Abstract
Glucagon-like peptide-1 (GLP-1) receptor agonists have been used extensively in the clinic and have an established safety profile in cardiovascular disease settings. For the treatment of peptide-secreting enteroendocrine cells, most research has focused on developing peptide multi-agonists as most of these cells are multihormonal. Among the various peptides secreted by enteroendocrine cells, the combination of GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) is an attractive strategy for treating type 2 diabetes mellitus (T2DM) because both of these hormones have glucose-lowering actions. Tirzepatide, a synthetic peptide composed of 39 amino acids, functions as a dual receptor agonist of both the GIP and GLP-1 receptors. This unique mechanism of action has earned tirzepatide the nickname “twincretin.” Tirzepatide’s dual agonist activity may be the mechanism by which tirzepatide significantly reduces glycated hemoglobin levels and body weight in patients with T2DM as observed in phase 3 clinical trials. Besides its glucose-lowering and anti-obesity effects, tirzepatide has been reported to have potential cardiovascular benefits. In this review, we discuss the cardiovascular effects of tirzepatide based on the available preclinical and clinical data.
Keywords: Tirzepatide, GLP-1, Glucose-dependent insulinotropic peptide, Cardiovascular risk factors
INTRODUCTION
Tirzepatide is a synthetic peptide composed of 39 amino acids, which functions as a dual receptor agonist (RA) binding both the glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptors and this unique mechanism of action resulted in the nickname “twincretin.” The structure of tirzepatide is based on that of the native GIP with the addition of a 20-carbon fatty diacid moiety, which increases its half-life to 5 days allowing for once-weekly administration.1
Studies using animal models of obesity and diabetes have shown that the co-administration of GIP and GLP-1 has synergistic effects in reducing body weight, food consumption, and fat mass.1 Tirzepatide’s dual agonist activity may result in these benefits in humans as well, as evidenced the significant reductions in glycated hemoglobin (HbA1c) levels and body weight in patients with type 2 diabetes mellitus (T2DM) observed in phase 3 clinical trials (such as, SURPASS; A Study of Tirzepatide [LY3298176] in Participants With T2DM and SURMOUNT; A Study of Tirzepatide (LY3298176) in Participants With Obesity or Overweight).2,3,4,5,6,7 Tirzepatide has been reported to have potential cardiovascular benefits in addition to its glucose-lowering effects.
In this review, we provide an overview of the available preclinical and clinical data describing the cardiovascular effects of tirzepatide.
GLP-1 AND GIP
Given the extensive clinical experience with GLP-1 RAs and the established safety profile in cardiovascular disease settings, peptide multi-agonists have been developed to target multihormonal peptide-secreting enteroendocrine cells.8 Due to their glucose-lowering functions, the combination of GLP-1 and GIP hormones is one of the most attractive strategies for treating T2DM.8,9
GLP-1 was first identified as a cleavage product of preproglucagon generated by L cells in the small intestine and functions to increase postprandial insulin production and lower blood glucose.10,11,12,13 Since its discovery, efforts have focused on extending GLP-1 agonist activity through structural changes.14 Liraglutide and semaglutide, two long-acting GLP-1 RAs, have been authorized for the treatment of T2DM and obesity. While GLP-1 RAs were the first to reach the market, primarily due to the serendipitous discovery of exendin-4, GLP-1 is only one of many hormones that modulate glucose metabolism and regulate appetite. GIP is another hormone that regulates glucose metabolism. GIP was initially isolated from intestinal extracts and shown to have a potent insulinotropic effect.10,15,16 However, clinical studies demonstrated that, in hyperglycemic states, the insulinotropic action of GIP is inhibited, while GLP-1’s function remains intact.17,18 Recent research indicates that GIP is largely responsible for postprandial insulin secretion in T2DM, rather than the opposite.19 In preclinical studies, GIP receptor deficiency in mice leads to impaired glucose tolerance with reduced β-cell function,20 while GIP-overexpressing mice demonstrate reduced diet-induced obesity and steatosis, and improved beta cell function and glucose homeostasis.21 Collectively, GLP-1 and GIP seem to work in tandem with GLP-1 inhibiting glucagon secretion when the plasma glucose concentration is high, while GIP acts at lower glucose levels.17,22 Thus, these two hormones could work together in T2DM as the consistent glucose-lowering effect of GLP-1 could potentiate the role of GIP, whose action is dependent upon the glycemic status. Based on these findings, the first unimolecular GIP/GLP1 receptor co-agonist was introduced in 2013.23 This peptide exhibited enhanced antihyperglycemic and insulinotropic efficacy, as well as a weight loss effect.23 Dual GIP/GLP-1 receptor co-agonists have been demonstrated to be safe and efficacious in humans,24 and in 2022, tirzepatide was the first GIP/GLP-1 receptor co-agonist to be approved for the treatment of T2DM.
The detailed physiology of GLP-1 and GIP is not the primary objective of this review. Please refer to some excellent review papers for the detailed information on this issue.25,26
METABOLIC AND CARDIOVASCULAR EFFECTS OF TIRZEPATIDE
1. HbA1c
Once-weekly subcutaneous administration of tirzepatide dose-dependently reduced HbA1c levels across all studies using the combination of GLP-1 and GIP.2,3,4,5,6 The results of the SURPASS-2 study showed that individuals who had established T2DM and were treated with tirzepatide at doses ranging from 5 to 15 mg for a duration of 40 weeks experienced a reduction in HbA1c levels ranging from 2.09% to 2.46%.3 This reduction was higher than that observed in individuals treated with semaglutide (1 mg), who experienced a 1.86% reduction in HbA1c levels.3 Similarly, in the SURPASS-3 study, individuals who received tirzepatide at doses ranging from 5 to 15 mg for 52 weeks experienced a reduction in HbA1c levels ranging from 1.93% to 2.37%, which was higher than that observed in individuals treated with insulin degludec, who experienced a decrease of 1.34%.4
In Japanese individuals with T2DM, after 52 weeks of treatment, tirzepatide at doses ranging from 5 to 15 mg reduced HbA1c levels by 2.4%–3.02%.27 Additionally, in this population, tirzepatide was more effective in controlling blood glucose levels than dulaglutide administered once weekly at a dose of 0.75 mg.27 Collectively, tirzepatide elicited a much stronger HbA1c-lowering effect in all the SURPASS programs compared with placebo or active comparators, including GLP-1 RA and basal insulin.2,3,4,5,6,27 Table 1 summarizes the main findings of the SURPASS programs and details the HbA1c lowering efficacy of tirzepatide.
Table 1. The main results of tirzepatide in phase 3 clinical trials.
| Study name | Enrolled patients | Study arms | Duration (wk) | ΔHbA1c (%) | ΔBW (kg) | ΔSBP (mmHg) | ΔDBP (mmHg) | ΔLDL-C (%) | ΔTG (%) | ΔHDL-C (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| SURPASS 12 | T2DM | Placebo | 40 | 0.04 | −0.7 | −2.0 | −1.4 | −1.6 | 4.7 | −3.8 |
| Tirzepatide (5, 10, 15 mg) | −1.87, −1.89, −2.07 | −7.0, −7.8, −9.5 | −4.7, −4.7, −5.2 | −2.-, −3.1, −3.4 | −6.7, −7.6, −12.4 | −18.5, −18.2, −21.0 | 4.8, 3.2, 7.5 | |||
| SURPASS 23 | T2DM | Semaglutide 1 mg | 40 | −1.86 | −5.7 | −3.6 | −1.0 | −6.4 | −11.5 | 4.4 |
| Tirzepatide (5, 10, 15 mg) | −2.01, −2.24, −2.30 | −7.6, −9.3, −11.2 | −4.8, −5.3, −6.5 | −1.9, −2.5, −2.9 | −7.7, −5.6, −5.2 | −19.0, −24.1, −24.8 | 6.8, 7.9, 7.1 | |||
| SURPASS 34 | T2DM | Insulin Degludec | 52 | −1.34 | 2.3 | 0.5 | 0.4 | −2.71 | −12.2 | 1.03 |
| Tirzepatide (5, 10, 15 mg) | −1.93, −2.20, −2.37 | −7.5, −10.7, −12.9 | −4.9, −6.6, −5.5 | −2.0, −2.5, −1.9 | −6.01, −5.70, −6.55 | −15.4, −26.7, −25.2 | 5.49, 10.22, 10.20 | |||
| SURPASS 45 | T2DM | Insulin Glargine | 52 | −1.44 | 1.9 | 1.3 | 0.7 | 1.4 | −6.4 | 2.9 |
| Tirzepatide (5, 10, 15 mg) | −2.24, −2.43, −2.58 | −7.1, −9.5, −11.7 | −2.8, −3.7, −4.8 | −1.0, −0.8, −1.0 | −6.8, −8.3, −7.9 | −16.3, −20.1, −22.5 | 6.7, 9.7, 10.8 | |||
| SURPASS 56 | T2DM* | Placebo | 40 | −0.86 | 1.6 | −1.7 | −2.1 | 2.8 | −6.8 | 1.7 |
| Tirzepatide (5, 10, 15 mg) | −2.11, −2.40, −2.34 | −5.4, −7.5, −8.8 | −6.1, −8.3, −12.6 | −2.0, −3.3, −4.5 | −8.9, −12.8, −15.5 | −15.2, −19.3, −24.9 | 2.1, 1.8, 0.9 | |||
| SURPASS J-Mono27 | T2DM | Dulaglutide 0.75 mg | 52 | −1.3 | −0.5 | −1.4 | 0.1 | −4.8 | −8.2 | 0 |
| Tirzepatide (5, 10, 15 mg) | −2.4, −2.6, −2.8 | −5.8, −8.5, −10.7 | −6.5, −8.8, −11.0 | −3.2, −4.0, −5.6 | −12.0, −12.8, −19.3 | −27.1, −32.8, −37.7 | 3.8, 5.4, 5.9 | |||
| SURPASS J-Combo28 | T2DM | Tirzepatide (5, 10, 15 mg) | 52 | −2.5, −3.0, −3.0 | −3.8, −7.5, −10.2 | −5.1, −8.7, −10.2 | −2.7, −3.4, −3.6 | −13.6, −15.1, −18.0 | −21.9, −31.1, −37.4 | 0.9, 2.4, 5.1 |
| SURMOUNT 17 | Non-T2DM | Placebo | 72 | N/A | −3.1 | 1.2 | −1.0 | −0.9 | −6.3 | 0.2 |
| Tirzepatide (5, 10, 15 mg) | N/A | −15.0, −19.5, −20.9 | −7.0, −7.6, −8.2 | −4.6, −5.2, −5.5 | −5.3, −6.6, −8.6 | −24.3, −27.0, −31.4 | 7.0, 8.6, 8.2 |
All values indicate the changes from the baseline (Δ).
HbA1c, glycated hemoglobin; BW, body weight; SBP, systolic blood pressure; DBP, diastolic blood pressure; LDL-C, low-density lipoprotein cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; T2DM, type 2 diabetes mellitus; N/A, not available.
*Treated with insulin glargine.
2. Body weight
Tirzepatide has also been shown to significantly reduce body weight in clinical trials. In the SURPASS-3 trial, once-weekly subcutaneous tirzepatide administration reduced body weight by 9.8 kg at the dose of 5 mg and 15.2 kg at the dose of 15 mg compared with insulin degludec in patients with T2DM.4 In the SURPASS-5 trial, tirzepatide reduced body weight by 7.1 kg at the once-weekly dose of 5 mg and 10.5 kg at the once-weekly dose of 15 mg compared with placebo when added to insulin glargine in patients with T2DM.6
Subsequently, in June 2022, the results of the SURMOUNT-1 trial, a phase 3 trial including only obese patients, were announced.7 The trial was a randomized, double-blind, placebo-controlled study in which 2,539 patients with a body mass index (BMI) ≥30 kg/m2 or ≥27 kg/m2 with at least one comorbidity other than T2DM were assigned to receive subcutaneous tirzepatide at a dose of 5 mg, 10 mg, or 15 mg or placebo once a week for 72 weeks.7 The mean weight and BMI at baseline were 104.8 kg and 38.0 kg/m2, respectively, and after 72 weeks, the weight loss rates in patients who received tirzepatide at doses of 5 mg, 10 mg, and 15 mg were 15%, 19.5%, and 20.9%, respectively, which were significantly higher than the 3.1% weight loss rate observed in patients who received placebo.7 The percentage of patients who lost >20% of their body weight was over 50% in the tirzepatide 10-mg and 15-mg groups, whereas it was only 3% in the placebo group.7 The most common adverse effect observed was gastrointestinal symptoms, which were generally mild to moderate, and the drug discontinuation rate due to adverse effects was 2.6% in the placebo group and 6.2% in the tirzepatide 15-mg group.7 Table 1 summarizes the main findings of the SURPASS programs and SURMOUNT-1 regarding the body weight-reducing effect of tirzepatide.
The weight loss effects of tirzepatide are notable, as obesity is a major risk factor for the development of T2DM and cardiovascular diseases. The reduction in body weight achieved with tirzepatide may also improve cardiovascular risk factors as observed in clinical trials.
Besides its profound body weight-reducing effect, tirzepatide also showed a significant ability to reduce liver fat content (measured by magnetic resonance imaging-proton density fat fraction) compared with insulin degludec in the subpopulation of patients with T2DM in the SURPASS-3 study.29 These data provide additional evidence on the metabolic effects of this novel GLP-1/GIP dual RA. The impact of tirzepatide in individuals with nonalcoholic steatohepatitis will be assessed in the ongoing A Study of Tirzepatide (LY3298176) in Participants With Nonalcoholic Steatohepatitis (SYNERGY-NASH) trial (NCT04166773).
Although the reduced food intake stimulated by tirzepatide is considered to be the main contributor to the reduced body weight,30 other mechanisms, including increased energy expenditure induced by tirzepatide, may contribute to the reduced body weight and liver fat content.1,31 An ongoing clinical trial whose main purpose is to investigate the effect of tirzepatide on energy expenditure and food intake in obese patients (NCT04081337) will provide more information on the mechanism by which tirzepatide treatment reduces body weight.
3. Lipid profiles
Tirzepatide has been shown to improve lipid profiles in clinical studies. In humans, tirzepatide, whether used as monotherapy or add-on therapy, lowered the percentage change of total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and triglycerides (TGs) and significantly increased high-density lipoprotein cholesterol (HDL-C). For instance, in the SURPASS-4 trial, where tirzepatide was compared with insulin glargine in patients with T2DM and a high cardiovascular risk, 15 mg of tirzepatide reduced the levels of TC, LDL-C, and TGs by 5.6%, 7.9%, and 22.5%, respectively, and increased the levels of HDL-C by 10.8%.5 In the SURPASS-5 trial, where tirzepatide was added to basal insulin treatment, tirzepatide also showed favorable effects on lipid profile parameters compared with placebo in patients with T2DM.6 Specifically, tirzepatide reduced the levels of TC, LDL-C, and TGs by 12.9%, 15.5%, and 24.9%, respectively, and increased the levels of HDL-C by 0.9%.6 In both trials, the effects of tirzepatide on lipid profile were dose-dependent, with greater improvements observed at higher doses and these improvements were sustained over the duration of the trials.5,6 Table 1 summarizes the main findings of the SURPASS programs regarding the ability of tirzepatide to improve atherogenic dyslipidemia.
Although GLP-1 RAs have been shown to reduce the production of lipoproteins, chylomicrons, and postprandial TGs,32 the effect of chronic treatment of GIP RAs on plasma lipids is not well understood. Considering the findings that the activation of the GIP receptor increases adipose tissue blood flow and promotes adipose tissue lipid uptake, GIP receptor agonism in adipocytes may be a key regulator of postprandial lipid clearance and overall lipid homeostasis.26 Further studies on how GLP-1/GIP dual agonism improves atherogenic dyslipidemia are needed.
4. Blood pressure (BP)
Tirzepatide has also been shown to reduce BP in clinical studies. The patients treated with tirzepatide showed significantly lowered systolic and diastolic BP compared with patients in the control group, regardless of whether tirzepatide was used as a monotherapy or add-on therapy. Overall, tirzepatide treatment reduced systolic and diastolic BP by 4.8 and 1.7 mmHg, respectively.33
Tirzepatide appears to have a more significant impact on BP in Japanese individuals with T2DM.27,28 At a dose of 15 mg, tirzepatide reduced systolic BP by 11.0 mmHg and diastolic BP by 5.6 mmHg from baseline.27 Further research is necessary to determine which factors of tirzepatide treatment are responsible for the more pronounced decrease in BP observed in Asian populations. Table 1 summarizes the main findings of the SURPASS programs regarding the BP-lowering effect of tirzepatide.
The effect of tirzepatide on 24-hour mean systolic BP, diastolic BP, and heart rate (HR) measured during 24-hour ambulatory BP monitoring (ABPM) in individuals living with obesity without T2DM, has been evaluated in the SURMOUNT-1 ABPM sub-study.34 After 36 weeks of treatment, tirzepatide was associated with statistically significant and clinically meaningful reductions in the mean 24-hour systolic BP at all doses and reduced diastolic BP at the 2 lower doses.34 Simultaneously, tirzepatide treatment was associated with a dose-dependent increase in the mean 24-hour HR.34 Fortunately, there is no clear evidence that tirzepatide increases the risk of atrial fibrillation.35
5. Inflammatory markers
Low-grade inflammation has been identified as a significant risk factor for cardiovascular diseases.36 Tirzepatide treatment has been shown to have favorable effects on inflammatory markers in clinical trials. In a 26-week phase 2 study involving patients with obesity and T2DM, treatment with tirzepatide dose-dependently decreased the circulating levels of specific biomarkers of inflammation and endothelial dysfunction previously associated with cardiovascular events, suggesting a net improvement in the cardiovascular risk profile of these patients.37 In this post hoc analysis, inflammation, endothelial dysfunction, and cellular stress biomarkers were measured at baseline and 4, 12, and 26 weeks to evaluate the additional effects of tirzepatide on cardiovascular risk factors.37 After 26 weeks, tirzepatide treatment at doses of 10 and 15 mg decreased the levels of YKL-40 (also known as chitinase-3 like-protein-1), intercellular adhesion molecule 1 (ICAM-1), leptin, and growth differentiation factor 15 compared with baseline. In addition, tirzepatide administration reduced the levels of YKL-40 and leptin versus placebo and dulaglutide treatment.37 Tirzepatide treatment at a dose of 15 mg also decreased ICAM-1 levels compared with placebo and dulaglutide administration and reduced high-sensitivity C-reactive protein levels compared with baseline and placebo treatment, but not dulaglutide treatment.37
The mechanism by which tirzepatide reduces these inflammatory markers is not fully understood; however, it may be related to the compound’s dual agonist activity. Both GLP-1 and GIP have anti-inflammatory effects,38 and it is possible that the combination of these 2 hormones in tirzepatide leads to a greater reduction in inflammation than either hormone alone.
6. Major adverse cardiovascular events (MACEs)
An ongoing cardiovascular outcome trial of tirzepatide, called A Study of Tirzepatide (LY3298176) Compared With Dulaglutide on Major Cardiovascular Events in Participants With Type 2 Diabetes (SURPASS-CVOT), aims to evaluate the potential benefits of dual GIP and GLP-1 receptor agonism, which has been shown to have greater glucose-lowering and weight loss effects compared to GLP-1 receptor agonism alone. The study is a randomized, double-blind, active comparator-controlled trial that enrolled 13,299 patients with T2DM and established cardiovascular disease or high risk of cardiovascular disease. Patients were randomized to receive either tirzepatide or dulaglutide once weekly for up to 236 weeks. The primary outcome measure of the study is a composite of MACEs, including cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke. Secondary outcome measures include the expanded analysis of cardiovascular-related death, myocardial infarction, stroke, coronary revascularization, hospitalization for unstable angina, or heart failure. Changes from baseline in HbA1c levels, body weight, urinary albumin to creatinine ratio, and lipid profiles, are also included as secondary outcomes.
Although the clinical relevance of the aforementioned cardiovascular risk factor alterations by tirzepatide will be assessed in the planned cardiovascular outcome study SURPASS-CVOT (NCT04255433), a pre-specified cardiovascular meta-analysis indicated that tirzepatide did not increase the risk of MACEs in participants with T2DM compared with controls.39 This pre-specified meta-analysis included all seven randomized controlled trials with a duration of at least 26 weeks included in the tirzepatide T2DM clinical development program, SURPASS.39 The pre-specified primary objective of this meta-analysis was the comparison of the time to the first occurrence of confirmed 4-component MACEs (MACE-4) (i.e., cardiovascular death, myocardial infarction, stroke, and hospitalization for unstable angina) between the pooled tirzepatide and control groups.39 The hazard ratios comparing tirzepatide versus controls were 0.80 (95% confidence interval [CI], 0.57–1.11) for MACE-4, 0.90 (95% CI, 0.50–1.61) for cardiovascular death, and 0.80 (95% CI, 0.51–1.25) for all-cause death.39
The effect of tirzepatide treatment on cardiovascular events has been studied in SURPASS-4, a trial involving patients with known coronary, peripheral arterial, or cerebrovascular diseases or those who were at a high risk of these diseases.5 In this study, no tirzepatide dose numerically increased the risk of cardiovascular events.5 Instead, for patients treated with the highest dose of tirzepatide (15 mg per week), the risk of developing any MACE (e.g., myocardial infarction, stroke, hospitalization for angina, or all-cause death) was estimated to be 0.50 (95% CI, 0.26–0.95).5 However, this was based on only 11 events in the tirzepatide 15 mg per week group (and 62 events with insulin glargine treatment).5 Collectively, tirzepatide did not increase the risk of MACEs in participants with T2DM compared with controls. The above-mentioned ongoing long-term cardiovascular outcome trials using tripeptide will provide clear answers on whether tirzepatide can protect patients with T2DM at a high cardiovascular risk from recurrent cardiovascular events.
CONCLUSION
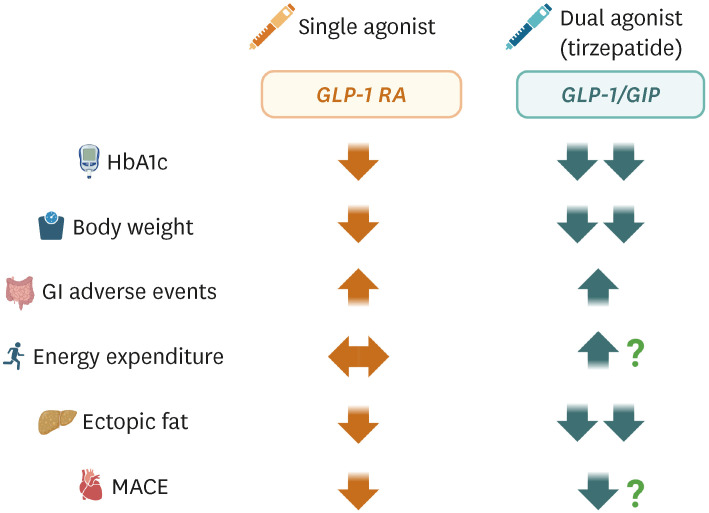
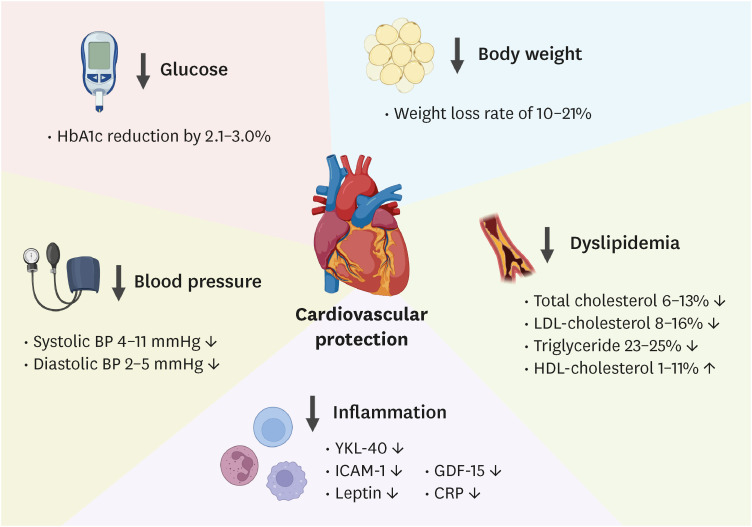
Fig. 1 summarizes the several characteristics between single agonism using GLP-1 and dual agonism of GLP-1/GIP (i.e., tirzepatide) treatment. Tirzepatide is a unique dual RA of both the GLP-1 and GIP receptors, which significantly reduces HbA1c levels and body weight in patients with T2DM and obesity. Its once-weekly dosing and potential cardiovascular benefits make tirzepatide an attractive option for treating these conditions (Fig. 2). Tirzepatide’s ability to co-administer GIP and GLP-1 has synergistic effects on reducing body weight, food consumption, and fat mass in animal models, and this benefit has been observed in humans as well. Although gastrointestinal symptoms have been reported to be the most common adverse effects, they were mild to moderate. Further research is needed to understand the long-term effects of tirzepatide on cardiovascular outcomes.
Fig. 1. Comparison between GLP-1 RA and the GLP-1/GIP dual agonist (tirzepatide).
GLP-1, glucagon-like peptide-1; GIP, glucose-dependent insulinotropic polypeptide; RA, receptor agonist; HbA1c, glycated hemoglobin; GI, gastrointestinal; MACE, major adverse cardiovascular event.
Fig. 2. Potential mechanisms by which tirzepatide imparts cardiovascular benefits.
HbA1c, glycated hemoglobin; BP, blood pressure; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; ICAM-1, intercellular adhesion molecule 1; GDF-15, growth differentiation factor 15; CRP, C-reactive protein.
Footnotes
Funding: This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (grant numbers: NRF-2020R1A2C1101977: Chang Hee Jung).
Conflict of Interest: Yun Kyung Cho is editor of Journal of LIpid and Atherosclerosis. However, she was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Data Availability Statement: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
- Conceptualization: Jung CH.
- Visualization: Cho YK.
- Writing - original draft: Jung CH.
- Writing - review & editing: Cho YK, Lee YL.
References
- 1.Coskun T, Sloop KW, Loghin C, Alsina-Fernandez J, Urva S, Bokvist KB, et al. LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: From discovery to clinical proof of concept. Mol Metab. 2018;18:3–14. doi: 10.1016/j.molmet.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenstock J, Wysham C, Frías JP, Kaneko S, Lee CJ, Fernández Landó L, et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet. 2021;398:143–155. doi: 10.1016/S0140-6736(21)01324-6. [DOI] [PubMed] [Google Scholar]
- 3.Frías JP, Davies MJ, Rosenstock J, Pérez Manghi FC, Fernández Landó L, Bergman BK, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385:503–515. doi: 10.1056/NEJMoa2107519. [DOI] [PubMed] [Google Scholar]
- 4.Ludvik B, Giorgino F, Jódar E, Frias JP, Fernández Landó L, Brown K, et al. Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): a randomised, open-label, parallel-group, phase 3 trial. Lancet. 2021;398:583–598. doi: 10.1016/S0140-6736(21)01443-4. [DOI] [PubMed] [Google Scholar]
- 5.Del Prato S, Kahn SE, Pavo I, Weerakkody GJ, Yang Z, Doupis J, et al. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): a randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet. 2021;398:1811–1824. doi: 10.1016/S0140-6736(21)02188-7. [DOI] [PubMed] [Google Scholar]
- 6.Dahl D, Onishi Y, Norwood P, Huh R, Bray R, Patel H, et al. Effect of subcutaneous tirzepatide vs placebo added to titrated insulin glargine on glycemic control in patients with type 2 diabetes: the SURPASS-5 randomized clinical trial. JAMA. 2022;327:534–545. doi: 10.1001/jama.2022.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jastreboff AM, Aronne LJ, Ahmad NN, Wharton S, Connery L, Alves B, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387:205–216. doi: 10.1056/NEJMoa2206038. [DOI] [PubMed] [Google Scholar]
- 8.Baggio LL, Drucker DJ. Glucagon-like peptide-1 receptor co-agonists for treating metabolic disease. Mol Metab. 2021;46:101090. doi: 10.1016/j.molmet.2020.101090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nauck MA, Meier JJ. GIP and GLP-1: stepsiblings rather than monozygotic twins within the incretin family. Diabetes. 2019;68:897–900. doi: 10.2337/dbi19-0005. [DOI] [PubMed] [Google Scholar]
- 10.Bass J, Tschöp MH, Beutler LR. Dual gut hormone receptor agonists for diabetes and obesity. J Clin Invest. 2023;133:e167952. doi: 10.1172/JCI167952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holst JJ, Orskov C, Nielsen OV, Schwartz TW. Truncated glucagon-like peptide I, an insulin-releasing hormone from the distal gut. FEBS Lett. 1987;211:169–174. doi: 10.1016/0014-5793(87)81430-8. [DOI] [PubMed] [Google Scholar]
- 12.Mojsov S, Weir GC, Habener JF. Insulinotropin: glucagon-like peptide I (7-37) co-encoded in the glucagon gene is a potent stimulator of insulin release in the perfused rat pancreas. J Clin Invest. 1987;79:616–619. doi: 10.1172/JCI112855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kreymann B, Williams G, Ghatei MA, Bloom SR. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet. 1987;2:1300–1304. doi: 10.1016/s0140-6736(87)91194-9. [DOI] [PubMed] [Google Scholar]
- 14.Knudsen LB, Lau J. The discovery and development of liraglutide and semaglutide. Front Endocrinol (Lausanne) 2019;10:155. doi: 10.3389/fendo.2019.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown JC, Mutt V, Pederson RA. Further purification of a polypeptide demonstrating enterogastrone activity. J Physiol. 1970;209:57–64. doi: 10.1113/jphysiol.1970.sp009155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dupre J, Ross SA, Watson D, Brown JC. Stimulation of insulin secretion by gastric inhibitory polypeptide in man. J Clin Endocrinol Metab. 1973;37:826–828. doi: 10.1210/jcem-37-5-826. [DOI] [PubMed] [Google Scholar]
- 17.Rizvi AA, Rizzo M. The emerging role of dual GLP-1 and GIP receptor agonists in glycemic management and cardiovascular risk reduction. Diabetes Metab Syndr Obes. 2022;15:1023–1030. doi: 10.2147/DMSO.S351982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elahi D, McAloon-Dyke M, Fukagawa NK, Meneilly GS, Sclater AL, Minaker KL, et al. The insulinotropic actions of glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (7-37) in normal and diabetic subjects. Regul Pept. 1994;51:63–74. doi: 10.1016/0167-0115(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 19.Holst JJ, Rosenkilde MM. Recent advances of GIP and future horizons. Peptides. 2020;125:170230. doi: 10.1016/j.peptides.2019.170230. [DOI] [PubMed] [Google Scholar]
- 20.Yamada Y, Seino Y. Physiology of GIP--a lesson from GIP receptor knockout mice. Horm Metab Res. 2004;36:771–774. doi: 10.1055/s-2004-826162. [DOI] [PubMed] [Google Scholar]
- 21.Kim SJ, Nian C, Karunakaran S, Clee SM, Isales CM, McIntosh CH. GIP-overexpressing mice demonstrate reduced diet-induced obesity and steatosis, and improved glucose homeostasis. PLoS One. 2012;7:e40156. doi: 10.1371/journal.pone.0040156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holst JJ. The incretin system in healthy humans: the role of GIP and GLP-1. Metabolism. 2019;96:46–55. doi: 10.1016/j.metabol.2019.04.014. [DOI] [PubMed] [Google Scholar]
- 23.Finan B, Ma T, Ottaway N, Müller TD, Habegger KM, Heppner KM, et al. Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans. Sci Transl Med. 2013;5:209ra151. doi: 10.1126/scitranslmed.3007218. [DOI] [PubMed] [Google Scholar]
- 24.Frias JP, Bastyr EJ, 3rd, Vignati L, Tschöp MH, Schmitt C, Owen K, et al. The sustained effects of a dual GIP/GLP-1 receptor agonist, NNC0090-2746, in patients with type 2 diabetes. Cell Metab. 2017;26:343–352.e2. doi: 10.1016/j.cmet.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 25.Nauck MA, Meier JJ. Incretin hormones: their role in health and disease. Diabetes Obes Metab. 2018;20(Suppl 1):5–21. doi: 10.1111/dom.13129. [DOI] [PubMed] [Google Scholar]
- 26.Nauck MA, Quast DR, Wefers J, Pfeiffer AF. The evolving story of incretins (GIP and GLP-1) in metabolic and cardiovascular disease: a pathophysiological update. Diabetes Obes Metab. 2021;23(Suppl 3):5–29. doi: 10.1111/dom.14496. [DOI] [PubMed] [Google Scholar]
- 27.Inagaki N, Takeuchi M, Oura T, Imaoka T, Seino Y. Efficacy and safety of tirzepatide monotherapy compared with dulaglutide in Japanese patients with type 2 diabetes (SURPASS J-mono): a double-blind, multicentre, randomised, phase 3 trial. Lancet Diabetes Endocrinol. 2022;10:623–633. doi: 10.1016/S2213-8587(22)00188-7. [DOI] [PubMed] [Google Scholar]
- 28.Kadowaki T, Chin R, Ozeki A, Imaoka T, Ogawa Y. Safety and efficacy of tirzepatide as an add-on to single oral antihyperglycaemic medication in patients with type 2 diabetes in Japan (SURPASS J-combo): a multicentre, randomised, open-label, parallel-group, phase 3 trial. Lancet Diabetes Endocrinol. 2022;10:634–644. doi: 10.1016/S2213-8587(22)00187-5. [DOI] [PubMed] [Google Scholar]
- 29.Gastaldelli A, Cusi K, Fernández Landó L, Bray R, Brouwers B, Rodríguez Á. Effect of tirzepatide versus insulin degludec on liver fat content and abdominal adipose tissue in people with type 2 diabetes (SURPASS-3 MRI): a substudy of the randomised, open-label, parallel-group, phase 3 SURPASS-3 trial. Lancet Diabetes Endocrinol. 2022;10:393–406. doi: 10.1016/S2213-8587(22)00070-5. [DOI] [PubMed] [Google Scholar]
- 30.Heise T, DeVries JH, Urva S, Li J, Pratt EJ, Thomas MK, et al. Tirzepatide reduces appetite, energy intake, and fat mass in people with type 2 diabetes. Diabetes Care. 2023;46:998–1004. doi: 10.2337/dc22-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samms RJ, Zhang G, He W, Ilkayeva O, Droz BA, Bauer SM, et al. Tirzepatide induces a thermogenic-like amino acid signature in brown adipose tissue. Mol Metab. 2022;64:101550. doi: 10.1016/j.molmet.2022.101550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoffman S, Alvares D, Adeli K. GLP-1 attenuates intestinal fat absorption and chylomicron production via vagal afferent nerves originating in the portal vein. Mol Metab. 2022;65:101590. doi: 10.1016/j.molmet.2022.101590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guan R, Yang Q, Yang X, Du W, Li X, Ma G. Efficacy and safety of tirzepatide in patients with type 2 diabetes mellitus: a bayesian network meta-analysis. Front Pharmacol. 2022;13:998816. doi: 10.3389/fphar.2022.998816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lemos JA. Abstract 10370: Effects of tirzepatide on 24-hour ambulatory blood pressure and heart rate in adults with obesity - results from the SURMOUNT-1 ambulatory blood pressure monitoring sub-study. Circulation. 2022;146(Suppl_1):A10370 [Google Scholar]
- 35.Patoulias D, Doumas M, Papadopoulos C. Meta-analysis assessing the effect of tirzepatide on the risk for atrial fibrillation in patients with type 2 diabetes mellitus. Am J Cardiol. 2022;173:157–158. doi: 10.1016/j.amjcard.2022.03.042. [DOI] [PubMed] [Google Scholar]
- 36.Sharif S, Van der Graaf Y, Cramer MJ, Kapelle LJ, de Borst GJ, Visseren FLJ, et al. Low-grade inflammation as a risk factor for cardiovascular events and all-cause mortality in patients with type 2 diabetes. Cardiovasc Diabetol. 2021;20:220. doi: 10.1186/s12933-021-01409-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson JM, Lin Y, Luo MJ, Considine G, Cox AL, Bowsman LM, et al. The dual glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 receptor agonist tirzepatide improves cardiovascular risk biomarkers in patients with type 2 diabetes: a post hoc analysis. Diabetes Obes Metab. 2022;24:148–153. doi: 10.1111/dom.14553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mori Y, Matsui T, Hirano T, Yamagishi SI. GIP as a potential therapeutic target for atherosclerotic cardiovascular disease-a systematic review. Int J Mol Sci. 2020;21:1509. doi: 10.3390/ijms21041509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sattar N, McGuire DK, Pavo I, Weerakkody GJ, Nishiyama H, Wiese RJ, et al. Tirzepatide cardiovascular event risk assessment: a pre-specified meta-analysis. Nat Med. 2022;28:591–598. doi: 10.1038/s41591-022-01707-4. [DOI] [PMC free article] [PubMed] [Google Scholar]