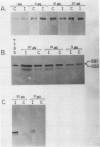
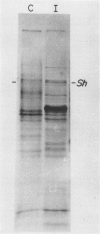
Abstract
This report examines the effect of anaerobic stress on the expression of sucrose synthase in maize (Zea mays L.). Following 24 hours of anaerobic treatment, alcohol dehydrogenase displayed the classical characteristics of induction: increased mRNA and protein levels. However, there was no detectable increase in sucrose synthase specific proteins by either native or denaturing Western blot analysis nor was there an increase in sucrose synthase activity. Anaerobic treatment did induce significantly higher steady state levels of sucrose synthase mRNA. Even though previous work has implicated sucrose synthase as an anaerobically induced protein, the data in this report suggest that sucrose synthase is not inducible at the protein level by anaerobic treatment.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baird W. V., Meagher R. B. A complex gene superfamily encodes actin in petunia. EMBO J. 1987 Nov;6(11):3223–3231. doi: 10.1002/j.1460-2075.1987.tb02639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry J. O., Nikolau B. J., Carr J. P., Klessig D. F. Transcriptional and post-transcriptional regulation of ribulose 1,5-bisphosphate carboxylase gene expression in light- and dark-grown amaranth cotyledons. Mol Cell Biol. 1985 Sep;5(9):2238–2246. doi: 10.1128/mcb.5.9.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr B., Burr F. A. Controlling-element events at the shrunken locus in maize. Genetics. 1981 May;98(1):143–156. doi: 10.1093/genetics/98.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echt C. S., Chourey P. S. A Comparison of Two Sucrose Synthetase Isozymes from Normal and shrunken-1 Maize. Plant Physiol. 1985 Oct;79(2):530–536. doi: 10.1104/pp.79.2.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamine G., Nash B., Weissbach H., Brot N. Light regulation of the synthesis of the large subunit of ribulose-1,5-bisphosphate carboxylase in peas: Evidence for translational control. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5690–5694. doi: 10.1073/pnas.82.17.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley P. M., Freeling M. Anaerobic expression of maize fructose-1,6-diphosphate aldolase. J Biol Chem. 1984 Nov 25;259(22):14180–14183. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lizardi P. M., Engelberg A. Rapid isolation of RNA using proteinase K and sodium perchlorate. Anal Biochem. 1979 Sep 15;98(1):116–122. doi: 10.1016/0003-2697(79)90714-0. [DOI] [PubMed] [Google Scholar]
- McCarty D. R., Shaw J. R., Hannah L. C. The cloning, genetic mapping, and expression of the constitutive sucrose synthase locus of maize. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9099–9103. doi: 10.1073/pnas.83.23.9099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poutre C. G., Fox T. D. PET111, a Saccharomyces cerevisiae nuclear gene required for translation of the mitochondrial mRNA encoding cytochrome c oxidase subunit II. Genetics. 1987 Apr;115(4):637–647. doi: 10.1093/genetics/115.4.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland L. J., Strommer J. N. Anaerobic treatment of maize roots affects transcription of Adh1 and transcript stability. Mol Cell Biol. 1986 Oct;6(10):3368–3372. doi: 10.1128/mcb.6.10.3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs M. M., Freeling M., Okimoto R. The anaerobic proteins of maize. Cell. 1980 Jul;20(3):761–767. doi: 10.1016/0092-8674(80)90322-0. [DOI] [PubMed] [Google Scholar]
- Scott M. P., Pardue M. L. Translational control in lysates of Drosophila melanogaster cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3353–3357. doi: 10.1073/pnas.78.6.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer B., Werr W., Starlinger P., Bennett D. C., Zokolica M., Freeling M. The Shrunken gene on chromosome 9 of Zea mays L is expressed in various plant tissues and encodes an anaerobic protein. Mol Gen Genet. 1986 Dec;205(3):461–468. doi: 10.1007/BF00338083. [DOI] [PubMed] [Google Scholar]
- Thireos G., Griffin-Shea R., Kafatos F. C. Untranslated mRNA for a chorion protein of Drosophila melanogaster accumulates transiently at the onset of specific gene amplification. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5789–5793. doi: 10.1073/pnas.77.10.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]