Abstract
Posttraumatic stress disorder (PTSD) significantly impacts many veterans. Although PTSD has been linked to alterations in the fear brain network, the disorder likely involves alterations in both the fear and anxiety networks. Fear involves responses to imminent, predictable threat and is driven by the amygdala, whereas anxiety involves responses to potential, unpredictable threat and engages the bed nucleus of the stria terminalis (BNST). The BNST has been implicated in PTSD, but the role of the BNST in combat veterans with PTSD has yet to be examined. Identifying alterations in BNST responses to unpredictable threat could provide important new targets for treatment. The current study examined whether veterans with PTSD have altered BNST or amygdala responses (function and connectivity) to unpredictable and predictable threat. The fMRI task involved viewing predictable threat cues followed by threat images, predictable neutral cues followed by neutral images, and unpredictable threat cues followed by either a threat or neutral image. Participants included 32 combat-exposed veterans with PTSD and 13 combat-exposed controls without PTSD. Across all conditions, veterans with PTSD had heightened BNST activation and displayed stronger BNST and amygdala connectivity with multiple fear and anxiety regions (hypothalamus, hippocampus, insula, ventromedial prefrontal cortex) relative to controls. In contrast, combat controls showed a pattern of stronger connectivity during neutral conditions (e.g., BNST-vmPFC), which may suggest a neural signature of resilience to developing PTSD ηp2 = .087–.527, ps < .001. These findings have implications for understanding fear and anxiety networks that may contribute to the development and maintenance of PTSD.
Posttraumatic stress disorder (PTSD) is a common, disabling disorder that significantly impacts many combat veterans. In the soldiers returning from deployment in Afghanistan and Iraq, PTSD rates range from 15% to 23% (Fulton et al., 2015), at least twice the rate of lifetime PTSD in the general population (Kessler et al., 2005). PTSD is associated with numerous negative outcomes, including substantial disability; increased rates of mortality; and a heightened risk of depression, substance abuse, and suicide (Edmondson et al., 2013; Fanning & Pietrzak, 2013; Kessler et al., 1995). Although significant progress has been made on evidence-based treatments for PTSD, many patients continue to report substantial residual symptoms posttreatment (Bradley et al., 2005). One possibility for the lack of treatment success is that the neurobiology of PTSD is complex, with alterations that extend beyond the amygdala-based fear neurocircuitry to, for example, include bed nucleus of the stria terminalis (BNST)–based anxiety networks. First-line PTSD treatments, including selective serotonin reuptake inhibitors (SSRIs) and exposure-based therapies (Bisson et al., 2013), are effective in reducing symptoms and are thought to work by normalizing fear circuits, either by dampening fear reactivity or enhancing fear extinction learning (Felmingham et al., 2007; Hauner et al., 2012). However, many veterans with PTSD fail to remit completely, and the rate of relapse is high (Eftekhari et al., 2013). Therefore, expanding the focus of neuroscience research on PTSD to include additional networks may be beneficial for identifying novel treatment targets.
A growing body of work suggests that PTSD results from alterations in both the fear and anxiety networks. Although fear and anxiety are often treated as interchangeable constructs, decades of rodent studies provide evidence that fear and anxiety are separable and mediated by distinct neural networks. For example, findings from translational studies suggest that fear involves responses to a predictable or immediate threat, produces a fight-or-flight response, and is mediated by the amygdala, whereas anxiety occurs in response to unpredictable threat, is sustained over time, produces hypervigilance and hyperarousal, and is mediated by the BNST (see reviews by Avery et al., 2016; Lebow & Chen, 2016). Although there is also emerging evidence of overlapping roles for the amygdala and BNST during threat processing (Fox & Shackman, 2019; Hur et al., 2020). Foundational work exploring the BNST’s role in threat processing was conducted in rodents; however, recent advances in imaging methods have laid the groundwork for mapping the structural and functional connectivity of the BNST in humans (Avery et al., 2014; McMenamin et al., 2014; Tillman et al., 2018). Building on that foundation, studies have explored the role of the BNST in anxiety disorders. For example, individuals with anxiety disorders display heightened BNST response during unpredictable threat anticipation (Figel et al., 2019) as well as altered BNST connectivity (Torrisi et al., 2019). The degree of BNST activation and connectivity has been shown to be correlated with trait anxiety (Somerville et al., 2010) and anxiety symptoms (Andreescu et al., 2015; Clauss et al., 2019).
Despite emerging research implicating the BNST in threat processing, most PTSD research in humans to date has focused on fear neural circuits based on the notion that alterations in fear processing contribute to the development of PTSD (Pitman et al., 2012). Numerous studies have found that individuals with PTSD display increased amygdala activity to trauma-related (Liberzon et al., 1999; Shin et al., 1997) and aversive stimuli (Protopopescu et al., 2005; Rauch et al., 2000; Stevens et al., 2013). Individuals with PTSD also demonstrate increased amygdala connectivity with regions involved in fear processing (Brown et al., 2014; Rabinak et al., 2011), and the degree of connectivity has been shown to be correlated with PTSD symptom severity (Liu et al., 2021; Sun et al., 2020). Together, these findings highlight alterations in the fear network in individuals with PTSD.
Although a fear-centric view of PTSD has implicated the amygdala, converging lines of rodent and nonhuman primate research suggest the BNST also plays a role in PTSD. In rodents, the BNST has been shown to mediate symptoms similar to those characteristic of PTSD, including hypervigilance, anxiety, arousal, and stress-enhanced learning (Miles & Maren, 2019). In nonhuman primates, increased BNST activation during potential threat has been found to be related to more anxious behavior (Fox et al., 2008). The few studies in humans with PTSD have reported alterations in BNST activation during the anticipation of threat (Brinkmann, Buff, Neumeister, et al., 2017) or processing of trauma-related words (Awasthi et al., 2020), as well as altered BNST connectivity at rest (Rabellino et al., 2018). However, the role of the BNST in response to uncertain or unpredictable threat remains unclear and has yet to be examined in combat veterans. Evidence of alterations in BNST responses to unpredictable threat could provide important new targets for treatment.
The goal of the current study was to determine whether amygdala or BNST responses to unpredictable or predictable threat are altered in combat veterans with PTSD relative to those without PTSD. We hypothesized that veterans with PTSD would display heightened BNST activation in response to unpredictable threat relative to combat-exposed controls (CCs). In addition, we predicted that veterans with PTSD would display increased BNST connectivity within the brain regions associated with reactivity (i.e., amygdala, insula, hypothalamus) and decreased connectivity with regulatory brain regions (i.e., ventromedial prefrontal cortex; vmPFC) that would be specific to unpredictable threat. Aligning with previous studies of PTSD, we predicted that veterans with PTSD would demonstrate increased amygdala activation and altered connectivity during predictable threat relative to combat controls.
METHOD
Participants and procedure
Veterans between 18 and 50 years of age who were exposed to combat while deployed in support of recent military operations in Afghanistan and Iraq were recruited from a Veteran Affairs (VA) medical center in Nashville, Tennessee. Individuals were not eligible for the study if they had a diagnosis of or received treatment for a psychotic disorder or bipolar disorder, moderate-to-severe traumatic brain injury (TBI), current alcohol or substance abuse disorder, or major medical illness, or if they failed a magnetic resonance imaging (MRI) safety screen. In addition, all participants were screened for drug and alcohol use on the day of the scan. This research was conducted in accordance with the VA Human Research Protection Program. All participants provided written informed consent and received financial compensation $125 (USD).
Participants were categorized as combat-exposed veterans with PTSD (n = 36) or CCs without PTSD (n = 13). Individuals within the CC group met the following criteria: the absence of a current or past PTSD diagnosis, determined using the Clinician-Administered PTSD Scale for DSM-5 (CAPS-5; Weathers et al., 2014); the absence of subthreshold symptoms (i.e., a CAPS-5 score less than 15); and no use of psychoactive medications in the past 6 weeks, excluding gabapentin and SSRIs. Participants in the PTSD group were required to have a current PTSD diagnosis related to combat-related trauma, as determined using the CAPS-5. The Structured Clinical Interview of the DSM-IV-TR (SCID; First et al., 2002) was used to diagnose other psychiatric disorders.
Participants also completed self-report measures of depressive, anxiety, and PTSD symptoms; combat experience; and childhood trauma. Details of the self-report measures are presented in the Supplementary Materials. Participant characteristics are reported in Table 1.
TABLE 1.
Participant characteristics, by diagnostic status
| Variable | CC (n = 13) | PTSD (n = 32) | CC vs. PTSDa | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| % | M | SD | % | M | SD | p | |
|
| |||||||
| Male | 84.6 | 100.0 | .023 | ||||
| Race | .237 | ||||||
| White | 92.3 | 78.1 | |||||
| Black | 0.0 | 12.5 | |||||
| Asian | 7.7 | 0.0 | |||||
| American Indian | 0 | 3.1 | |||||
| Other | 0 | 6.3 | |||||
| Hispanic ethnicity | 7.7 | 15.6 | .478 | ||||
| Mild TBI | 15.4 | 15.6 | 1.00 | ||||
| Medication use | 7.7 | 53.1 | .047 | ||||
| Comorbid diagnoses | |||||||
| Depressive disorder | 7.7 | 40.6 | .03 | ||||
| Anxiety disorder | 7.7 | 37.5 | .05 | ||||
| Age (years) | 38.00 | 6.03 | 34.78 | 6.45 | .129 | ||
| Months of active duty | 94.23 | 79.36 | 105.78 | 61.71 | .603 | ||
| Total service months | 152.31 | 83.68 | 125.50 | 71.73 | .285 | ||
| Combat exposure score (CES) | 17.54 | 10.60 | 21.31 | 9.49 | .249 | ||
| Clinician-rated PTSD symptoms (CAPS-5) | 1.62 | 3.61 | 42.56 | 2.41 | < .001 | ||
| Self-reported PTSD symptoms (PCL-5) | 1.08 | 2.47 | 32.09 | 7.11 | < .001 | ||
| Anxiety symptom score (STAI) | 23.23 | 2.68 | 47.06 | 10.31 | < .001 | ||
| Depressive symptom score (BDI-II) | 1.62 | 2.79 | 20.84 | 9.39 | < .001 | ||
| Childhood trauma exposure score (CTQ) | 38.62 | 7.58 | 57.81 | 19.99 | .002 | ||
Note: CC = combat-exposed controls; PTSD = posttraumatic stress disorder; TBI = traumatic brain injury; CES = Combat Exposure Scale; CAPS-5 = Clinician-Administered PTSD Scale for DSM-5; PCL-5 = PTSD Checklist for DSM-5; STAI = State–Trait Anxiety Inventory; BDI-II = Beck Depression Inventory–II; CTQ = Childhood Trauma Questionnaire.
Significance for the comparison between the two groups. Continuous variables were compared using t tests (i.e., age, combat time and exposure, symptoms, trauma); categorical variables were compared using chi-square tests (i.e., sex, race, ethnicity, medication, TBI, comorbid diagnosis).
Measures
Self-report measures
PTSD symptoms.
The 20-item, self-report PTSD Checklist for DSM-5 (PCL-5; Weathers et al., 2013) was used to assess symptoms of PTSD. Participants were asked to rate how much they were bothered by each symptom in the past month, using a 5-point scale ranging from 0 (not at all) to 4 (extremely). Total scores range from 0 to 80, with higher scores indicating more severe symptoms. In the present sample, Cronbach’s alpha was 0.97.
Combat exposure.
The seven-item Combat Exposure Scale (CES) Keane et al., 1989) was used to assess war zone experience. Items are scored on a scale of 1 to 5, with higher scores indicative of a higher degree of combat exposure. In the present sample, Cronbach’s alpha was 0.83.
Depressive symptoms.
Symptoms of depression were assessed using the Beck Depression Inventory–II (BDI-II; Beck et al., 1996). Participants were asked to rate each of the 21 items on a scale of 0 to 3. Scores are summed, with higher scores indicating more severe depressive symptoms (range: 0–63). In the present sample, Cronbach’s alpha was 0.95.
Anxiety symptoms.
The 40-item State–Trait Anxiety Inventory (STAI; Spielberger et al., 1983) was used to assess anxiety. Participants were asked to rate each item on a scale of 1 to 4, with higher scores indicating more severe and higher trait anxiety. In the present sample, Cronbach’s alpha was 0.96.
Childhood trauma.
Trauma exposure during childhood was assessed using the Childhood Trauma Questionnaire (CTQ; Bernstein et al., 2003). Respondents were asked to score 28 items on a scale of 1 to 5, with higher scores indicating higher levels of trauma exposure. In the current sample, Cronbach’s alpha was 0.96.
Threat anticipation task
To study the effect of unpredictable threat, we used a cued anticipation threat task with unpredictable and predictable conditions. Participants were trained to associate three different cues with different events: (a) an unpredictable threat cue followed by either a combat-related or neutral image, (b) a predictable threat cue followed by a combat-related image, and (c) a predictable neutral cue followed by a neutral image (Supplementary Figure S1). Details of the task are provided in the Supplementary Materials.
MRI data acquisition, data processing, and statistical analysis
Structural and functional MRI (fMRI) data were acquired on a 3T scanner. Data were processed using standard methods in statistical parametric mapping (SPM). Individual participant generalized linear models were estimated using SPM12 software with cue (unpredictable, predictable threat, predictable neutral) and image (unpredictable threat, unpredictable neutral, predictable threat, predictable neutral) conditions.
To measure BNST and amygdala response during the anticipation (i.e., cue) and image viewing phases (i.e., images), MarsBar (Brett et al., 2002) was used to compute activation for each cue or image condition as the average percentage of signal change. Four individuals from the PTSD group were excluded: two for button-push accuracy, one for motion, and one for motion and accuracy. Eight participants had one run removed for motion (n = 7 PTSD, n = 1 CC).
Task connectivity was computed using beta-series correlations, a method of estimating functional connectivity by computing correlations of trial-by-trial variability (Rissman et al., 2004). This method provides correlations for each set of brain regions for each condition; functional connectivity between conditions is then compared as contrasts. Although this method is similar to psychophysiological interaction (PPI) methods, it may provide a more sensitive measure of connectivity for event-related designs (Cisler et al., 2014). Estimates of functional connectivity (i.e., correlations) were computed for both the anticipation and image viewing phases separately. The seed regions were the BNST and amygdala. The target regions of interest (ROIs) were selected based on regions with established structural connections to the BNST and amygdala and included the anterior hippocampus, anterior insula, hypothalamus, and vmPFC. Additional details about the methods and ROI masks, as well as descriptive statistics, are presented in the Supplementary Materials.
Data analysis
For this initial investigation of BNST and amygdala responses to unpredictable and predictable threat in PTSD, we used a planned comparisons approach. When there are specific a priori hypotheses, planned contrasts provide more Type II error while maintaining Type I error protection. The planned contrasts tested (a) unpredictable threat anticipation, defined as unpredictable cue–predictable neutral cue; (b) predictable threat anticipation, defined as predictable threat cue–predictable neutral cue; (c) unpredictable threat image viewing, defined as unpredictable threat image–unpredictable neutral image; and (d) predictable threat image viewing, defined as predictable threat image–predictable neutral image. Separate linear mixed models were used to assess group differences for each of the contrasts. The p values for the primary outcome (i.e., group effect for the contrast, calculated as the Group x Condition interaction) are provided and were followed by post hoc analyses. The p values for the main effects of group and condition are provided in the Supplementary Materials. Effect sizes were calculated for these analyses using partial eta-squared for the fixed effects in the model and are provided in the Supplementary Materials. Exploratory analyses were conducted to test correlations between the BNST and amygdala and self-report measures. Post hoc subgroup analyses were performed to explore the potential impact of comorbid depression diagnosis, childhood trauma, and medication on the significant findings; these results are reported in the Supplementary Materials along with additional details about statistical analyses.
RESULTS
Participant characteristics
The CC and PTSD groups did not significantly differ with regard to age, race, ethnicity, active duty time, total service time, combat exposure, or proportion of participants with mild TBI (Table 1). The PTSD group included significantly more male veterans and individuals with higher levels of self-reported PTSD symptoms, higher clinician-rated PTSD symptom severity, higher percentage with a comorbid anxiety diagnosis, higher percentage with a comorbid depression diagnosis, more severe anxiety symptoms, higher levels of depressive symptoms, more childhood trauma, and more medication use than the CC group.
Activation
Anticipation phase
For unpredictable threat contrast (i.e., unpredictable cue–predictable neutral cue), veterans with PTSD showed stronger BNST activation to both types of cues (i.e., main effect of group) relative to CCs (see Figure 1 and Supplementary Table S1). For predictable threat contrast (predictable threat cue–predictable neutral cue), veterans with PTSD displayed a pattern of stronger BNST activation to the predictable threat and predictable neutral cues relative to CCs; however, the main effect of group was not significant, p = .053 (Figure 1; Supplementary Table S1).
FIGURE 1. Bed nucleus of the stria terminalis (BNST) and amygdala activation during anticipation.
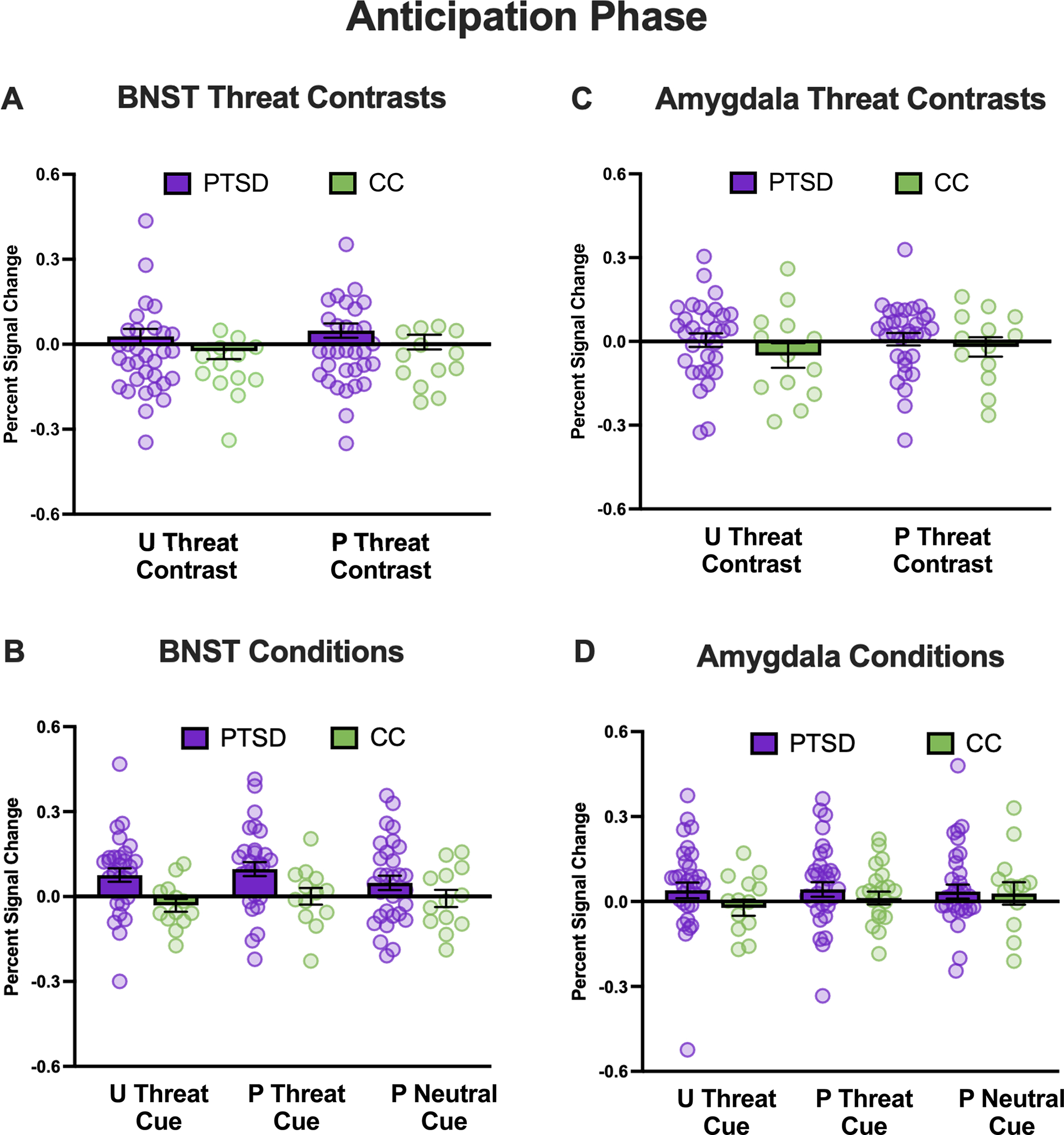
Note: Percentages of signal change for the BNST (left column) and amygdala (right column) during cues for the posttraumatic stress disorder (PTSD) group are shown in purple, and combat controls are shown in green. Panels A and C show the percentage of signal change during cues for the unpredictable threat (unpredictable–predictable neutral) and predictable threat (predictable threat–predictable neutral) contrasts. Panels B and D show the percentage of signal change for individual cues. U = unpredictable; P = predictable.
Image viewing phase
For unpredictable threat contrast (unpredictable threat image–unpredictable neutral image), participants in the PTSD group showed stronger BNST activation than CCs for both the unpredictable threat and unpredictable neutral images (i.e., main effect of group; see Figure 2 and Supplementary Table S1). Amygdala activation was significantly stronger for the unpredictable threat relative to the unpredictable neutral images for both groups (i.e., main effect of condition; see Figure 2 and Supplementary Table S1). For predictable threat contrast (predictable threat image–predictable neutral image), veterans with PTSD showed stronger BNST activation than CCs for both the predictable threat and predictable neutral images (i.e., main effect of group; see Figure 2 and Supplementary Table S1). Across both groups, there was also stronger BNST and amygdala activation to predictable threat relative to predictable neutral images (i.e., main effect of condition; see Figure 2 and Supplementary Table S1).
FIGURE 2. Bed nucleus of the stria terminalis (BNST) and amygdala activation during image viewing.
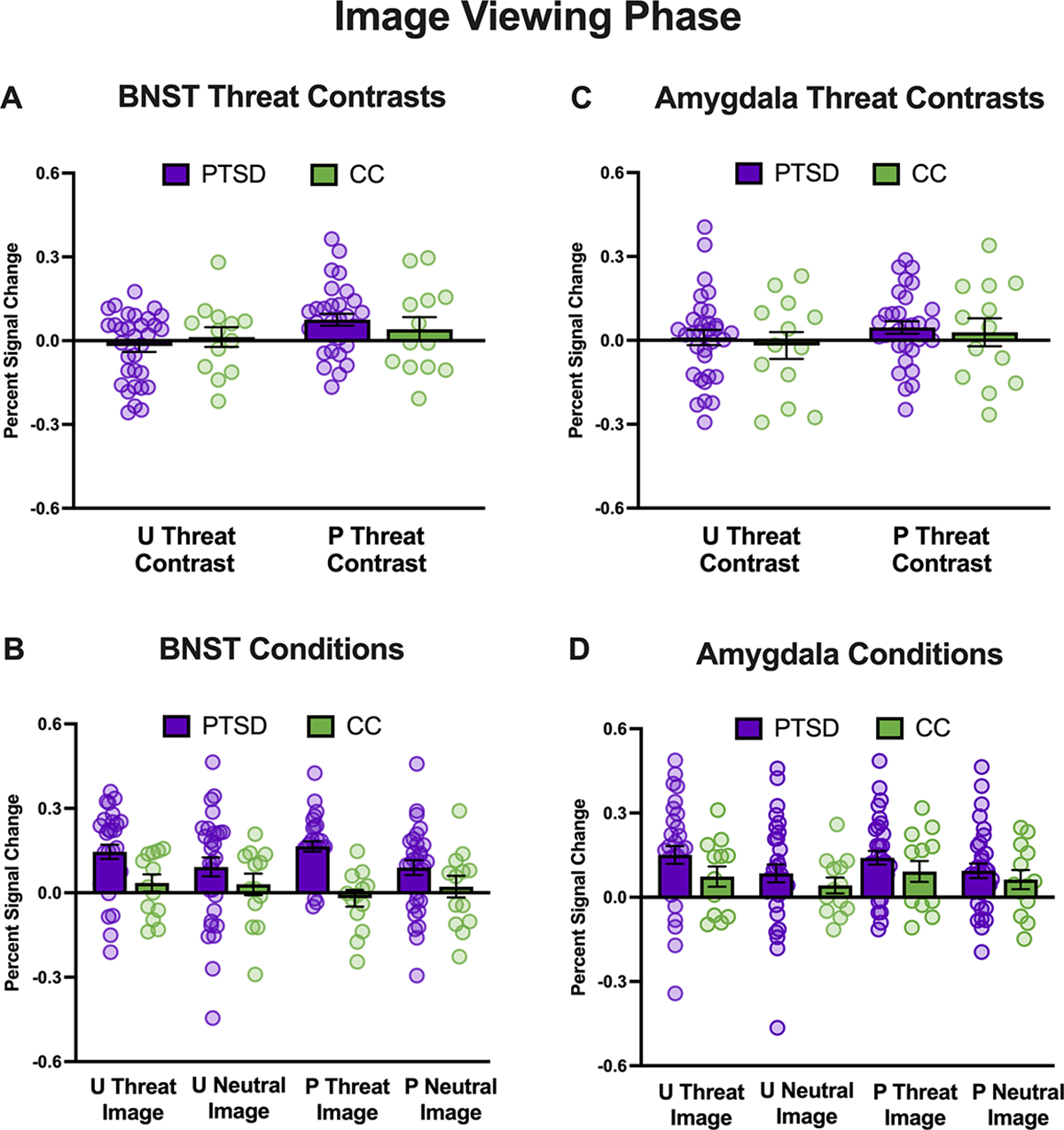
Note: Percentages of signal change for the BNST (left column) and amygdala (right column) during cues for the posttraumatic stress disorder (PTSD) group are shown in purple, and combat controls are shown in green. Panels A and C depict the percentage of signal change during images for the unpredictable threat (unpredictable threat–unpredictable neutral) and predictable threat (predictable threat–predictable neutral) contrasts. Panels B and D depict the percentage of signal change for the individual images. U = unpredictable; P = predictable.
Connectivity
Anticipation phase: Unpredictable cue–predictable neutral cue
BNST.
For unpredictable threat contrast (i.e., unpredictable cue–predictable neutral cue) the PTSD group demonstrated significantly stronger BNST–insula connectivity relative to CCs for the unpredictable threat contrast, p = .018 (Figure 3, Panel A; Supplementary Tables S2 and S4); post hoc analyses examining group differences showed that BNST–insula connectivity was significantly stronger for the unpredictable cue relative to the neutral cue for veterans in the PTSD group, p = .018, but was not significantly different for CCs. There were significant group differences in BNST–vmPFC connectivity, p = .012 (Figure 3, Panel A; Supplementary Tables S2 and S4); post hoc analyses showed that BNST–vmPFC connectivity was significantly weaker for unpredictable cue relative to predictable neutral cue for CCs, p = .041, and did not differ for the PTSD group. Finally, BNST–hypothalamus connectivity was stronger for the unpredictable cue relative to the predictable neutral cue for both groups (i.e., main effect of condition; Supplementary Tables S2 and S4).
FIGURE 3. Bed nucleus of the stria terminalis (BNST) and amygdala connectivity during anticipation.
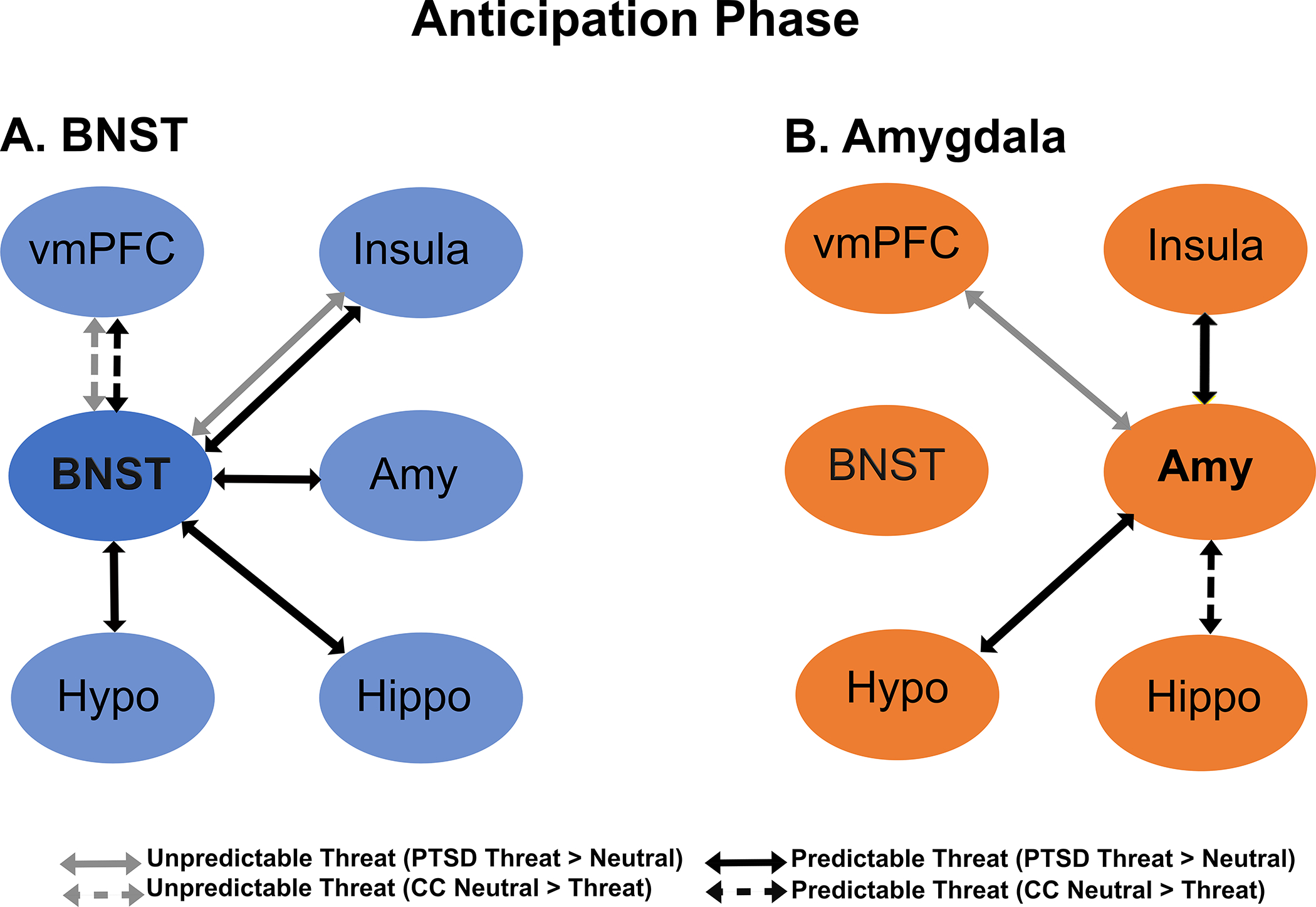
Note: The figure depicts significant Group x Condition interactions for connectivity during cues during unpredictable threat (gray arrows) and predictable threat (black arrows). Solid lines indicate increased connectivity to threat versus neutral conditions in the posttraumatic stress disorder (PTSD) group, whereas dashed lines indicate increased connectivity to neutral versus threat conditions in combat controls (CCs). Panel A shows BNST connectivity, and Panel B shows amygdala connectivity. vmPFC = ventromedial prefrontal cortex; amy = amygdala; hippo = hippocampus; Hypo: hypothalamus.
Amygdala.
Relative to the combat controls, individuals in the PTSD group had stronger amygdala–vmPFC connectivity to the unpredictable versus predictable neutral cue, p = .013 (Figure 3, Panel B; Supplementary Table S2). Post hoc analyses showed this was driven by differences in the PTSD group, p <.001, with no differences among CCs. The PTSD group also demonstrated weaker amygdala–hippocampus connectivity across both cue types relative to CCs (i.e., main effect of group, Supplementary Table S2). Across both groups, amygdala connectivity was stronger for the unpredictable cue relative to the predictable neutral cue for the BNST, hypothalamus, insula, and vmPFC (i.e., main effect of condition; Supplementary Tables S2 and S4). Amygdala–hippocampus connectivity showed a similar trend but did not reach the criterion for significance.
Anticipation Phase: Predictable threat contrast (predictable threat cue–predictable neutral cue)
BNST.
Relative to combat CCs, veterans with PTSD had significantly stronger BNST connectivity with the amygdala, p = .022; hippocampus, p < .001; hypothalamus, p < .001; and insula, p = .003, for the predictable threat cue relative to the predictable neutral cue (Figure 3, Panel A; Supplementary Tables S2 and S4). Post hoc analyses showed that BNST connectivity with the hippocampus, hypothalamus, and insula demonstrated the same pattern, with significantly stronger connectivity during the predictable threat cue relative to the predictable neutral cue in the PTSD group and significantly weaker connectivity for the controls, p < .001–p = .049. The results of post hoc analyses for BNST–amygdala connectivity showed significantly stronger connectivity for the predictable threat cue versus the neutral cue for veterans with PTSD, p = .033, with no difference for CCs. Finally, there were significant group differences in BNST–vmPFC connectivity. Veterans in the CC group had stronger BNST–vmPFC connectivity for the predictable neutral cue relative to the predictable threat cue, p = .021, with no differences for those in the PTSD group.
Amygdala.
For the predictable threat contrast, there were significant group differences in amygdala connectivity with the hippocampus, p < .001; hypothalamus, p = .021; and insula, p = .034, but not the vmPFC, p = .054 (Figure 3, Panel B; Supplementary Tables S2 and S4). Post hoc analyses performed to examine interactions showed that for CCs, amygdala–hippocampus connectivity was significantly stronger in response to the predictable neutral cue versus the predictable threat cue, p = .003, with no differences for the PTSD group. For the amygdala and hypothalamus, veterans with PTSD demonstrated stronger connectivity to the predictable threat cue versus the predictable neutral cue, p = .021, with no differences for CCs. For amygdala–insula connectivity, post hoc tests were not significant for either group. Finally, for both groups, amygdala-hippocampus connectivity was weaker during predictable threat cues relative to neutral cues (i.e., main effect of condition; Supplementary Table S2).
Image viewing phase: Unpredictable threat contrast (unpredictable threat image–unpredictable neutral image)
BNST.
During image viewing, there was a significant group difference in BNST–insula connectivity for unpredictable threat images relative to unpredictable neutral images, p = .002 (Figure 4, Panel A; Supplementary Tables S3 and S5). Post hoc analysis showed that veterans with PTSD group had significantly stronger connectivity during unpredictable threat relative to unpredictable neutral images, p < .001, but there were no differences among CCs. Across both groups, there was stronger BNST–amygdala, BNST–hypothalamus, and BNST–vmPFC connectivity during unpredictable threat relative to unpredictable neutral images (i.e., main effect of condition; Supplementary Tables S3 and S5).
FIGURE 4. Bed nucleus of the stria terminalis (BNST) and amygdala connectivity during imaging viewing.
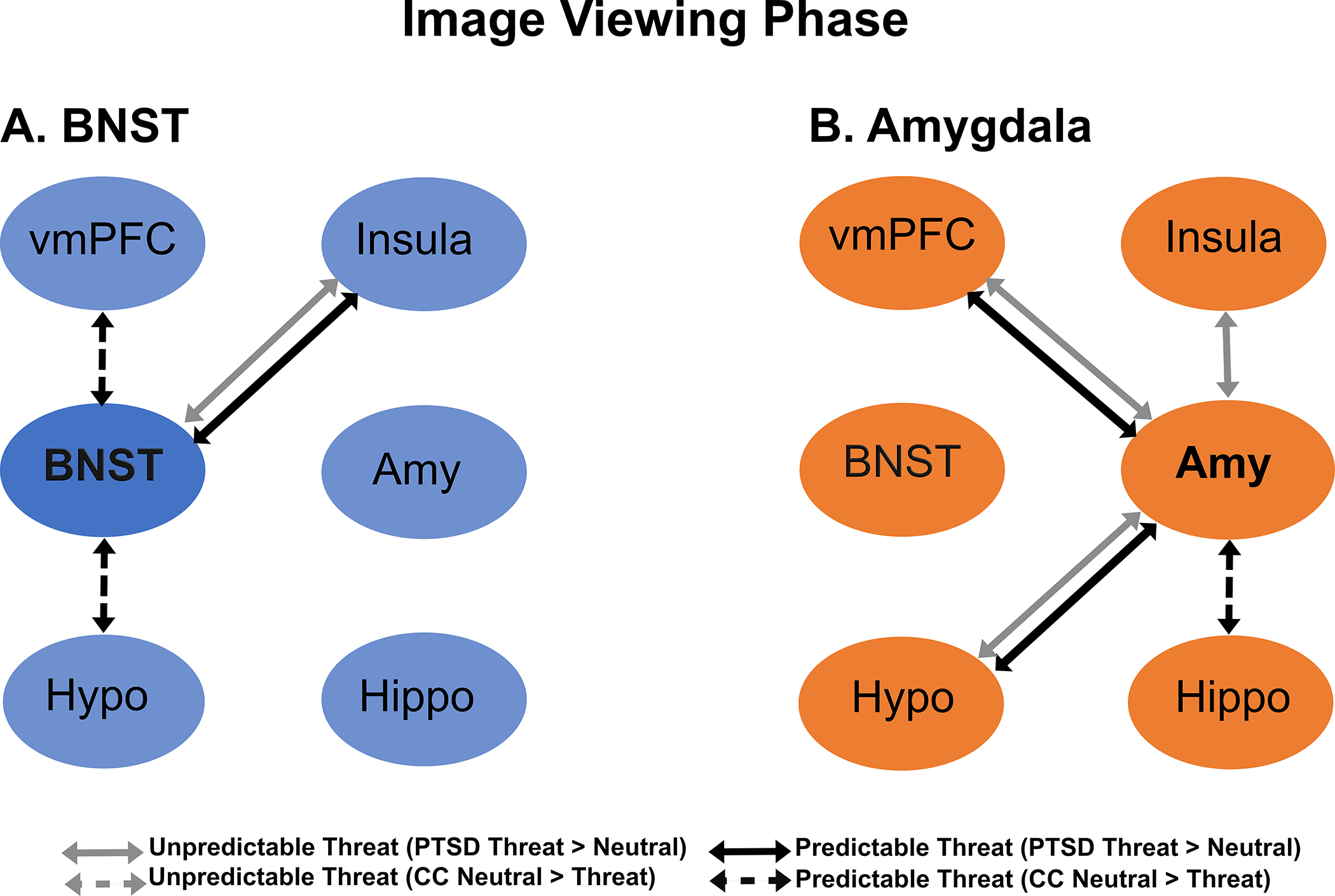
Note: Figure depicts significant Group x Condition interactions for connectivity during cues during unpredictable threat (gray arrows) and predictable threat (black arrows). Solid lines indicate increased connectivity to threat versus neutral conditions in the posttraumatic stress disorder (PTSD) group, whereas dashed lines indicate increased connectivity to neutral versus threat conditions in the combat controls (CC). Panel A shows BNST connectivity, and Panel B shows amygdala connectivity vmPFC = ventromedial prefrontal cortex; amy = amygdala; hippo = hippocampus; Hypo: hypothalamus.
Amygdala.
There were significant group differences in the unpredictable threat contrast for amygdala connectivity with the hypothalamus, p = .008; insula, p = .044; and vmPFC, p = .030 (Figure 4, Panel B and Supplement Tables S3 and S5). Post hoc analyses showed that participants in the PTSD group showed the same pattern of amygdala connectivity with the hypothalamus and vmPFC such that connectivity was significantly stronger for the unpredictable threat cue compared to unpredictable neutral images, hypothalamus: p = .001, vmPFC: p =.002; there were no differences for CCs. Post hoc analyses did not demonstrate differences in amygdala–insula connectivity. In addition, amygdala–BNST connectivity was stronger for unpredictable threat relative to unpredictable neutral images (i.e., main effect of condition).
Image viewing phase: Predictable threat contrast (predictable threat image–predictable neutral image)
BNST.
There were significant group differences for the predictable threat contrast for BNST connectivity with the hypothalamus, p <. 001; insula, p <. 001; and vmPFC, p = .002 (Figure 4, Panel A; Supplementary Tables S3 and S5). In post hoc analyses, veterans with PTSD demonstrated stronger BNST–insula connectivity to predictable threat relative to predictable neutral images, p = .004, whereas CCs showed stronger connectivity to predictable neutral images compared to predictable threat images, p < .001. CCs had significantly stronger connectivity during predictable neutral images relative to predictable threat images for both BNST–hypothalamus connectivity, p = .003, and BNST–vmPFC connectivity, p = .005, with no differences observed for the PTSD group. Finally, across both groups, BNST–hypothalamus and BNST–insula connectivity was stronger during predictable neutral images relative to predictable threat images (i.e., main effect of condition).
Amygdala.
Veterans with PTSD and CCs demonstrated significantly different connectivity for the predictable threat contrast for amygdala connectivity with the hippocampus, p = .001; hypothalamus, p < .001; and vmPFC, p = .033 (Figure 4, Panel B; Supplementary Table S3 and S5). Post hoc analyses showed that amygdala–hypothalamus connectivity was significantly stronger for veterans with PTSD while viewing predictable threat images versus predictable neutral images, p = .002; CCs showed stronger connectivity when viewing predictable neutral images, p < .001. For amygdala–hippocampus connectivity, CCs showed stronger connectivity when viewing predictable neutral images compared to predictable threat images, p = .003, with no differences for the PTSD group. For amygdala–vmPFC connectivity, neither group demonstrated significant differences in post hoc analyses. Overall, CCs displayed stronger amygdala–hippocampus connectivity across conditions (i.e., main effect of group).
Individual differences
Exploratory analyses were conducted to test correlations between brain responses and self-report measures for veterans with PTSD. Of these analyses, only childhood trauma and trait anxiety demonstrated significant correlations, with no significant differences for the other measures (see Supplementary Materials). Post hoc subgroup analyses were performed within the PTSD group to determine the potential impact of comorbid depression diagnosis, childhood trauma, and medication use on the study findings. Significant subgroup differences were observed in 15.4% of analyses for depression, 38.5% of analyses for childhood trauma, and 15.4% of analyses for medication use. In general, the results suggested stronger activation or connectivity in individuals with PTSD and co-occurring depression, lower childhood trauma scores, and no medication use; however, the findings were distributed across the main study findings and showed no specific pattern.
DISCUSSION
The current study examined whether combat veterans with PTSD display altered BNST and amygdala responses to threat relative to those without PTSD. The findings highlight novel alterations in BNST activation, BNST connectivity, and amygdala connectivity in veterans with PTSD. First, veterans with PTSD displayed significantly elevated BNST activation to most cues and images presented in this study. Second, veterans with PTSD showed stronger connectivity in both BNST and amygdala networks during threat anticipation and threat image viewing. Third, combat controls showed stronger BNST and amygdala connectivity with other brain regions when viewing neutral relative to threatening combat images. In addition, the study findings add to the substantial literature showing altered amygdala responses in PTSD, with new findings showing stronger amygdala connectivity during unpredictable and predictable threat. Taken together, these findings suggest that the neurobiological mechanisms of PTSD extend beyond the amygdala-mediated fear network and indicate potential benefits of augmenting current conceptual models to include the BNST-mediated anxiety network in combat veterans with PTSD.
Although we hypothesized that veterans with PTSD would show heightened BNST activation to unpredictable threat, the findings revealed that PTSD was associated with heightened BNST activation relative to all conditions. That is, the veterans with PTSD demonstrated heightened BNST activation in response to unpredictable threat, predictable threat, and neutral conditions across the anticipation and image viewing phases. Previous studies of PTSD in civilian samples have found heightened BNST responses to unpredictable threat specificially (Brinkmann, Buff, Neumeister, et al., 2017). However, unlike the previous studies that demonstrated a separation between unpredictable and predictable threat, our findings may indicate that veterans with PTSD have higher baseline BNST reactivity independent of context. Although the task used in the present study was modified to be relevant to veterans and included threatening war-related stimuli, BNST activation was not modulated by threat. One interpretation of these findings is that veterans with PTSD may be generally more reactive to all stimuli, akin to a hyperarousal across all conditions, and not specifically sensitive to threatening or aversive stimuli. This view is consistent with prior research showing that compared to individuals with anxiety disorders, those with PTSD evidence higher levels of self-reported arousal (Badour & Feldner, 2013). Another explanation is that this study was the first to compare BNST responses in veterans with PTSD to those in combat-exposed rather than healthy controls, which provides a different comparison. Previous studies examining the BNST in PTSD have used a healthy control group (Awasthi et al., 2020); thus, a strength of the current study was the ability to control for combat exposure to isolate the impact of PTSD on BNST activation.
The present study findings revealed prominent group differences in BNST and amygdala connectivity. Although the analyses did not directly test this, the connectivity data show that there were more significant differences for BNST connectivity during the anticipation phase compared to the image viewing phase. First, veterans with PTSD displayed stronger BNST–anterior insula connectivity to both types of threat across both the anticipation and viewing phases. There are known structural and functional connections between the anterior insula and the BNST (Flook et al., 2020), and heightened BNST–insula connectivity has been found during threat anticipation in healthy individuals (Carlson et al., 2011). Neuroimaging studies of PTSD have also shown increased insula activation to traumatic stimuli (Garfinkel & Liberzon, 2009), threat anticipation (Brinkmann, Buff, Feldker, et al., 2017), and alterations in insula connectivity at rest (Zhang et al., 2016). Second, connectivity with the hypothalamus was stronger for both the BNST and amygdala in veterans with PTSD during threat across both the anticipation and viewing phases. Both the BNST and amygdala influence the stress system through connections with the paraventricular nucleus of the hypothalamus, the starting point for the hypothalamic–pituitary–adrenal axis, which produces cortisol. In rodents, BNST lesions have been shown to reduce stress-responsive corticosterone release (Sullivan et al., 2004), and studies have shown increased cortisol responses in veterans with PTSD (Liberzon et al., 1999). Thus, stronger BNST and amygdala connectivity with the hypothalamus may reflect heightened stress signaling during predictable threat. Third, veterans with PTSD showed increased BNST–hippocampus connectivity during predictable threat anticipation. The hippocampus is a core region for integrating episodic memories, including memories with emotional significance (Phelps, 2004). There is substantial evidence implicating the hippocampus in PTSD: Individuals with PTSD have smaller hippocampal volumes, which research suggests may be a risk factor for PTSD (Gilbertson et al., 2002). Individuals with PTSD also demonstrate increased hippocampal activation during encoding and remembering negative stimuli (Brohawn et al., 2010) and display altered resting-state connectivity of hippocampal subregions (Malivoire et al., 2018). Fourth, veterans with PTSD displayed stronger amygdala–vmPFC connectivity during unpredictable and predictable threat across phases. The vmPFC plays a critical role in determining whether a stimulus is threatening, regulating threat responses, and fear conditioning and other emotional tasks (Milad et al., 2007).
The present study suggests that veterans with PTSD have stronger connectivity within multiple regions of the fear and anxiety networks relative to those without PTSD. These findings may indicate that compared with task activation, task-based connectivity was more sensitive in detecting differences in this study, consistent with the potential advantage of task-based connectivity proposed by other researchers (Feola et al., 2021; Finn, 2021; Greene et al., 2020). Relatedly, CCs without PTSD displayed a unique pattern of connectivity, with stronger connectivity during the neutral conditions relative to veterans with PTSD. Relative to veterans with PTSD, those who experienced combat exposure but did not develop PTSD demonstrated stronger BNST–vmPFC connectivity and amygdala–hippocampus connectivity when viewing neutral stimuli. These findings were intriguing and may reflect postcombat response or even a pretrauma resilient response in combat-exposed individuals who do not go on to develop PTSD. Therefore, the findings may represent protective or resilient responses that reflect altered connectivity in the fear and anxiety networks in neutral states among individuals with combat exposure who do not develop PTSD.
Although the focus of this initial study was on group differences, the exploratory brain–behavior correlations and subgroup analyses (see Supplementary Materials) highlight that PTSD is a heterogeneous, complex disorder. PTSD has numerous factors that are highly intertwined—including depression, trait anxiety, childhood trauma, and medication use—and our results suggest that these important factors may influence how the brain responds to threat. Therefore, it is important that future studies investigate how individual differences in these factors contribute to brain function and connectivity in PTSD.
Although the present study suggests alterations in the BNST and amygdala connectivity in response to threat among veterans with PTSD, several limitations should be noted. First, although this was the first investigation of the BNST in combat PTSD, the sample sizes were modest, and it is important to replicate these findings with a larger sample. Second, the study sample was predominantly male, and the results may not generalize to female veterans. It is common for samples of combat-exposed veterans to be predominantly male because fewer women than men in the military are exposed to combat. Nevertheless, understanding how male and female veterans with PTSD differ is a critical next step with important treatment implications. Third, we used a targeted ROI approach based on a priori brain regions that are structurally and functionally connected with the BNST. Although this approach is hypothesis-driven, using a whole-brain approach to examine BNST connectivity may identify additional brain regions with altered BNST connectivity. Future studies with larger samples and increased statistical power should aim to use whole-brain approaches. Future research along these lines will advance knowledge regarding the neurobiological mechanisms of fear and anxiety in PTSD that may inform the development of more targeted treatments.
Supplementary Material
OPEN PRACTICES STATEMENT.
The study reported in this article was not formally preregistered. Neither the data nor the materials have been made available on a permanent third-party archive; requests for the data or materials can be sent via email to the lead author at jblackford@unmc.edu.
Acknowledgments
This material is based upon work supported by the Department of Veterans Affairs (VA), Veterans Health Administration, Office of Research and Development and Clinical Sciences Research and Development (MERIT CX001226 to Jennifer Urbano Blackford); the National Institutes of Health (NIH; T32MH018921; Brandee Feola, Elizabeth A. Flook); and the Vanderbilt Institute for Clinical and Translational Research from the National Center for Research Resources/NIH (1-UL-1TR000445).
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the VA or the United States government.
REFERENCES
- Andreescu C, Mennin D, Tudorascu D, Sheu LK, Walker S, Banihashemi L, & Aizenstein H (2015). The many faces of anxiety-neurobiological correlates of anxiety phenotypes. Psychiatry Research–Neuroimaging, 234(1), 96–105. 10.1016/j.pscychresns.2015.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery SN, Clauss JA, Winder DG, Woodward N, Heckers S, & Blackford JU (2014). BNST neurocircuitry in humans. NeuroImage, 91, 311–323. 10.1016/j.neuroimage.2014.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery SN, Clauss JA, & Blackford JU (2016). The human BNST: Functional role in anxiety and addiction. Neuropsychopharmacology, 41(1), 126–141. 10.1038/npp.2015.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasthi S, Pan H, LeDoux JE, Cloitre M, Altemus M, McEwen B, Silbersweig D, & Stern E (2020). The bed nucleus of the stria terminalis and functionally linked neurocircuitry modulate emotion processing and HPA axis dysfunction in posttraumatic stress disorder. NeuroImage: Clinical, 28, 102442. 10.1016/j.nicl.2020.102442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badour CL, & Feldner MT (2013). Trauma-related reactivity and regulation of emotion: Associations with posttraumatic stress symptoms. Journal of Behavior Therapy and Experimental Psychiatry, 44(1), 69–76. 10.1016/j.jbtep.2012.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, & Brown GK (1996). Manual for Beck Depression Inventory–II (BDI-II). Psychology Corporation. [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Stokes J, Handelsman L, Medrano M, Desmond D, & Zule W (2003). Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse and Neglect, 27(2), 169–190. 10.1016/S0145-2134(02)00541-0 [DOI] [PubMed] [Google Scholar]
- Bisson JI, Roberts NP, Andrew M, Cooper R, & Lewis C (2013). Psychological therapies for chronic post-traumatic stress disorder (PTSD) in adults. Cochrane Database of Systematic Reviews, 12, CD003388. 10.1002/14651858.CD003388.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley R, Greene J, Russ E, Dutra L, & Westen D (2005). Reviews and overviews a multidimensional meta-analysis of psychotherapy for PTSD. American Journal of Psychiatry, 162(2), 214–227. 10.1176/appi.ajp.162.2.214 [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, & Poline J-B (2002). Region of interest analysis using an SPM toolbox. NeuroImage, 16(2), 497s. [Google Scholar]
- Brinkmann L, Buff C, Feldker K, Tupak S, Becker M, Herrmann M, & Straube T (2017). Distinct phasic and sustained brain responses and connectivity of amygdala and bed nucleus of the stria terminalis during threat anticipation in panic disorder. Psychological Medicine, 47(15), 2675–2688. 10.1017/S0033291717001192 [DOI] [PubMed] [Google Scholar]
- Brinkmann Leonie, Buff C, Neumeister P, Tupak SV, Becker MPI, Herrmann MJ, & Straube T (2017). Dissociation between amygdala and bed nucleus of the stria terminalis during threat anticipation in female post-traumatic stress disorder patients. Human Brain Mapping, 38(4), 2190–2205. 10.1002/hbm.23513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brohawn KH, Offringa R, Pfaff DL, Hughes KC, & Shin LM (2010). The neural correlates of emotional memory in posttraumatic stress disorder. Biological Psychiatry, 68(11), 1023–1030. 10.1016/j.biopsych.2010.07.018 [DOI] [PubMed] [Google Scholar]
- Brown VM, Labar KS, Haswell CC, Gold AL, Beall SK, Van Voorhees E, Marx CE, Calhoun PS, Fairbank JA, Green KT, Tupler LA, Weiner RD, Beckham JC, Brancu M, Hoerle JM, Pender M, Kudler H, Swinkels CM, Nieuwsma JA, … Morey RA (2014). Altered resting-state functional connectivity of basolateral and centromedial amygdala complexes in posttraumatic stress disorder. Neuropsychopharmacology, 39(2), 351–359. 10.1038/npp.2013.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson JM, Greenberg T, Rubin D, & Mujica-Parodi LR (2011). Feeling anxious: Anticipatory amygdala-insular response predicts the feeling of anxious anticipation. Social Cognitive and Affective Neuroscience, 6(1), 74–81. 10.1093/scan/nsq017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler JM, Bush K, & Steele JS (2014). A comparison of statistical methods for detecting context-modulated functional connectivity in fMRI. NeuroImage, 84, 1042–1052. 10.1016/j.neuroimage.2013.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss JA, Avery SN, Benningfield MM, & Blackford JU (2019). Social anxiety is associated with BNST response to unpredictability. Depression and Anxiety, 36(8), 666–675. 10.1002/da.22891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson D, Kronish IM, Shaffer JA, Falzon L, Burg MM, & York N (2013). Posttraumatic stress disorder and risk for coronary heart disease: A meta-analytic review. American Heart Journal, 166(5), 806–814. 10.1016/j.ahj.2013.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eftekhari A, Ruzek JI, Crowley JJ, Rosen CS, Greenbaum M. a, & Karlin BE (2013). Effectiveness of national implementation of prolonged exposure therapy in Veterans Affairs care. JAMA Psychiatry, 70(9), 949–955. 10.1001/jamapsychiatry.2013.36 [DOI] [PubMed] [Google Scholar]
- Fanning JR, & Pietrzak RH (2013). Suicidality among older male veterans in the United States: Results from the National Health and Resilience in Veterans Study. Journal of Psychiatric Research, 47(11), 1766–1775. 10.1016/j.jpsychires.2013.07.015 [DOI] [PubMed] [Google Scholar]
- Felmingham K, Kemp A, Williams L, Das P, Hughes G, Peduto A, & Bryant R (2007). Changes in anterior cingulate and amygdala after cognitive behavior therapy of posttraumatic stress disorder. Psychological Science, 18(2), 127–129. 10.1111/j.1467-9280.2007.01860.x [DOI] [PubMed] [Google Scholar]
- Feola B, McHugo M, Armstrong K, Noall MP, Flook EA, Woodward ND, Heckers S, & Blackford JU (2021). BNST and amygdala connectivity are altered during threat anticipation in schizophrenia. Behavioural Brain Research, 412, 113428. 10.1016/j.bbr.2021.113428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figel B, Brinkmann L, Buff C, Heitmann CY, Hofmann D, Bruchmann M, Becker MPI, Herrmann MJ, & Straube T (2019). Phasic amygdala and BNST activation during the anticipation of temporally unpredictable social observation in social anxiety disorder patients. NeuroImage: Clinical, 22, 101735. 10.1016/j.nicl.2019.101735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn ES (2021). Is it time to put rest to rest? Trends in Cognitive Sciences, 25(12), 1021–1032. 10.1016/j.tics.2021.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, & Williams JBW (2002). Structured Clinical Interview For DSM-IV-TR Axis I Disorders, Research version, patient edition (SCID-I/P). Biometrics Research, New York State Psychiatric Institute. [Google Scholar]
- Flook EA, Feola B, Avery SN, Winder DG, Woodward ND, Heckers S, & Blackford JU (2020). NeuroImage BNST-insula structural connectivity in humans. NeuroImage, 210, 116555. 10.1016/j.neuroimage.2020.116555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, & Shackman AJ (2019). The central extended amygdala in fear and anxiety: Closing the gap between mechanistic and neuroimaging research. Neuroscience Letters, 693, 58–67. 10.1016/j.neulet.2017.11.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, Shelton SE, Oakes TR, Davidson RJ, & Kalin NH (2008). Trait-like brain activity during adolescence predicts anxious temperament in primates. PLoS ONE, 3(7), e2570. 10.1371/journal.pone.0002570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton JJ, Calhoun PS, Wagner HR, Schry AR, Hair LP, Feeling N, Elbogen E, & Beckham JC (2015). The prevalence of posttraumatic stress disorder in Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) veterans: A meta-analysis. Journal of Anxiety Disorders, 31, 98–107. 10.1016/j.janxdis.2015.02.003 [DOI] [PubMed] [Google Scholar]
- Garfinkel SN, & Liberzon I (2009). Neurobiology of PTSD: A review of neuroimaging findings. Psychiatric Annals, 39(6), 370–381. 10.3928/00485713-20090527-01 [DOI] [Google Scholar]
- Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, & Pitman RK (2002). Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nature Neuroscience, 5(11), 1242–1247. 10.1038/nn958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode TD, & Maren S (2017). Role of the bed nucleus of the stria terminalis in aversive learning and memory. Learning and Memory, 24(9), 480–491. 10.1101/lm.044206.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene AS, Gao S, Noble S, Scheinost D, & Constable RT (2020). How tasks change whole-brain functional organization to reveal brain-phenotype relationships. Cell Reports, 32(8), 108066. 10.1016/j.celrep.2020.108066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauner KK, Mineka S, Voss JL, & Paller K. a. (2012). Exposure therapy triggers lasting reorganization of neural fear processing. Proceedings of the National Academy of Sciences of the United States of America, 109(23), 9203–9208. 10.1073/pnas.1205242109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur J, Smith JF, DeYoung KA, Anderson AS, Kuang J, Kim HC, Tillman RM, Kuhn M, Fox AS, & Shackman AJ (2020). Anxiety and the neurobiology of temporally uncertain threat anticipation. Journal of Neuroscience, 40(41), 7949–7964. 10.1523/JNEUROSCI.0704-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane T, Fairbank J, Caddell J, Zimering R, Taylor K, & Mora C (1989). Clinical evaluation of a measure to assess combat exposure. Psychological Assessment, 1(1), 53–55. [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, & Walters EE (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry, 62(6), 593–602. 10.1001/archpsyc.62.6.593 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Nelson CB, Bromet E, & Hughes M (1995). Posttraumatic stress disorder in the National Comorbidity Survey. Archives of General Psychiatry, 52(12), 1048–1060. 10.1001/archpsyc.1995.03950240066012 [DOI] [PubMed] [Google Scholar]
- Lebow MA, & Chen A (2016). Overshadowed by the amygdala: The bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Molecular Psychiatry, 21(4), 450–463. 10.1038/mp.2016.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I, Abelson JL, Flagel SB, Raz J, & Young EA (1999). Neuroendocrine and psychophysiologic responses in PTSD: A symptom provocation study. Neuropsychopharmacology, 21(1), 40–50. 10.1016/S0893-133X(98)00128-6 [DOI] [PubMed] [Google Scholar]
- Liu T, Ke J, Qi R, Zhang L, Zhang Z, Xu Q, Zhong Y, Lu G, & Chen F (2021). Altered functional connectivity of the amygdala and its subregions in typhoon-related post-traumatic stress disorder. Brain and Behavior, 11(1), e01952. 10.1002/brb3.1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malivoire BL, Girard TA, Patel R, & Monson CM (2018). Functional connectivity of hippocampal subregions in PTSD: Relations with symptoms. BMC Psychiatry, 18(1), 129. 10.1186/s12888-018-1716-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMenamin BW, Langeslag SJE, Sirbu M, Padmala S, & Pessoa XL (2014). Network organization unfolds over time during periods of anxious anticipation. Journal of Neuroscience, 34(34), 11261–11273. 10.1523/JNEUROSCI.1579-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, & Rauch SL (2007). Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biological Psychiatry, 62(5), 446–454. 10.1016/j.biopsych.2006.10.011 [DOI] [PubMed] [Google Scholar]
- Miles OW, & Maren S (2019). Role of the bed nucleus of the stria terminalis in PTSD: Insights from preclinical models. Frontiers in Behavioral Neuroscience, 13, 68. 10.3389/fnbeh.2019.00068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA (2004). Human emotion and memory: Interactions of the amygdala and hippocampal complex. Current Opinion in Neurobiology, 14(2), 198–202. 10.1016/j.conb.2004.03.015 [DOI] [PubMed] [Google Scholar]
- Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, Milad MR, & Liberzon I (2012). Biological studies of post-traumatic stress disorder. Nature Reviews Neuroscience, 13(11), 769–787. 10.1038/nrn3339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protopopescu X, Pan H, Tuescher O, Cloitre M, Goldstein M, Engelien W, Epstein J, Yang Y, Gorman J, LeDoux J, Silbersweig D, & Stern E (2005). Differential time courses and specificity of amygdala activity in posttraumatic stress disorder subjects and normal control subjects. Biological Psychiatry, 57(5), 464–473. 10.1016/j.biopsych.2004.12.026 [DOI] [PubMed] [Google Scholar]
- Rabellino D, Densmore M, Harricharan S, Jean T, McKinnon MC, & Lanius RA (2018). Resting-state functional connectivity of the bed nucleus of the stria terminalis in post-traumatic stress disorder and its dissociative subtype. Human Brain Mapping, 39(3), 1367–1379. 10.1002/hbm.23925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinak CA, Angstadt M, Welsh RC, Kenndy AE, Lyubkin M, Martis B, & Luan Phan K (2011). Altered amygdala resting-state functional connectivity in post-traumatic stress disorder. Frontiers in Psychiatry, 2, 62. 10.3389/fpsyt.2011.00062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, Mcinerney SC, Macklin ML, Lasko NB, Orr SP, & Pitman RK (2000). Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: A functional MRI study. Biological Psychiatry, 47(9), 769–776. 10.1016/s0006-3223(00)00828-3 [DOI] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, & D’Esposito M (2004). Measuring functional connectivity during distinct stages of a cognitive task. NeuroImage, 23(2), 752–763. 10.1016/j.neuroimage.2004.06.035 [DOI] [PubMed] [Google Scholar]
- Shin LM, Kosslyn SM, McNally RJ, Alpert NM, Thompson WL, Rauch SL, Macklin ML, & Pitman RK (1997). Visual imagery and perception in posttraumatic stress disorder: A positron emission tomographic investigation. Archives of General Psychiatry, 54(3), 233–241. 10.1001/archpsyc.1997.01830150057010 [DOI] [PubMed] [Google Scholar]
- Somerville LH, Whalen PJ, & Kelley WM (2010). Human bed nucleus of the stria terminalis indexes hypervigilant threat monitoring. Biological Psychiatry, 68(5), 416–424. 10.1016/j.biopsych.2010.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, & Jacobs GA (1983). Manual for the State–Trait Anxiety Inventory. Consulting Psychologists Press. [Google Scholar]
- Stevens JS, Jovanovic T, Fani N, Ely TD, Glover EM, Bradley B, & Ressler KJ (2013). Disrupted amygdala-prefrontal functional connectivity in civilian women with posttraumatic stress disorder. Journal of Psychiatric Research, 47(10), 1469–1478. 10.1016/j.jpsychires.2013.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan GM, Apergis J, Bush DEA, Johnson LR, Hou M, & Ledoux JE (2004). Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience, 128(1), 7–14. 10.1016/j.neuroscience.2004.06.015 [DOI] [PubMed] [Google Scholar]
- Sun D, Gold AL, Swanson CA, Haswell CC, Brown VM, Stjepanovic D, Beckham JC, Brancu M, Calhoun PS, Dedert E, Elbogen EB, Green KT, Kimbrel N, Kirby A, McCarthy G, Moore SD, Runnals JJ, Swinkels C, Tupler LA, … Morey RA (2020). Threat-induced anxiety during goal pursuit disrupts amygdala–prefrontal cortex connectivity in posttraumatic stress disorder. Translational Psychiatry, 10(1), 61. 10.1038/s41398-020-0739-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillman RM, Stockbridge MD, Nacewicz BM, Torrisi S, Fox AS, Smith JF, & Shackman AJ (2018). Intrinsic functional connectivity of the central extended amygdala. Human Brain Mapping, 39(3), 1291–1312. 10.1002/hbm.23917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrisi S, Alvarez GM, Gorka AX, Fuchs B, Geraci M, Grillon C, & Ernst M (2019). Resting-state connectivity of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in clinical anxiety. Journal of Psychiatry and Neuroscience, 44(5), 313–323. 10.1503/jpn.180150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers FW, Blake DD, Schnurr PP, Kaloupek DG, Marx BP, & Keane TM (2013). The Clinician-Administered PTSD Scale for DSM-5 (CAPS-5). https://www.ptsd.va.gov/professional/assessment/adult-int/caps.asp [DOI] [PMC free article] [PubMed]
- Weathers FW, Litz BT, Keane TM, Palmier PA, Marx BP, & Schnurr PP (2013). The PTSD Checklist for DSM-5 (PCL-5). https://www.ptsd.va.gov/professional/assessment/adult-sr/ptsd-checklist.asp
- Zhang Y, Xie B, Chen H, Li M, Guo X, & Chen H (2016). Disrupted resting-state insular subregions functional connectivity in post-traumatic stress disorder. Medicine (United States), 95(27), 1–5. 10.1097/MD.0000000000004083 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


