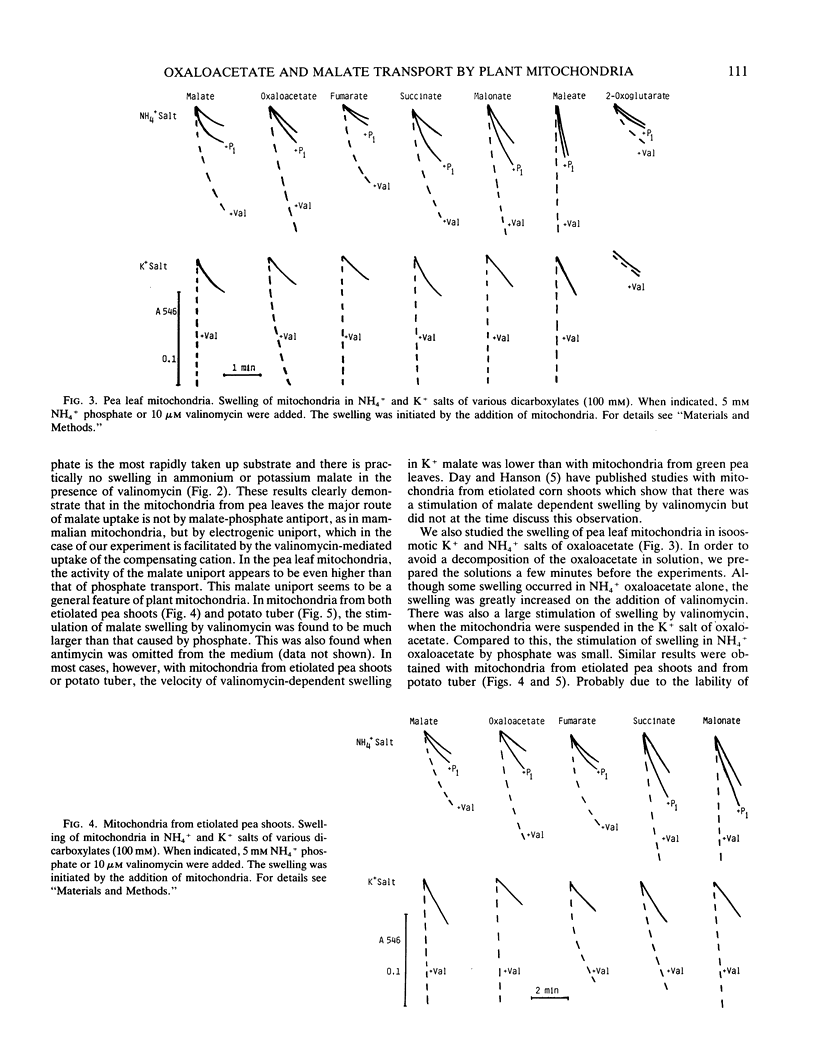
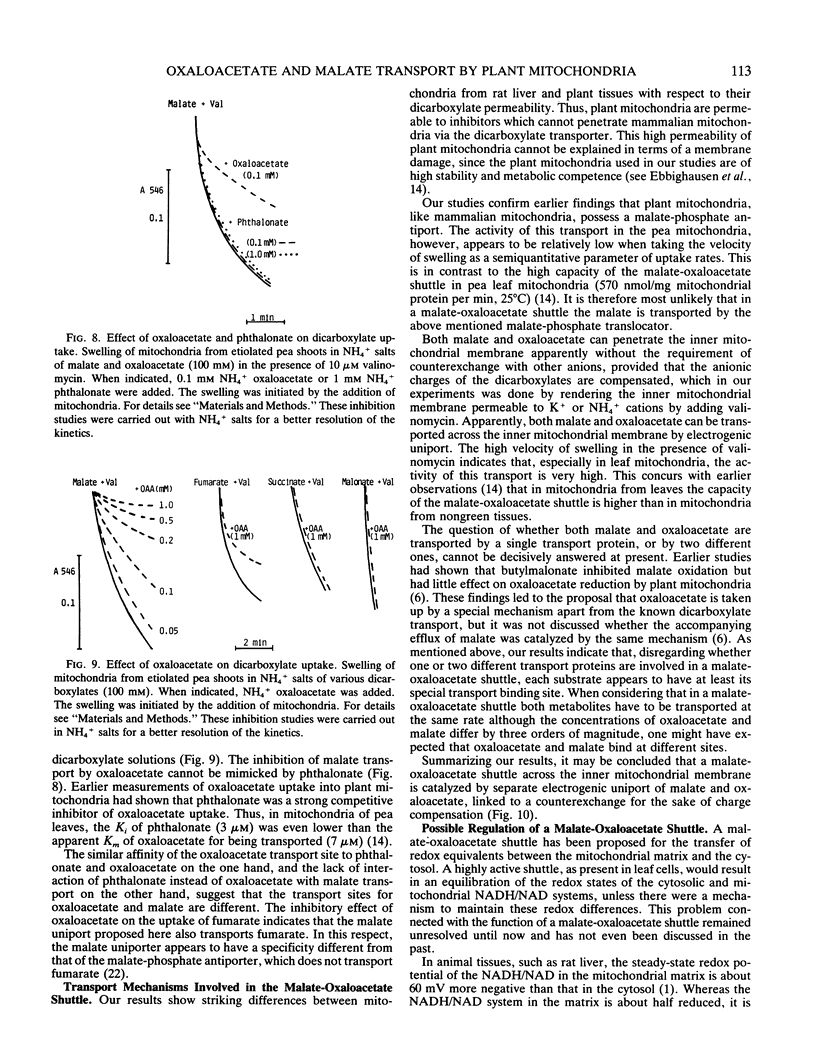
Abstract
The permeability of mitochondria from pea (Pisum sativum L. var Kleine Rheinländerin) leaves, etiolated pea shoots, and potato (Solanum tuberosum) tuber for malate, oxaloacetate, and other dicarboxylates was investigated by measurement of mitochondrial swelling in isoosmolar solutions of the above mentioned metabolites. For the sake of comparison, parallel experiments were also performed with rat liver mitochondria. Unlike the mammalian mitochondria, the plant mitochondria showed only little swelling in ammonium malate plus phosphate media but a dramatic increase of swelling on the addition of valinomycin. Similar results were obtained with oxaloacetate, maleate, fumarate, succinate, and malonate. n-Butylmalonate and phenylsuccinate, impermeant inhibitors of malate transport in mammalian mitochondria, had no marked inhibitory effect on valinomycin-dependent malate and oxaloacetate uptake of the plant mitochondria. The swelling of plant mitochondria in malate plus valinomycin was strongly inhibited by oxaloacetate, at a concentration ratio of oxaloacetate/malate of 10−3. From these findings it is concluded: (a) In a malate-oxaloacetate shuttle transferring redox equivalents from the mitochondrial matrix to the cytosol, malate and oxaloacetate are each transported by electrogenic uniport, probably linked to each other for the sake of charge compensation. (b) The transport of malate between the mitochondrial matrix and the cytosol is controlled by the oxaloacetate level in such a way that a redox gradient can be maintained between the NADH/NAD systems in the matrix and the cytosol. (c) The malate-oxaloacetate shuttle functions mainly in the export of malate from the mitochondria, whereas the import of malate as a respiratory substrate may proceed by the classical malate-phosphate antiport.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chappell J. B. Systems used for the transport of substrates into mitochondria. Br Med Bull. 1968 May;24(2):150–157. doi: 10.1093/oxfordjournals.bmb.a070618. [DOI] [PubMed] [Google Scholar]
- Day D. A., Hanson J. B. Pyruvate and malate transport and oxidation in corn mitochondria. Plant Physiol. 1977 Apr;59(4):630–635. doi: 10.1104/pp.59.4.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day D. A., Wiskich J. T. Effect of phthalonic acid on respiration and metabolite transport in higher plant mitochondria. Arch Biochem Biophys. 1981 Oct 1;211(1):100–107. doi: 10.1016/0003-9861(81)90434-3. [DOI] [PubMed] [Google Scholar]
- Day D. A., Wiskich J. T. Glycine metabolism and oxalacetate transport by pea leaf mitochondria. Plant Physiol. 1981 Aug;68(2):425–429. doi: 10.1104/pp.68.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douce R., Bonner W. D., Jr Oxalacetate control of Krebs cycle oxidations in purified plant mitochondria. Biochem Biophys Res Commun. 1972 May 12;47(3):619–624. doi: 10.1016/0006-291x(72)90923-0. [DOI] [PubMed] [Google Scholar]
- Douce R., Moore A. L., Neuburger M. Isolation and oxidative properties of intact mitochondria isolated from spinach leaves. Plant Physiol. 1977 Oct;60(4):625–628. doi: 10.1104/pp.60.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Journet E. P., Neuburger M., Douce R. Role of Glutamate-oxaloacetate Transaminase and Malate Dehydrogenase in the Regeneration of NAD for Glycine Oxidation by Spinach leaf Mitochondria. Plant Physiol. 1981 Mar;67(3):467–469. doi: 10.1104/pp.67.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung D. W., Laties G. G. Citrate and succinate uptake by potato mitochondria. Plant Physiol. 1979 Apr;63(4):591–597. doi: 10.1104/pp.63.4.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingenberg M. Kinetic study of the tricarboxylate carrier in rat liver mitochondria. Eur J Biochem. 1972 Apr 24;26(4):587–594. doi: 10.1111/j.1432-1033.1972.tb01801.x. [DOI] [PubMed] [Google Scholar]
- LaNoue K. F., Meijer A. J., Brouwer A. Evidence for electrogenic aspartate transport in rat liver mitochondria. Arch Biochem Biophys. 1974 Apr 2;161(2):544–550. doi: 10.1016/0003-9861(74)90337-3. [DOI] [PubMed] [Google Scholar]
- Meijer A. J., Groot G. S.P., Tager J. M. Effect of sulphydryl-blocking reagents on mitochondrial anion-exchange reactions involving phosphate. FEBS Lett. 1970 May 11;8(1):41–44. doi: 10.1016/0014-5793(70)80220-4. [DOI] [PubMed] [Google Scholar]
- Oliver D. J., Walker G. H. Characterization of the transport of oxaloacetate by pea leaf mitochondria. Plant Physiol. 1984 Oct;76(2):409–413. doi: 10.1104/pp.76.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri F., Prezioso G., Quagliariello E., Klingenberg M. Kinetic study of the dicarboxylate carrier in rat liver mitochondria. Eur J Biochem. 1971 Sep 13;22(1):66–74. doi: 10.1111/j.1432-1033.1971.tb01515.x. [DOI] [PubMed] [Google Scholar]
- Palmieri F., Quagliariello E., Klingenberger M. Kinetics and specificity of the oxoglutarate carrier in rat-liver mitochondria. Eur J Biochem. 1972 Sep 25;29(3):408–416. doi: 10.1111/j.1432-1033.1972.tb02003.x. [DOI] [PubMed] [Google Scholar]
- Passarella S., Atlante A., Quagliariello E. Oxaloacetate permeation in rat kidney mitochondria: pyruvate/oxaloacetate and malate/oxaloacetate translocators. Biochem Biophys Res Commun. 1985 May 31;129(1):1–10. doi: 10.1016/0006-291x(85)91394-4. [DOI] [PubMed] [Google Scholar]
- Passarella S., Palmieri F., Quagliariello E. The transport of oxaloacetate in isolated mitochondria. Arch Biochem Biophys. 1977 Apr 15;180(1):160–168. doi: 10.1016/0003-9861(77)90020-0. [DOI] [PubMed] [Google Scholar]
- Phillips M. L., Williams G. R. Anion transporters in plant mitochondria. Plant Physiol. 1973 Apr;51(4):667–670. doi: 10.1104/pp.51.4.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STERN J. R., OCHOA S., LYNEN F. Enzymatic synthesis of citric acid. V. Reaction of acetyl coenzyme A. J Biol Chem. 1952 Sep;198(1):313–321. [PubMed] [Google Scholar]
- Tyler D. D. The inhibition of phosphate entry into rat liver mitochondria by organic mercurials and by formaldehyde. Biochem J. 1968 Mar;107(1):121–123. doi: 10.1042/bj1070121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo K. C., Jokinen M., Canvin D. T. Reduction of Nitrate via a Dicarboxylate Shuttle in a Reconstituted System of Supernatant and Mitochondria from Spinach Leaves. Plant Physiol. 1980 Mar;65(3):433–436. doi: 10.1104/pp.65.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]


