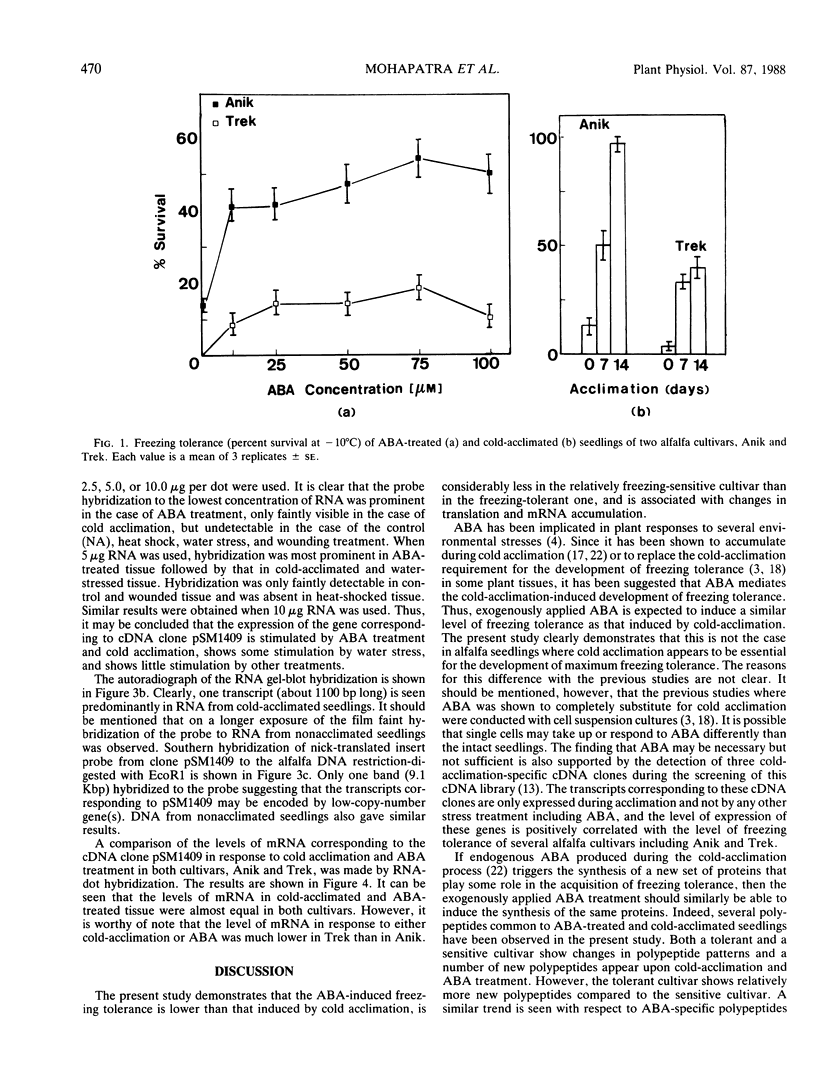
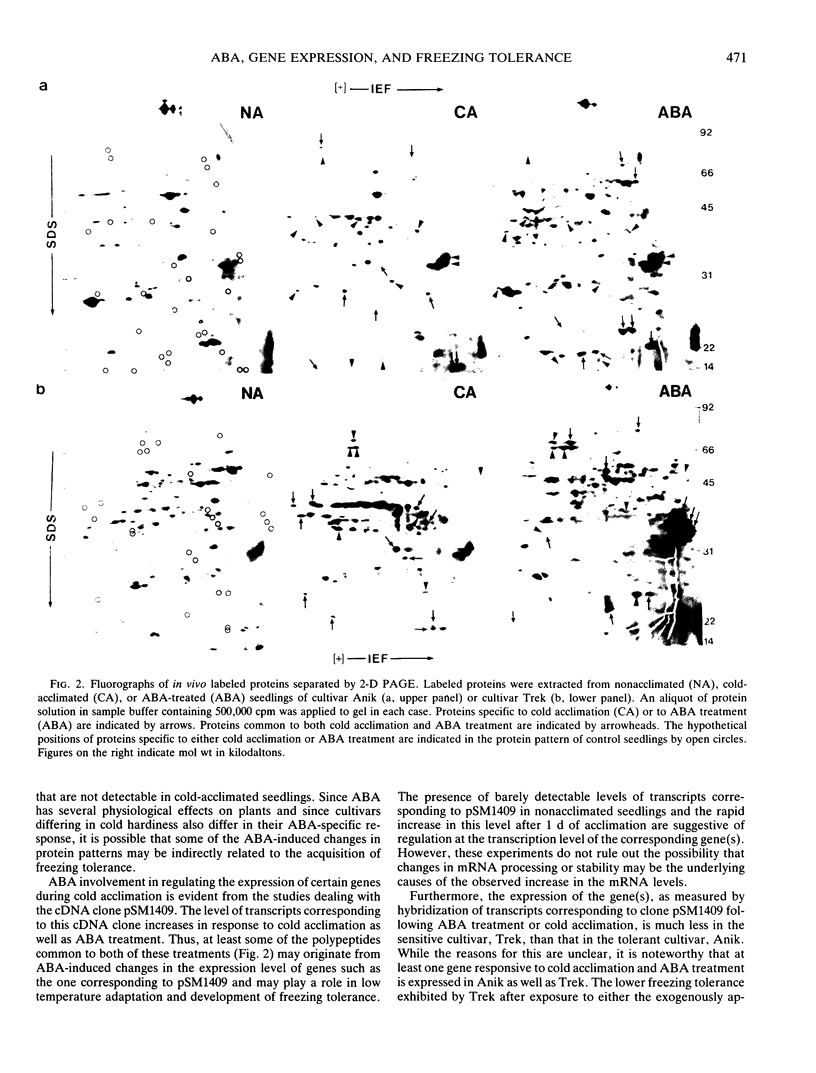
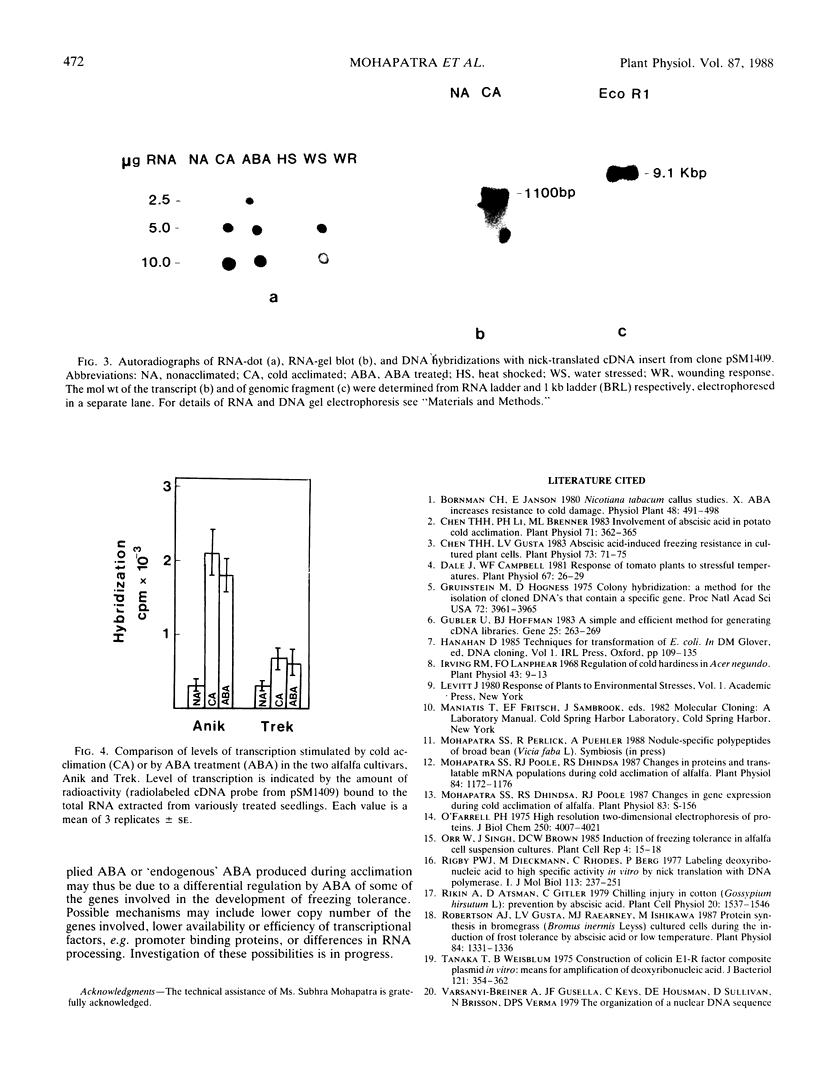
Abstract
A comparison of abscisic acid (ABA)-induced and cold-acclimation-induced freezing tolerance in two alfalfa cultivars (Medicago falcata cv Anik and Medicago sativa v Trek) indicates that ABA alone can increase freezing tolerance to some extent, but for the development of maximum tolerance, cold acclimation is essential. Analysis of in vivo-labeled proteins of ABA-treated seedlings reveals that ABA causes several changes in the pattern of protein synthesis. While some of these changes appear to be similar to those induced by cold acclimation, others seem to be specific to ABA treatment. From a cDNA library constructed against poly(A+) RNA of a freezing-tolerant alfalfa cultivar, Anik, a cDNA clone, pSM1409, has been isolated. Expression of the gene corresponding to this clone, as determined by northern hybridization, is regulated most likely at the transcriptional level by cold acclimation and exogenously supplied ABA. However, the increase in the transcript level is much greater in the freezing-tolerant cultivar Anik than in the relatively freezing-sensitive cultivar, Trek. The role of ABA in the acquisition of freezing tolerance is discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen H. H., Li P. H., Brenner M. L. Involvement of abscisic Acid in potato cold acclimation. Plant Physiol. 1983 Feb;71(2):362–365. doi: 10.1104/pp.71.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. H., Gusta L. V. Abscisic Acid-induced freezing resistance in cultured plant cells. Plant Physiol. 1983 Sep;73(1):71–75. doi: 10.1104/pp.73.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daie J., Campbell W. F. Response of Tomato Plants to Stressful Temperatures : INCREASE IN ABSCISIC ACID CONCENTRATIONS. Plant Physiol. 1981 Jan;67(1):26–29. doi: 10.1104/pp.67.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Irving R. M., Lanphear F. O. Regulation of Cold Hardiness in Acer negundo. Plant Physiol. 1968 Jan;43(1):9–13. doi: 10.1104/pp.43.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra S. S., Poole R. J., Dhindsa R. S. Changes in Protein Patterns and Translatable Messenger RNA Populations during Cold Acclimation of Alfalfa. Plant Physiol. 1987 Aug;84(4):1172–1176. doi: 10.1104/pp.84.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Robertson A. J., Gusta L. V., Reaney M. J., Ishikawa M. Protein Synthesis in Bromegrass (Bromus inermis Leyss) Cultured Cells during the Induction of Frost Tolerance by Abscisic Acid or Low Temperature. Plant Physiol. 1987 Aug;84(4):1331–1336. doi: 10.1104/pp.84.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Weisblum B. Construction of a colicin E1-R factor composite plasmid in vitro: means for amplification of deoxyribonucleic acid. J Bacteriol. 1975 Jan;121(1):354–362. doi: 10.1128/jb.121.1.354-362.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa-Komaroff L., Efstratiadis A., Broome S., Lomedico P., Tizard R., Naber S. P., Chick W. L., Gilbert W. A bacterial clone synthesizing proinsulin. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3727–3731. doi: 10.1073/pnas.75.8.3727. [DOI] [PMC free article] [PubMed] [Google Scholar]




