Abstract
Objective
This phase IV, multicenter, randomized controlled, open-label, and parallel clinical trial aimed to compare the efficacy and safety of ezetimibe and moderate intensity rosuvastatin combination therapy to that of high intensity rosuvastatin monotherapy in patients with atherosclerotic cardiovascular disease (ASCVD).
Methods
This study enrolled patients with ASCVD and after a four-week screening period, patients were randomly assigned to receive either rosuvastatin and ezetimibe (RE 10/10 group) or high-intensity rosuvastatin (R20 group) only in a 1:1 ratio. The primary outcome was the difference in the percent change in the mean low-density lipoprotein cholesterol (LDL-C) level from baseline to 12 weeks between two groups after treatment.
Results
The study found that after 12 and 24 weeks of treatment, the RE10/10 group had a greater reduction in LDL-C level compared to the R20 group (−22.9±2.6% vs. −15.6 ± 2.5% [p=0.041] and −24.2±2.5% vs. −12.9±2.4% [p=0.001] at 12 and 24 weeks, respectively). Moreover, a greater number of patients achieved the target LDL-C level of ≤70 mg/dL after the treatment period in the combination group (74.6% vs. 59.9% [p=0.012] and 76.2% vs. 50.8% [p<0.001] at 12 and 24 weeks, respectively). Importantly, there were no significant differences in the occurrence of overall adverse events and adverse drug reactions between two groups.
Conclusion
Moderate-intensity rosuvastatin and ezetimibe combination therapy had better efficacy in lowering LDL-C levels without increasing adverse effects in patients with ASCVD than high-intensity rosuvastatin monotherapy.
Trial Registration
ClinicalTrials.gov Identifier: NCT03494270
Keywords: Rosuvastatin calcium; Ezetimibe; Cholesterol, LDL; Cardiovascular diseases
INTRODUCTION
Atherosclerotic cardiovascular diseases (ASCVD), including coronary artery disease, ischemic stroke, and peripheral arterial disease, are the leading causes of death worldwide.1,2 Lipid-lowering therapy, especially low-density lipoprotein cholesterol (LDL-C)-lowering therapy, is an important strategy for secondary prevention of ASCVD.3,4 Contemporary treatment guidelines recommend high-intensity statin titrated up to the maximum tolerable dose.5
Despite the widespread use of high-intensity statin therapy, achieving target LDL-C levels remains challenging, with LDL-C goal attainment rates reported to be lower than 50% in high-risk patients with cardiovascular disease in Korea, as shown by Yang et al.6 Furthermore, intensive statin therapy is frequently associated with adverse events including muscle symptoms and elevated liver enzymes, with the risk of transaminase elevation significantly higher with statin therapy compared to placebo, particularly with high-dose statins.7 In fact, up to 10% of patients treated with high-dose statins may experience muscle symptoms within one month of the initial treatment.8 Additionally, there is another concern regarding with new-onset diabetes associated with statin therapy.9
Ezetimibe, a selective inhibitor of the Niemann-Pick C1 like 1 cholesterol transporter protein, blocks dietary cholesterol absorption into the jejunal enterocyte.10 Combination therapy with ezetimibe and statins has been shown to inhibit cholesterol absorption as well as synthesis, resulting in an additional 13%–20% reduction in LDL-C and greater attainment of LDL-C targets than with statin alone. Moreover, co-administration of ezetimibe with statins is generally well tolerated.11,12 However, it is considered a second-line therapy and is used when the target LDL-C goal is unachievable at the maximum tolerated statin dose.13,14
In this study, we compared the LDL-C-lowering efficacy and safety of combined ezetimibe and moderate-intensity statin with that of high-intensity statin alone in patients with ASCVD. We hypothesized that the combination therapy would achieve a non-inferior LDL-C-lowering efficacy and cause fewer adverse events than high-intensity statin monotherapy. The study drug was administered as a fixed-dose single-pill combination, which has been shown to enhance drug adherence compared to two separate pills.15
MATERIALS AND METHODS
1. Study design
This was a multicenter, randomized, controlled, open-label, parallel, phase IV trial conducted at six hospitals in the Republic of Korea between March 2018 and September 2019 (Supplementary Table 1) comparing two lipid-lowering treatment strategies for patients with ASCVD. We compared the efficacy and safety of moderate-intensity statin and ezetimibe combination therapy with high-intensity statin therapy. The study conformed to the protocols and principles of the latest version of the Declaration of Helsinki (revised version 2013). The Institutional Review Board (IRB) of each participating center approved this study (IRB No.: B-1712/439-002). Written informed consent was obtained from all eligible participants. The study protocol was registered at ClinicalTrials.gov (ID: NCT03494270).
2. Study participants
The inclusion and exclusion criteria for this study are shown in Supplementary Table 2. The key inclusion criterion was the presence of ASCVD requiring optimal statin therapy. The presence of ASCVD was determined based on clinical diagnosis, and confirmed by International Classification of Diseases 10th Revision code. ASCVD included coronary artery disease, stroke, transient ischemic attack, and peripheral artery disease (Supplementary Table 2). The participants were aged between 19 and 75 years. Patients over 75 years were not included because there is insufficient evidence from randomized controlled trials for the use of high-intensity statins in this population.3,16 Patients with severe renal impairment or abnormalities in liver enzyme or creatine phosphokinase (CPK) levels were excluded.
3. Randomization, treatment, and study protocol
The enrolled participants underwent a screening phase of four weeks. Those on lipid-lowering agents for ≥ four weeks without significant side effects were randomized via a sealed envelope to either the rosuvastatin and ezetimibe combination therapy (RE10/10) group or intensive statin therapy (R20) group, in a 1:1 manner. An independent statistician generated the randomization numbers. Block randomization was performed stratified by the participating centers and presence of diabetes.
Participants in the RE10/10 group (combination therapy) were administered a fixed-dose single-pill (Rosuvamibe Tab. 10/20 mg; Yuhan Corporation, Seoul, Korea) that contained a combination of moderate-intensity statin and ezetimibe (rosuvastatin 10 mg plus ezetimibe 10 mg) once daily. Patients in the R20 group (intensive statin therapy) received high-intensity statin therapy (rosuvastatin 20 mg) once daily (Monorova Tab. 20 mg; Yuhan Corporation). Study drugs were provided in an open-label manner. The overall study duration was 24 weeks. Follow-up visits occurred at 12 and 24 weeks after randomization. All clinically relevant side effects were assessed, and treatment adherence was checked at each study visit. The first patient enrollment date was March 29, 2018, and follow-up of the final patient was completed on September 26, 2019.
Blood samples were collected by venous sampling on the day of enrollment and at 12 and 24 weeks. Samples were drawn while fasting, at least 8 hours after the last meal. Baseline screening tests were performed at the participating centers. Meanwhile, samples for the effectiveness test were collected at the time of randomization (visit 1) and follow-up visits (visits 2 and 3) and analyzed at the central lab (Global Clinical Central Lab [GCCL] Co., Ltd., Yongin, Korea). LDL-C levels were measured directly, and non-high-density lipoprotein cholesterol (non-HDL-C) levels were calculated.
4. Study endpoints
The primary endpoint was the percent change in the LDL-C level after 12 weeks, which was calculated using the following equation:
The secondary endpoints were mean percent changes in the following: (1) total cholesterol, HDL cholesterol, triglyceride, non-HDL cholesterol, apolipoprotein A1, apolipoprotein B, high sensitivity C-reactive protein, fasting plasma glucose, and homeostatic model assessment for insulin resistance (HOMA-IR) after 12 and 24 weeks; and (2) LDL cholesterol level after 24 weeks of treatment. Moreover, the proportion of patients with LDL cholesterol levels below 70 mg/dL after 12 and 24 weeks was compared. The number of statin-associated muscle symptoms, such as elevated CPK levels, hepatic enzyme abnormalities, and major adverse cardiac and cerebrovascular events (MACCE), were compared after the treatment period (see Supplementary Table 3 for the definition of study endpoints). Statin-associated muscle symptoms, such as muscle aches/cramps, myositis, rhabdomyolysis, and CPK elevation > four times the upper limit of normal, were defined as muscular symptoms requiring discontinuation or disruption of the study drugs.
5. Study hypothesis and sample size calculation
The hypothesis was non-inferiority of the RE10/10 treatment to the R20 treatment based on the percent change in LDL-C levels after 12 weeks. A total of 272 patients (136 in each group) were required to provide 80% statistical power when assuming a one-sided α level of 0.025 and an attrition rate of 10%. A previous study showed a −23.7% and −5.6% reduction in LDL-C with daily rosuvastatin 10 mg and ezetimibe 10 mg combination therapy and rosuvastatin 10 mg monotherapy, respectively.17 The non-inferiority margin was set at half of the difference (9%) because a higher dose of statin was administered in the R20 group. The standard deviation was assumed to be 5% based on a previous study.18 If the upper limit of the 95% confidence interval for the difference in the percent change in LDL-C was lower than 9%, the null hypothesis was rejected. Subsequent superiority testing was performed if the statistical criterion for non-inferiority was satisfied.
6. Statistical analysis
The full analysis (FA) set included all randomized patients who received at least one dose of the study medication and who provided at least one LDL cholesterol measurement after randomization. The main study analyses were performed using the FA set, which followed the intention-to-treat principle. Patients and study endpoints were analyzed based on the allocated patient groups, regardless of subsequent crossover or non-adherence to the assigned treatment. The per-protocol (PP) set included study patients adhering to the trial instructions specified in the study protocol. Major protocol violations included a dropout, any missing LDL-C values at baseline and 12 weeks, poor adherence (<80%) or crossover, and other crucial violations as determined by the steering committee. The safety set included any study subjects who received ≥ one study drug.
Baseline characteristics are presented as the mean ± standard deviation or median (minimum to maximum) for continuous variables and as proportions (%) for categorical variables. Continuous variables were compared using Student’s t-test or the Wilcoxon rank sum test as appropriate. Categorical variables were compared using the χ2 test or Fisher’s exact test. Differences within groups were compared using a paired t-test or Wilcoxon signed-rank test.
An efficacy analysis was conducted on the FA and PP sets. The primary endpoint was compared via analysis of covariance (ANCOVA), adjusting for the baseline LDL-C level and diabetes. The least-squares means are presented with standard errors, 95% confidence intervals, and p-values.
The incidence of MACCE during the clinical trial was presented and the differences between groups tested using the χ2 test (or Fisher’s exact test). P-values of less than 0.05 were considered statistically significant. All statistical analyses were performed using SAS 9.4 (Statistical Analysis Software 9.4; SAS Institute Inc, Cary, NC, USA).
RESULTS
1. Study flow and baseline characteristics
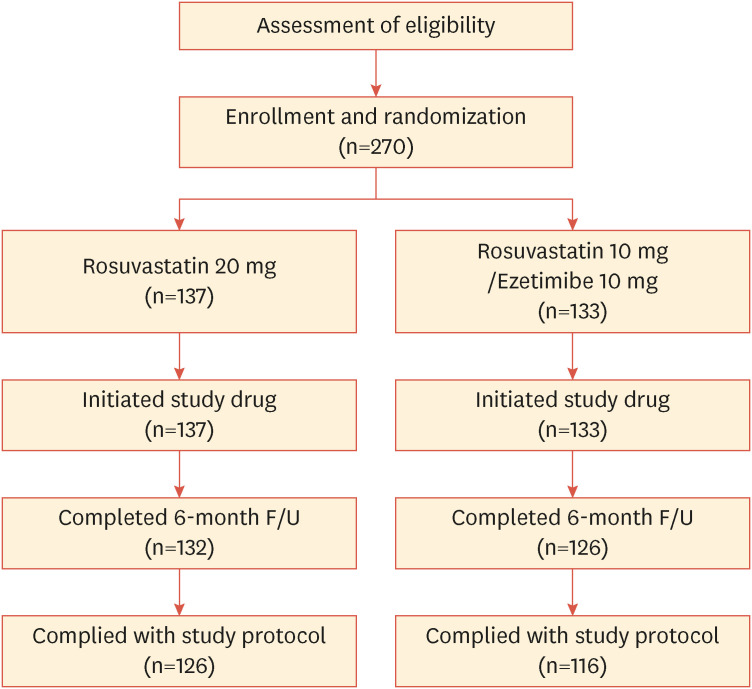
Fig. 1 shows a flow diagram depicting the screening, randomization, and follow-up of study participants. Among the 274 patients screened, one did not meet the inclusion criteria and three withdrew consent after the four-week rule-in period. Therefore, 270 patients were enrolled and randomly assigned to either the RE10/10 (n=133) or R20 group (n=137). After six months, 254 patients completed the study visits and 16 patients dropped out for the following reasons: 12 withdrew consent and one each due to loss to follow-up, adverse events, death unrelated to the study drugs, and researchers’ discretion (Supplementary Fig. 1). Table 1 shows the baseline demographics and clinical characteristics of the 258 patients in the FA set, which are similar between the treatment groups. Approximately three-fourths of the patients were men, the mean age was 62 years, 21.7% smoked cigarettes, 32.6% had diabetes, and 51.2% had hypertension. The mean duration of hypercholesterolemia was 75.8 months.
Fig. 1. Flow chart of the study design.
F/U, follow up.
Table 1. Demographic and baseline characteristics of enrolled patients.
| Variables | R20 (n=132) | RE10/10 (n=126) | Total (n=258) | p-value | |
|---|---|---|---|---|---|
| Male, No. (%) | 104 (78.8) | 91 (72.2) | 195 (75.6) | 0.220* | |
| Age (yr), median [Min, Max] | 63.5, [25.0, 74.0] | 63.5, [39.0, 75.0] | 63.5, [25.0, 75.0] | 0.478† | |
| Current smoker, No. (%) | 30 (22.7) | 26 (20.6) | 56 (21.7) | 0.684* | |
| Height (cm), median [Min, Max] | 165.0, [146.0, 185.0] | 165.0, [141.0, 182.0] | 165.0, [141.0, 185.0] | 0.465† | |
| Weight (kg), median [Min, Max] | 70.8, [44.0, 121.1] | 68.8, [46.0, 95.7] | 69.8, [44.0, 121.1] | 0.546† | |
| Duration of hypercholesterolemia (mon), median [Min, Max] | 58.5, [0.0, 247] | 61.0, [1.0, 253.0] | 59.0, [0.0, 253.0] | 0.821† | |
| Diabetes mellitus | 44 (33.3) | 40 (31.8) | 84 (32.6) | 0.786* | |
| Hypertension | 69 (52.3) | 63 (50.0) | 132 (51.2) | 0.810* | |
| Hypothyroidism | 3 (2.3) | 5 (4.0) | 8 (3.1) | 0.492* | |
| Clinical indications, No. (%) | |||||
| Acute myocardial infarction | 18 (13.6) | 27 (21.4) | 45 (17.4) | 0.138* | |
| Angina pectoris | 46 (34.9) | 29 (23.0) | 75 (29.1) | 0.051* | |
| Angina unstable | 36 (27.3) | 46 (36.5) | 82 (31.8) | 0.145* | |
R20, high intensity statin group which received 20 mg of rosuvastatin once daily; RE10/10, combination therapy group which received 10 mg rosuvastatin and 10 mg ezetimibe once daily.
*χ2 test; †Wilcoxon rank sum test.
2. Efficacy
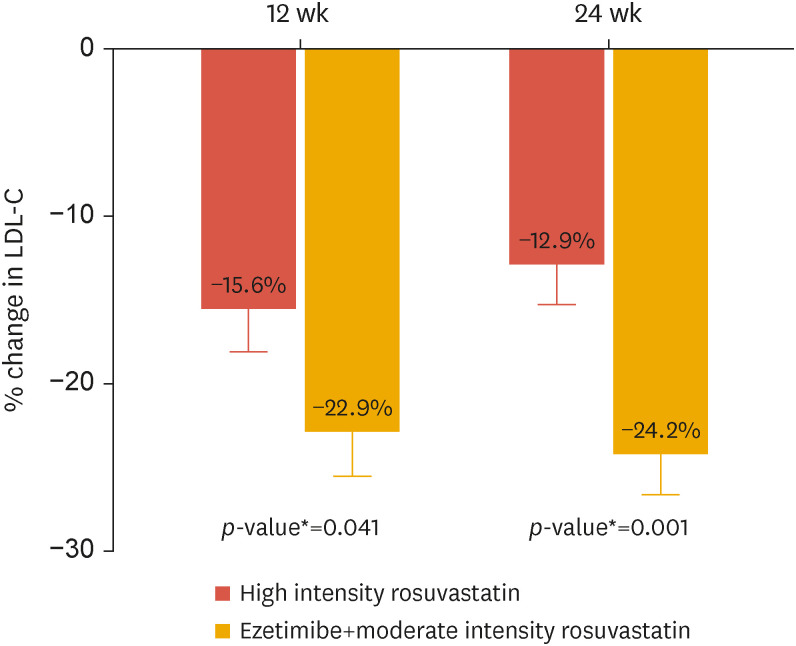
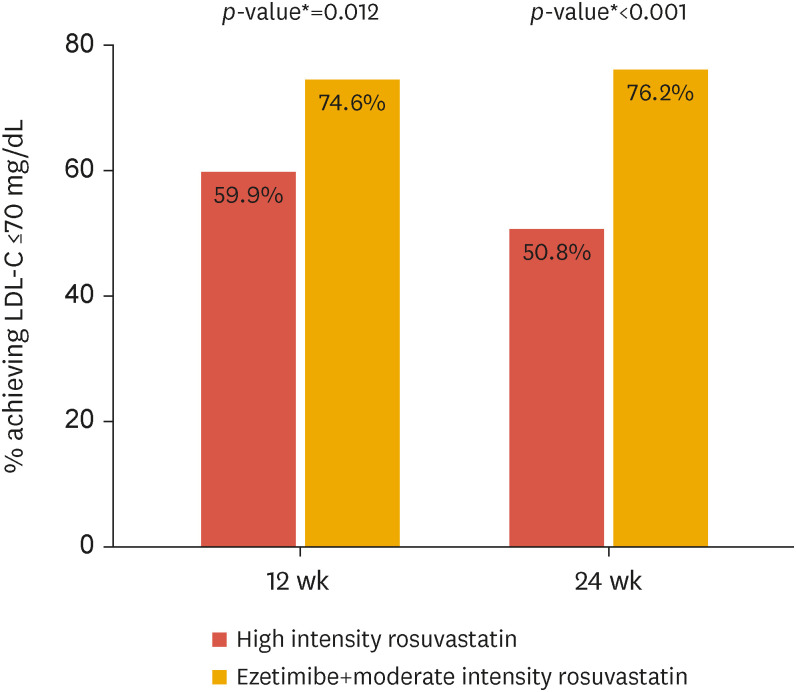
Table 2 and Fig. 2 depict the results of the main analysis performed on the FA set. At baseline, LDL-C was 80.9 mg/dL and 83.8 mg/dL in the R20 and RE10/10 groups, respectively. After 12 weeks, LDL-C decreased by −15.6 ± 2.5% and −22.9 ± 2.6% in the R20 and RE10/10 groups, respectively. The mean difference was −7.35%, and the upper limit of the 95% confidence interval was −0.30%, which was significantly lower than the predefined non-inferiority margin of 9%. Moreover, the subsequent superiority test revealed a greater reduction in LDL-C in the RE10/10 group compared to that in the R20 group (p=0.041). The difference in LDL-C further diverged after 24 weeks of treatment: −12.9±2.4% vs. −24.2±2.5% (p=0.001) in the R20 and RE10/10 groups, respectively. Therefore, the proportion of responders, which was defined as patients with LDL-C controlled at a level equal to or less than 70 mg/dL, was significantly higher in the RE10/10 combination group after 12 and 24 weeks (p=0.012 and <0.001, respectively) (Fig. 3). To create a more robust analysis, a sensitivity analysis was performed using the ANCOVA model with post-treatment LDL as the outcome and baseline LDL and diabetes as the covariates. Additionally, we performed a linear model using natural log-transformed pre-and post-treatment LDL. The results of all analyses were similar (Supplementary Tables 4 and 5).
Table 2. LDL cholesterol levels at baseline and after treatment with rosuvastatin monotherapy or rosuvastatin/ezetimibe combination therapy.
| Variables | R20 (n=132) | RE10/10 (n=126) | Mean difference (standard error) | 95% confidence interval | p-value | |
|---|---|---|---|---|---|---|
| LDL-C at baseline (mg/dL), median [Min, Max] | 78.5, [34, 192] | 79.5, [32, 209] | 0.425* | |||
| At 12 wk | ||||||
| LDL -C at 12 wk (mg/dL) | 66.7 (20.5) | 60.5 (24.1) | ||||
| Absolute changes (mg/dL) | −14.3 (22.9) | −22.9 (38.1) | ||||
| Diabetes status | 0.086† | |||||
| % change‡ (%) | −15.6 (2.5) | −22.9 (2.6) | −7.4 (3.6) | −14.4 to −0.3 | 0.041§ | |
| At 24 wk | ||||||
| LDL-C at 24 wk (mg/dL) | 68.6 (22.19) | 60.9 (25.8) | ||||
| Absolute changes (mg/dL) | −11.4 (28.6) | −23.8 (31.4) | ||||
| Diabetes status | 0.016† | |||||
| % change∥ (%) | −12.9 (2.4) | −24.2 (2.5) | −11.3 (3.5) | −18.1 to −4.5 | 0.001§ | |
R20, high intensity statin group which received 20 mg of rosuvastatin once daily; RE10/10, combination therapy group which received 10 mg rosuvastatin and 10 mg ezetimibe once daily; LDL-C, low-density lipoprotein cholesterol.
*Difference between control and treatment groups (Wilcoxon rank sum test); †Difference between diabetic and nondiabetic patients using the ANCOVA model including baseline LDL-C as a covariate; ‡Percent change from baseline at week 12(%) = {(LDL-C after 12 weeks)−(LDL-C at baseline)}/(LDL-C at baseline)×100; §Difference between control and treatment groups using the ANCOVA model including baseline LDL-C and diabetic status as covariates; ∥Percent change from baseline at week 24(%) = {(LDL-C after 24 weeks)−(LDL-C at baseline)}/(LDL-C at baseline)×100.
Fig. 2. Least squares mean of the percent change in LDL-C after 12 and 24 weeks of treatment with ezetimibe/rosuvastatin or rosuvastatin.
LDL-C, low-density lipoprotein cholesterol.
*p-values from analysis of covariance test.
Fig. 3. LDL-C target achievement rates after 12 and 24 weeks of treatment with ezetimibe/rosuvastatin or rosuvastatin. The LDL-C target was 70 mg/dL or less.
LDL-C, low-density lipoprotein cholesterol.
*p-values from logistic regression model.
Analysis of the PP set showed results similar to those for the FA set (Supplementary Table 6). After 12 weeks, the percent change in LDL-C was −15.9% and −21.7% in the R20 and RE10/10 groups, respectively. The difference and upper limit of the 95% confidence intervals were −5.8% and 1.6%, respectively, and met the non-inferiority criteria. Superiority testing showed that the difference after 12 weeks was not significant (p=0.124). However, the difference further widened to −10.0% after 24 weeks, which achieved significance (p=0.006).
Changes in the secondary study endpoints after 12 and 24 weeks compared to those at baseline are shown in Supplementary Table 7. The changes in total cholesterol and non-HDL cholesterol were significantly greater in the RE10/10 group than those in the R20 group after 12 and 24 weeks. Moreover, apolipoprotein B levels decreased greatly after 24 weeks in the RE10/10 group. Insulin resistance, measured using HOMA-IR, increased in both groups after study drug administration; however, the difference between groups did not change (Supplementary Table 8).
3. Adverse events and safety
There were no definite cases of statin-associated muscle symptoms in either group during the study period, which were defined as muscular symptoms and elevated CPK levels. One patient reported muscular weakness and one reported myalgia in the R20 group; however, neither showed significant CPK elevations. The proportion of patients with elevated CPK levels was numerically lower in the RE10/10 group, which was not significant. Only one patient showed liver enzyme levels >4 times the upper limit of normal in the R20 group (Table 3). There was no significant difference in the MACCE during the study period (p=1.0); however, one patient in each group developed unstable angina (Supplementary Table 9). Therefore, the occurrence of adverse events did not differ between groups, and serious adverse events were rare (Supplementary Table 10).
Table 3. Creatine phosphokinase level and liver function test results.
| Variables | R20 (n=132) | RE10/10 (n=126) | p-value | ||
|---|---|---|---|---|---|
| After 12 wk | |||||
| Creatine phosphokinase | |||||
| >ULN | 13 (9.9) | 7 (5.6) | 0.197* | ||
| >4×ULN | 0 (0.0) | 0 (0.0) | - | ||
| >10×ULN | 0 (0.0) | 0 (0.0) | - | ||
| Liver enzymes | |||||
| AST or ALT >4×ULN | 0 (0.0) | 0 (0.0) | - | ||
| AST or ALT >10×ULN | 0 (0.0) | 0 (0.0) | - | ||
| After 24 wk | |||||
| Creatine phosphokinase | |||||
| >ULN | 16 (12.1) | 10 (7.9) | 0.264* | ||
| >4×ULN | 2 (1.5) | 0 (0.0) | 0.498† | ||
| >10×ULN | 0 (0.0) | 0 (0.0) | - | ||
| Liver enzymes | |||||
| AST or ALT >4×ULN | 1 (0.8) | 0 (0.0) | 1.000† | ||
| AST or ALT >10×ULN | 1 (0.8) | 0 (0.0) | 1.000† | ||
Values are presented as number (%).
R20, high-intensity statin group which received 20 mg of rosuvastatin once daily; RE10/10, combination therapy group which received 10 mg rosuvastatin and 10 mg ezetimibe once daily; ULN, upper limit of normal; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
*χ2 test; †Fisher’s exact test.
DISCUSSION
This multicenter, randomized controlled trial showed that combination therapy with moderate-intensity statin and ezetimibe is non-inferior to the high-intensity rosuvastatin monotherapy regimen in reducing LDL-C levels. Moreover, the combination therapy significantly lowered LDL-C, total cholesterol, non-HDL cholesterol, and apolipoprotein B levels compared to the high-intensity statin therapy without any increase in adverse events. A higher proportion of patients achieved the LDL-C target level following the combination therapy.
Statin therapy is generally tolerable and safe. However, high-intensity statin therapy is associated with a higher prevalence of adverse events.19,20,21 Statin-associated muscle symptoms are the most commonly encountered side effects. Approximately 7%–29% of patients taking statins report muscle symptoms such as myalgia; however, the prevalence is higher among patients on high-dose statin therapy.22 Statins are frequently associated with an increase in the liver function test values, with an incidence rate ranging from 0.5%–2.0%.23 Clinically relevant elevation of alanine aminotransferase (ALT) levels may lead to discontinuation of treatment. Statin treatment increases the risk of diabetes,24 and the risk increases in a dose-dependent manner.25
Lowering LDL cholesterol levels in patients with ASCVD has been consistently associated with favorable clinical outcomes, including a risk of further cardiovascular events.26,27,28 On this basis, recent guidelines recommend a stricter LDL cholesterol target of <55 mg/dL for very high-risk individuals as part of their secondary prevention approach.5 However, generally attaining a more aggressive LDL cholesterol target using statin therapy alone can be challenging. Moreover, the benefits associated with reducing LDL cholesterol are not limited to statin therapy alone.12,29 Therefore, the addition of ezetimibe to moderate-intensity statins was expected to be an effective strategy in achieving the desired LDL cholesterol levels while mitigating the risk of side effects associated with high-intensity statin regimens in patients with ASCVD.
Two small, randomized studies have previously reported on the efficacy and safety of combining moderate-intensity statin with ezetimibe compared to high-intensity statin therapy.30,31 Our findings are in line with previous studies. We showed that statin and ezetimibe combination therapy effectively decreased LDL-C levels in ASCVD patients according to the current guidelines. Moreover, the fixed-dose combination containing 10 mg rosuvastatin and 10 mg ezetimibe significantly reduced LDL-C levels compared to 20 mg rosuvastatin. Although insignificant, elevated CPK and AST or ALT levels requiring attention were noted only in patients receiving high-intensity statin therapy. The incidence of other adverse events and drug adverse reactions did not differ statistically between the groups. Therefore, in our study, the combination of ezetimibe and moderate-intensity rosuvastatin was optimal for lipid-lowering and achieving the goals recommended in the guidelines. Moreover, the combination was safe to use in ASCVD patients with hypercholesterolemia.
A recent randomized comparison of efficacy and safety of lipid lowering with statin monotherapy vs. statin–ezetimibe combination for high-risk cardiovascular disease (RACING) trial, provided robust evidence supporting the effectiveness of a combination strategy involving ezetimibe in lipid-lowering therapy. The study investigated the long-term clinical outcomes of combining a moderate-intensity statin with ezetimibe compared to using high-intensity statin monotherapy, rather than focusing on the change in LDL cholesterol levels. Over a three-year follow-up period, the study demonstrated that the combination therapy was non-inferior to monotherapy in terms of cardiovascular mortality and major cardiovascular events. Additionally, the combination therapy resulted in a higher rate of achieving target LDL cholesterol levels and lower rates of side effects.32
There had been studies that evaluated the effect of ezetimibe simply in addition to statin.18,33,34,35 However, our study, similar to the RACING trial, aimed to demonstrate the non-inferiority and potential superiority of ezetimibe combination therapy with moderate-intensity statins as a substitute for high-intensity statin therapy. Our study observed rapid and safe attainment of target LDL cholesterol levels over a 12-week period, which further supports the results of the RACING trial. These findings strengthen the evidence for the short- and long-term effectiveness and stability of combination therapy, providing the basis of considering early initiation of combination therapy in high-risk ASCVD patients requiring strong lipid-lowering effects.
One major limitation of this study was that it was not designed to test clinical outcomes. Although the ezetimibe and moderate-intensity statin combination therapy effectively lowered LDL-C levels, future studies are required to test whether this leads to fewer incidences of recurrent cardiovascular events. Moreover, larger sample sizes and longer follow-up periods are needed. It is worth mentioning that the RACING trial, which overcame this limitation, demonstrated impressive results in terms of long-term clinical outcomes. In the future, if the results of combination therapy can be evaluated in a wider and more diverse patient population with demographic characteristics and clinical environments, the possibility of generalization will be higher. Furthermore, it should be noted that a fixed-dose combination of two different lipid-lowering agents was used in this study. A single-pill combination (fixed-dose combination) has been shown to improve adherence by reducing the daily dosage.15 Therefore, the findings cannot be extrapolated to the administration of two separate pills. Finally, the results suggest that the RE10/10 combination is superior to R20; however, this study does not possess the statistical power required to prove this superiority.
This trial demonstrated that the efficacy of the ezetimibe and moderate-intensity statin combination therapy in lowering LDL-C was better compared to high-intensity statin. Both strategies were safe. Adverse events were not frequent and mostly mild. Moreover, their occurrence did not differ significantly between groups. Future studies are needed to determine whether this fixed-dose combination is an equivalent alternative to high-intensity statin therapy.
Footnotes
Funding: None.
Conflict of Interest: The authors have no conflicts of interest to declare.
Data Availability Statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
- Conceptualization: Kang SH, Jeong SW, Yoon CH, Youn TJ, Song WH, Jeon DW, Lim SW, Lee JH, Cho SW, Kim CH, Chae IH.
- Data curation: Kang SH, Jeong SW, Yoon CH, Youn TJ, Song WH, Jeon DW, Lim SW, Lee JH, Cho SW, Kim CH.
- Formal analysis: Kang SH, Chae IH.
- Investigation: Kang SH, Chae IH.
- Project administration: Kang SH, Jeong SW, Yoon CH, Chae IH.
- Resources: Kang SH.
- Software: Kang SH.
- Supervision: Kang SH, Chae IH.
- Writing - original draft: Choi H, Kang SH, Chae IH.
- Writing - review & editing: Choi H, Kang SH.
SUPPLEMENTARY MATERIALS
List of centers and local principal investigators
Inclusion and exclusion criteria
Primary and secondary study endpoints
LDLpost-treatment ~ ANCOVA(LDLbaseline + DM) model
ln (LDLpost-treatment) ~ lm(ln (LDLbaseline) + DM) model
LDL cholesterol levels at baseline and after treatment with rosuvastatin monotherapy or rosuvastatin/ezetimibe combination therapy (PP set)
Changes in secondary study endpoints at 12-weeks and 24-weeks after treatment with rosuvastatin monotherapy or rosuvastatin/ezetimibe combination therapy
Changes in insulin resistance at 12-weeks and 24-weeks after treatment with rosuvastatin monotherapy or rosuvastatin/ezetimibe combination therapy
The incidence of MACCE during the clinical trial period
Overall summary of AEs
Flow chart showing the number of patients who participated in and dropped out of the study.
References
- 1.Naghavi M, Abajobir AA, Abbafati C, Abbas KM, Abd-Allah F, Abera SF, et al. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1151–1210. doi: 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017;70:1–25. doi: 10.1016/j.jacc.2017.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;39:e1082–e1143. doi: 10.1161/CIR.0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38:2459–2472. doi: 10.1093/eurheartj/ehx144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 6.Yang YS, Yang BR, Kim MS, Hwang Y, Choi SH. Low-density lipoprotein cholesterol goal attainment rates in high-risk patients with cardiovascular diseases and diabetes mellitus in Korea: a retrospective cohort study. Lipids Health Dis. 2020;19:5. doi: 10.1186/s12944-019-1158-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naci H, Brugts J, Ades T. Comparative tolerability and harms of individual statins: a study-level network meta-analysis of 246 955 participants from 135 randomized, controlled trials. Circ Cardiovasc Qual Outcomes. 2013;6:390–399. doi: 10.1161/CIRCOUTCOMES.111.000071. [DOI] [PubMed] [Google Scholar]
- 8.Bruckert E, Hayem G, Dejager S, Yau C, Bégaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients--the PRIMO study. Cardiovasc Drugs Ther. 2005;19:403–414. doi: 10.1007/s10557-005-5686-z. [DOI] [PubMed] [Google Scholar]
- 9.Dormuth CR, Filion KB, Paterson JM, James MT, Teare GF, Raymond CB, et al. Higher potency statins and the risk of new diabetes: multicentre, observational study of administrative databases. BMJ. 2014;348:g3244. doi: 10.1136/bmj.g3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sudhop T, Lütjohann D, Kodal A, Igel M, Tribble DL, Shah S, et al. Inhibition of intestinal cholesterol absorption by ezetimibe in humans. Circulation. 2002;106:1943–1948. doi: 10.1161/01.cir.0000034044.95911.dc. [DOI] [PubMed] [Google Scholar]
- 11.Ballantyne CM, Blazing MA, King TR, Brady WE, Palmisano J. Efficacy and safety of ezetimibe co-administered with simvastatin compared with atorvastatin in adults with hypercholesterolemia. Am J Cardiol. 2004;93:1487–1494. doi: 10.1016/j.amjcard.2004.02.060. [DOI] [PubMed] [Google Scholar]
- 12.Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397. doi: 10.1056/NEJMoa1410489. [DOI] [PubMed] [Google Scholar]
- 13.Khan SU, Khan MU, Rahman H, Khan MS, Riaz H, Novak M, et al. A Bayesian network meta-analysis of preventive strategies for contrast-induced nephropathy after cardiac catheterization. Cardiovasc Revasc Med. 2019;20:29–37. doi: 10.1016/j.carrev.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Koskinas KC, Siontis GC, Piccolo R, Mavridis D, Räber L, Mach F, et al. Effect of statins and non-statin LDL-lowering medications on cardiovascular outcomes in secondary prevention: a meta-analysis of randomized trials. Eur Heart J. 2018;39:1172–1180. doi: 10.1093/eurheartj/ehx566. [DOI] [PubMed] [Google Scholar]
- 15.de Cates AN, Farr MR, Wright N, Jarvis MC, Rees K, Ebrahim S, et al. Fixed-dose combination therapy for the prevention of cardiovascular disease. Cochrane Database Syst Rev. 2014;4:CD009868. doi: 10.1002/14651858.CD009868.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Bays HE, Davidson MH, Massaad R, Flaim D, Lowe RS, Tershakovec AM, et al. Safety and efficacy of ezetimibe added on to rosuvastatin 5 or 10 mg versus up-titration of rosuvastatin in patients with hypercholesterolemia (the ACTE Study) Am J Cardiol. 2011;108:523–530. doi: 10.1016/j.amjcard.2011.03.079. [DOI] [PubMed] [Google Scholar]
- 18.Kim KJ, Kim SH, Yoon YW, Rha SW, Hong SJ, Kwak CH, et al. Effect of fixed-dose combinations of ezetimibe plus rosuvastatin in patients with primary hypercholesterolemia: MRS-ROZE (Multicenter Randomized Study of ROsuvastatin and eZEtimibe) Cardiovasc Ther. 2016;34:371–382. doi: 10.1111/1755-5922.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 20.LaRosa JC, Grundy SM, Waters DD, Shear C, Barter P, Fruchart JC, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–1435. doi: 10.1056/NEJMoa050461. [DOI] [PubMed] [Google Scholar]
- 21.Pedersen TR, Faergeman O, Kastelein JJ, Olsson AG, Tikkanen MJ, Holme I, et al. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA. 2005;294:2437–2445. doi: 10.1001/jama.294.19.2437. [DOI] [PubMed] [Google Scholar]
- 22.Stroes ES, Thompson PD, Corsini A, Vladutiu GD, Raal FJ, Ray KK, et al. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. 2015;36:1012–1022. doi: 10.1093/eurheartj/ehv043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen DE, Anania FA, Chalasani N National Lipid Association Statin Safety Task Force Liver Expert Panel. An assessment of statin safety by hepatologists. Am J Cardiol. 2006;97:77C–81C. doi: 10.1016/j.amjcard.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 24.Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735–742. doi: 10.1016/S0140-6736(09)61965-6. [DOI] [PubMed] [Google Scholar]
- 25.Preiss D, Seshasai SR, Welsh P, Murphy SA, Ho JE, Waters DD, et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA. 2011;305:2556–2564. doi: 10.1001/jama.2011.860. [DOI] [PubMed] [Google Scholar]
- 26.Fulcher J, O’Connell R, Voysey M, Emberson J, Blackwell L, Mihaylova B, et al. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet. 2015;385:1397–1405. doi: 10.1016/S0140-6736(14)61368-4. [DOI] [PubMed] [Google Scholar]
- 27.Silverman MG, Ference BA, Im K, Wiviott SD, Giugliano RP, Grundy SM, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA. 2016;316:1289–1297. doi: 10.1001/jama.2016.13985. [DOI] [PubMed] [Google Scholar]
- 28.Navarese EP, Robinson JG, Kowalewski M, Kolodziejczak M, Andreotti F, Bliden K, et al. Association between baseline LDL-C level and total and cardiovascular mortality after LDL-C lowering: a systematic review and meta-analysis. JAMA. 2018;319:1566–1579. doi: 10.1001/jama.2018.2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bays HE, Averna M, Majul C, Muller-Wieland D, De Pellegrin A, Giezek H, et al. Efficacy and safety of ezetimibe added to atorvastatin versus atorvastatin uptitration or switching to rosuvastatin in patients with primary hypercholesterolemia. Am J Cardiol. 2013;112:1885–1895. doi: 10.1016/j.amjcard.2013.08.031. [DOI] [PubMed] [Google Scholar]
- 30.Wu NQ, Guo YL, Zhu CG, Gao Y, Zhao X, Sun D, et al. Comparison of statin plus ezetimibe with double-dose statin on lipid profiles and inflammation markers. Lipids Health Dis. 2018;17:265. doi: 10.1186/s12944-018-0909-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ran D, Nie HJ, Gao YL, Deng SB, Du JL, Liu YJ, et al. A randomized, controlled comparison of different intensive lipid-lowering therapies in Chinese patients with non-ST-elevation acute coronary syndrome (NSTE-ACS): ezetimibe and rosuvastatin versus high-dose rosuvastatin. Int J Cardiol. 2017;235:49–55. doi: 10.1016/j.ijcard.2017.02.099. [DOI] [PubMed] [Google Scholar]
- 32.Kim BK, Hong SJ, Lee YJ, Hong SJ, Yun KH, Hong BK, et al. Long-term efficacy and safety of moderate-intensity statin with ezetimibe combination therapy versus high-intensity statin monotherapy in patients with atherosclerotic cardiovascular disease (RACING): a randomised, open-label, non-inferiority trial. Lancet. 2022;400:380–390. doi: 10.1016/S0140-6736(22)00916-3. [DOI] [PubMed] [Google Scholar]
- 33.Hong SJ, Jeong HS, Ahn JC, Cha DH, Won KH, Kim W, et al. A phase III, multicenter, randomized, double-blind, active comparator clinical trial to compare the efficacy and safety of combination therapy with ezetimibe and rosuvastatin versus rosuvastatin monotherapy in patients with hypercholesterolemia: I-ROSETTE (Ildong Rosuvastatin & Ezetimibe for Hypercholesterolemia) randomized controlled trial. Clin Ther. 2018;40:226–241.e4. doi: 10.1016/j.clinthera.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 34.Ballantyne CM, Weiss R, Moccetti T, Vogt A, Eber B, Sosef F, et al. Efficacy and safety of rosuvastatin 40 mg alone or in combination with ezetimibe in patients at high risk of cardiovascular disease (results from the EXPLORER study) Am J Cardiol. 2007;99:673–680. doi: 10.1016/j.amjcard.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 35.Yang YJ, Lee SH, Kim BS, Cho YK, Cho HJ, Cho KI, et al. Combination therapy of rosuvastatin and ezetimibe in patients with high cardiovascular risk. Clin Ther. 2017;39:107–117. doi: 10.1016/j.clinthera.2016.11.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of centers and local principal investigators
Inclusion and exclusion criteria
Primary and secondary study endpoints
LDLpost-treatment ~ ANCOVA(LDLbaseline + DM) model
ln (LDLpost-treatment) ~ lm(ln (LDLbaseline) + DM) model
LDL cholesterol levels at baseline and after treatment with rosuvastatin monotherapy or rosuvastatin/ezetimibe combination therapy (PP set)
Changes in secondary study endpoints at 12-weeks and 24-weeks after treatment with rosuvastatin monotherapy or rosuvastatin/ezetimibe combination therapy
Changes in insulin resistance at 12-weeks and 24-weeks after treatment with rosuvastatin monotherapy or rosuvastatin/ezetimibe combination therapy
The incidence of MACCE during the clinical trial period
Overall summary of AEs
Flow chart showing the number of patients who participated in and dropped out of the study.