Abstract
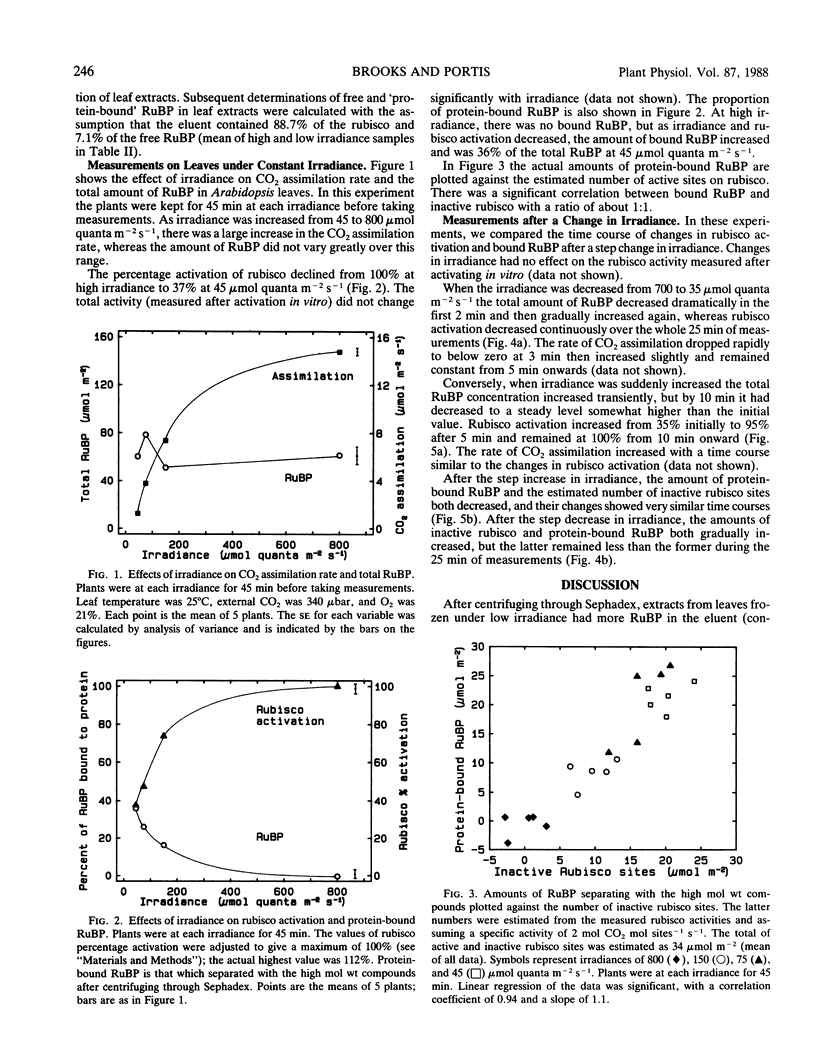
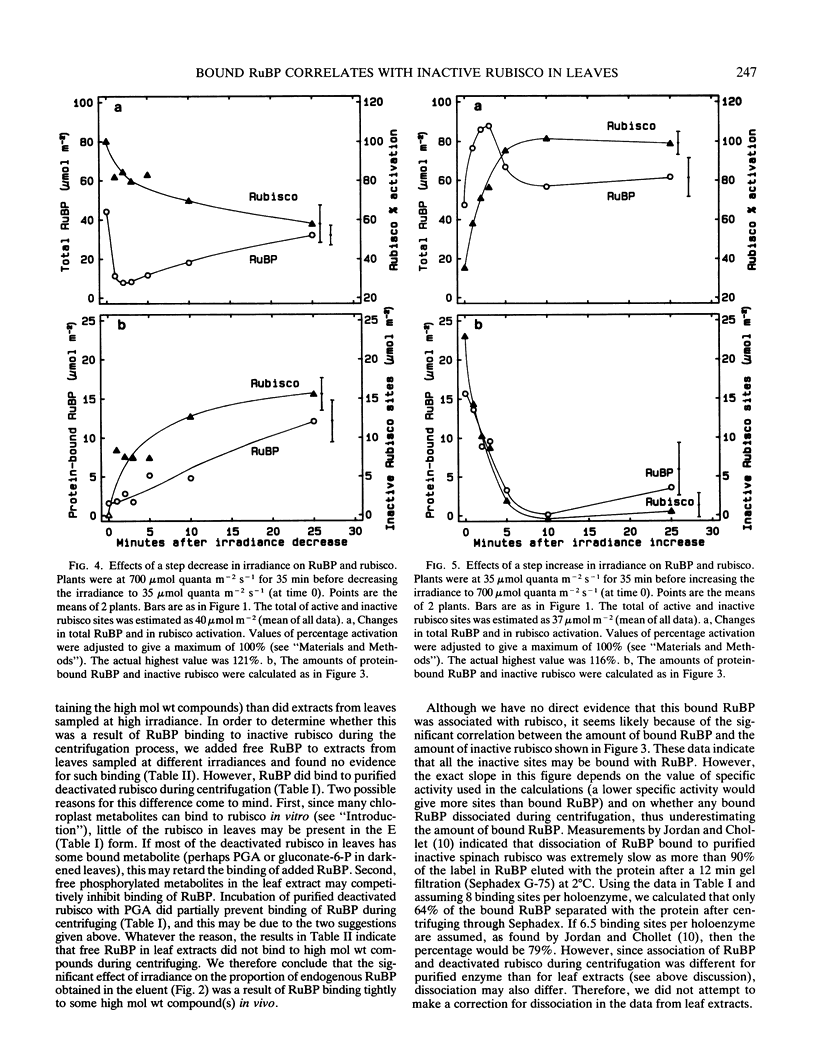
Previous reports indicate that ribulose 1,5-bisphosphate (RuBP) binds very tightly to inactive ribulose bisphosphate carboxylase (rubisco) in vitro. Therefore, we decided to investigate whether there was evidence for tight binding of RuBP associated with deactivation of rubisco in vivo. We modified a technique for rapidly separating `free' metabolites from those bound to high molecular compounds. Arabidopsis thaliana plants were illuminated at various irradiances before freezing the leaves in liquid N2 and assaying rubisco activity and RuBP. The percentage activation of rubisco varied from 37% at low irradiance (45 micromoles quanta per square meter per second) to 100% at high irradiance (800 micromoles quanta per square meter per second). The total amount of RuBP did not vary much with irradiance, but bound RuBP changed from 36% of the total at low irradiance to none at high irradiance. Bound RuBP was significantly correlated with the estimated number of inactive rubisco sites, with a ratio of about 1:1. After a step increase in irradiance, rubisco activation increased and total RuBP increased transiently, but steady levels of both occurred by 10 minutes. The amount of bound RuBP decreased with a similar time course to the estimated decrease in inactive rubisco sites. After a step decrease in irradiance, rubisco deactivated slowly for at least 25 minutes. Bound RuBP increased gradually but did so more slowly than the estimated increase in inactive rubisco sites.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belknap W. R., Portis A. R. Exchange Properties of the Activator CO(2) of Spinach Ribulose-1,5-Bisphosphate Carboxylase/Oxygenase. Plant Physiol. 1986 Mar;80(3):707–710. doi: 10.1104/pp.80.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu D. K., Bassham J. A. Regulation of ribulose 1,5-diphosphate carboxylase by substrates and other metabolites: further evidence for several types of binding sites. Plant Physiol. 1975 Apr;55(4):720–726. doi: 10.1104/pp.55.4.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan D. B., Chollet R. Inhibition of ribulose bisphosphate carboxylase by substrate ribulose 1,5-bisphosphate. J Biol Chem. 1983 Nov 25;258(22):13752–13758. [PubMed] [Google Scholar]
- Jordan D. B., Ogren W. L. A Sensitive Assay Procedure for Simultaneous Determination of Ribulose-1,5-bisphosphate Carboxylase and Oxygenase Activities. Plant Physiol. 1981 Feb;67(2):237–245. doi: 10.1104/pp.67.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing W. A., Christeller J. T. A model for the kinetics of activation and catalysis of ribulose 1,5-bisphosphate carboxylase. Biochem J. 1976 Dec 1;159(3):563–570. doi: 10.1042/bj1590563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorimer G. H., Badger M. R., Andrews T. J. The activation of ribulose-1,5-bisphosphate carboxylase by carbon dioxide and magnesium ions. Equilibria, kinetics, a suggested mechanism, and physiological implications. Biochemistry. 1976 Feb 10;15(3):529–536. doi: 10.1021/bi00648a012. [DOI] [PubMed] [Google Scholar]
- Mott K. A., Berry J. A. Effects of pH on Activity and Activation of Ribulose 1,5-Bisphosphate Carboxylase at Air Level CO(2). Plant Physiol. 1986 Sep;82(1):77–82. doi: 10.1104/pp.82.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott K. A., Jensen R. G., O'leary J. W., Berry J. A. Photosynthesis and Ribulose 1,5-Bisphosphate Concentrations in Intact Leaves of Xanthium strumarium L. Plant Physiol. 1984 Dec;76(4):968–971. doi: 10.1104/pp.76.4.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penefsky H. S. Reversible binding of Pi by beef heart mitochondrial adenosine triphosphatase. J Biol Chem. 1977 May 10;252(9):2891–2899. [PubMed] [Google Scholar]
- Perchorowicz J. T., Jensen R. G. Photosynthesis and Activation of Ribulose Bisphosphate Carboxylase in Wheat Seedlings : Regulation by CO(2) and O(2). Plant Physiol. 1983 Apr;71(4):955–960. doi: 10.1104/pp.71.4.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portis A. R., Salvucci M. E., Ogren W. L. Activation of Ribulosebisphosphate Carboxylase/Oxygenase at Physiological CO(2) and Ribulosebisphosphate Concentrations by Rubisco Activase. Plant Physiol. 1986 Dec;82(4):967–971. doi: 10.1104/pp.82.4.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S. P., Portis A. R. Involvement of stromal ATP in the light activation of ribulose-1,5-bisphosphate carboxylase/oxygenase in intact isolated chloroplasts. Plant Physiol. 1988 Jan;86(1):293–298. doi: 10.1104/pp.86.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvucci M. E., Portis A. R., Jr, Ogren W. L. Purification of ribulose-1, 5-bisphosphate carboxylase/oxygenase with high specific activity by fast protein liquid chromatography. Anal Biochem. 1986 Feb 15;153(1):97–101. doi: 10.1016/0003-2697(86)90066-7. [DOI] [PubMed] [Google Scholar]
- Seemann J. R., Badger M. R., Berry J. A. Variations in the Specific Activity of Ribulose-1,5-bisphosphate Carboxylase between Species Utilizing Differing Photosynthetic Pathways. Plant Physiol. 1984 Apr;74(4):791–794. doi: 10.1104/pp.74.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M. Limitation of Photosynthesis by Carbon Metabolism : I. Evidence for Excess Electron Transport Capacity in Leaves Carrying Out Photosynthesis in Saturating Light and CO(2). Plant Physiol. 1986 Aug;81(4):1115–1122. doi: 10.1104/pp.81.4.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streusand V. J., Portis A. R. Rubisco Activase Mediates ATP-Dependent Activation of Ribulose Bisphosphate Carboxylase. Plant Physiol. 1987 Sep;85(1):152–154. doi: 10.1104/pp.85.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S. E., Terry N. Limiting Factors in Photosynthesis: V. Photochemical Energy Supply Colimits Photosynthesis at Low Values of Intercellular CO(2) Concentration. Plant Physiol. 1984 May;75(1):82–86. doi: 10.1104/pp.75.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishnick M., Lane M. D., Scrutton M. C. The interaction of metal ions with ribulose 1,5-diphosphate carboxylase from spinach. J Biol Chem. 1970 Oct 10;245(19):4939–4947. [PubMed] [Google Scholar]


