Abstract
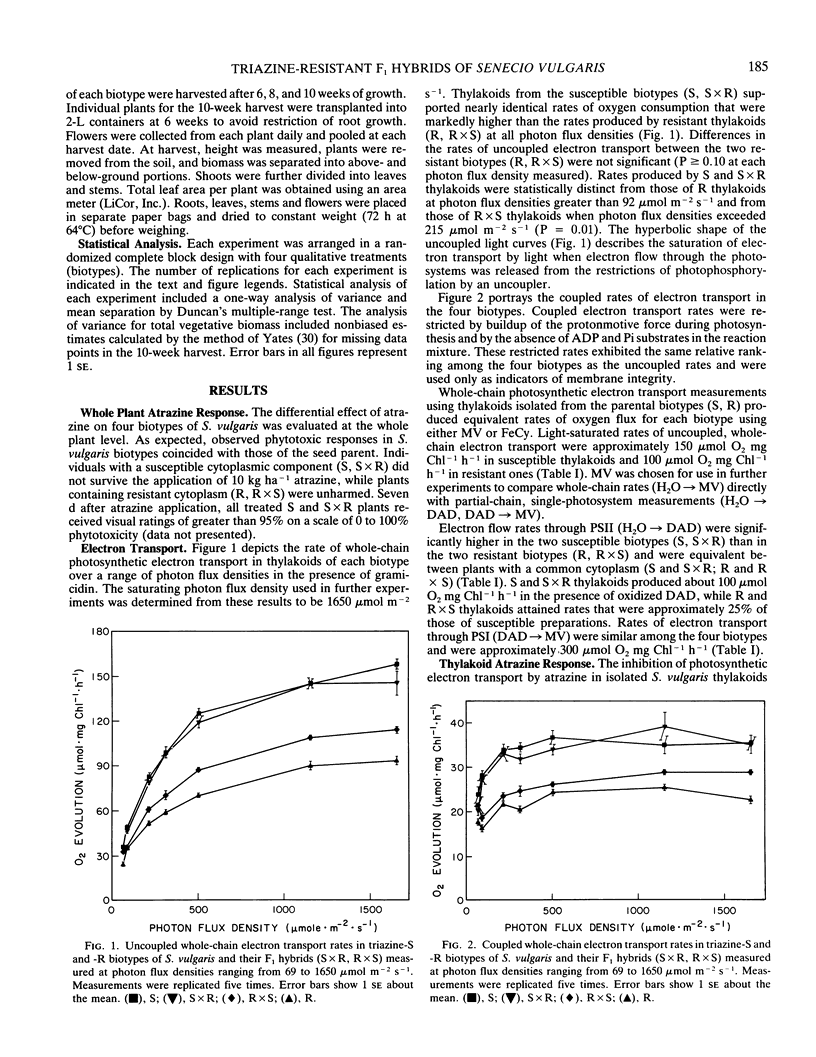
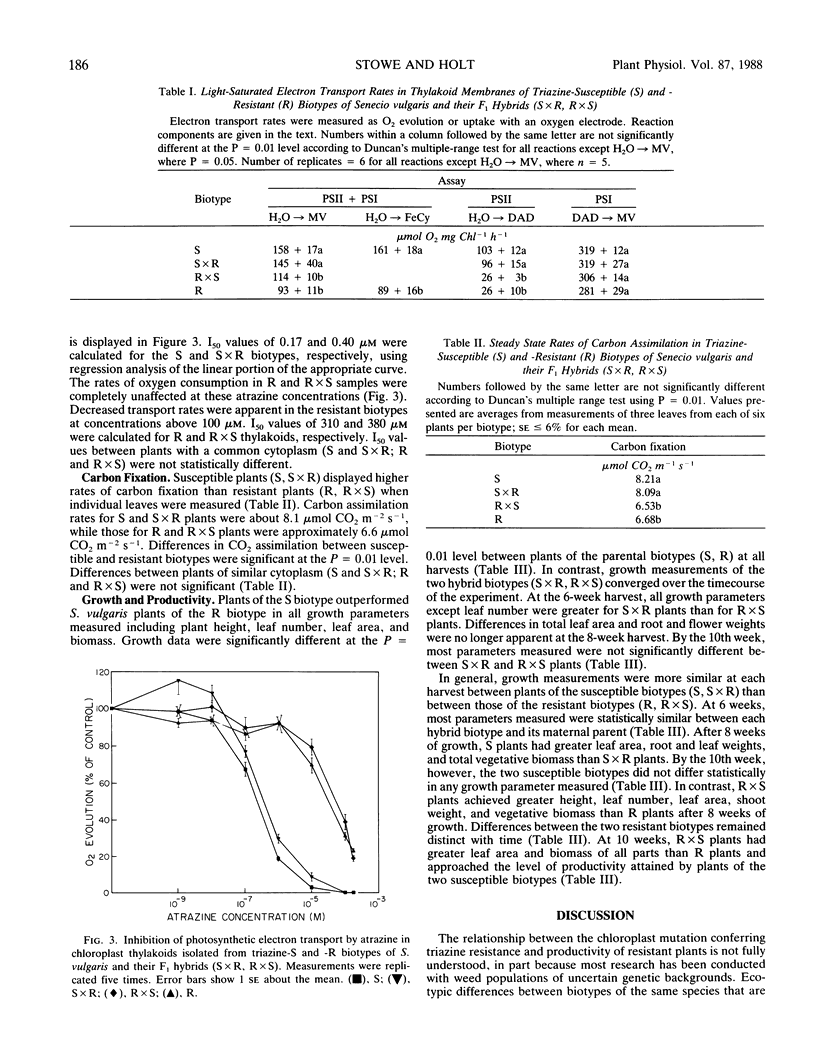
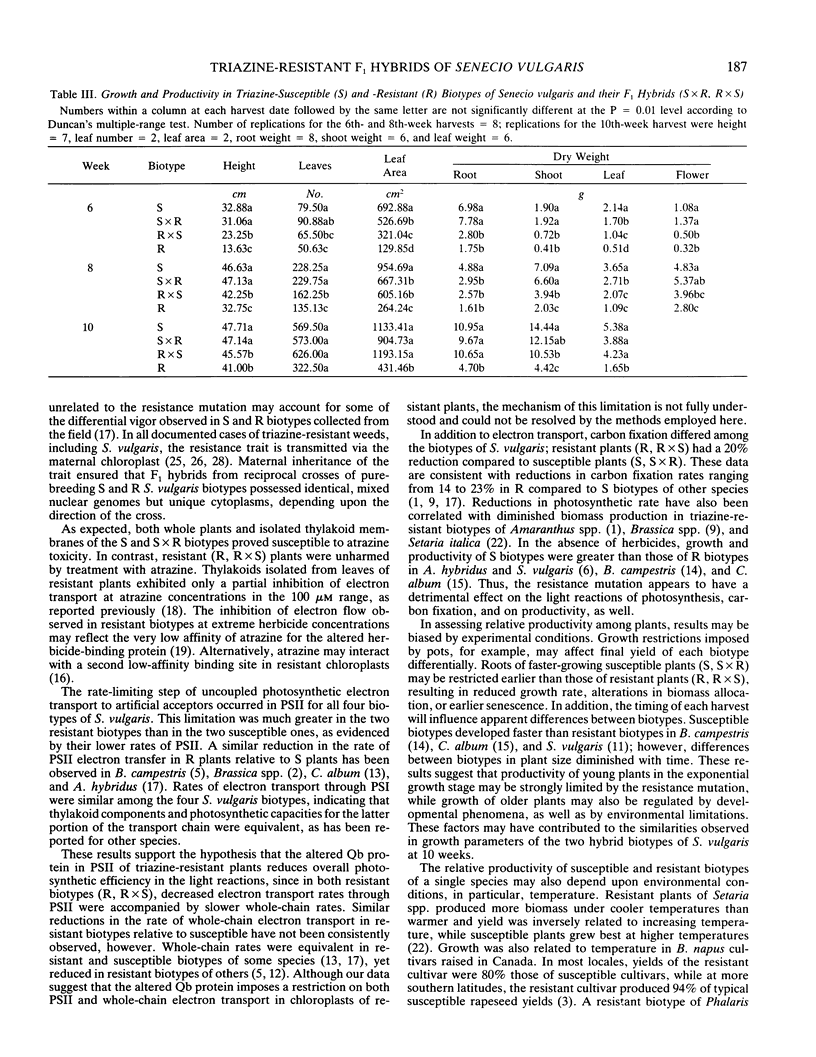
The relationship of triazine resistance to decreased plant productivity was investigated in Senecio vulgaris L. F1 reciprocal hybrids were developed from pure-breeding susceptible (S) and resistant (R) lines. The four biotypes (S, S × R, R, R × S) were compared in terms of atrazine response, electron transport, carbon fixation, and biomass production. Atrazine response, carbon fixation rate, and PSII and whole-chain electron transport rates of hybrids were nearly identical to those of their respective maternal parents. Significant differences occurred between the two susceptible (S, S × R) and two resistant (R, R × S) biotypes in atrazine response (I50), carbon fixation rate, and PSII and whole-chain electron transport rates; PSI rates were identical in all four biotypes. Coupled and uncoupled, whole-chain electron transport rates of thylakoids of the two susceptible biotypes were approximately 50% greater than those of the two resistant biotypes at photon flux densities greater than 215 micromoles per square meter per second. Carbon exchange rates of the two susceptible biotypes were 23% greater than those of the two resistant biotypes. Hybrid biotypes (S × R, R × S) were not identical to their maternal parents in biomass production. The S, S × R, and R × S plants all achieved greater biomass than R plants. These results suggest that while the resistance mutation influences thylakoid performance, reduced productivity of triazine-resistant plants cannot be ascribed solely to decreases in electron transport or carbon assimilation rates brought about by the altered binding protein. Since the F1 hybrids differed from their maternal parents only in nuclear genes, it appears that the detrimental effects of the triazine resistance mutation on plant growth may be attenuated by interactions of the plastid and nuclear genomes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali A., Fuerst E. P., Arntzen C. J., Machado V. S. Stability of chloroplastic triazine resistance in rutabaga backcross generations. Plant Physiol. 1986 Feb;80(2):511–514. doi: 10.1104/pp.80.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade Z. A., Andrade S. G., Susin M. Pathological changes due to massive schistosomal infection in man (a case presentation). Rev Inst Med Trop Sao Paulo. 1974 May-Jun;16(3):171–177. [PubMed] [Google Scholar]
- Bowes J., Crofts A. R., Arntzen C. J. Redox Reactions on the reducing side of photosystem II in chloroplasts with altered herbicide binding properties. Arch Biochem Biophys. 1980 Apr 1;200(2):303–308. doi: 10.1016/0003-9861(80)90359-8. [DOI] [PubMed] [Google Scholar]
- Burke J. J., Wilson R. F., Swafford J. R. Characterization of Chloroplasts Isolated from Triazine-Susceptible and Triazine-Resistant Biotypes of Brassica campestris L. Plant Physiol. 1982 Jul;70(1):24–29. doi: 10.1104/pp.70.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt J. S., Goffner D. P. Altered Leaf Structure and Function in Triazine-Resistant Common Groundsel (Senecio vulgaris). Plant Physiol. 1985 Nov;79(3):699–705. doi: 10.1104/pp.79.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt J. S., Stemler A. J., Radosevich S. R. Differential Light Responses of Photosynthesis by Triazine-resistant and Triazine-susceptible Senecio vulgaris Biotypes. Plant Physiol. 1981 Apr;67(4):744–748. doi: 10.1104/pp.67.4.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ort D. R., Ahrens W. H., Martin B., Stoller E. W. Comparison of Photosynthetic Performance in Triazine-Resistant and Susceptible Biotypes of Amaranthus hybridus. Plant Physiol. 1983 Aug;72(4):925–930. doi: 10.1104/pp.72.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister K., Steinback K. E., Gardner G., Arntzen C. J. Photoaffinity labeling of an herbicide receptor protein in chloroplast membranes. Proc Natl Acad Sci U S A. 1981 Feb;78(2):981–985. doi: 10.1073/pnas.78.2.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai P., John J. B. Lipid composition of chloroplast membranes from weed biotypes differentially sensitive to triazine herbicides. Plant Physiol. 1981 Sep;68(3):585–587. doi: 10.1104/pp.68.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönfeld M., Yaacoby T., Michael O., Rubin B. Triazine Resistance without Reduced Vigor in Phalaris paradoxa. Plant Physiol. 1987 Feb;83(2):329–333. doi: 10.1104/pp.83.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]


