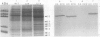Abstract
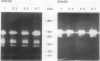
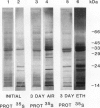
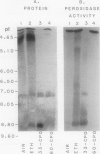
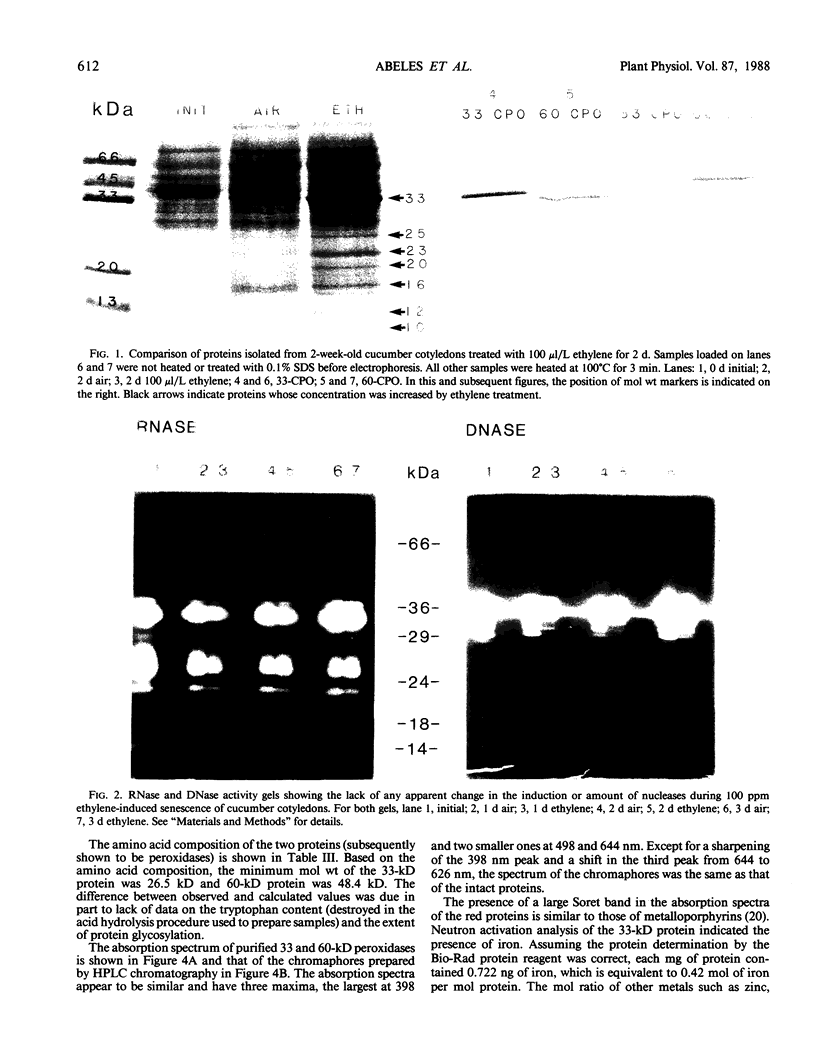
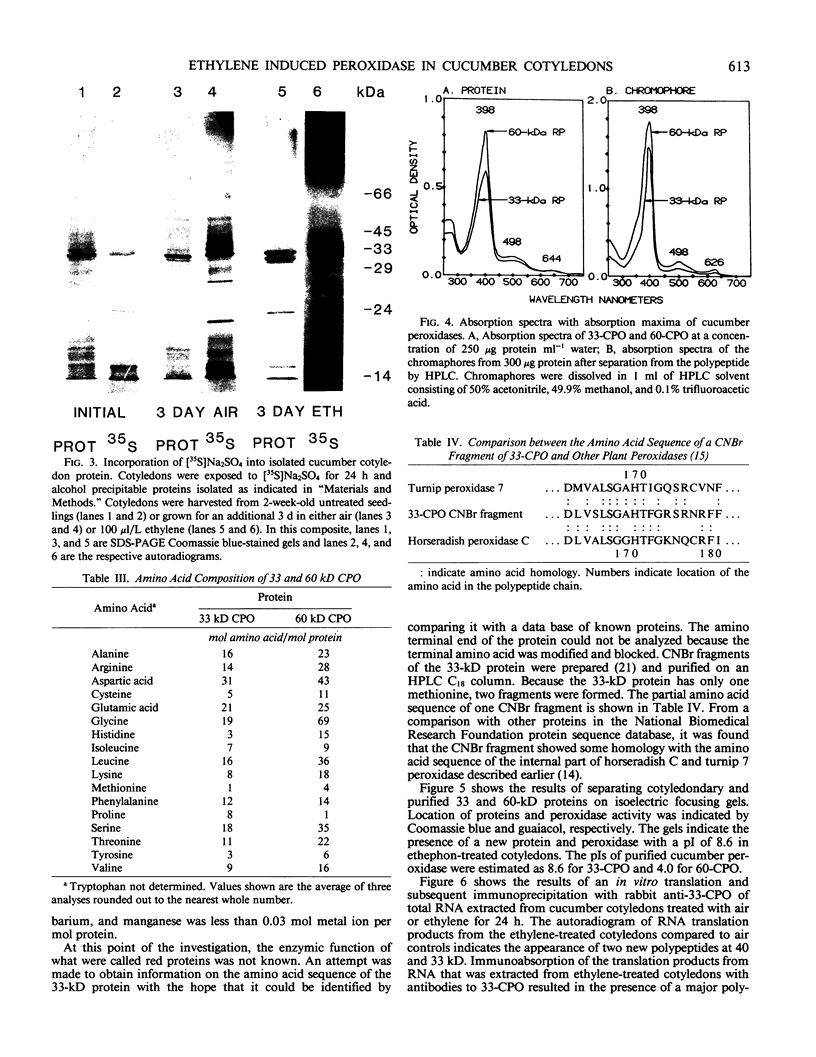
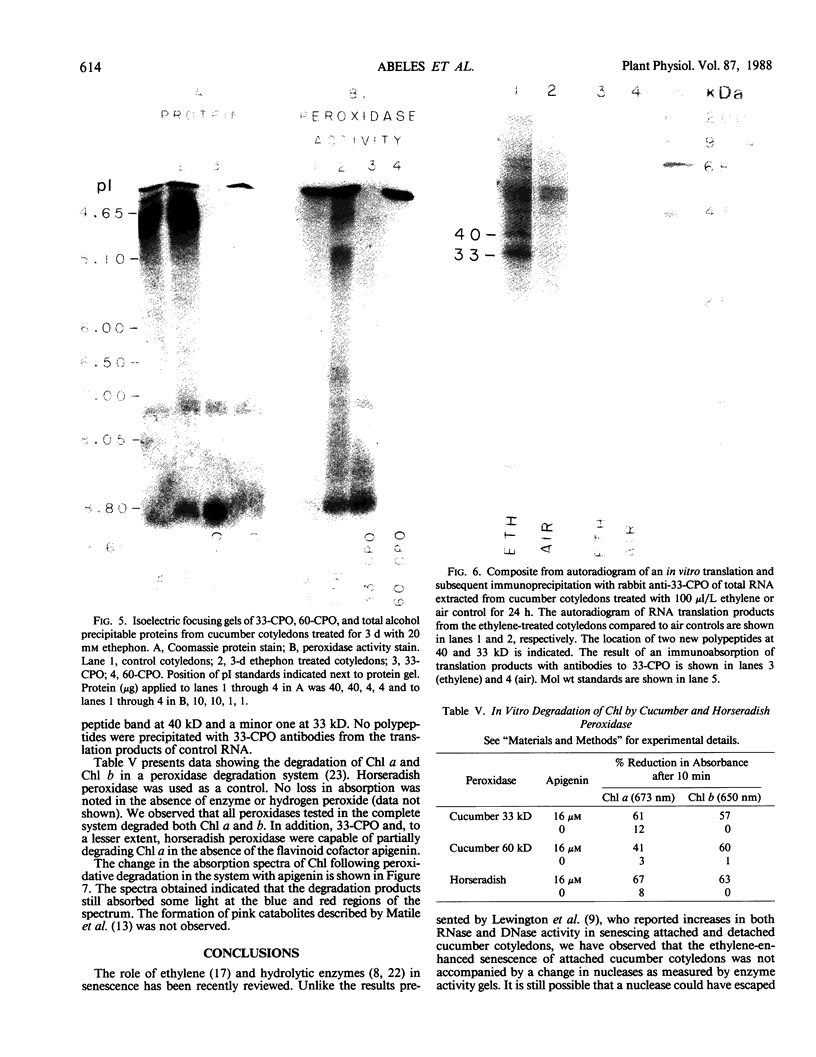
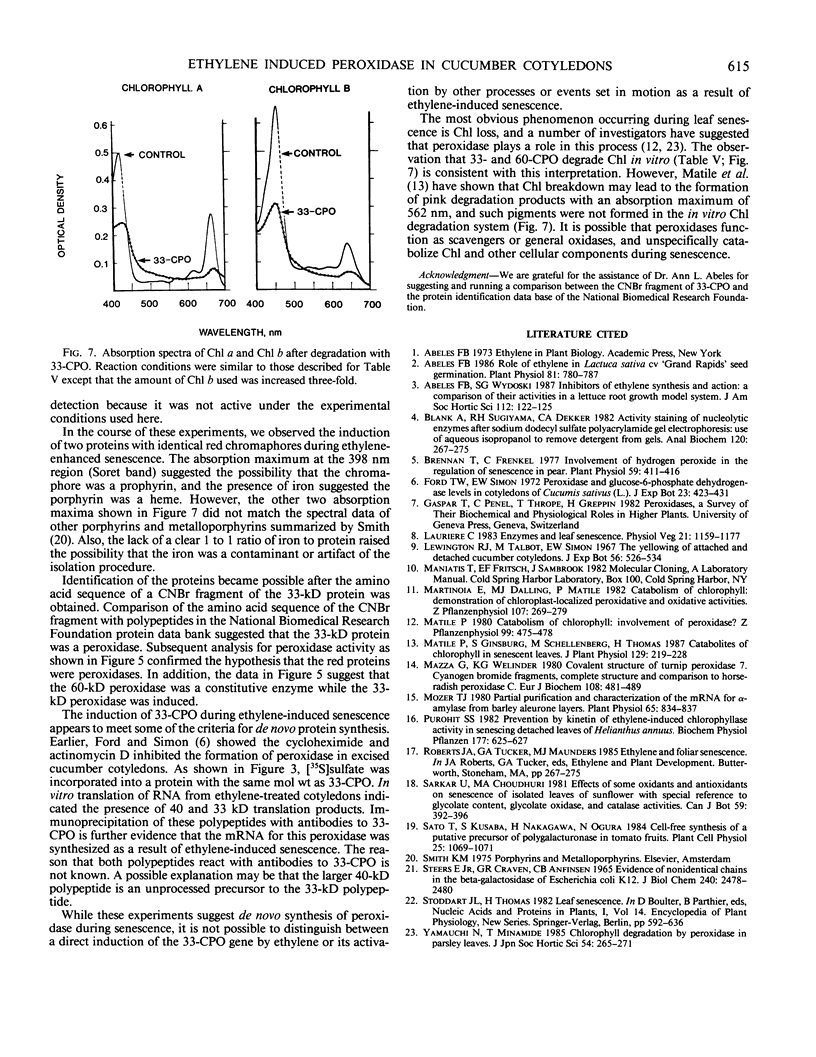
Ethylene enhanced the senescence of cucumber (Cucumis sativus L. cv `Poinsett 76') cotyledons. The effect of 10 microliters per liter ethylene was inhibited by 1 millimolar silver thiosulfate, an inhibitor of ethylene action. An increase in proteins with molecular weights of 33 to 30 kilodaltons and lower molecular weights (25, 23, 20, 16, 12, and 10 kilodaltons) were observed in sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels after ethylene enhanced senescence. The measurement of DNase and RNase activity in gels indicated that these new proteins were not nucleases. Two proteins from ethylene-treated cotyledons were purified on the basis of their association with a red chromaphore and subsequently were identified as peroxidases. The molecular weights and isoelectric points (pI) of two of these peroxidases were 33 kilodaltons (cationic, pI = 8.9) and 60 kilodaltons (anionic, pI = 4.0). The observation that [35S]Na2SO4 was incorporated into these proteins during ethylene-enhanced senescence suggests that these peroxidases represent newly synthesized proteins. Antibodies to the 33-kilodalton peroxidase precipitated two in vitro translation products from RNA isolated from ethylene-treated but not from control cucumber seedlings. This indicates that the increase in 33-kilodalton peroxidase activity represents de novo protein synthesis. Both forms of peroxidase degraded chlorophyll in vitro, which is consistent with the hypothesis that peroxidases have catabolic or scavenging functions in senescent tissues.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeles F. B. Role of Ethylene in Lactuca sativa cv ;Grand Rapids' Seed Germination. Plant Physiol. 1986 Jul;81(3):780–787. doi: 10.1104/pp.81.3.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank A., Sugiyama R. H., Dekker C. A. Activity staining of nucleolytic enzymes after sodium dodecyl sulfate-polyacrylamide gel electrophoresis: use of aqueous isopropanol to remove detergent from gels. Anal Biochem. 1982 Mar 1;120(2):267–275. doi: 10.1016/0003-2697(82)90347-5. [DOI] [PubMed] [Google Scholar]
- Brennan T., Frenkel C. Involvement of hydrogen peroxide in the regulation of senescence in pear. Plant Physiol. 1977 Mar;59(3):411–416. doi: 10.1104/pp.59.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza G., Welinder K. G. Covalent structure of turnip peroxidase 7. Cyanogen bromide fragments, complete structure and comparison to horseradish peroxidase C. Eur J Biochem. 1980 Jul;108(2):481–489. doi: 10.1111/j.1432-1033.1980.tb04745.x. [DOI] [PubMed] [Google Scholar]
- Mozer T. J. Partial purification and characterization of the mRNA for alpha-amylase from barley aleurone layers. Plant Physiol. 1980 May;65(5):834–837. doi: 10.1104/pp.65.5.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEERS E., Jr, CRAVEN G. R., ANFINSEN C. B., BETHUNE J. L. EVIDENCE FOR NONIDENTICAL CHAINS IN THE BETA-GALACTOSIDASE OF ESCHERICHIA COLI K12. J Biol Chem. 1965 Jun;240:2478–2484. [PubMed] [Google Scholar]