Abstract
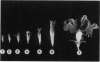
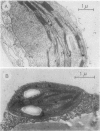
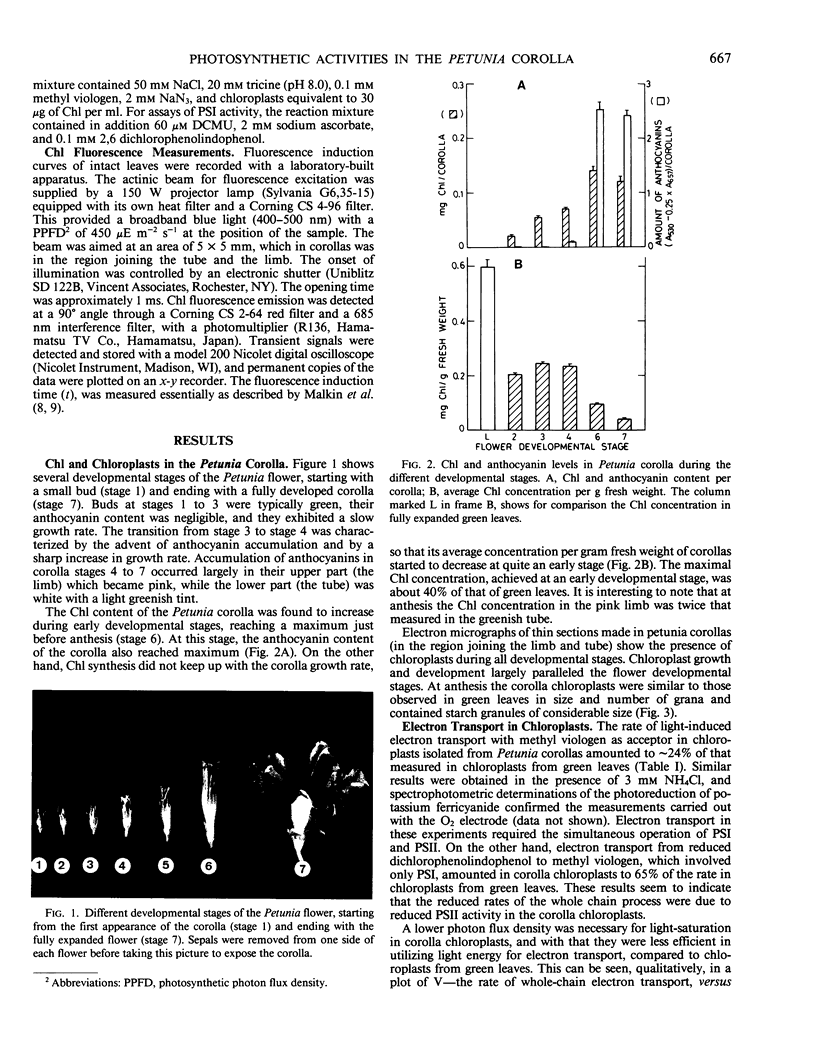
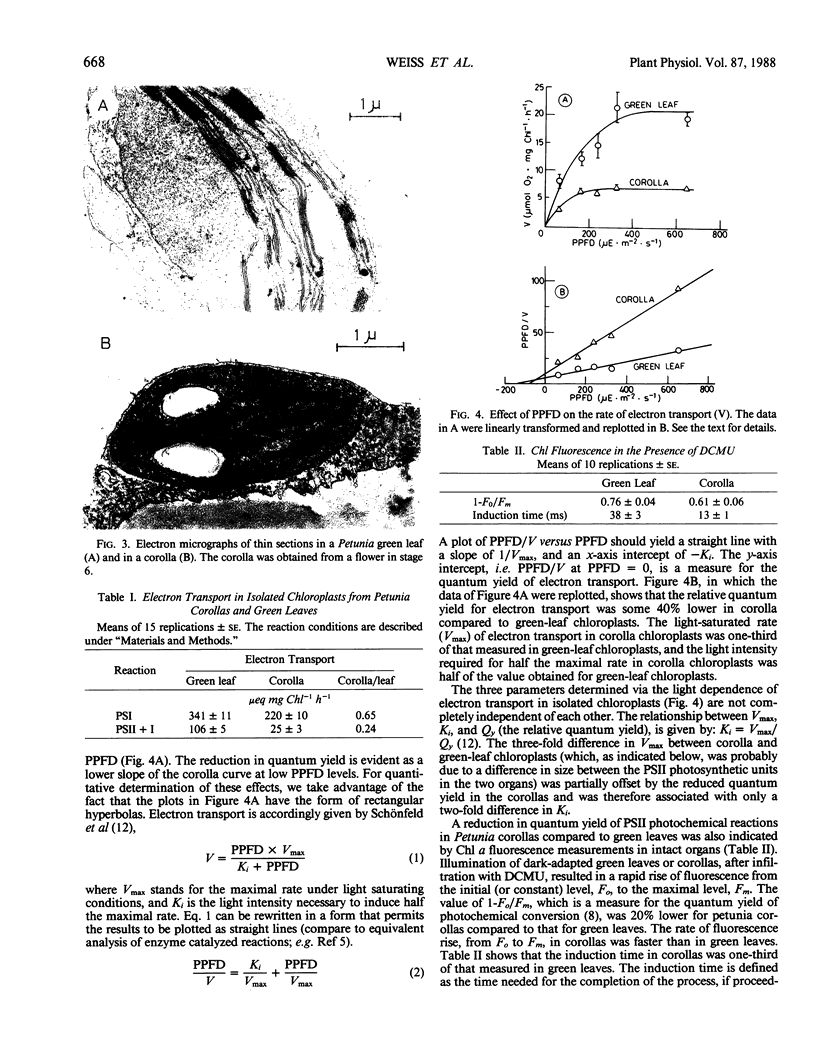
Pink Petuniahybrida (cv Hit Parade Rosa) corollas were found to contain photosynthetically active chloroplasts. The corolla chloroplasts were similar to those of green leaves in size and structure. The chlorophyll (Chl) content of Petunia corollas increased during early stages of flower development, reaching a maximum just before anthesis. Chloroplasts isolated from corollas at this stage, carried out photosystem I-dependent electron transport at rates which were two-thirds of those measured in chloroplasts from green leaves, but full chain electron transport at only one-quarter of the rate carried out by chloroplasts from green leaves. Both the light saturated rate and the quantum yield for electron transport were lower in corolla chloroplasts, which also required lower intensities for light saturation. Reduced efficiency of photosystem II photoreactions in the corolla was also indicated by the ratio between variable and constant components of Chl fluorescence, which was lower in corollas compared to green leaves. The induction time of Chl fluorescence was at least three times shorter in corollas compared to green leaves, indicating a smaller number of functional photosystem II centers (per Chl) in the corolla. It is suggested that corolla chloroplasts of Petunia might have a role in flower developmental processes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black J. L., Collins D. W., De Roach J. N., Zubrick S. R. Dyslexia: saccadic eye movements. Percept Mot Skills. 1984 Jun;58(3):903–910. doi: 10.2466/pms.1984.58.3.903. [DOI] [PubMed] [Google Scholar]
- Dueker J., Arditti J. Photosynthetic CO(2) Fixation by Green Cymbidium (Orchidaceae) Flowers. Plant Physiol. 1968 Jan;43(1):130–132. doi: 10.1104/pp.43.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkin S., Armond P. A., Mooney H. A., Fork D. C. Photosystem II Photosynthetic Unit Sizes from Fluorescence Induction in Leaves : CORRELATION TO PHOTOSYNTHETIC CAPACITY. Plant Physiol. 1981 Mar;67(3):570–579. doi: 10.1104/pp.67.3.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkin S., Fork D. C. Photosynthetic units of sun and shade plants. Plant Physiol. 1981 Mar;67(3):580–583. doi: 10.1104/pp.67.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancinelli A. L., Yang C. P., Lindquist P., Anderson O. R., Rabino I. Photocontrol of Anthocyanin Synthesis: III. The Action of Streptomycin on the Synthesis of Chlorophyll and Anthocyanin. Plant Physiol. 1975 Feb;55(2):251–257. doi: 10.1104/pp.55.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M. J., Stimson W. R. Contributions of photosynthesis and phytochrome to the formation of anthocyanin in turnip seedlings. Plant Physiol. 1971 Sep;48(3):312–315. doi: 10.1104/pp.48.3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönfeld M., Yaacoby T., Michael O., Rubin B. Triazine Resistance without Reduced Vigor in Phalaris paradoxa. Plant Physiol. 1987 Feb;83(2):329–333. doi: 10.1104/pp.83.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu J. C., Yelenosky G., Bausher M. G. Photosynthetic Activity in the Flower Buds of ;Valencia' Orange (Citrus sinensis [L.] Osbeck). Plant Physiol. 1985 Jun;78(2):420–423. doi: 10.1104/pp.78.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]