Abstract
Antigen-presenting cells (APCs) orchestrate immune responses and are therefore of interest for the targeted delivery of therapeutic vaccines. Dendritic cells (DCs) are professional APCs that excel in presentation of exogenous antigens toward CD4+ T helper cells, as well as cytotoxic CD8+ T cells. DCs are highly heterogeneous and can be divided into subpopulations that differ in abundance, function, and phenotype, such as differential expression of endocytic receptor molecules. It is firmly established that targeting antigens to DC receptors enhances the efficacy of therapeutic vaccines. While most studies emphasize the importance of targeting a specific DC subset, we argue that the differential intracellular routing downstream of the targeted receptors within the DC subset should also be considered. Here, we review the mouse and human receptors studied as target for therapeutic vaccines, focusing on antibody and ligand conjugates and how their targeting affects antigen presentation. We aim to delineate how targeting distinct receptors affects antigen presentation and vaccine efficacy, which will guide target selection for future therapeutic vaccine development.
Keywords: cancer vaccine, immunotherapy, cross-presentation, dendritic cells, endocytic receptor
1. Introduction to Vaccines
Vaccines are immunological tools to boost the immune system of the recipient. The aim of prophylactic vaccination is to generate immunological memory after exposure to an antigenic challenge. Vaccines drastically improved global healthcare playing a crucial role in the eradication of smallpox and rinderpest, and controlling many other pathogenic diseases.1,2 In principle, a vaccine contains two fundamental components: antigens to confer specificity and adjuvants to induce antigen-presenting cell (APC) maturation and immunity.
APCs are key regulators of adaptive immunity. APCs take up and process antigens into epitopes that are presented via major histocompatibility complexes (MHCs). They express pathogen recognition receptors (PRRs) to discriminate between harmless and hazardous antigens.3 Engagement of PRRs such as toll-like receptors (TLRs), by damage associated molecular patterns (DAMPs) or pathogen associated molecular patterns (PAMPs) leads to APC maturation. Mature APCs downregulate antigen uptake, while enhancing antigen preservation and presentation on MHC.4 Moreover, APC maturation causes an increase in the expression levels of costimulatory molecules such as CD40, CD80 (B7–1), or CD86 (B7–2) and an inflammatory cytokine profile characterized by IL-2, IL-12, and IFN-γ.5−7 In this state, APCs license B cells, CD4+ T cells, and CD8+ T cells to induce an immune response against the presented antigen. Antigen uptake without engagement of PRRs causes the APC to present the antigen in an immature state, which can induce anergy in reactive CD4+ or CD8+ T cells or activate peripherally induced regulatory T cells (iTregs).8 Among APCs, dendritic cells (DCs) are considered the most specialized in antigen processing and presentation.
Researchers are investigating therapeutic vaccination strategies with the goal to replicate the success of prophylactic vaccines.9−11 Therapeutic cancer vaccines aim to induce or reactivate adaptive immune responses toward an established tumor. They are highly promising in the field of cancer immunotherapy, because they can induce antigen-specific memory responses that could prevent cancer relapse by maintaining long-term immune surveillance against cancer cells. The initial response of cancer vaccines is directed against a restricted set of antigens present in the vaccine. This can be either tumor-associated antigens (TAAs) or tumor-specific antigens (TSAs). TAAs are commonly overexpressed or aberrantly expressed proteins on tumors, for instance, glycoprotein 100 (gp100) or tyrosine related protein-2 (TRP-2). As TAAs are often shared across patients and cancer types, their incorporation in vaccines could benefit a larger group of patients and ease the manufacturing process. However, their efficacy is threatened by pre-existing central and peripheral tolerance, which could limit their immunogenicity. In contrast, TSAs originating from, for example, point or frame shift mutations are highly immunogenic. Since TSAs are unique to each patient, a personalized approach is necessary for their identification and production of the vaccine. This presents a manufacturing challenge. Second, tumors develop an immunosuppressive microenvironment, which could induce peripheral tolerance toward TSAs in the absence of strong immunostimulatory agents to drive the immune response. After the initial tumor cell killing by effector immune cells, other tumor antigens are released to induce epitope spreading. This can expand the range of tumor antigens recognized by the immune system and improves responses against the tumor.12
Two main strategies exist in therapeutic vaccination focused on antigen presentation by DCs: 1) Ex vivo DC therapy consists of activating and loading patient-derived DCs ex vivo and the subsequent transfusion of the matured DCs into the patient. 2) In vivo cancer vaccination depends on targeted delivery of antigens and immunostimulatory adjuvants to DCs in vivo. Subsequently, the trained DCs will activate antigen-specific responses via CD8+ cytotoxic and CD4+ helper T cells to elicit antitumoral immunity.13 The following paragraphs introduce these vaccination strategies and their associated advantages.
Ex vivo DC therapy relies on differentiating and maturing patient-derived DCs or DC progenitors ex vivo while pulsing them with tumor lysate or antigens. Tumor-primed DCs are transfused back in the patient and can migrate to the lymph nodes where they prime tumor-specific T cells.14 Most studies have relied on monocyte-derived DCs (moDCs), which are easily generated in large numbers. After over two decades of evaluation, it is evident that patients almost always respond to the cell therapy by generating vaccine-specific T cells but only show modest clinical benefits.15−17 A recent murine study demonstrated that host DCs are essential for T cell priming after vaccination with ex vivo loaded moDCs. It is theorized that most transfused moDCs cannot migrate to lymph nodes and die upon injection. Upon cell death, they release their tumor antigens to host DCs instead of priming T cells themselves. This suggests that moDCs are not the optimal choice for ex vivo DC therapies.18,19 Clinical studies have recently shown that the use of naturally present DCs outperforms moDCs.20 Additionally, it was demonstrated that ex vivo loaded type 1 conventional DCs (DC1s), but not type 2 conventional DCs (cDC2s) or moDCs, can drive tumor rejection in mice independently of host DCs.21 As such, DC1-based ex vivo DC therapy has an increased potential to function as cancer therapy.22,23 While DC1s are scarce in peripheral blood (<0.05% of PBMCs), technical advances such as cell reprogramming or differentiation from stem cells to generate larger numbers of functional DC1s will allow clinical studies utilizing autologous DC1s.24−26
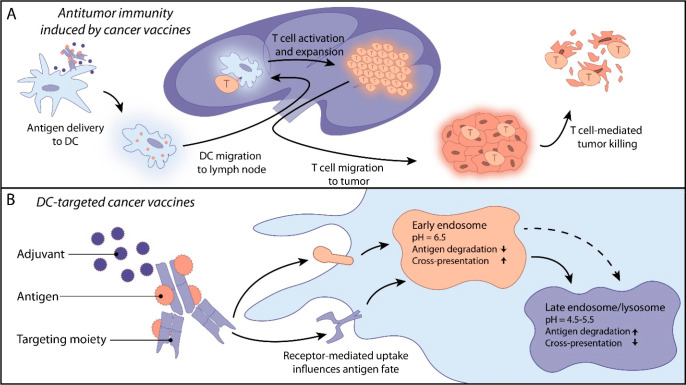
In vivo cancer vaccines deliver antigenic epitopes to APCs to elicit antitumor immunity (Figure 1a). This can be promoted by targeting APC surface molecules. TAAs or TSAs can be delivered as full proteins, peptides, or antigen-encoding nucleotides.13 Immunostimulatory adjuvants can be administered systemically or incorporated into the vaccine.27−29 Moreover, the antigen itself can be intrinsically immunostimulatory, such as antigen-encoding DNA or mRNA, that can bind to TLRs 3, 7, 8 or 9.30 The manufacturing of in vivo vaccines is relatively affordable and scalable and does not require the isolation and culturing of DCs from the patient. This could result in readily available off-the-shelf therapies and is a major advantage in comparison to ex vivo DC therapy.31 Several in vivo cancer vaccines are currently in clinical trials. NEO-PV-1 is a neoantigen peptide-based vaccine adjuvanted with poly-ICLC, which was demonstrated to be safe in a phase I study.32 Combination of NEO-PV-1 with nivolumab, an anti-PD-L1 immune checkpoint inhibitor, increased T cell reactivity and epitope spreading in a phase Ib study.33 Lipo-MERIT (BNT111, FixVac) and mRNA-4157 are mRNA-formulated tumor antigens encapsulated in liposomes. These mRNA/liposomes assemblies have demonstrated an acceptable safety profile, with promising immunological and clinical responses in phase I/II trials.34−36 Human papillomavirus-16 (HPV-16)-derived synthetic long peptides conjugated to Amplivant, a synthetic TLR2 agonist, were administered intradermally in a phase I study. This vaccine was capable of inducing T cell responses as measured by IFN-γ ELISpot assays.29 In this trial, most patients developed CD4+ T cell responses, while CD8+ T cell responses were observed less frequently. These recent trials showed promising results, yet none of these vaccines actively target APCs. This while the nonspecific uptake of these vaccines by other cells could decrease their effectiveness and could potentially cause harmful off-target responses.
Figure 1.
A) Schematic representation of mechanism of action from targeted antigen delivery to T cell mediated tumor killing. B) Schematic representation of DC-targeted cancer vaccines and the influence of receptor targeting on antigen fate. This review covers antibody-, antibody fragments, and nanobody and ligand conjugates. For image clarity only an antibody is depicted as a targeting moiety.
DCs are ideal candidates for targeted vaccine delivery. Pioneering studies by Steinman and colleagues showed that selective delivery of antigens to DCs improves T cell priming.37,38 These results have been confirmed by a myriad of follow-up studies, which reinforces that targeting antigens toward DCs improves antigen presentation, adaptive immune responses and tumor rejection.38,39 DCs can engage CD4+ T helper cells as well as cytotoxic CD8+ T cells, both of which are required to induce long lasting antitumor immune responses.40,41
DCs display a large array of specialized surface receptors functioning as pathogen sensors. Downstream signaling of these receptors culminates in an immune response mechanistically biased to the elimination of the given pathogen. Sensing of viruses results in type I interferon release, which promotes cross-presentation and induces a CD8+ T cell-bias.42 Detection of bacteria or helminths induces a Th1- or Th2-bias, respectively. It may be possible to exploit these biases by selecting specific DC surface receptors to control the immune responses generated by vaccination. In line with this, preclinical studies have reported different immunological and clinical outcomes when comparing DC surface markers as vaccine target. This holds true for receptors expressed by distinct DC subpopulations and also for receptors expressed by the same DC subpopulation.43−46
Selecting specific cell surface targets can enhance the effectiveness of therapeutic vaccines by determining the subpopulation of DCs targeted, affecting the endosomal routing and antigen fate, and ultimately steering the immune response (Figure 1b).47,48 Decoupling the influence of the DC subset from that of the cell surface receptor is difficult, and few comparative studies have set out to investigate this issue. In this Review, we aim to delineate the impact of target receptor choice on the efficacy of DC-targeted therapeutic vaccines, in a perspective focused on endosomal routing inherent to the receptor. We set out to compare the effects of antibody and ligand conjugates on antigen presentation and immune response, particularly in the context of cancer vaccines. Ultimately, we aim to guide the target selection for the development of therapeutic cancer vaccines.
2. General Considerations on Immune Cells Involved in Therapeutic Vaccines and Antigen Processing Pathways
2.1. Types of Immune Cells Induced by Therapeutic Vaccines
2.1.1. Dendritic Cells
DCs can be divided into several subpopulations: plasmacytoid DCs (pDCs) are involved in antiviral immunity via secretion of type I and type III interferons;49 DC1s are known to preferentially induce CD8+ T cell mediated immune responses but also can induce CD4+ T cells, such as T helper type 1 (Th1);50,51 DC2s represent 90% of the conventional DCs (cDCs) and excel in the induction of CD4+ T cells;52,53 DC3s, macrophages, and Langerhans cells can also scavenge antigens.54 DC3s have only recently been described as separate subset and are mainly considered to be immunosuppressive.55 In such, they might hamper the efficacy of vaccines that target DC subsets with an overlapping receptor expression.56 As discussed above, moDCs are DC-like cells often used as in vitro models in DC biology, but they are also present in circulation during the course of inflammation. The unique expression profile of receptors on DC subpopulations enables targeting of specific subtypes with specialized properties and determines the encountered endocytic pathway (Table 1). This offers an opportunity to steer the immune system toward the desired response.
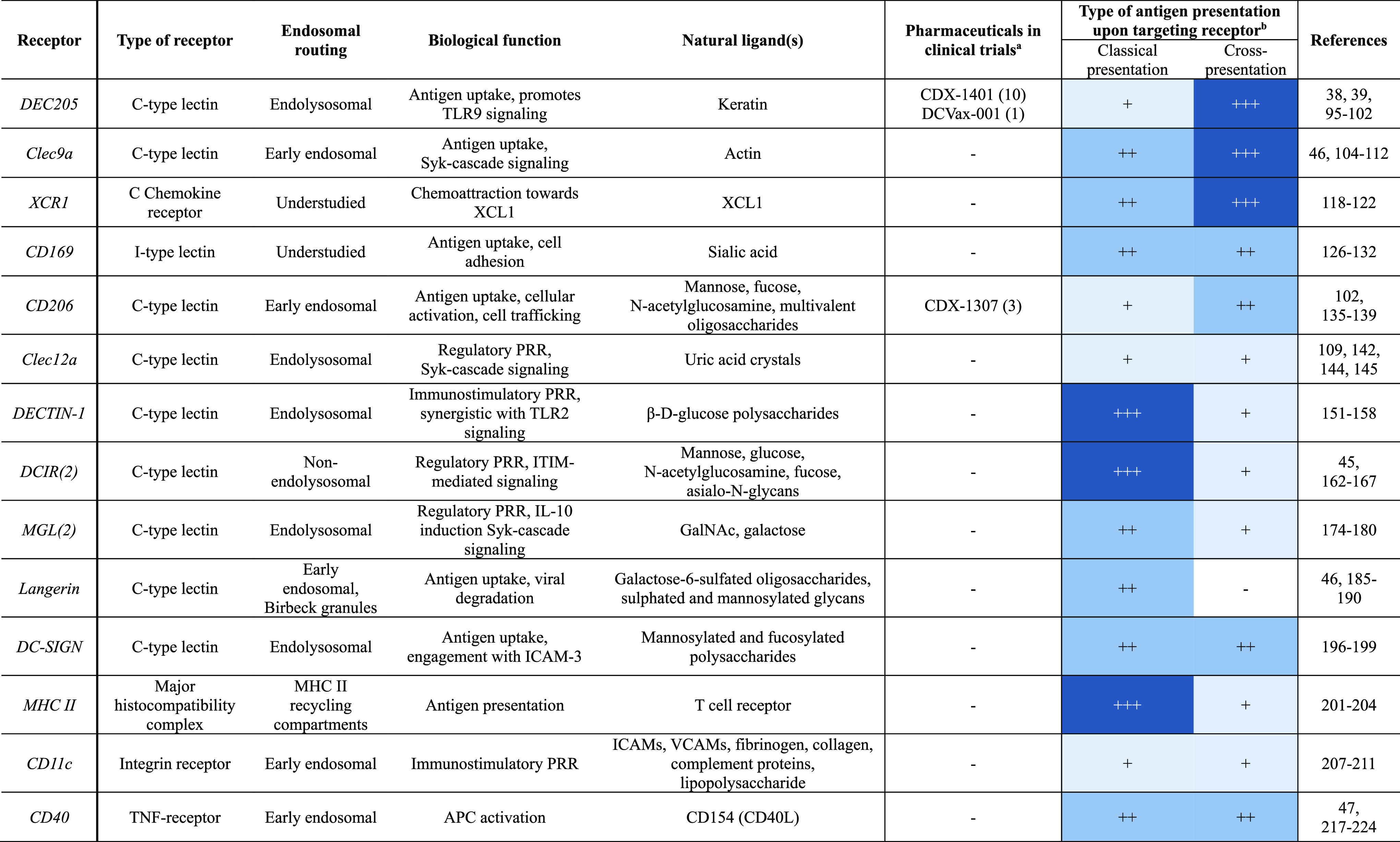
Table 1. Receptor Expression on Human and Mouse APCs of the Main Vaccine Targets Discussed in This Reviewa.
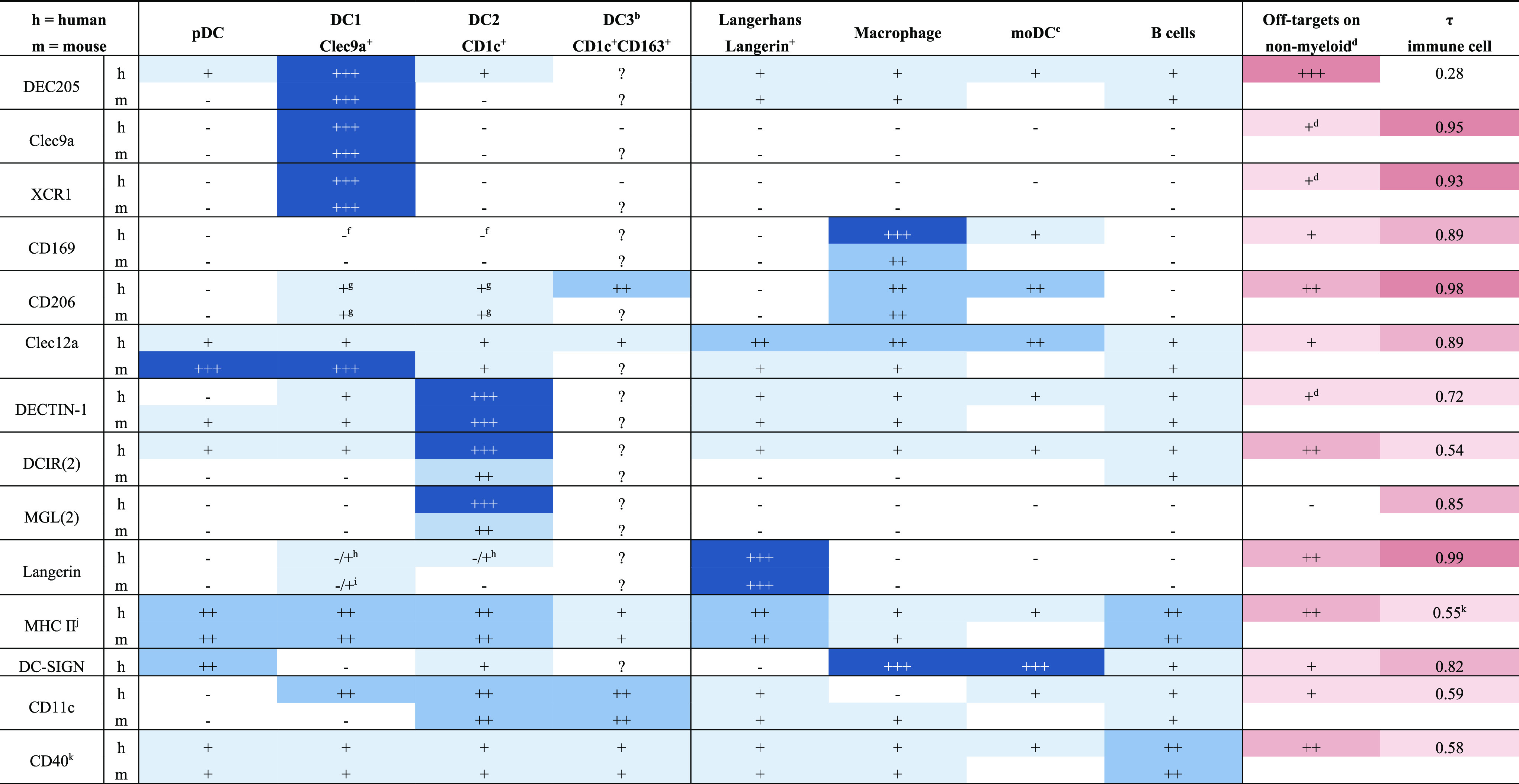
The relative expression is indicated by −, +, ++, and +++.
Receptor expression on DC3s is not yet studied for cancer vaccine targets but expected to be comparable to DC2s.
Murine equivalent of moDCs are poorly defined; thus, no receptor expression could be determined.
Receptor expression is determined by the protein atlas.57 Tissue specificity index (τ) of receptor expression in human immune cells as determined by the protein atlas. τ ranges between 0 and 1, where 0 indicates broad expression of the receptor on different immune cells and 1 indicates highly specific immune cell type expression.58 No such database exists for receptor expression in mouse.
Main off-target on nonmyeloid cells are Schwann cells.
CD169 is expressed on the pre-DC progenitor of cDCs.
CD206 is downregulated upon APC maturation.
Conflicting results have been published.
Langerin expression is mouse strain-dependent.
MHC II is upregulated upon DC maturation.
Multiple genes exist for the different MHC types in human: CIITA is used as representative gene for MHC II expression, because expression of all MHC types is under control by CIITA.
Expression of CD40 is upregulated on mature APCs.
2.1.2. CD8+ T Cells
MHC I restricted T cell responses have received the most attention in cancer vaccine design. CD8+ T cells can lyse tumor cells directly by releasing cytotoxic granules or by inducing apoptosis via the Fas/FasL pathway.59 Incorporation of CD8+ T cell-specific epitopes in cancer vaccines is feasible. Epitopes are well described for the most common TAAs in multiple MHC subclasses, and algorithms have been developed to predict putative MHC I epitopes originating from TSAs. Either a too strong or too weak antigenic stimulation during the initial priming can drive CD8+ T cell dysfunction and lack of tumor control.60 The potential impact of the strength of TCR stimuli on CD8+ T cell responses is currently not understood fully.
2.1.3. CD4+ T Cells
CD4+ T helper cells provide support to other immune cells during priming and activation by producing large amounts of immunostimulatory cytokines. In the context of antitumor responses, CD4+ T cells license DCs and improve priming of CD8+ T cells,40 whereas CD8+ T cell priming in absence of CD4+ T cells leads to T cell exhaustion.61 Moreover, CD4+ T cells support B cell development and affinity maturation. CD4+ T cells have repeatedly been shown to be crucial for antitumor responses.62,40 Results of a trial using peptides selected to encompass MHC I TSAs adjuvanted with poly-ICLC noted that most patients mounted TSA-specific CD4+ T cells responses instead of the expected CD8+ T cell responses.63,64 We suggest that the predisposition of CD4+ T cells to respond to tumors should be harnessed by including MHC II epitopes in vaccination strategies.40 However, MHC II epitopes are often insufficiently characterized for TAAs. Algorithms to identify TSA-derived MHC II epitopes are not as accurate due to less stringent factors determining the binding of MHC II epitopes in comparison with MHC I epitopes. To bypass the need to identify MHC II epitopes, Sahin and colleagues have postulated that point mutations are intrinsically immunogenic and aimed to generate a diverse pool of epitope variants by placing mutations strategically within epitopes.65,66 Yet, the elimination of MHC II-deficient tumors is still dependent on CD4+ T cells.62 These findings indicate that CD4+ T cells may not necessarily need to recognize tumor-specific antigens.67 Instead, pre-existing memory CD4+ T cells directed against universal epitopes, such as those found in diphtheria and tetanus, might provide the necessary support to the immune system. Therefore, the inclusion of universal epitopes in cancer vaccines could enhance antitumoral effectiveness while simplifying the manufacturing process.
2.1.4. B Cells
It remains elusive whether humoral immunity to cancer antigens is important for antitumor responses. In principle, antibodies can recognize antigens aberrantly expressed by tumor cells and mediate antibody-dependent cytotoxicity by natural killer (NK) cells or phagocytosis by macrophages. Tumor-reactive antibodies also promote antigen uptake by DCs via immune complexes and broaden the tumor-reactive T cell repertoire.68,69 Nevertheless, humoral immunity is often overlooked in cancer vaccination strategies as well as the antigen-presenting capacity of B cells.
2.2. Antigen Cross-Presentation
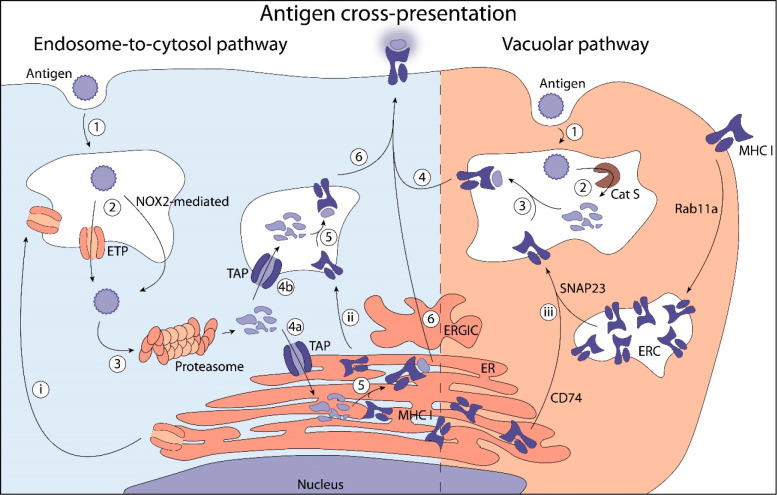
Antigenic epitopes are present to T cells on DC surfaces via MHC molecules. In principle, exogenous antigens are presented via MHC II to CD4+ T cells, whereas endogenous antigens are presented via MHC I to CD8+ T cells.70 Yet, exogenous antigens can be presented on MHC I, a process referred to as cross-presentation (XP).71−73 XP is crucial to inducing a cytotoxic immune response against virally infected or malignant cells. In particular DC1s excel at XP and subsequent induction of cytotoxic immune responses.74 The current paradigm proposes two main pathways for XP (Figure 2).
Figure 2.
Overview of the XP pathways. Endosome-to-cytosol pathway: 1) Antigen uptake by endocytosis or phagocytosis. 2) Endosomal escape is mediated by active transport through ETPs as HRD1, Derlin-1, and Sec66b or through lipid peroxidation mediated by NOX2.75−78 3) Cytosolic cleavage of antigen by proteasome. 4) TAP-mediated uptake of antigen into the ER (a) or endosomes (b). 5) Loading of antigen onto MHC I. 6) Transport of MHC I to the cell surface for antigen presentation. (i) Transport of ETPs originating in the ER to endosomes is most likely mediated by Sec22b.79 (ii) MHC I originating in ER are transported to endosomes. Vacuolar pathway: 1) Antigen uptake by endocytosis or phagocytosis. 2) Proteolytic processing of antigen in early endosomes is presumably mediated by cathepsin S.83 3) Loading of antigen onto MHC I. 4) Transport of MHC class I to the cell surface for antigen presentation. (ii) MHC I is transported to endosomal recycling compartments (ERC) from cell surface mediated by Rab11a and subsequently SNAP23 to fuse with endosomes, or MHC I is transported to endosomes from the ER mediated by CD74.84,85
2.2.1. Endosome-to-Cytosol Cross-Presentation
The endosome-to-cytosol pathway describes the escape of antigens from endocytic compartments to the cytosol, their processing by the proteasome, and the transport to the endoplasmic reticulum (ER) or endosomes, where epitopes are loaded onto MHC I. Antigen escape can be passive, following membrane lipid peroxidation induced by NOX2;75 or active, via endosome transporter proteins (ETPs) such as Sec61, Derlin-1, and HRD1.76−78 Sec22b likely regulates antigen transport by ETPs.79 After they escape the endocytic compartment, antigens are processed by the ubiquitin-proteasome system (UPS), an elaborate catalytic machinery able to efficiently hydrolyze cytosolic and nuclear proteins. Antigenic peptides released by the proteasome are translocated to the ER or endosomes by the transporter associated with antigen processing (TAP). The endosome-to-cytosol pathway strictly requires TAP activity.80,81 In the ER or endosomes, antigenic epitopes are loaded onto MHC I and transported via the Golgi apparatus to the cell surface.
2.2.2. Vacuolar Cross-Presentation
The vacuolar pathway is TAP- and proteasome-independent.82 In this second main XP pathway, antigen processing and epitope loading take place directly inside early endosomes. Cathepsin S is presumably largely responsible for the antigen processing, as its inhibition leads to major deficiencies in the vacuolar pathway.83 MHC I is transported to endosomes in vesicles originating from the ER under regulation of CD74, or via endosomal recycling compartments (ERCs) under control of Rab11a and SNAP23.84,85 The recruitment of MHC I from the ERCs is TLR-dependent, and promoted by the simultaneous presence of TLR stimulating signals and antigens inside individual phagosomes.86−88 This is thought to be a mechanism to transiently enhance XP in response to an antigenic threat.
3. Promoting Antigen Presentation on MHC I to Induce Cytotoxic Immunity
In the next sections, we introduce receptors described to target therapeutic vaccines to DCs. For each receptor, we highlight what has been reported in murine and human studies and briefly summarize the influence of targeting this receptor on the downstream response.
3.1. DEC205
DEC205 (CD205, LY75, Clec13b) binds keratin at slightly acidic pH to recognize apoptotic or necrotic cell debris.89−91 DEC205 can mediate the internalization and trafficking to late endosomes/lysosomes of CpG oligonucleotides promoting TLR9 signaling.92 In the mouse, DEC205 is expressed mainly by CD8+ DCs (DC1s). In humans, besides its expression on DC1s, DEC205 is also expressed at low levels on DC2s and pDCs, and in high levels on epithelial cells (Table 1).93,94 In a pioneering work, Steinman and colleagues targeted antigens to DEC205 and established that delivering antigens specifically to DCs could enhance antigen-specific T cell responses and improve the therapeutic window.38,39
3.1.1. DEC205–Mouse
Targeting of mouse DEC205 is extensively described in literature. In Steinman’s initial studies, ovalbumin (OVA) protein was chemically conjugated to the anti-DEC205 monoclonal antibody (mAb). Conjugation of OVA to anti-DEC205 increased CD8+ T cell responses over 1000-fold, and CD4+ T cell responses are over 50-fold compared to soluble OVA.39 Co-administration of immunostimulatory anti-CD40 mAb was shown to be crucial for tumor rejection and prevented the induction of tolerance.38 These promising results encouraged further study of the DEC205 receptor: Many studies have reported enhanced XP of TAAs (gp100, HER2/neu, mesothelin) and strong CD8+ T cell responses upon targeting DEC205.95−97 Antigens targeted to DEC205 mainly accumulated in late endosomes, which should bias antigen processing toward proteolysis, MHC II presentation, and CD4+ T cell activation.98 MHC II antigen presentation was not increased as much as XP. We attribute the enhanced CD8+ T cell activation to the combination of DEC205-mediated intracellular trafficking with an increased antigen uptake by DC1s, which upregulate Tap1/2 and Sec61 thereby promoting the endosome-to-cytosol XP pathway.45,81
3.1.2. DEC205–Human
The broad expression profile of DEC205 in human cells complicates interspecies comparison.93 The first in-human targeted cancer vaccine consisted of an anti-DEC205 mAb fused to the cancer testis antigen NY-ESO-1 adjuvanted with coadministered poly-I:C (CDX-1401). Its clinical evaluation in phase I and II trials demonstrated a favorable safety profile and moderate CD4+ and CD8+ T cell responses without induction of Tregs.99,100 Pretreatment with systemic fms-like tyrosine kinase 3 ligand (Flt3l) 1 week before CDX-1401 administration increased the abundance of DCs prior to vaccination and improved T cell responses to CDX-1401.101 This preliminary study did not examine the specific contribution of different DC subsets to T cell activation. In a different study, PBMCs isolated from NY-ESO-1-expressing cancer patients were treated with anti-DEC205 or anti-CD206 mAbs fused to NY-ESO-1.102 While receptor targeting improved expansion of antigen specific CD4+ and CD8+ T cells in vitro, no significant differences in targeting DEC205 or CD206 were observed. Targeting influenza peptide to isolated human DC1s in vitro via DEC205 resulted in lower levels of XP in comparison with targeting to CD40 and CD11c, both known to direct antigens to early endosomes. Strikingly, early endosomal targeting resulted in comparable levels of XP in DC1s and DC2s. This emphasizes the importance of taking into account antigen routing to optimize processing in cancer vaccines.47 In total, ten clinical trials using CDX-1401 have been initiated (Table 2). These pioneering trials in DEC205-targeted antigen delivery could provide valuable insight into the translation of targeted vaccines. Yet, targeting human DEC205 might not be as effective at inducing antigen XP and subsequent CD8+ T cell activation due to routing to late endosomes. Moreover, the broad expression profile of DEC205 in myeloid and nonmyeloid human cells may result in undesirable off-targeting and could decrease the efficacy of DEC205 targeted vaccines in vivo.
Table 2. Comprehensive Overview of the Receptors Discussed in This Review38,39,45−47,95−102,104−112,118−122,126−132,135−139,142,144,145,151−158,162−167,174−180,185−190,196−199,201−204,207−211,217−224.
Clinical trial identifiers: CDX1401: NCT03358719, NCT01834248, NCT01522820, NCT02661100, NCT02129075, NCT03206047, NCT02413827, NCT02495636, NCT02166905, NCT00948961; DCVax-001: NCT01127464; CDX-1307: NCT00648102, NCT01094496, NCT00709462.
The relative propensity of antigen presentation is indicated by −, + , ++, or +++, based on the studies described in this review.
Cross-presentation by targeting CD169 occurs indirectly through antigen transfer to DCs.
3.2. Clec9a
Clec9a (DNGR-1, CD370) recognizes actin exposed to damaged cells. It binds filamentous actin in complex with cytoskeletal proteins and signals through the Syk-cascade.103 Clec9a is selectively expressed on DC1s in mouse and human (Table 1).104 Clec9a and DEC205 differ in their intracellular routing. In an in vitro study, fluorescently labeled anti-DEC205 mAbs but not anti-Clec9a mAbs colocalized with lysotracker. Instead, anti-Clec9a colocalized with necrotic cell material away from lysosomes.105 In addition, engagement of Clec9a signals for phagosomal rupture, resulting in antigen escape into the cytosol, which, in turn, enhances XP. These features make Clec9a a compelling target to promote XP.
3.2.1. Clec9a–Mouse
In B6 mice, delivery of CD8+ and CD4+ T cell epitopes to DC1s using anti-Clec9a mAb results in enhanced XP and MHC II presentation, respectively, in comparison with untargeted controls.104,106 In CB6.F1 mice, HIV gag-p24 peptide targeted to either Clec9a or DEC205 resulted in similar CD8+ T cell proliferation and comparable IFN-γ levels in an restimulation assay.46 Targeting antigens with CBP12, a 12-mer peptide specific to Clec9a, also demonstrated enhanced XP and increased specific T cell responses.107,108 CBP12-mediated delivery of the peptides of the peptides of the OVA257–264 and gp100 under adjuvant-free conditions demonstrated potent antitumor responses in anti-PD1-resistant B16-OVA and B16 melanoma, respectively. Mechanistically, the authors demonstrated that CBP12 binding triggered IL-21 signaling, which was crucial for vaccine activity. Targeting Clec9a with mAb-OVA fusion constructs increased XP in comparison with Clec12a targeting. Targeting Clec9a significantly enhanced humoral responses compared to DEC205 and Clec12a, even in adjuvant-free conditions.109 Altogether, this indicates that Clec9a is a promising target for the delivery of antigen to murine DC1s.
3.2.2. Clec9a–Human
CLEC9a has restricted expression on human DC1s, unlike DEC205. Viral antigen (pp65), NY-ESO-1 polypeptide, or Wilms’ tumor 1 antigen delivery to Clec9a or DEC205 was compared using human PBMCs.110−112 Naïve and memory CD8+ T cell activation was enhanced upon antigen delivery to Clec9a compared with DEC205. Yet, in humanized NSG-A2 mice, equally potent XP of pp65 was observed for both targets.110 Unlike humans, NSG mice express do not express DEC205 on nonlymphatic endothelial cells. The authors speculate that this leads to a higher persistence of anti-hDEC205 mAb in NSG mice than is expected in humans, effectively increasing the specificity of anti-hDEC205 to DC1s. Due to the highly specific expression profile of Clec9a on human DC1s, it is expected that a Clec9a-targeted vaccine will demonstrate an increased activity compared to DEC205 in a clinical setting.113
3.3. XCR1
X-C motif chemokine receptor 1 (XCR1, GPR5) mediates chemoattraction toward lymphotactin (XCL1), a chemokine secreted by activated T cells and NK cells.114,115 This interaction promotes antigen uptake in inflammation sites and interaction with CD8+ T cells.116 Similar to Clec9a, XCR1 expression is restricted to DC1s (Table 1).114,116,117 Because of the lack of publicly available mAbs against human XCR1, studies on XCR1 have been limited to XCL1-mediated targeting.
3.3.1. XCR1–Mouse
XCL1 fused to OVA or influenza antigens induces strong CD8+ T cell cytotoxicity in B6 mice.118,119 Fossum and colleagues performed a thorough comparative study of XCR1, Clec9a and DEC205 as potential DC1 vaccine targets.118 Immunization with plasmid DNA encoding XCR1 or Clec9a targeted hemagglutinin (HA) protected mice in a lethal viral challenge, whereas DEC205 targeted HA did not. Clec9a and DEC205 were targeted by nanobodies, whereas XCR1 was targeted via XCL1. The strongest immune responses were noted for XCR1, irrespective of the antigen or the mouse strain. This study is one of the few that compared the activities of vaccines targeting receptors specifically expressed by the same DC subpopulation. Future studies should confirm whether this also holds true for protein-based immunization and investigate the influence of the adjuvant. Taken together, these results emphasize that intrinsic factors of receptors, such as expression levels and endosomal routing, influence antigen presentation and subsequent T cell activation.
3.3.2. XCR1–Human
XCL1–antigen fusions induced stronger antigen XP in comparison with free antigen or vehicle control in human PBMCs.120−122 Human XCL1–antigen fusions conserved its targeting ability in vivo, as confirmed in transgenic mice expressing human XCR1, and induces potent CD8+ T cell activation.120 Moreover, XCL1-NY-ESO-1-peptide-PEG5k constructs retained their activity as DC1 chemoattractant, which could potentiate DC1 activation and XP by attracting DC1s toward the injection site.121 In summary, XCL1 has chemotactic properties and retains its DC1-specific targeting capability upon C-terminal modification. Further engineering of XCL1 constructs to increase its affinity, stability and agonistic activity could yield optimized targeting agents to explore in a therapeutic setting.119,123
3.4. CD169
The sialic acid binding immunoglobulin-type lectin 1 (SIGLEC-1, Sn, CD169) is involved in uptake and presentation of dead cell-associated antigens, including tumor antigens.124 CD169 is expressed on a subpopulation of macrophages located in the marginal zone of the spleen and the lymphatic sinuses of secondary lymph nodes. This strategic location allows CD169+ macrophages to filter and capture circulating antigens, pathogens or cellular debris.125 CD169+ macrophages act as an antigen reservoir for the splenic and sinusoidal resident lymphohematopoietic systems by retaining and releasing these antigens gradually. CD169 itself mediates antigen transfer to DCs, and especially DC1s, by binding to sialic acid containing glycans on the surface of DCs.126
3.4.1. CD169–Mouse
Targeted delivery using anti-CD169 or CD169-specific ligands conjugated to TAA peptides resulted in antitumor responses in B6 mice.127,128 Mice that lack DCs but not CD169+ macrophages were as capable as WT mice at priming CD8+ T cells upon CD169-mediated delivery of OVA peptide.129 In a more physiological setup, CD169+ macrophages transferred antigens to DC1s to promote cross-priming of CD8+ T cells in vivo.126 This plausible mechanism may be exploited to improve the XP in targeted therapeutic vaccines.
3.4.2. CD169–Human
Antigen delivery to CD169 resulted in a slower uptake compared to antigen delivery to DC-SIGN in moDCs.128 This correlated with murine data and hints toward a pathway favoring antigen retention for transfer to DC1s.130 Delivery of the tumor antigens gp100 and WT1 using anti-CD169 resulted in specific CD8+ T cell expansion possibly mediated through cross-talk between CD169+ macrophages and DC1s.131 Sialic acid covered liposomes were shown to induce antigen-specific CD8+ T cell activation and proliferation in human PBMCs.132 Targeting CD169+ macrophages could be an efficient way of indirectly targeting the relatively low number of DC1s by harnessing the higher prevalence of CD169+ macrophages and their capacity to retain antigens.
3.5. CD206
CD206 (MR, MCR1, MMR) is involved in numerous processes such as cellular activation, clearance of glycosylated molecules, promotion of antigen presentation, cell trafficking and collagen internalization.133 CD206 binds to mannose, fucose, and N-acetylglucosamine, but it is especially known for its high affinity for multivalent oligosaccharides.134 In DCs, soluble antigen uptake by CD206 leads to transport to early endosomes, which increases XP via the vacuolar pathway.135,136
3.5.1. CD206–Mouse
CD206 appears to be involved in the uptake of soluble antigens by DCs. Mannan, an inhibitor of CD206-mediated endocytosis, completely blocked uptake of soluble OVA in mouse bone marrow-derived DCs (BMDCs) but not of cell-associated OVA. In line with this, OVA-specific CD8+ OT-I cells activation upon vaccination with OVA was hampered in CD206–/– B6 mice.135 This finding prompted a study on trafficking and processing of OVA in BMDCs of WT or CD206–/– B6 mice.136 MHC II antigen presentation of the CD4-epitope was unaltered in CD206–/– BMDCs, whereas XP of the CD8-epitope was strongly reduced in CD206–/– BMDCs, as measured by OT-II and OT-I cell proliferation, respectively. Microscopy revealed that CD206-mediated uptake delivered antigens toward early endosomes and away from lysosomes, thus facilitating antigen preservation and XP. Mannosylation of antigenic peptides enhanced XP in B6 mice and resulted in stronger T cell proliferation compared to nonmannosylated peptides.137 This was not observed in CD206–/– B6 mice, indicating a functional dependency on CD206.
3.5.2. CD206–Human
Human chorionic gonadotropin β (hCG-β) is a TAA that is expressed in epithelial cancers. The targeted cancer vaccine CDX-1307, anti-CD206 mAb fused to hCG-β, demonstrated tolerable safety profiles in a phase I trial of bladder cancer patients. Patients developed T and B cell responses even if they presented hCG-β high serum levels pretreatment. This was especially pronounced upon coadministration of the TLR-agonists resiquimod and/or poly-ICLC.138,139 These trials demonstrate the feasibility of breaking tolerance against a self-antigen by targeting CD206. Unfortunately, the subsequent phase II trial (NCT01094496) was terminated due to portfolio prioritization. An in vitro comparative study of NY-ESO-1 delivery via anti-CD206 or anti-DEC205 was conducted using moDCs and patient-derived T cells.102 Targeting to either DEC-205 or CD206 increased XP of NY-ESO-1 compared to untargeted protein, but the MHC II presentation was comparable in all conditions. This suggests that targeted delivery of NY-ESO-1 to late (via DEC-205) or early (via CD206) endosomal compartments increased XP. These findings should be confirmed using isolated DC1s and DC2s in a similar setup. Of note, CD206 is highly expressed on tumor-associated macrophages (TAMs), which opens up the possibility to repolarize TAMs by targeting TLR7/8 agonists toward CD206.140 Simultaneous antigen delivery to TAMs is two-edged: If repolarization is successful, then it could remodel the TME and harness the antigen-presenting capacity of prevalent macrophages. However, if repolarization is unsuccessful, then it could strengthen peripheral tolerance.
3.6. Clec12a
Clec12a (MICL, CD371, DCAL-2) is an inhibitory PRR that recognizes uric acid crystals formed upon release of intracellular uric acid by dying cells.141 Clec12a inhibits the Syk-cascade and hampers ROS production to limit inflammatory responses. Clec12a is highly expressed on murine pDCs and DC1s, but equally expressed among human DC subpopulations (Table 1).142,143
3.6.1. Clec12a–Mouse
Murine splenic DCs targeted ex vivo with anti-Clec12a mAb-OVA fusions displayed superior cross-presenting capabilities in comparison with Clec9a-targeting fusions as measured by proliferation of OT-I cells.144,145 Yet, in a head-to-head comparison of OVA protein fused to anti-Clec12a mAb, anti-Clec9a mAb, or anti-DEC205 mAb, Clec12a-targeting fusions induced lower proliferation of CD8+ T cells in B6 mice compared to the latter two.109 This emphasizes that in vitro studies of XP do not always accurately predict in vivo efficacy. CD4+ T cell proliferation was not observed for Clec12a-targeting fusions, whereas fusions targeting Clec9a or DEC205 resulted in both CD8+ and CD4+ T cell proliferation.109
3.6.2. Clec12a–Human
Both Clec12a and DEC205 direct antigens to lysosomes via early endosomes. The retention of antigen in early endosomes has been shown slightly longer upon targeting Clec12a, thus promoting XP.142 Clec12a-mediated delivery of keyhole limpet hemocyanin (KLH) to moDCs, pDCs, DC2s, or DC1s led to enhanced XP, IFN-γ production, and CD4+ T cell proliferation compared to untargeted controls.142 These findings indicate a potential use for Clec12a as a target in cancer vaccines. However, mouse studies demonstrate no to little beneficial effects and challenge these conclusions.109 In summary, conflicting results prevent one from concluding on the potential of Clec12a as target to enhance XP.
4. Promoting MHC II Antigen Presentation to Improve CD4+ T Helper Responses
4.1. DECTIN-1
DECTIN-1 (Clec7a, CD369) initiates the release of antifungal cytokines and chemokines upon recognition of β-d-glucose polysaccharides, often present on pathogens.146−148 Its signaling is inherently immunostimulatory and is synergistic with TLR2 signaling.149,150 DECTIN-1 can be used to target DC2s (Table 1).
4.1.2. DECTIN-1–Mouse
Notable differences in T cell activation were observed upon delivery of the OVA using anti-DECTIN 1 and anti-DEC205. Targeting DECTIN-1 increased CD4+ T cell proliferation and antibody production, whereas targeting DEC205 increased CD8+ T cell proliferation.151 β-glucan functionalization of nanoparticles increased CD4+ T cell proliferation compared to untargeted controls, and reduced tumor growth by elicitation of CD4+ Th1 cells, and to a lesser extent, of CD4+ Th9 cells.152−155 However, the dependency on DECTIN-1 remains to be demonstrated as particle functionalization can alter charge, uptake properties, and pharmacokinetics.
4.1.3. DECTIN-1–Human
In humans, targeting DECTIN-1 via β-glucans or mAbs was shown to be immunostimulatory.156,157 This finding has been used to target moDCs with MART-1 peptide without extra adjuvant to activate T cells in vitro.156 The selective expansion of CD4+ Th17 cells in vitro may be explained by the involvement of DECTIN-1 in antifungal immunity.158 The intrinsic immunostimulatory capacity of β-glucans is being explored in phase I clinical trials to enhance the efficacy of anti-GD2 immunotherapy against neuroblastoma.157 The dual use of β-glucans as both DECTIN-1-targeting and immunostimulatory adjuvants should be further explored in cancer vaccine formulations.
4.2. DCIR(2)
DCIR (Clec4a, CD367) regulates inflammation and T cell immunity.159 The mouse homologue DCIR2 is uniquely expressed on DC2s, whereas DCIR is expressed less restrictively in humans (Table 1).45 DCIR(2) binds mannose, glucose, N-acetylglucosamine, fucose, and asialo-N-glycan(s). The latter is crucial in DC regulation processes.160 A single glycosylation site in its carbohydrate binding pocket controls the specificity of DCIR for its ligand.161
4.2.1. DCIR2–Mouse
Dudziak and colleagues compared DCIR2 and DEC205 as vaccine targets.45 In vivo delivery of the OVA protein to DCIR2 resulted in a faster class switching to IgG2b/c, indicating a Th1-oriented response in B6 mice, whereas delivery to DEC205 increased antigen presentation on MHC I. Both vaccines elicited similar CD4+ T cell responses. Even though DEC205 targeting formulations led to increased CD8+ T cell responses, both formulations were capable of protection in a tumor challenge.162 In contrast, in BALB/c mice, immunization with viral NS1 protein delivered to DCIR2 did not provide protection in a lethal challenge, whereas delivery to DEC205 conferred partial protection.163 This reflects a heavy dependence on CD8+ T responses during a dengue challenge, while antitumor immunity benefits from the combined action of CD4+ and CD8+ T cells.40,62 It is possible that the strain in which these studies were made influenced the nature of the immune response, as B6 mice are Th1 prone while BALB/c are Th2-prone.164,165 Performing similar experiments in CB6.F1 mice (B6 × BALB/c (H2Kb+/d+)) could help unify these findings. In addition, DCIR2 and DEC205 are expressed on distinct DC subsets, and it is thus not possible to dissect the effect of the receptor and routing from the inherent physiological differences among the DC subsets. Nevertheless, these studies demonstrate the possibility of directing the immune response by targeting different DC receptors.
4.2.2. DCIR–Human
The less restricted expression of DCIR in humans enables a comparison of antigen delivery to the same receptor on different subsets. Myeloid DCs, pDCs, and Langerhans cells isolated from human PBMCs showed similar CD8+ T cell priming ability when targeted with anti-DCIR-MART-1 peptide or anti-DCIR-influenza matrix protein fusions.166 DCIR had a different intracellular routing than other C-type lectins and did not preferentially colocalize with either early, late, or recycling endosomes, nor with the ER or Golgi.167 In line with the poor association with the endolysosomal system, low CD4+ T cell responses were measured upon ligand internalization. These results should be translated with caution, as DCs might act differently in an in vivo setting.
4.3. MGL(2)
MGL (Clec10a, DC-ASGPR, CD301) is a galactose-binding lectin that is mainly targeted using its ligands, α- or β-linked N-acetyl galactosamine (GalNAc) or galactose.168−170 Activation of MGL leads to production of IL-10 via Syk signaling.171 MGL is primarily expressed on DC2s and downregulated upon DC activation.172
4.3.1. MGL2–Mouse
MGL1 and MGL2 homologues have been identified in mice.173 Murine MGL2 can be specifically targeted by modifying antigens with GalNAc, which has been shown to enhance CD4+ T cell responses.174,175
4.3.2. MGL–Human
Abnormal O-glycosylation of mucins is a trait often shared by tumors. The Thomsen-nouveau (Tn) antigen, α-linked GalNAc carried on serine or threonine residues, is often found on mucin-type glycoproteins such as MUC1 at the surface of carcinoma cells.176 Because MUC1 is a tumor antigen, the capacity of Tn to bind human MGL has been used to increase TAA delivery to DCs.177−179 Tn-MUC1 glycoprotein was detected only in MHC II compartments, while a short Tn-MUC1 glycopeptide was found in both MHC I and MHC II compartments. This suggests that antigen size or physicochemical properties can influence intracellular routing and that using short peptide antigens could favor XP. This remains to be confirmed with an increased sample size.178 As Tn-glycosylation can negatively impair antigen processing, it should be carefully studied to what degree Tn-glycosylation can be introduced to increase uptake via MGL without interfering with antigen presentation.179 Rhesus macaques vaccinated with Tn-MUC1 showed significantly higher IFN-γ+ T cell responses by ELISpot than those vaccinated with Tn-negative MUC1. Unfortunately, the authors did not investigate whether these were CD4+ or CD8+ T cells.180 In a different study, Tn-MUC6 glycoprotein was found to induce lower IFN-γ but higher IL-17 secretion by CD4+ T cells compared to nonglycosylated MUC6. This suggests that targeting of Tn via MGL can promote a Th17 phenotype.179
4.4. Langerin
Langerin (Clec4k, CD207) is specifically expressed on Langerhans cells (LCs), a skin resident cell population of monocytic origin that displays DC-like properties. LCs patrol dermal tissue, take up antigens and migrate to lymph nodes upon activation.54,181 LCs can be targeted by transdermal vaccines through simple application on the skin, which has the advantage to be noninvasive.182 Langerin is also expressed at low levels on a subset of murine DC1s and human DC2s. Antigens taken up via Langerin are internalized into Birbeck granules, which are unusual organelles involved in viral degradation.183 Langerin has a high affinity for galactose-6-sulfated oligosaccharides and recognizes sulfated and mannosylated glycans.184
4.4.1. Langerin–Mouse
In a comparative study, subcutaneous delivery of OVA conjugated to anti-Langerin, anti-DEC205, or anti-DCIR2 revealed that LCs can induce systemic CD4+ and CD8+ T cell responses.185 Anti-Langerin fusions induced fewer CD8+ T cells but more CD4+ T cells compared to anti-DEC205 fusions upon subcutaneous vaccination, while anti-DCIR2 delivery induced even more pronounced CD4+ T cell responses. Moreover, anti-Langerin targeting resulted in a prolonged antigen presentation for several days. Taken together, this indicates that Langerin mediates efficient OVA antigen presentation on both MHC I and MHC II, however this should be confirmed in more clinically relevant settings.185 In a different setup, HIV gag-p24 protein was delivered intraperitoneally via anti-Langerin, anti-Clec9a, anti-DCIR2, or anti-DEC205 in combination with anti-CD40 and polyI:C. In comparison with Langerin-targeting vaccines, DC1-targeting vaccines (anti-Clec9a, anti-DEC205) induced comparable IFN-γ-producing CD4+ Th1 and CD8+ T cells in CB6.F1 mice and outperformed DC2-targeted vaccines (anti-DCIR2).46 This suggests that targeting Langerin can be as potent as targeting Clec9a or DEC205. The authors speculate that the evident contradiction between these studies could be due to the model antigen, mouse strain, vaccination route, or adjuvant used. The multitude of possible factors emphasize the potential benefit of standardization of the mouse model (CB6.F1 mice) and vaccine components for comparative therapeutic vaccine studies.
4.4.2. Langerin–Human
Human skin is highly tolerogenic compared with murine skin. Skin resident human LCs are thus more prone to induced peripheral tolerance. Moreover, human LCs have been shown incapable of XP.186,187 Langerin can be targeted with glycomimetics of its ligand, which was shown to improve immune responses in various studies when compared to free cargo.188−190 However, it remains unclear whether improved pharmacokinetic properties are responsible for this increase or whether Langerin targeting itself played a role. In summary, Langerin might not be an ideal target to induce CD8+ T cell-oriented immunity in humans, but rather to direct transdermal vaccines toward LCs in combination with immunostimulatory adjuvants to improve classical antigen presentation to CD4+ T cells.
5. Promoting Pan-APC Antigen Presentation
5.1. DC-SIGN
The main function of DC-SIGN (CD209, Clec4L) is engagement of resting T cells via ICAM-3.191 DC-SIGN’s natural ligands are high mannose- and fucose-containing carbohydrates, such as Lewis X oligosaccharides.192 DC-SIGN has overlapping ligands with CD206, and displays a similar expression profile.133,193 Studying immunity induced by targeting DC-SIGN is complex in mouse models, as mice have eight homologues of human DC-SIGN.194,195
5.1.1. DC-SIGN–Human
Targeting antigens to DC-SIGN was shown to induce both CD4+ and CD8+ immune responses in humanized mice and in vitro models using moDCs.196−198 Targeting antigen with anti-DC-SIGN antibodies binding to the carbohydrate recognition domain (CRD) of DC-SIGN promoted routing toward late endosomes, whereas targeting antigens using antibodies binding to the neck region of DC-SIGN routed the antigen to early endosomes.199 The latter enhanced T cell proliferation compared to untargeted control; however, the CRD targeting mAb was not investigated for antigen delivery. Still, this study demonstrates that the targeted epitope can influence endosomal processing.
5.2. MHC II
MHC II is expressed by all professional APCs. Recycling of MHC II on the cell membrane is rapid in immature DCs but slower in mature DCs leading to a relative higher expression of MHC II on mature DCs.200 Targeting antigen to MHC II delivers the antigen to recycling compartments where antigen can be loaded directly on recycled MHC II complexes or cross-presented on MHC I.201
5.2.1. MHC II–Mouse
Nanobodies recognizing mouse MHC II conjugated to MUC1 or T cell epitopes of OVA were shown capable of inducing T cell proliferation in B6 mice when administered with immunostimulatory anti-CD40 mAbs and polyI:C.201,202 Targeting MHC II increased CD4+ T cell activation compared to targeting DEC205. In addition, a MHC II targeted vaccine induced potent humoral immune responses against the SARS-CoV-2 spike protein.203 Due to its broad expression on DCs, B cells and other APCs, MHC II might be one of the best ways to reach a large population of immune cells.204
5.3. CD11c
CD11c (integrin αx, ITGAX) recognizes a variety of ligands, including bacterial lipopolysaccharide (LPS) and several adhesion molecules.205,206 It is expressed on all conventional DC subsets as well as several other immune cells such as macrophages, neutrophils, and B cells (Table 1). Conflicting reports have been published on the efficacy of CD11c as vaccine target.207−211
5.3.1. CD11c–Mouse
In a comparative study, OVA was targeted to CD11c, DEC205, MHC II, CD40, TLR2, and FcγRII/III by chemical conjugation to the respective Fab′ fragments.207 Anti-CD11c conjugates outperformed all others in inducing CD4+ and CD8+ T cell proliferation in B6 mice. Humoral responses were induced without adjuvant upon targeting CD11c in BALB/c mice.208 Furthermore, tumor-reactive CD4+ and CD8+ T cells were observed in BALB/c mice upon delivery of HER2/neu using anti-CD11c antibody fragments.209 In contrast, plasmid DNA vaccination of B6 mice with CD11c-targeted tuberculosis antigen did not increase T cell activation compared to a nontargeting variant, whereas DEC205 targeting did.210 It is hypothesized that the vaccine format, as well as the mouse strain, can influence the outcome. We emphasize again the importance of conducting major comparative studies in H2Kb+/d+ CB6.F1 mice.
5.4. CD40
CD40 (Bp50, TNFRSF5) is part of the TNF-receptor superfamily and an important regulator of antigen processing.212 Interaction with its ligand CD154 (CD40L) promotes the antigen presentation and maturation of APCs. Agonistic anti-CD40 antibodies have been developed and can be used to mimic the CD40-CD154 interaction. They are utilized as immunostimulatory adjuvant or stand-alone cancer immunotherapy.213,214 CD40 is expressed on all APCs and upregulated during maturation.215 Similar to DEC205, CD40 is expressed on endothelial cells, which may lead to more off-targeting in comparison with restrictedly expressed DC surface markers.216
5.4.1. CD40–Mouse
Agonistic anti-CD40 mAb antigen fusions could be an interesting avenue to combine antigens and adjuvants into a single construct. Fusions of the T cell epitope λ2315 of myeloma protein M315 with CD40-targeting single-chain variable fragments (scFvs) induced stronger M315-specific T cell proliferation compared to untargeted fusions.217 The agonistic capability of the CD40-targeting moiety was not altered by the conjugation of the antigen in these in vitro experiments. In BALB/c mice, strong IgG2a humoral responses were induced by targeting CD40.218 High IgG2a levels are associated with Th1-mediated immunity; thus, targeting antigens to CD40 might have induced a Th1-biased response. Nanoparticles targeted to CD40 induced stronger CD8+ T cell responses in comparison with nanoparticles targeted toward DEC205 or CD11c.219,220 However, as nanoparticles have pharmacokinetic properties distinct from those of antibody-based vaccines, it would be interesting to repeat this comparative study using antibody-based formulations. Special attention should be given to the intrinsic immunostimulatory capacities of CD40-targeting, as this might provide new opportunities for cancer vaccines without the requirement of systemic adjuvants.
5.4.2. CD40–Human
Enhancement of T cell proliferation and humoral responses by targeting CD40 has been shown for distinct types of antigens (FluM1, HIV, HPV) in vitro.221−223 Careful dissection of endosomal routing revealed that targeting CD40 directed antigen to early endosomes, resulting in equally potent XP in comparison with targeting DEC205 on primary DC1s.47 Yin et al. performed an extensive comparative study on targeted delivery of MART-1 peptide conjugated to mAb in moDCs.224 They observed enhanced MART-1 specific CD4+ and CD8+ T cell proliferation upon targeting CD40 in comparison to LOX-1 and DECTIN-1. MoDCs were cultured with IL-2 and IL-7 during the T cell activation assay, but no adjuvant was provided. Therefore, it cannot be concluded whether antigen fate or DC maturation induced by CD40 targeting was the main determinant for the enhanced T cell proliferation. Of note, an exciting prospect in the context of CD40 targeting are bispecific antibodies recognizing CD40 and a TAA.225,226 These constructs bring tumor debris in proximity to CD40+ cells, which can take up, process, and present the tumor antigens, while simultaneously being stimulated via CD40. This results in antitumor responses surpassing immune reactivity toward the initial targeted epitope.226
5.5. Fc Receptors
Fc receptors (FcRs) are expressed in a variety of immune cells. FcRs recognize specific glycosylation patterns on the Fc-region of mAbs and notably mediate the uptake of opsonized antigens.227 They can be actively targeted using anti-CD16, anti-CD32 or anti-CD64 mAbs, or FcR specific ligands. Moreover, FcRs are also passively targeted when mAbs containing functional Fc regions. FcRs are usually specific for a set of isotypes and can trigger inflammatory (e.g., FcγRI, FcγRIIa) or tolerogenic (FcγRIIb) responses.228 FcR biology is complex because of their varying affinity for different isotypes and expression patterns on different APCs, and it remains difficult to identify general intracellular pathways. Furthermore, interspecies differences between mouse and human complicate comparison, although signaling cascades and effector function are reasonably conserved.229 Unlike most endocytic receptors mentioned in this review, FcRs generally do not release their ligand upon internalization, which has been suggested to target antigens toward lysosomes.230
5.5.1. Fc Receptors–Mouse
Formyl peptide receptor-like 1 inhibitor (FLIPr) is secreted by bacteria to evade opsonization by binding antagonistically to FcRs.231 Fusions of FLIPr with OVA conferred a significant survival benefit in tumor-bearing B6 mice, even in the absence of an adjuvant.232,233 These effects were not observed in TAP–/– B6 mice highlighting the necessity for XP to obtain antitumor effects.233
5.5.2. Fc Receptors–Human
Active targeting of FcγRII on moDCs with a peptide derived from human cytomegalovirus enhanced XP compared to nontargeting variants in vitro, demonstrating the possibilities of actively targeting FcRs.234 In contrast, antigen delivery via FcεRI-bound IgE targets antigens to a cathepsin S-dependent MHC class II pathway is expected to favor CD4+ T cell responses.235 The diverse physiological functions of FcRs are reflected in the various types of vaccines targeting them.236−238 Examples include vaccines targeting FcγRI for protection against dengue, vaccines recruiting intracellular FcR TRIM21 to induce XP in moDCs, and vaccines targeting multiple FcRs (CD16, CD32, CD64) to dampen autoimmunity. FcRs can be targeted to promote classical antigen presentation or XP, but the specific pathways activated by targeting different FcRs require further investigation for the therapeutic vaccine development.
6. Future Perspectives and Recommendations for Therapeutic Vaccine Design
6.1. Controlling the Immune Response by Targeting Specific DC Receptors
Therapeutic vaccines have yet to achieve the same impact on modern healthcare as prophylactic vaccines. While it is firmly established that targeting vaccines to DC receptors enhances therapeutic efficacy, the optimal target remains elusive. Here, we reviewed the literature regarding distinct receptors for their effect on antigen presentation and immune response (Table 2).
Targeting therapeutic vaccines to DC1-specific receptors harnesses the superior ability of DC1s at XP and improves CD8+ T cell responses.50 The first-in-human DC-targeted vaccine CDX-1401 demonstrated a reasonable safety profile and a proof-of-concept for feasibility. It elicited NY-ESO-1-specific T cell responses in patients, but yielded only modest clinical benefits.99,101 The observed T cell responses are likely not a result of increased XP directly but rather increased uptake by DCs in general. The broad expression pattern of DEC205 on myeloid and nonmyeloid cells may have hindered efficient delivery of NY-ESO-1 to DC1s. Targeting of the DC1-specific receptors Clec9a or XCR1 induced stronger XP in preclinical studies in comparison with human DEC205, which we hypothesize is due to fundamental differences in intracellular routing.44,106,111,112 We envision that targeting Clec9a or XCR1, both exclusively expressed on cross-presenting DC1s, could strongly improve the efficacy of therapeutic cancer vaccines through the promotion of potent XP of the delivered antigen. Importantly, CDX-1401 efficacy was potentiated by pretreatment with Flt3l, which increased the abundance of peripheral conventional DCs and monocytes.101 Other studies including Flt3l have shown its ability to induce differentiation and recruitment of DC1s.239 Therefore, pretreatment with Flt3l could be especially beneficial to vaccines designed to improve XP by targeting of DC1s. Yet, the low numbers of circulating DC1s (<0.05% of PBMCs) could restrict the absolute antigen uptake, and therefore targeting a larger fraction of APCs may overall be more beneficial. Antigen retention and transfer to DC1s by CD169+ macrophages is a promising strategy to improve antigen XP.126,128 Finally, targeting CD206 was shown to promote XP by delivering antigens to early endosomes.135,136 Because CD206 is also expressed on TAMs, antigens should only be targeted to CD206 in combination with TAM repolarization agents such as TLR7/8-agonists.140 Clinical studies exploring antigen targeting to CD206 (e.g., CDX-1307) may especially benefit from codelivery of adjuvants.138,139
Antigen routing toward late endosomes or direct delivery in the MHC II loading compartment improves CD4+ Th responses and humoral immunity. This can be promoted by targeting DC receptors such as DCIR(2), MGL(2), or MHC II. Incorporating CD4+ T cell epitopes in vaccine design is expected to support cytotoxic immune responses by means of CD4+ T cell help.40,62 Finally, the immunostimulatory signaling inherent by receptors as DECTIN-1 or CD40 could be advantageous when trying to break tolerance to self-antigens and should be further explored in the context of therapeutic cancer vaccines.138,156,158
6.2. Specific Targeting of DC Subpopulations or Broad Targeting of pAPCs?
Targeting FcRs or MHC II allows for the targeting of a broad range of APCs, and this approach has been shown to significantly enhance immune responses.202,203,234 Targeting a larger pool of APCs could potentially elicit an immune response of greater magnitude compared to targeting less abundant DC subpopulations.24 Yet, broad targeting approaches could be more prone to induce Tregs if antigens are delivered to immune suppressive APCs such as TAMs. So far, there have been no reports of a direct comparison between therapeutic cancer vaccines that target broadly expressed receptors, such as MHC II or FcR and vaccines that target receptors with restricted expression, such as XCR1 or Clec9a. Such comparative studies would help define whether strict targeting or broader targeting induces optimal antitumor immune responses to explore for clinical translation. Lastly, combined targeting of CD8 epitopes to XP-enhancing receptors and CD4 epitopes to receptors specialized in class II antigen presentation may be of interest to pursue in the field of therapeutic cancer vaccines.
6.3. Extensive Characterization of Adjuvants, Immune Responses, and Formulation
Immunostimulatory adjuvants are essential in therapeutic cancer vaccines to induce immune responses toward the presented epitope. Antigen presentation, in absence of immunostimulatory adjuvants, by immature DCs induces tolerance and tumor progression.240,241 Preferably, antigen and adjuvant should be delivered simultaneously to DCs, as this increased antigen presentation in multiple models.87,88 A premature maturation of DCs could impair vaccine uptake and cause autoimmunity in response to systemic adjuvant encounter.4 Covalent incorporation of adjuvants into DC-targeted vaccines could improve the therapeutic window, ensure simultaneous delivery of both antigen and adjuvant and limit off-target effects.27,29,242,243 Much like interspecies differences in DC surface receptors, variations in TLR expression patterns affect the clinical translation of murine studies.3,244 For instance, murine DC1s do not express TLR7, yet human DC1s are highly responsive to imidazoquinolines, a class of TLR7-agonists with promising therapeutic potential.245 TLR3 is mainly expressed by DC1s, which would indicate that TLR3-agonists as poly-I:C can be more effective than other adjuvants to promote XP and CD8+ T cell activation by DC1s. A combination therapy of TLR3-agonist, Flt3l, and radiotherapy recruited cross-presenting DCs in the tumor, resulting in cytotoxic T cell responses and tumor clearance in lymphoma patients.239 These promising results emphasize the potential of Flt3l to stimulate DCs, which could also be used in combination with therapeutic vaccines.
The significant role of CD4+ T cells in driving antitumor responses is increasingly evident.40,41,62,246 While most mechanistic studies focus on individual pathways, we recommend including the characterization of T helper responses and humoral responses along with cytotoxic T cell responses. Such studies will be crucial to delineate whether a single receptor on DCs can optimally induce XP as well as classical presentation or whether combination therapies should be investigated as an ideal targeting strategy. In mouse studies, the strain should be considered carefully.164,165 We recommend the use of CB6.F1 mice to unify previous findings, as this hybrid mouse strain neutralizes the difference between the commonly used B6 and BALB/c mice. While moDCs are easy to generate at moderate costs and well-suited for initial studies, they have inherent differences in antigen processing and presentation capacity, and are therefore not fully reflective of circulating primary DCs.21,22 Therefore, in vitro studies should include naturally occurring DC subsets to confirm findings on moDCs. Finally, the influence of the isotype in the case of a targeting mAb should be studied to account for FcR-mediated effects.227,228,247 We emphasize the importance of including isotype controls in DC targeted vaccine studies. Improved circulation time has been shown to positively affect targeting and improve immunological outcome of targeted vaccines.110 Fc-isotype engineering could offer opportunities for improving circulation time, for example through incorporation of Fc-silent mutations or increasing affinity to neonatal FcR to promote recycling of mAbs.248−250
This review focuses on targeted antigen delivery through antibody and ligand conjugates. Targeting of DCs has also been explored using other delivery vehicles, for instance, nanoparticles or viral vectors. Nanoparticle encapsulation of antigens is an attractive strategy to extend half-life and allow codelivery of antigens and adjuvants. The particulate nature renders them especially susceptible to phagocytosis by APCs. However, this nature also promotes clearance via neutrophils or macrophages, reducing antigen presentation. Although DC targeting of nanoparticles can be improved by conjugation of antibodies or ligands recognized by DC receptors, skewing the biodistribution of nanoparticles is difficult, as it is mostly dictated by particle size and surface composition.251 Larger nanoparticles (>150 nm in diameter) are cleared by the reticuloendothelial system and taken up by phagocytic cells in proximity of the injection site or in the liver. In contrast, smaller nanoparticles (<150 nm in diameter) can evade clearance by the reticuloendothelial system and reach lymph nodes.252 A positive surface charge has been reported to improve the uptake of nanoparticles in vivo.253,254 For more in-depth reading on nanoparticles vaccination strategies, we refer to reviews covering this subject.255−258
An approach different from nanoparticles or antibody or ligand conjugates is viral vectors. Lentiviral vectors are a commonly used type of vector to deliver antigens to DCs, which are inherently immunogenic, thereby also serving as adjuvant. Engineering of the Sindbis viral vector by removing the heparan sulfate recognition site, while preserving the glycoprotein recognized by DC-SIGN, enables targeting of these vectors toward DCs.259 Phase I/II clinical trials for the DC-SIGN targeted lentiviral vector LV305 containing a NY-ESO-1 TAA demonstrated the feasibility of this approach, as no undesired mutagenesis and viral persistence were noted.260,261 Direct comparisons between delivery vehicles are seldom performed. This is an interesting avenue to explore and could provide new insights into the field of targeted antigen delivery.
6.4. Recommendations for Designing Targeted Therapeutic Cancer Vaccines
In this Review, we suggest that selecting the appropriate target may surpass selecting a specific DC subset. Distinct receptors on a single DC subset engage specific endosomal pathways, resulting in different levels of antigen presentation. An ideal therapeutic cancer vaccine achieves sufficient XP to prime CD8+ T cells, while maintaining adequate CD4+ T helper activation. The next step in targeted therapeutic vaccination is the translation of preclinical studies into the clinic. We recommend the use of Clec9a or XCR1 as target for therapeutic vaccines in combination with Flt3l to boost DC1 abundance.101 Targeting Clec9a or XCR1 results in superior CD8+ T cell mediated antitumor immunity over targeting DEC205, while sustaining CD4+ T helper cell responses in preclinical models.44,46,110−112 Moreover, Clec9a and XCR1 are specifically expressed on DC1s, thereby limiting off-target engagement. Flt3l coadministration could augment DC1-targeted therapies by increasing the natural low abundance of DC1s (<0.05% of PBMCs), as demonstrated by clinical trials administering CDX-1401 in combination with Flt3l.101 To further enhance vaccine efficacy, we would recommend incorporation of immunostimulatory adjuvants, for example through encapsulation in nanoparticles, antigen-adjuvant conjugates, or self-adjuvating mRNA vaccines.29,242 This will ensure simultaneous delivery of adjuvant and antigen to DCs, which strongly improves antigen presentation, reduces tolerance induction, and may improve the therapeutic window of the adjuvant by reducing systemic immune activation.87,88
Results of clinical trials have shown the safety and feasibility of in vivo cancer vaccination (Table 2). Thorough preclinical evaluation and comparison of different vaccines and targets is required to move toward clinical translation. It will require head-to-head comparisons of different vaccine targets and experimental standardization, for example, in terms of the use of mouse strain, adjuvant, and isotype controls. Studies on human targets should be performed on primary DCs as much as possible to aid the clinical translation of the vaccine formulation. It is recommended that future studies focus on a broad readout of the immune response including phenotypic characterization of CD4+ and CD8+ T cell responses.62,246 An interesting comparative study would be to investigate side-by-side targeting of broadly expressed receptors, such as MHC II or FcRs, and restrictedly expressed receptors, such as Clec9a or XCR1. This will help to establish the most optimal target to pursue in therapeutic cancer vaccines. It is well established that targeting MHC I epitopes toward receptors promoting XP enhances CD8 T cell responses in a preclinical setting. However, analyzing this in a clinical setting remains challenging. Humanized mouse models offer an opportunity to assess the rationale of steering the immune system through receptor targeting, yet capturing the full complexity of the human immune system will be difficult. Therefore, the true benefits of novel targeted antigen delivery strategies ought to be assessed in clinical trials, which is an exciting prospect. To conclude, ample suitable surface markers on DCs exist for targeted therapeutic cancer vaccines, and proper selection will undoubtedly enhance future vaccine efficacy.
Glossary
Abbreviations
- APC
antigen-presenting cell
- DC
dendritic cell
- MHC
major histocompatibility complex
- PRR
pathogen recognition receptor
- TLR
Toll-like receptors
- DAMP
damage associated molecular pattern
- PAMP
pathogen associated molecular pattern
- iTregs
induced regulatory T cells
- TAA
tumor-associated antigen
- gp100
glycoprotein 100
- TRP-2
tyrosine related protein 2
- TSA
tumor-specific antigen
- NK
natural killer
- moDC
monocyte-derived dendritic cell
- DC1
type 1 conventional dendritic cell
- DC2
type 2 conventional dendritic cell
- HPV
human papillomavirus
- XP
cross-presentation
- ER
endoplasmic reticulum
- ETP
endosome transporter protein
- UPS
ubiquitin-proteasome system
- TAP
transporter associated with antigen processing
- ERC
endosomal recycling compartment
- pDC
plasmacytoid dendritic cell
- Th1
T helper type 1
- Th2
T helper type 2
- cDC
conventional dendritic cell
- OVA
ovalbumin
- mAb
monoclonal antibody
- Flt3L
fms-like tyrosine kinase 3 ligand
- HA
hemagglutinin
- BMDC
bone marrow-derived dendritic cell
- hCG-β
Human chorionic gonadotropin β
- TAM
tumor-associated macrophage
- KLH
keyhole limpet hemocyanin
- GalNAc
galactosamine
- Tn
Thomsen nouveau
- LC
Langerhans cell
- CRD
carbohydrate recognition domain
- LPS
lipopolysaccharide
- scFv
single chain variable fragment
- FLIPr
formyl peptide receptor-like 1 inhibitor
Author Present Address
§ Program in Cellular and Molecular Medicine, 1 Blackfan Circle, Boston Children’s Hospital, 02115, Boston, Massachusetts, United States of America
Author Contributions
∥ C.M.L. and M.V. contributed equally. The manuscript was written by Z.W., F.J.D., C.M.L., and M.V. Figures were designed by Z.W. and F.J.D. Conceptualization was done by Z.W., F.J.D., C.M.L., and M.V. All authors have approved the final version of the manuscript.
This work was supported by the NWO Gravity Program, Institute for Chemical Immunology (ICI-00031 and ICI-00201), ERC Starting grant CHEMCHECK (679921) and ERC PoC grant CNECT-VAX (101069163). The APC was funded by the NWO Gravity Program, Institute for Chemical Immunology (ICI-00031)
The authors declare no competing financial interest.
References
- Fenner F. A Successful Eradication Campaign. Global Eradication of Smallpox. Rev. Infect Dis 1982, 4 (5), 916–930. 10.1093/clinids/4.5.916. [DOI] [PubMed] [Google Scholar]
- Mariner J. C.; House J. A.; Mebus C. A.; Sollod A. E.; Chibeu D.; Jones B. A.; Roeder P. L.; Admassu B.; van ’t Klooster G. G. M. Rinderpest Eradication: Appropriate Technology and Social Innovations. Science 2012, 337, 1309. 10.1126/science.1223805. [DOI] [PubMed] [Google Scholar]
- Iwasaki A.; Medzhitov R. Toll-like Receptor Control of the Adaptive Immune Responses. Nat. Immunol 2004, 5 (10), 987–995. 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- Reis e Sousa C. Dendritic Cells in a Mature Age. Nat. Rev. Immunol 2006, 6 (6), 476–483. 10.1038/nri1845. [DOI] [PubMed] [Google Scholar]
- Schoenberger S. P.; Toes R. E. M.; van der Voort E. I. H.; Offringa R.; Melief C. J. M. T-Cell Help for Cytotoxic T Lymphocytes Is Mediated by CD40-CD40L Interactions. Nature 1998, 393 (6684), 480–483. 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- Lanier L. L.; O’Fallon S.; Somoza C.; Phillips J. H.; Linsley P. S.; Okumura K.; Ito D.; Azuma M. CD80 (B7) and CD86 (B70) Provide Similar Costimulatory Signals for T Cell Proliferation, Cytokine Production, and Generation of CTL. J. Immunol. 1995, 154 (1), 97–105. 10.4049/jimmunol.154.1.97. [DOI] [PubMed] [Google Scholar]
- Propper D. J.; Balkwill F. R. Harnessing Cytokines and Chemokines for Cancer Therapy. Nat. Rev. Clin Oncol 2022, 19 (4), 237–253. 10.1038/s41571-021-00588-9. [DOI] [PubMed] [Google Scholar]
- Steinman R. M.; Turley S.; Mellman I.; Inaba K. The Induction of Tolerance by Dendritic Cells That Have Captured Apoptotic Cells. Journal of Experimental Medicine 2000, 191 (3), 411–416. 10.1084/jem.191.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palucka K.; Banchereau J. Cancer Immunotherapy via Dendritic Cells. Nat. Rev. Cancer 2012, 12 (4), 265–277. 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bol K. F.; Schreibelt G.; Gerritsen W. R.; de Vries I. J. M.; Figdor C. G. Dendritic Cell-Based Immunotherapy: State of the Art and Beyond. Clin. Cancer Res. 2016, 22 (8), 1897–1906. 10.1158/1078-0432.CCR-15-1399. [DOI] [PubMed] [Google Scholar]
- Sellars M. C.; Wu C. J.; Fritsch E. F. Cancer Vaccines: Building a Bridge over Troubled Waters. Cell 2022, 185 (15), 2770–2788. 10.1016/j.cell.2022.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brossart P. The Role of Antigen Spreading in the Efficacy of Immunotherapies. Clin. Cancer Res. 2020, 26 (17), 4442–4447. 10.1158/1078-0432.CCR-20-0305. [DOI] [PubMed] [Google Scholar]
- Kreutz M.; Tacken P. J.; Figdor C. G. Targeting Dendritic Cells—Why Bother?. Blood 2013, 121 (15), 2836–2844. 10.1182/blood-2012-09-452078. [DOI] [PubMed] [Google Scholar]
- Perez C. R.; De Palma M. Engineering Dendritic Cell Vaccines to Improve Cancer Immunotherapy. Nat. Commun. 2019, 10 (1), 5408. 10.1038/s41467-019-13368-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmers F.; Schreibelt G.; Sköld A. E.; Figdor C. G.; De Vries I. J. M. Paradigm Shift in Dendritic Cell-Based Immunotherapy: From in Vitro Generated Monocyte-Derived DCs to Naturally Circulating DC Subsets. Front. Immunol. 2014, 5, 00165. 10.3389/fimmu.2014.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himoudi N.; Wallace R.; Parsley K. L.; Gilmour K.; Barrie A.-U.; Howe K.; Dong R.; Sebire N. J.; Michalski A.; Thrasher A. J.; Anderson J. Lack of T-Cell Responses Following Autologous Tumour Lysate Pulsed Dendritic Cell Vaccination, in Patients with Relapsed Osteosarcoma. Clin Transl Oncol 2012, 14 (4), 271–279. 10.1007/s12094-012-0795-1. [DOI] [PubMed] [Google Scholar]
- Kantoff P. W.; Higano C. S.; Shore N. D.; Berger E. R.; Small E. J.; Penson D. F.; Redfern C. H.; Ferrari A. C.; Dreicer R.; Sims R. B.; Xu Y.; Frohlich M. W.; Schellhammer P. F. Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer. New England Journal of Medicine 2010, 363 (5), 411–422. 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- Yewdall A. W.; Drutman S. B.; Jinwala F.; Bahjat K. S.; Bhardwaj N. CD8+ T Cell Priming by Dendritic Cell Vaccines Requires Antigen Transfer to Endogenous Antigen Presenting Cells. PLoS One 2010, 5 (6), e11144 10.1371/journal.pone.0011144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleindienst P.; Brocker T. Endogenous Dendritic Cells Are Required for Amplification of T Cell Responses Induced by Dendritic Cell Vaccines In Vivo. J. Immunol. 2003, 170 (6), 2817–2823. 10.4049/jimmunol.170.6.2817. [DOI] [PubMed] [Google Scholar]
- van Beek J. J. P.; Flórez-Grau G.; Gorris M. A. J.; Mathan T. S. M.; Schreibelt G.; Bol K. F.; Textor J.; de Vries I. J. M. Human PDCs Are Superior to CDC2s in Attracting Cytolytic Lymphocytes in Melanoma Patients Receiving DC Vaccination. Cell Reports 2020, 30 (4), 1027–1038. 10.1016/j.celrep.2019.12.096. [DOI] [PubMed] [Google Scholar]
- Ferris S. T.; Ohara R. A.; Ou F.; Wu R.; Huang X.; Kim S.; Chen J.; Liu T.-T.; Schreiber R. D.; Murphy T. L.; Murphy K. M. CDC1 Vaccines Drive Tumor Rejection by Direct Presentation Independently of Host CDC1. Cancer Immunology Research 2022, 10 (8), 920–931. 10.1158/2326-6066.CIR-21-0865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreibelt G.; Bol K. F.; Westdorp H.; Wimmers F.; Aarntzen E. H. J. G.; Duiveman-de Boer T.; van de Rakt M. W. M. M.; Scharenborg N. M.; de Boer A. J.; Pots J. M.; Olde Nordkamp M. A. M.; van Oorschot T. G. M.; Tel J.; Winkels G.; Petry K.; Blokx W. A. M.; van Rossum M. M.; Welzen M. E. B.; Mus R. D. M.; Croockewit S. A. J.; Koornstra R. H. T.; Jacobs J. F. M.; Kelderman S.; Blank C. U.; Gerritsen W. R.; Punt C. J. A.; Figdor C. G.; de Vries I. J. M. Effective Clinical Responses in Metastatic Melanoma Patients after Vaccination with Primary Myeloid Dendritic Cells. Clin. Cancer Res. 2016, 22 (9), 2155–2166. 10.1158/1078-0432.CCR-15-2205. [DOI] [PubMed] [Google Scholar]
- Zhou Y.; Slone N.; Chrisikos T. T.; Kyrysyuk O.; Babcock R. L.; Medik Y. B.; Li H. S.; Kleinerman E. S.; Watowich S. S. Vaccine Efficacy against Primary and Metastatic Cancer with in Vitro-Generated CD103+ Conventional Dendritic Cells. J. Immunother Cancer 2020, 8 (1), e000474 10.1136/jitc-2019-000474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P.; Rosendahl N.; Radford K. J. Conventional Type 1 Dendritic Cells (CDC1) as Cancer Therapeutics: Challenges and Opportunities. Expert Opinion on Biological Therapy 2022, 22 (4), 465–472. 10.1080/14712598.2022.1994943. [DOI] [PubMed] [Google Scholar]
- Balan S.; Ollion V.; Colletti N.; Chelbi R.; Montanana-Sanchis F.; Liu H.; Vu Manh T.-P.; Sanchez C.; Savoret J.; Perrot I.; Doffin A.-C.; Fossum E.; Bechlian D.; Chabannon C.; Bogen B.; Asselin-Paturel C.; Shaw M.; Soos T.; Caux C.; Valladeau-Guilemond J.; Dalod M. Human XCR1+ Dendritic Cells Derived In Vitro from CD34+ Progenitors Closely Resemble Blood Dendritic Cells, Including Their Adjuvant Responsiveness, Contrary to Monocyte-Derived Dendritic Cells. J. Immunol. 2014, 193 (4), 1622–1635. 10.4049/jimmunol.1401243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa F. F.; Pires C. F.; Kurochkin I.; Ferreira A. G.; Gomes A. M.; Palma L. G.; Shaiv K.; Solanas L.; Azenha C.; Papatsenko D.; Schulz O.; Reis e Sousa C.; Pereira C.-F. Direct Reprogramming of Fibroblasts into Antigen-Presenting Dendritic Cells. Science Immunology 2018, 3 (30), eaau4292 10.1126/sciimmunol.aau4292. [DOI] [PubMed] [Google Scholar]
- Reintjens N. R. M.; Tondini E.; de Jong A. R.; Meeuwenoord N. J.; Chiodo F.; Peterse E.; Overkleeft H. S.; Filippov D. V.; van der Marel G. A.; Ossendorp F.; Codée J. D. C. Self-Adjuvanting Cancer Vaccines from Conjugation-Ready Lipid A Analogues and Synthetic Long Peptides. J. Med. Chem. 2020, 63 (20), 11691–11706. 10.1021/acs.jmedchem.0c00851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn G. M.; Sedlik C.; Baharom F.; Zhu Y.; Ramirez-Valdez R. A.; Coble V. L.; Tobin K.; Nichols S. R.; Itzkowitz Y.; Zaidi N.; Gammon J. M.; Blobel N. J.; Denizeau J.; de la Rochere P.; Francica B. J.; Decker B.; Maciejewski M.; Cheung J.; Yamane H.; Smelkinson M. G.; Francica J. R.; Laga R.; Bernstock J. D.; Seymour L. W.; Drake C. G.; Jewell C. M.; Lantz O.; Piaggio E.; Ishizuka A. S.; Seder R. A. Peptide-TLR-7/8a Conjugate Vaccines Chemically Programmed for Nanoparticle Self-Assembly Enhance CD8 T-Cell Immunity to Tumor Antigens. Nat. Biotechnol. 2020, 38 (3), 320–332. 10.1038/s41587-019-0390-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speetjens F. M; Welters M. J. P.; Slingerland M.; van Poelgeest M. I. E.; de Vos van Steenwijk P. J.; Roozen I.; Boekestijn S.; Loof N. M.; Zom G. G.; Valentijn A. R. P. M.; et al. Intradermal Vaccination of HPV-16 E6 Synthetic Peptides Conjugated to an Optimized Toll-like Receptor 2 Ligand Shows Safety and Potent T Cell Immunogenicity in Patients with HPV-16 Positive (Pre-)Malignant Lesions. J. Immunother. Cancer 2022, 10 (10), e005016 10.1136/jitc-2022-005016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardi N.; Hogan M. J.; Porter F. W.; Weissman D. MRNA Vaccines — a New Era in Vaccinology. Nat. Rev. Drug Discovery 2018, 17 (4), 261–279. 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldin A. V.; Savvateeva L. V.; Bazhin A. V.; Zamyatnin A. A. Dendritic Cells in Anticancer Vaccination: Rationale for Ex Vivo Loading or In Vivo Targeting. Cancers 2020, 12 (3), 590. 10.3390/cancers12030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott P. A.; Govindan R.; Naing A.; Friedlander T. W.; Margolin K.; Lin J. J.; Bhardwaj N.; Hellman M. D.; Srinivasan L.; Greshock J.; Moles M.; Gaynor R. B.; Goldstein M. J.; Hu-Lieskovan S. A Personal Neoantigen Vaccine, NEO-PV-01, with Anti-PD1 Induces Broad de Novo Anti-Tumor Immunity in Patients with Metastatic Melanoma, NSCLC, and Bladder Cancer. Annals of Oncology 2018, 29, viii400. 10.1093/annonc/mdy288. [DOI] [Google Scholar]
- Ott P. A.; Hu-Lieskovan S.; Chmielowski B.; Govindan R.; Naing A.; Bhardwaj N.; Margolin K.; Awad M. M.; Hellmann M. D.; Lin J. J.; Friedlander T.; Bushway M. E.; Balogh K. N.; Sciuto T. E.; Kohler V.; Turnbull S. J.; Besada R.; Curran R. R.; Trapp B.; Scherer J.; Poran A.; Harjanto D.; Barthelme D.; Ting Y. S.; Dong J. Z.; Ware Y.; Huang Y.; Huang Z.; Wanamaker A.; Cleary L. D.; Moles M. A.; Manson K.; Greshock J.; Khondker Z. S.; Fritsch E.; Rooney M. S.; DeMario M.; Gaynor R. B.; Srinivasan L. A Phase Ib Trial of Personalized Neoantigen Therapy Plus Anti-PD-1 in Patients with Advanced Melanoma, Non-Small Cell Lung Cancer, or Bladder Cancer. Cell 2020, 183 (2), 347–362. 10.1016/j.cell.2020.08.053. [DOI] [PubMed] [Google Scholar]
- Bauman J.; Burris H.; Clarke J.; Patel M.; Cho D.; Gutierrez M.; Julian R.; Scott A.; Cohen P.; Frederick J. 798 Safety, Tolerability, and Immunogenicity of MRNA-4157 in Combination with Pembrolizumab in Subjects with Unresectable Solid Tumors (KEYNOTE-603): An Update. J. Immunother. Cancer 2020, 8, 0798. 10.1136/jitc-2020-SITC2020.0798. [DOI] [Google Scholar]
- Burris H. A.; Patel M. R.; Cho D. C.; Clarke J. M.; Gutierrez M.; Zaks T. Z.; Frederick J.; Hopson K.; Mody K.; Binanti-Berube A.; et al. A Phase I Multicenter Study to Assess the Safety, Tolerability, and Immunogenicity of MRNA-4157 Alone in Patients with Resected Solid Tumors and in Combination with Pembrolizumab in Patients with Unresectable Solid Tumors. J. Clin. Oncol. 2019, 37, 2523–2523. 10.1200/JCO.2019.37.15_suppl.2523. [DOI] [Google Scholar]
- Sahin U.; Oehm P.; Derhovanessian E.; Jabulowsky R. A.; Vormehr M.; Gold M.; Maurus D.; Schwarck-Kokarakis D.; Kuhn A. N.; Omokoko T.; Kranz L. M.; Diken M.; Kreiter S.; Haas H.; Attig S.; Rae R.; Cuk K.; Kemmer-Brück A.; Breitkreuz A.; Tolliver C.; Caspar J.; Quinkhardt J.; Hebich L.; Stein M.; Hohberger A.; Vogler I.; Liebig I.; Renken S.; Sikorski J.; Leierer M.; Müller V.; Mitzel-Rink H.; Miederer M.; Huber C.; Grabbe S.; Utikal J.; Pinter A.; Kaufmann R.; Hassel J. C.; Loquai C.; Türeci Ö. An RNA Vaccine Drives Immunity in Checkpoint-Inhibitor-Treated Melanoma. Nature 2020, 585 (7823), 107–112. 10.1038/s41586-020-2537-9. [DOI] [PubMed] [Google Scholar]
- Liu H.; Moynihan K. D.; Zheng Y.; Szeto G. L.; Li A. V.; Huang B.; Van Egeren D. S.; Park C.; Irvine D. J. Structure-Based Programming of Lymph Node Targeting in Molecular Vaccines. Nature 2014, 507 (7493), 519–522. 10.1038/nature12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifaz L.; Bonnyay D.; Mahnke K.; Rivera M.; Nussenzweig M. C.; Steinman R. M. Efficient Targeting of Protein Antigen to the Dendritic Cell Receptor DEC-205 in the Steady State Leads to Antigen Presentation on Major Histocompatibility Complex Class I Products and Peripheral CD8+ T Cell Tolerance. J. Exp Med. 2002, 196 (12), 1627–1638. 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifaz L. C.; Bonnyay D. P.; Charalambous A.; Darguste D. I.; Fujii S.-I.; Soares H.; Brimnes M. K.; Moltedo B.; Moran T. M.; Steinman R. M. In Vivo Targeting of Antigens to Maturing Dendritic Cells via the DEC-205 Receptor Improves T Cell Vaccination. J. Exp Med. 2004, 199 (6), 815–824. 10.1084/jem.20032220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris S. T.; Durai V.; Wu R.; Theisen D. J.; Ward J. P.; Bern M. D.; Davidson J. T.; Bagadia P.; Liu T.; Briseño C. G.; Li L.; Gillanders W. E.; Wu G. F.; Yokoyama W. M.; Murphy T. L.; Schreiber R. D.; Murphy K. M. CDC1 Prime and Are Licensed by CD4 + T Cells to Induce Anti-Tumour Immunity. Nature 2020, 584 (7822), 624–629. 10.1038/s41586-020-2611-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossendorp F.; Mengedé E.; Camps M.; Filius R.; Melief C. J. M. Specific T Helper Cell Requirement for Optimal Induction of Cytotoxic T Lymphocytes against Major Histocompatibility Complex Class II Negative Tumors. Journal of Experimental Medicine 1998, 187 (5), 693–702. 10.1084/jem.187.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bon A.; Etchart N.; Rossmann C.; Ashton M.; Hou S.; Gewert D.; Borrow P.; Tough D. F. Cross-Priming of CD8+ T Cells Stimulated by Virus-Induced Type I Interferon. Nat. Immunol 2003, 4 (10), 1009–1015. 10.1038/ni978. [DOI] [PubMed] [Google Scholar]
- Reuter A.; Panozza S. E.; Macri C.; Dumont C.; Li J.; Liu H.; Segura E.; Vega-Ramos J.; Gupta N.; Caminschi I.; Villadangos J. A.; Johnston A. P. R.; Mintern J. D. Criteria for Dendritic Cell Receptor Selection for Efficient Antibody-Targeted Vaccination. J. Immunol. 2015, 194 (6), 2696–2705. 10.4049/jimmunol.1402535. [DOI] [PubMed] [Google Scholar]
- Fossum E.; Tesfaye D. Y.; Bobic S.; Gudjonsson A.; Braathen R.; Lahoud M. H.; Caminschi I.; Bogen B. Targeting Antigens to Different Receptors on Conventional Type 1 Dendritic Cells Impacts the Immune Response. J. Immunol. 2020, 205 (3), 661–673. 10.4049/jimmunol.1901119. [DOI] [PubMed] [Google Scholar]
- Dudziak D.; Kamphorst A. O.; Heidkamp G. F.; Buchholz V. R.; Trumpfheller C.; Yamazaki S.; Cheong C.; Liu K.; Lee H.-W.; Park C. G.; Steinman R. M.; Nussenzweig M. C. Differential Antigen Processing by Dendritic Cell Subsets in Vivo. Science 2007, 315, 107. 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- Idoyaga J.; Lubkin A.; Fiorese C.; Lahoud M. H.; Caminschi I.; Huang Y.; Rodriguez A.; Clausen B. E.; Park C. G.; Trumpfheller C.; Steinman R. M. Comparable T Helper 1 (Th1) and CD8 T-Cell Immunity by Targeting HIV Gag P24 to CD8 Dendritic Cells within Antibodies to Langerin, DEC205, and Clec9A. Proc. Natl. Acad. Sci. U. S. A. 2011, 108 (6), 2384–2389. 10.1073/pnas.1019547108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn L.; Chatterjee B.; Esselborn F.; Smed-Sörensen A.; Nakamura N.; Chalouni C.; Lee B.-C.; Vandlen R.; Keler T.; Lauer P.; Brockstedt D.; Mellman I.; Delamarre L. Antigen Delivery to Early Endosomes Eliminates the Superiority of Human Blood BDCA3+ Dendritic Cells at Cross Presentation. Journal of Experimental Medicine 2013, 210 (5), 1049–1063. 10.1084/jem.20121251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee B.; Smed-Sörensen A.; Cohn L.; Chalouni C.; Vandlen R.; Lee B.-C.; Widger J.; Keler T.; Delamarre L.; Mellman I. Internalization and Endosomal Degradation of Receptor-Bound Antigens Regulate the Efficiency of Cross Presentation by Human Dendritic Cells. Blood 2012, 120 (10), 2011–2020. 10.1182/blood-2012-01-402370. [DOI] [PubMed] [Google Scholar]
- Collin M.; Bigley V. Human Dendritic Cell Subsets: An Update. Immunology 2018, 154 (1), 3–20. 10.1111/imm.12888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongbloed S. L.; Kassianos A. J.; McDonald K. J.; Clark G. J.; Ju X.; Angel C. E.; Chen C.-J. J.; Dunbar P. R.; Wadley R. B.; Jeet V.; Vulink A. J. E.; Hart D. N. J.; Radford K. J. Human CD141+ (BDCA-3)+ Dendritic Cells (DCs) Represent a Unique Myeloid DC Subset That Cross-Presents Necrotic Cell Antigens. Journal of Experimental Medicine 2010, 207 (6), 1247–1260. 10.1084/jem.20092140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin L. F.; Salio M.; Griessinger E.; Anjos-Afonso F.; Craciun L.; Chen J.-L.; Keller A. M.; Joffre O.; Zelenay S.; Nye E.; Le Moine A.; Faure F.; Donckier V.; Sancho D.; Cerundolo V.; Bonnet D.; Reis e Sousa C. Characterization of Human DNGR-1+ BDCA3+ Leukocytes as Putative Equivalents of Mouse CD8α+ Dendritic Cells. Journal of Experimental Medicine 2010, 207 (6), 1261–1271. 10.1084/jem.20092618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merad M.; Sathe P.; Helft J.; Miller J.; Mortha A. The Dendritic Cell Lineage: Ontogeny and Function of Dendritic Cells and Their Subsets in the Steady State and the Inflamed Setting. Annu. Rev. Immunol. 2013, 31 (1), 563–604. 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sichien D.; Lambrecht B. N.; Guilliams M.; Scott C. L. Development of Conventional Dendritic Cells: From Common Bone Marrow Progenitors to Multiple Subsets in Peripheral Tissues. Mucosal Immunol 2017, 10 (4), 831–844. 10.1038/mi.2017.8. [DOI] [PubMed] [Google Scholar]
- Doebel T.; Voisin B.; Nagao K. Langerhans Cells - The Macrophage in Dendritic Cell Clothing. Trends in Immunology 2017, 38 (11), 817–828. 10.1016/j.it.2017.06.008. [DOI] [PubMed] [Google Scholar]
- Satoh T.; Toledo M. A. S.; Boehnke J.; Olschok K.; Flosdorf N.; Götz K.; Küstermann C.; Sontag S.; Seré K.; Koschmieder S. Human DC3 Antigen Presenting Dendritic Cells From Induced Pluripotent Stem Cells. Front. Cell Dev. Biol. 2021, 9, 667304. 10.3389/fcell.2021.667304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakdash G.; Buschow S. I.; Gorris M. A. J.; Halilovic A.; Hato S. V.; Sköld A. E.; Schreibelt G.; Sittig S. P.; Torensma R.; Duiveman-de Boer T.; Schröder C.; Smits E. L.; Figdor C. G.; de Vries I. J. M. Expansion of a BDCA1+CD14+ Myeloid Cell Population in Melanoma Patients May Attenuate the Efficacy of Dendritic Cell Vaccines. Cancer Res. 2016, 76 (15), 4332–4346. 10.1158/0008-5472.CAN-15-1695. [DOI] [PubMed] [Google Scholar]
- Uhlén M.; Fagerberg L.; Hallström B. M.; Lindskog C.; Oksvold P.; Mardinoglu A.; Sivertsson C.; Kampf C.; Sjöstedt E.; Asplund A.; et al. Tissue-Based Map of the Human Proteome. Science 2015, 347 (6220), 1260419. 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- Yanai I.; Benjamin H.; Shmoish M.; Chalifa-Caspi V.; Shklar M.; Ophir R.; Bar-Even A.; Horn-Saban S.; Safran M.; Domany E.; Lancet D.; Shmueli O. Genome-Wide Midrange Transcription Profiles Reveal Expression Level Relationships in Human Tissue Specification. Bioinformatics 2005, 21 (5), 650–659. 10.1093/bioinformatics/bti042. [DOI] [PubMed] [Google Scholar]
- Raskov H.; Orhan A.; Christensen J. P.; Gögenur I. Cytotoxic CD8+ T Cells in Cancer and Cancer Immunotherapy. Br. J. Cancer 2021, 124 (2), 359–367. 10.1038/s41416-020-01048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakiba M.; Zumbo P.; Espinosa-Carrasco G.; Menocal L.; Dündar F.; Carson S. E.; Bruno E. M.; Sanchez-Rivera F. J.; Lowe S. W.; Camara S.; Koche R. P.; Reuter V. P.; Socci N. D.; Whitlock B.; Tamzalit F.; Huse M.; Hellmann M. D.; Wells D. K.; Defranoux N. A.; Betel D.; Philip M.; Schietinger A. TCR Signal Strength Defines Distinct Mechanisms of T Cell Dysfunction and Cancer Evasion. Journal of Experimental Medicine 2022, 219 (2), e20201966 10.1084/jem.20201966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busselaar J.; Tian S.; van Eenennaam H.; Borst J. Helpless Priming Sends CD8+ T Cells on the Road to Exhaustion. Front. Immunol. 2020, 11, 592569. 10.3389/fimmu.2020.592569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alspach E.; Lussier D. M.; Miceli A. P.; Kizhvatov I.; DuPage M.; Luoma A. M.; Meng W.; Lichti C. F.; Esaulova E.; Vomund A. N.; Runci D.; Ward J. P.; Gubin M. M.; Medrano R. F. V.; Arthur C. D.; White J. M.; Sheehan K. C. F.; Chen A.; Wucherpfennig K. W.; Jacks T.; Unanue E. R.; Artyomov M. N.; Schreiber R. D. MHC-II Neoantigens Shape Tumour Immunity and Response to Immunotherapy. Nature 2019, 574 (7780), 696–701. 10.1038/s41586-019-1671-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott P. A.; Hu Z.; Keskin D. B.; Shukla S. A.; Sun J.; Bozym D. J.; Zhang W.; Luoma A.; Giobbie-Hurder A.; Peter L.; Chen C.; Olive O.; Carter T. A.; Li S.; Lieb D. J.; Eisenhaure T.; Gjini E.; Stevens J.; Lane W. J.; Javeri I.; Nellaiappan K.; Salazar A. M.; Daley H.; Seaman M.; Buchbinder E. I.; Yoon C. H.; Harden M.; Lennon N.; Gabriel S.; Rodig S. J.; Barouch D. H.; Aster J. C.; Getz G.; Wucherpfennig K.; Neuberg D.; Ritz J.; Lander E. S.; Fritsch E. F.; Hacohen N.; Wu C. J. An Immunogenic Personal Neoantigen Vaccine for Patients with Melanoma. Nature 2017, 547 (7662), 217–221. 10.1038/nature22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z.; Leet D. E.; Allesøe R. L.; Oliveira G.; Li S.; Luoma A. M.; Liu J.; Forman J.; Huang T.; Iorgulescu J. B.; Holden R.; Sarkizova S.; Gohil S. H.; Redd R. A.; Sun J.; Elagina L.; Giobbie-Hurder A.; Zhang W.; Peter L.; Ciantra Z.; Rodig S.; Olive O.; Shetty K.; Pyrdol J.; Uduman M.; Lee P. C.; Bachireddy P.; Buchbinder E. I.; Yoon C. H.; Neuberg D.; Pentelute B. L.; Hacohen N.; Livak K. J.; Shukla S. A.; Olsen L. R.; Barouch D. H.; Wucherpfennig K. W.; Fritsch E. F.; Keskin D. B.; Wu C. J.; Ott P. A. Personal Neoantigen Vaccines Induce Persistent Memory T Cell Responses and Epitope Spreading in Patients with Melanoma. Nat. Med. 2021, 27 (3), 515–525. 10.1038/s41591-020-01206-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiter S.; Vormehr M.; van de Roemer N.; Diken M.; Löwer M.; Diekmann J.; Boegel S.; Schrörs B.; Vascotto F.; Castle J. C.; Tadmor A. D.; Schoenberger S. P.; Huber C.; Türeci Ö.; Sahin U. Mutant MHC Class II Epitopes Drive Therapeutic Immune Responses to Cancer. Nature 2015, 520 (7549), 692–696. 10.1038/nature14426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin U.; Derhovanessian E.; Miller M.; Kloke B.-P.; Simon P.; Löwer M.; Bukur V.; Tadmor A. D.; Luxemburger U.; Schrörs B.; Omokoko T.; Vormehr M.; Albrecht C.; Paruzynski A.; Kuhn A. N.; Buck J.; Heesch S.; Schreeb K. H.; Müller F.; Ortseifer I.; Vogler I.; Godehardt E.; Attig S.; Rae R.; Breitkreuz A.; Tolliver C.; Suchan M.; Martic G.; Hohberger A.; Sorn P.; Diekmann J.; Ciesla J.; Waksmann O.; Brück A.-K.; Witt M.; Zillgen M.; Rothermel A.; Kasemann B.; Langer D.; Bolte S.; Diken M.; Kreiter S.; Nemecek R.; Gebhardt C.; Grabbe S.; Höller C.; Utikal J.; Huber C.; Loquai C.; Türeci Ö. Personalized RNA Mutanome Vaccines Mobilize Poly-Specific Therapeutic Immunity against Cancer. Nature 2017, 547 (7662), 222–226. 10.1038/nature23003. [DOI] [PubMed] [Google Scholar]
- Diethelm-Okita B. M.; Okita D. K.; Banaszak L.; Conti-Fine B. M. Universal Epitopes for Human CD4+ Cells on Tetanus and Diphtheria Toxins. Journal of Infectious Diseases 2000, 181 (3), 1001–1009. 10.1086/315324. [DOI] [PubMed] [Google Scholar]
- Yuen G. J.; Demissie E.; Pillai S. B Lymphocytes and Cancer: A Love-Hate Relationship. Trends in Cancer 2016, 2 (12), 747–757. 10.1016/j.trecan.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuschenbach M.; von Knebel Doeberitz M.; Wentzensen N. A Systematic Review of Humoral Immune Responses against Tumor Antigens. Cancer Immunol Immunother 2009, 58 (10), 1535–1544. 10.1007/s00262-009-0733-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombetta E. S.; Mellman I. Cell Biology of Antigen Processing in Vitro and in Vivo. Annu. Rev. Immunol. 2005, 23 (1), 975–1028. 10.1146/annurev.immunol.22.012703.104538. [DOI] [PubMed] [Google Scholar]
- Cruz F. M.; Colbert J. D.; Merino E.; Kriegsman B. A.; Rock K. L. The Biology and Underlying Mechanisms of Cross-Presentation of Exogenous Antigens on MHC-I Molecules. Annu. Rev. Immunol. 2017, 35 (1), 149–176. 10.1146/annurev-immunol-041015-055254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embgenbroich M.; Burgdorf S. Current Concepts of Antigen Cross-Presentation. Front. Immunol. 2018, 9, 01643. 10.3389/fimmu.2018.01643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbert J. D.; Cruz F. M.; Rock K. L. Cross-Presentation of Exogenous Antigens on MHC I Molecules. Current Opinion in Immunology 2020, 64, 1–8. 10.1016/j.coi.2019.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wculek S. K.; Cueto F. J.; Mujal A. M.; Melero I.; Krummel M. F.; Sancho D. Dendritic Cells in Cancer Immunology and Immunotherapy. Nature Reviews Immunology 2020, 20 (1), 7–24. 10.1038/s41577-019-0210-z. [DOI] [PubMed] [Google Scholar]
- Dingjan I.; Verboogen D. R.; Paardekooper L. M.; Revelo N. H.; Sittig S. P.; Visser L. J.; von Mollard G. F.; Henriet S. S.; Figdor C. G.; ter Beest M.; van den Bogaart G. Lipid Peroxidation Causes Endosomal Antigen Release for Cross-Presentation. Sci. Rep 2016, 6 (1), 22064. 10.1038/srep22064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiertz E. J. H. J.; Tortorella D.; Bogyo M.; Yu J.; Mothes W.; Jones T. R.; Rapoport T. A.; Ploegh H. L. Sec6l-Mediated Transfer of a Membrane Protein from the Endoplasmic Reticulum to the Proteasome for Destruction. Nature 1996, 384 (6608), 432–438. 10.1038/384432a0. [DOI] [PubMed] [Google Scholar]
- Zehner M.; Marschall A. L.; Bos E.; Schloetel J.-G.; Kreer C.; Fehrenschild D.; Limmer A.; Ossendorp F.; Lang T.; Koster A. J.; Dübel S.; Burgdorf S. The Translocon Protein Sec61 Mediates Antigen Transport from Endosomes in the Cytosol for Cross-Presentation to CD8+ T Cells. Immunity 2015, 42 (5), 850–863. 10.1016/j.immuni.2015.04.008. [DOI] [PubMed] [Google Scholar]
- Lilley B. N.; Ploegh H. L. A Membrane Protein Required for Dislocation of Misfolded Proteins from the ER. Nature 2004, 429 (6994), 834–840. 10.1038/nature02592. [DOI] [PubMed] [Google Scholar]
- Montealegre S.; van Endert P. MHC Class I Cross-Presentation: Stage Lights on Sec22b. Trends Immunol. 2017, 38 (9), 618–621. 10.1016/j.it.2017.07.002. [DOI] [PubMed] [Google Scholar]
- Kovacsovics-Bankowski M.; Rock K. L. A Phagosome-to-Cytosol Pathway for Exogenous Antigens Presented on MHC Class I Molecules. Science 1995, 267 (5195), 243–246. 10.1126/science.7809629. [DOI] [PubMed] [Google Scholar]
- Huang A. Y. C.; Bruce A. T.; Pardoll D. M.; Levitsky H. I. In Vivo Cross-Priming of MHC Class I-Restricted Antigens Requires the TAP Transporter. Immunity 1996, 4 (4), 349–355. 10.1016/S1074-7613(00)80248-4. [DOI] [PubMed] [Google Scholar]
- Tiwari N.; Garbi N.; Reinheckel T.; Moldenhauer G.; Hämmerling G. J.; Momburg F. A Transporter Associated with Antigen-Processing Independent Vacuolar Pathway for the MHC Class I-Mediated Presentation of Endogenous Transmembrane Proteins. J. Immunol. 2007, 178 (12), 7932–7942. 10.4049/jimmunol.178.12.7932. [DOI] [PubMed] [Google Scholar]
- Shen L.; Sigal L. J.; Boes M.; Rock K. L. Important Role of Cathepsin S in Generating Peptides for TAP-Independent MHC Class I Crosspresentation in Vivo. Immunity 2004, 21 (2), 155–165. 10.1016/j.immuni.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Basha G.; Omilusik K.; Chavez-Steenbock A.; Reinicke A. T.; Lack N.; Choi K. B.; Jefferies W. A. A CD74-Dependent MHC Class I Endolysosomal Cross-Presentation Pathway. Nat. Immunol 2012, 13 (3), 237–245. 10.1038/ni.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair-Gupta P.; Baccarini A.; Tung N.; Seyffer F.; Florey O.; Huang Y.; Banerjee M.; Overholtzer M.; Roche P. A.; Tampé R.; Brown B. D.; Amsen D.; Whiteheart S. W.; Blander J. M. TLR Signals Induce Phagosomal MHC-I Delivery from the Endosomal Recycling Compartment to Allow Cross-Presentation. Cell 2014, 158 (3), 506–521. 10.1016/j.cell.2014.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloatti A.; Kotsias F.; Pauwels A.-M.; Carpier J.-M.; Jouve M.; Timmerman E.; Pace L.; Vargas P.; Maurin M.; Gehrmann U.; Joannas L.; Vivar O. I.; Lennon-Duménil A.-M.; Savina A.; Gevaert K.; Beyaert R.; Hoffmann E.; Amigorena S. Toll-like Receptor 4 Engagement on Dendritic Cells Restrains Phago-Lysosome Fusion and Promotes Cross-Presentation of Antigens. Immunity 2015, 43 (6), 1087–1100. 10.1016/j.immuni.2015.11.006. [DOI] [PubMed] [Google Scholar]
- Hoffmann E.; Kotsias F.; Visentin G.; Bruhns P.; Savina A.; Amigorena S. Autonomous Phagosomal Degradation and Antigen Presentation in Dendritic Cells. Proc. Natl. Acad. Sci. U. S. A. 2012, 109 (36), 14556–14561. 10.1073/pnas.1203912109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blander J. M.; Medzhitov R. Toll-Dependent Selection of Microbial Antigens for Presentation by Dendritic Cells. Nature 2006, 440 (7085), 808–812. 10.1038/nature04596. [DOI] [PubMed] [Google Scholar]
- Gully B. S.; Venugopal H.; Fulcher A. J.; Fu Z.; Li J.; Deuss F. A.; Llerena C.; Heath W. R.; Lahoud M. H.; Caminschi I.; Rossjohn J.; Berry R. The Cryo-EM Structure of the Endocytic Receptor DEC-205. J. Biol. Chem. 2021, 296, 100127. 10.1074/jbc.RA120.016451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L.; Chang H.; Shi X.; Peng C.; He Y. Keratin Mediates the Recognition of Apoptotic and Necrotic Cells through Dendritic Cell Receptor DEC205/CD205. Proc. Natl. Acad. Sci. U.S.A. 2016, 113 (47), 13438–13443. 10.1073/pnas.1609331113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrimpton R. E.; Butler M.; Morel A.-S.; Eren E.; Hue S. S.; Ritter M. A. CD205 (DEC-205): A Recognition Receptor for Apoptotic and Necrotic Self. Mol. Immunol 2009, 46 (6), 1229–1239. 10.1016/j.molimm.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahoud M. H.; Ahmet F.; Zhang J.-G.; Meuter S.; Policheni A. N.; Kitsoulis S.; Lee C.-N.; O’Keeffe M.; Sullivan L. C.; Brooks A. G.; Berry R.; Rossjohn J.; Mintern J. D.; Vega-Ramos J.; Villadangos J. A.; Nicola N. A.; Nussenzweig M. C.; Stacey K. J.; Shortman K.; Heath W. R.; Caminschi I. DEC-205 Is a Cell Surface Receptor for CpG Oligonucleotides. Proc. Natl. Acad. Sci. U. S. A. 2012, 109 (40), 16270–16275. 10.1073/pnas.1208796109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M.; McDonald K. J.; Khan S.; Ross I. L.; Vuckovic S.; Chen K.; Munster D.; MacDonald K. P. A.; Hart D. N. J. Expression of Human DEC-205 (CD205) Multilectin Receptor on Leukocytes. Int. Immunol. 2006, 18 (6), 857–869. 10.1093/intimm/dxl022. [DOI] [PubMed] [Google Scholar]
- Swiggard W. J.; Mirza A.; Nussenzweig M. C.; Steinman R. M. DEC-205, a 205-KDa Protein Abundant on Mouse Dendritic Cells and Thymic Epithelium That Is Detected by the Monoclonal Antibody NLDC-145: Purification, Characterization, and N-Terminal Amino Acid Sequence. Cellular Immunology 1995, 165 (2), 302–311. 10.1006/cimm.1995.1218. [DOI] [PubMed] [Google Scholar]
- Johnson T. S.; Mahnke K.; Storn V.; Schönfeld K.; Ring S.; Nettelbeck D. M.; Haisma H. J.; Le Gall F.; Kontermann R. E.; Enk A. H. Inhibition of Melanoma Growth by Targeting of Antigen to Dendritic Cells via an Anti-DEC-205 Single-Chain Fragment Variable Molecule. Clin. Cancer Res. 2008, 14 (24), 8169–8177. 10.1158/1078-0432.CCR-08-1474. [DOI] [PubMed] [Google Scholar]
- Wang B.; Zaidi N.; He L.-Z.; Zhang L.; Kuroiwa J. M.; Keler T.; Steinman R. M. Targeting of the Non-Mutated Tumor Antigen HER2/Neu to Mature Dendritic Cells Induces an Integrated Immune Response That Protects against Breast Cancer in Mice. Breast Cancer Research 2012, 14 (2), R39. 10.1186/bcr3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B.; Kuroiwa J. M. Y.; He L.-Z.; Charalambous A.; Keler T.; Steinman R. M. The Human Cancer Antigen Mesothelin Is More Efficiently Presented to the Mouse Immune System When Targeted to the DEC-205/CD205 Receptor on Dendritic Cells. Ann. N.Y. Acad. Sci. 2009, 1174, 6–17. 10.1111/j.1749-6632.2009.04933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahnke K.; Guo M.; Lee S.; Sepulveda H.; Swain S. L.; Nussenzweig M.; Steinman R. M. The Dendritic Cell Receptor for Endocytosis, DEC-205, Can Recycle and Enhance Antigen Presentation via Major Histocompatibility Complex Class II-Positive Lysosomal Compartments. J. Cell Biol. 2000, 151 (3), 673–684. 10.1083/jcb.151.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj N.; Pavlick A. C.; Ernstoff M. S.; Hanks B. A.; Albertini M. R.; Luke J. J.; Yellin M. J.; Keler T.; Davis T. A.; Crocker A.; et al. A Phase II Randomized Study of CDX-1401, a Dendritic Cell Targeting NY-ESO-1 Vaccine, in Patients with Malignant Melanoma Pre-Treated with Recombinant CDX-301, a Recombinant Human Flt3 Ligand. J. Clin. Oncol. 2016, 34, 9589–9589. 10.1200/JCO.2016.34.15_suppl.9589. [DOI] [Google Scholar]
- Griffiths E. A.; Srivastava P.; Matsuzaki J.; Brumberger Z.; Wang E. S.; Kocent J.; Miller A.; Roloff G. W.; Wong H. Y.; Paluch B. E.; Lutgen-Dunckley L. G.; Martens B. L.; Odunsi K.; Karpf A. R.; Hourigan C. S.; Nemeth M. J. NY-ESO-1 Vaccination in Combination with Decitabine Induces Antigen-Specific T-Lymphocyte Responses in Patients with Myelodysplastic Syndrome. Clin. Cancer Res. 2018, 24 (5), 1019–1029. 10.1158/1078-0432.CCR-17-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj N.; Friedlander P. A.; Pavlick A. C.; Ernstoff M. S.; Gastman B. R.; Hanks B. A.; Curti B. D.; Albertini M. R.; Luke J. J.; Blazquez A. B.; Balan S.; Bedognetti D.; Beechem J. M.; Crocker A. S.; D’Amico L.; Danaher P.; Davis T. A.; Hawthorne T.; Hess B. W.; Keler T.; Lundgren L.; Morishima C.; Ramchurren N.; Rinchai D.; Salazar A. M.; Salim B. A.; Sharon E.; Vitale L. A.; Wang E.; Warren S.; Yellin M. J.; Disis M. L.; Cheever M. A.; Fling S. P. Flt3 Ligand Augments Immune Responses to Anti-DEC-205-NY-ESO-1 Vaccine through Expansion of Dendritic Cell Subsets. Nat. Cancer 2020, 1 (12), 1204–1217. 10.1038/s43018-020-00143-y. [DOI] [PubMed] [Google Scholar]
- Tsuji T.; Matsuzaki J.; Kelly M. P.; Ramakrishna V.; Vitale L.; He L.-Z.; Keler T.; Odunsi K.; Old L. J.; Ritter G.; Gnjatic S. Antibody-Targeted NY-ESO-1 to Mannose Receptor or DEC-205 In Vitro Elicits Dual Human CD8+ and CD4+ T Cell Responses with Broad Antigen Specificity. J. Immunol. 2011, 186 (2), 1218–1227. 10.4049/jimmunol.1000808. [DOI] [PubMed] [Google Scholar]
- Zhang J.-G.; Czabotar P. E.; Policheni A. N.; Caminschi I.; San Wan S.; Kitsoulis S.; Tullett K. M.; Robin A. Y.; Brammananth R.; van Delft M. F.; Lu J.; O’Reilly L. A.; Josefsson E. C.; Kile B. T.; Chin W. J.; Mintern J. D.; Olshina M. A.; Wong W.; Baum J.; Wright M. D.; Huang D. C. S.; Mohandas N.; Coppel R. L.; Colman P. M.; Nicola N. A.; Shortman K.; Lahoud M. H. The Dendritic Cell Receptor Clec9A Binds Damaged Cells via Exposed Actin Filaments. Immunity 2012, 36 (4), 646–657. 10.1016/j.immuni.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Caminschi I.; Proietto A. I.; Ahmet F.; Kitsoulis S.; Shin Teh J.; Lo J. C. Y.; Rizzitelli A.; Wu L.; Vremec D.; van Dommelen S. L. H.; Campbell I. K.; Maraskovsky E.; Braley H.; Davey G. M.; Mottram P.; van de Velde N.; Jensen K.; Lew A. M.; Wright M. D.; Heath W. R.; Shortman K.; Lahoud M. H. The Dendritic Cell Subtype-Restricted C-Type Lectin Clec9A Is a Target for Vaccine Enhancement. Blood 2008, 112 (8), 3264–3273. 10.1182/blood-2008-05-155176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho D.; Joffre O. P.; Keller A. M.; Rogers N. C.; Martínez D.; Hernanz-Falcón P.; Rosewell I.; Sousa C. R. e. Identification of a Dendritic Cell Receptor That Couples Sensing of Necrosis to Immunity. Nature 2009, 458 (7240), 899–903. 10.1038/nature07750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho D.; Mourao-Sa D.; Joffre O. P.; Schulz O.; Rogers N. C.; Pennington D. J.; Carlyle J. R.; Reis e Sousa C. Tumor Therapy in Mice via Antigen Targeting to a Novel, DC-Restricted C-Type Lectin. J. Clin. Invest. 2008, 118 (6), 2098–2110. 10.1172/JCI34584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gou S.; Liu W.; Wang S.; Chen G.; Chen Z.; Qiu L.; Zhou X.; Wu Y.; Qi Y.; Gao Y. Engineered Nanovaccine Targeting Clec9a+ Dendritic Cells Remarkably Enhances the Cancer Immunotherapy Effects of STING Agonist. Nano Lett. 2021, 21 (23), 9939–9950. 10.1021/acs.nanolett.1c03243. [DOI] [PubMed] [Google Scholar]
- Gou S.; Wang S.; Liu W.; Chen G.; Zhang D.; Du J.; Yan Z.; Wang H.; Zhai W.; Sui X.; Wu Y.; Qi Y.; Gao Y. Adjuvant-Free Peptide Vaccine Targeting Clec9a on Dendritic Cells Can Induce Robust Antitumor Immune Response through Syk/IL-21 Axis. Theranostics 2021, 11 (15), 7308–7321. 10.7150/thno.56406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahoud M. H.; Ahmet F.; Kitsoulis S.; Wan S. S.; Vremec D.; Lee C.-N.; Phipson B.; Shi W.; Smyth G. K.; Lew A. M.; Kato Y.; Mueller S. N.; Davey G. M.; Heath W. R.; Shortman K.; Caminschi I. Targeting Antigen to Mouse Dendritic Cells via Clec9A Induces Potent CD4 T Cell Responses Biased toward a Follicular Helper Phenotype. J. Immunol. 2011, 187 (2), 842–850. 10.4049/jimmunol.1101176. [DOI] [PubMed] [Google Scholar]
- Tullett K. M.; Leal Rojas I. M.; Minoda Y.; Tan P. S.; Zhang J.-G.; Smith C.; Khanna R.; Shortman K.; Caminschi I.; Lahoud M. H.; Radford K. J. Targeting CLEC9A Delivers Antigen to Human CD141+ DC for CD4+ and CD8+T Cell Recognition. JCI Insight 2016, 1 (7), e87102 10.1172/jci.insight.87102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masterman K.-A.; Haigh O. L.; Tullett K. M.; Leal-Rojas I. M.; Walpole C.; Pearson F. E.; Cebon J.; Schmidt C.; O’Brien L.; Rosendahl N.; Daraj G.; Caminschi I.; Gschweng E. H.; Hollis R. P.; Kohn D. B.; Lahoud M. H.; Radford K. J. Human CLEC9A Antibodies Deliver NY-ESO-1 Antigen to CD141+ Dendritic Cells to Activate Naïve and Memory NY-ESO-1-Specific CD8+ T Cells. J. Immunother Cancer 2020, 8 (2), e000691 10.1136/jitc-2020-000691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson F. E.; Tullett K. M.; Leal-Rojas I. M.; Haigh O. L.; Masterman K.-A.; Walpole C.; Bridgeman J. S.; McLaren J. E.; Ladell K.; Miners K.; Llewellyn-Lacey S.; Price D. A.; Tunger A.; Schmitz M.; Miles J. J.; Lahoud M. H.; Radford K. J. Human CLEC9A Antibodies Deliver Wilms’ Tumor 1 (WT1) Antigen to CD141+ Dendritic Cells to Activate Naïve and Memory WT1-Specific CD8+ T Cells. Clinical & Translational Immunology 2020, 9 (6), e1141 10.1002/cti2.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreibelt G.; Klinkenberg L. J. J.; Cruz L. J.; Tacken P. J.; Tel J.; Kreutz M.; Adema G. J.; Brown G. D.; Figdor C. G.; de Vries I. J. M. The C-Type Lectin Receptor CLEC9A Mediates Antigen Uptake and (Cross-)Presentation by Human Blood BDCA3+ Myeloid Dendritic Cells. Blood 2012, 119 (10), 2284–2292. 10.1182/blood-2011-08-373944. [DOI] [PubMed] [Google Scholar]
- Crozat K.; Guiton R.; Contreras V.; Feuillet V.; Dutertre C.-A.; Ventre E.; Vu Manh T.-P.; Baranek T.; Storset A. K.; Marvel J.; Boudinot P.; Hosmalin A.; Schwartz-Cornil I.; Dalod M. The XC Chemokine Receptor 1 Is a Conserved Selective Marker of Mammalian Cells Homologous to Mouse CD8α+ Dendritic Cells. Journal of Experimental Medicine 2010, 207 (6), 1283–1292. 10.1084/jem.20100223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böttcher J. P.; Bonavita E.; Chakravarty P.; Blees H.; Cabeza-Cabrerizo M.; Sammicheli S.; Rogers N. C.; Sahai E.; Zelenay S.; Reis e Sousa C. NK Cells Stimulate Recruitment of CDC1 into the Tumor Microenvironment Promoting Cancer Immune Control. Cell 2018, 172 (5), 1022–1037. 10.1016/j.cell.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner B. G.; Dorner M. B.; Zhou X.; Opitz C.; Mora A.; Güttler S.; Hutloff A.; Mages H. W.; Ranke K.; Schaefer M.; Jack R. S.; Henn V.; Kroczek R. A. Selective Expression of the Chemokine Receptor XCR1 on Cross-Presenting Dendritic Cells Determines Cooperation with CD8+ T Cells. Immunity 2009, 31 (5), 823–833. 10.1016/j.immuni.2009.08.027. [DOI] [PubMed] [Google Scholar]
- Bachem A.; Güttler S.; Hartung E.; Ebstein F.; Schaefer M.; Tannert A.; Salama A.; Movassaghi K.; Opitz C.; Mages H. W.; Henn V.; Kloetzel P.-M.; Gurka S.; Kroczek R. A. Superior Antigen Cross-Presentation and XCR1 Expression Define Human CD11c+CD141+ Cells as Homologues of Mouse CD8+ Dendritic Cells. Journal of Experimental Medicine 2010, 207 (6), 1273–1281. 10.1084/jem.20100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossum E.; Grødeland G.; Terhorst D.; Tveita A. A.; Vikse E.; Mjaaland S.; Henri S.; Malissen B.; Bogen B. Vaccine Molecules Targeting Xcr1 on Cross-Presenting DCs Induce Protective CD8+ T-Cell Responses against Influenza Virus. Eur. J. Immunol. 2015, 45 (2), 624–635. 10.1002/eji.201445080. [DOI] [PubMed] [Google Scholar]
- Botelho N. K.; Tschumi B. O.; Hubbell J. A.; Swartz M. A.; Donda A.; Romero P. Combination of Synthetic Long Peptides and XCL1 Fusion Proteins Results in Superior Tumor Control. Front. Immunol. 2019, 10, 00294. 10.3389/fimmu.2019.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung E.; Becker M.; Bachem A.; Reeg N.; Jäkel A.; Hutloff A.; Weber H.; Weise C.; Giesecke C.; Henn V.; Gurka S.; Anastassiadis K.; Mages H. W.; Kroczek R. A. Induction of Potent CD8 T Cell Cytotoxicity by Specific Targeting of Antigen to Cross-Presenting Dendritic Cells In Vivo via Murine or Human XCR1. J. Immunol. 2015, 194 (3), 1069–1079. 10.4049/jimmunol.1401903. [DOI] [PubMed] [Google Scholar]
- Le Gall C.; Cammarata A.; de Haas L.; Ramos-Tomillero I.; Cuenca-Escalona J.; Schouren K.; Wijfjes Z.; Becker A. M. D.; Bödder J.; Dölen Y.; et al. Efficient Targeting of NY-ESO-1 Tumor Antigen to Human CDC1s by Lymphotactin Results in Cross-Presentation and Antigen-Specific T Cell Expansion. J. Immunother. Cancer 2022, 10 (4), e004309 10.1136/jitc-2021-004309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K.; Wu Z.; Zhao H.; Wang Y.; Ge Y.; Wang D.; Li Z.; An C.; Liu Y.; Wang F.; Bi X.; Wang H.; Cai J.; Ma C.; Qu C. XCL1/Glypican-3 Fusion Gene Immunization Generates Potent Antitumor Cellular Immunity and Enhances Anti-PD-1 Efficacy. Cancer Immunol Res. 2020, 8 (1), 81–93. 10.1158/2326-6066.CIR-19-0210. [DOI] [PubMed] [Google Scholar]
- Tuinstra R. L.; Peterson F. C.; Elgin E. S.; Pelzek A. J.; Volkman B. F. An Engineered Second Disulfide Bond Restricts Lymphotactin/XCL1 to a Chemokine-like Conformation with XCR1 Agonist Activity. Biochemistry 2007, 46 (10), 2564–2573. 10.1021/bi602365d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan S.; Paulson J. C. Siglecs as Immune Cell Checkpoints in Disease. Annu. Rev. Immunol. 2020, 38, 365–395. 10.1146/annurev-immunol-102419-035900. [DOI] [PubMed] [Google Scholar]
- Grabowska J.; Lopez-Venegas M. A.; Affandi A. J.; den Haan J. M. M. CD169+ Macrophages Capture and Dendritic Cells Instruct: The Interplay of the Gatekeeper and the General of the Immune System. Front. Immunol. 2018, 9, 02472. 10.3389/fimmu.2018.02472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dinther D.; Veninga H.; Iborra S.; Borg E. G. F.; Hoogterp L.; Olesek K.; Beijer M. R.; Schetters S. T. T.; Kalay H.; Garcia-Vallejo J. J.; Franken K. L.; Cham L. B.; Lang K. S.; van Kooyk Y.; Sancho D.; Crocker P. R.; den Haan J. M. M. Functional CD169 on Macrophages Mediates Interaction with Dendritic Cells for CD8+ T Cell Cross-Priming. Cell Reports 2018, 22 (6), 1484–1495. 10.1016/j.celrep.2018.01.021. [DOI] [PubMed] [Google Scholar]
- Edgar L. J.; Kawasaki N.; Nycholat C. M.; Paulson J. C. Targeted Delivery of Antigen to Activated CD169+ Macrophages Induces Bias for Expansion of CD8+ T Cells. Cell Chemical Biology 2019, 26 (1), 131–136. 10.1016/j.chembiol.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dinther D.; Lopez Venegas M.; Veninga H.; Olesek K.; Hoogterp L.; Revet M.; Ambrosini M.; Kalay H.; Stöckl J.; van Kooyk Y.; den Haan J. M. M. Activation of CD8+ T Cell Responses after Melanoma Antigen Targeting to CD169+ Antigen Presenting Cells in Mice and Humans. Cancers 2019, 11 (2), 183. 10.3390/cancers11020183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhard C. A.; Ried C.; Kochanek S.; Brocker T. CD169+ Macrophages Are Sufficient for Priming of CTLs with Specificities Left out by Cross-Priming Dendritic Cells. Proc. Natl. Acad. Sci. U.S.A. 2015, 112 (17), 5461–5466. 10.1073/pnas.1423356112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veninga H.; Borg E. G. F.; Vreeman K.; Taylor P. R.; Kalay H.; van Kooyk Y.; Kraal G.; Martinez-Pomares L.; den Haan J. M. M. Antigen Targeting Reveals Splenic CD169+ Macrophages as Promoters of Germinal Center B-Cell Responses. Eur. J. Immunol. 2015, 45 (3), 747–757. 10.1002/eji.201444983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Affandi A. J.; Olesek K.; Grabowska J.; Nijen Twilhaar M. K.; Rodríguez E.; Saris A.; Zwart E. S.; Nossent E. J.; Kalay H.; de Kok M. CD169 Defines Activated CD14+ Monocytes With Enhanced CD8+ T Cell Activation Capacity. Front. Immunol. 2021, 12, 697840. 10.3389/fimmu.2021.697840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Affandi A. J.; Grabowska J.; Olesek K.; Lopez Venegas M.; Barbaria A.; Rodríguez E.; Mulder P. P. G.; Pijffers H. J.; Ambrosini M.; Kalay H.; O’Toole T.; Zwart E. S.; Kazemier G.; Nazmi K.; Bikker F. J.; Stöckl J.; van den Eertwegh A. J. M.; de Gruijl T. D.; Storm G.; van Kooyk Y.; den Haan J. M. M. Selective Tumor Antigen Vaccine Delivery to Human CD169+ Antigen-Presenting Cells Using Ganglioside-Liposomes. Proc. Natl. Acad. Sci. U. S. A. 2020, 117 (44), 27528–27539. 10.1073/pnas.2006186117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Pomares L. The Mannose Receptor. Journal of Leukocyte Biology 2012, 92 (6), 1177–1186. 10.1189/jlb.0512231. [DOI] [PubMed] [Google Scholar]
- Taylor M. E.; Drickamer K. Structural Requirements for High Affinity Binding of Complex Ligands by the Macrophage Mannose Receptor. J. Biol. Chem. 1993, 268 (1), 399–404. 10.1016/S0021-9258(18)54164-8. [DOI] [PubMed] [Google Scholar]
- Burgdorf S.; Lukacs-Kornek V.; Kurts C. The Mannose Receptor Mediates Uptake of Soluble but Not of Cell-Associated Antigen for Cross-Presentation. J. Immunol. 2006, 176 (11), 6770–6776. 10.4049/jimmunol.176.11.6770. [DOI] [PubMed] [Google Scholar]
- Burgdorf S.; Kautz A.; Böhnert V.; Knolle P. A.; Kurts C. Distinct Pathways of Antigen Uptake and Intracellular Routing in CD4 and CD8 T Cell Activation. Science 2007, 316 (5824), 612–616. 10.1126/science.1137971. [DOI] [PubMed] [Google Scholar]
- Rauen J.; Kreer C.; Paillard A.; van Duikeren S.; Benckhuijsen W. E.; Camps M. G.; Valentijn A. R. P. M.; Ossendorp F.; Drijfhout J. W.; Arens R.; Burgdorf S. Enhanced Cross-Presentation and Improved CD8+ T Cell Responses after Mannosylation of Synthetic Long Peptides in Mice. PLoS One 2014, 9 (8), e103755 10.1371/journal.pone.0103755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celldex Therapeutics . A Phase II, Open-Label Study of the CDX-1307 Vaccine Regimen as Neoadjuvant and Adjuvant Therapy in Patients With Newly Diagnosed Muscle-Invasive Bladder Cancer Expressing HCG-β. ClinicalTrials.gov Identifier: NCT01094496, 2020. https://clinicaltrials.gov/ct2/show/NCT01094496 (accessed 2022–01–20).
- Morse M. A.; Chapman R.; Powderly J.; Blackwell K.; Keler T.; Green J.; Riggs R.; He L.-Z.; Ramakrishna V.; Vitale L.; Zhao B.; Butler S. A.; Hobeika A.; Osada T.; Davis T.; Clay T.; Lyerly H. K. Phase I Study Utilizing a Novel Antigen-Presenting Cell-Targeted Vaccine with Toll-like Receptor Stimulation to Induce Immunity to Self-Antigens in Cancer Patients. Clin. Cancer Res. 2011, 17 (14), 4844–4853. 10.1158/1078-0432.CCR-11-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodell C. B.; Arlauckas S. P.; Cuccarese M. F.; Garris C. S.; Li R.; Ahmed M. S.; Kohler R. H.; Pittet M. J.; Weissleder R. TLR7/8-Agonist-Loaded Nanoparticles Promote the Polarization of Tumour-Associated Macrophages to Enhance Cancer Immunotherapy. Nat. Biomed Eng. 2018, 2 (8), 578–588. 10.1038/s41551-018-0236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann K.; Castiñeiras-Vilariño M.; Höckendorf U.; Hannesschläger N.; Lemeer S.; Kupka D.; Meyermann S.; Lech M.; Anders H.-J.; Kuster B.; Busch D. H.; Gewies A.; Naumann R.; Groß O.; Ruland J. Clec12a Is an Inhibitory Receptor for Uric Acid Crystals That Regulates Inflammation in Response to Cell Death. Immunity 2014, 40 (3), 389–399. 10.1016/j.immuni.2013.12.015. [DOI] [PubMed] [Google Scholar]
- Hutten T. J. A.; Thordardottir S.; Fredrix H.; Janssen L.; Woestenenk R.; Tel J.; Joosten B.; Cambi A.; Heemskerk M. H. M.; Franssen G. M.; Boerman O. C.; Bakker L. B. H.; Jansen J. H.; Schaap N.; Dolstra H.; Hobo W. CLEC12A-Mediated Antigen Uptake and Cross-Presentation by Human Dendritic Cell Subsets Efficiently Boost Tumor-Reactive T Cell Responses. J. Immunol 2016, 197 (7), 2715–2725. 10.4049/jimmunol.1600011. [DOI] [PubMed] [Google Scholar]
- Lahoud M. H.; Proietto A. I.; Ahmet F.; Kitsoulis S.; Eidsmo L.; Wu L.; Sathe P.; Pietersz S.; Chang H.-W.; Walker I. D.; Maraskovsky E.; Braley H.; Lew A. M.; Wright M. D.; Heath W. R.; Shortman K.; Caminschi I. The C-Type Lectin Clec12A Present on Mouse and Human Dendritic Cells Can Serve as a Target for Antigen Delivery and Enhancement of Antibody Responses1. J. Immunol. 2009, 182 (12), 7587–7594. 10.4049/jimmunol.0900464. [DOI] [PubMed] [Google Scholar]
- Canton J.; Blees H.; Henry C. M.; Buck M. D.; Schulz O.; Rogers N. C.; Childs E.; Zelenay S.; Rhys H.; Domart M.-C.; Collinson L.; Alloatti A.; Ellison C. J.; Amigorena S.; Papayannopoulos V.; Thomas D. C.; Randow F.; Reis e Sousa C. The Receptor DNGR-1 Signals for Phagosomal Rupture to Promote Cross-Presentation of Dead-Cell-Associated Antigens. Nat. Immunol 2021, 22 (2), 140–153. 10.1038/s41590-020-00824-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macri C.; Dumont C.; Panozza S.; Lahoud M. H.; Caminschi I.; Villadangos J. A.; Johnston A. P. R.; Mintern J. D. Antibody-Mediated Targeting of Antigen to C-Type Lectin-like Receptors Clec9A and Clec12A Elicits Different Vaccination Outcomes. Molecular Immunology 2017, 81, 143–150. 10.1016/j.molimm.2016.12.010. [DOI] [PubMed] [Google Scholar]
- Brown G. D.; Gordon S. A New Receptor for β-Glucans. Nature 2001, 413 (6851), 36–37. 10.1038/35092620. [DOI] [PubMed] [Google Scholar]
- Brown G. D. Dectin-1: A Signalling Non-TLR Pattern-Recognition Receptor. Nat. Rev. Immunol 2006, 6 (1), 33–43. 10.1038/nri1745. [DOI] [PubMed] [Google Scholar]
- Kalia N.; Singh J.; Kaur M. The Role of Dectin-1 in Health and Disease. Immunobiology 2021, 226 (2), 152071. 10.1016/j.imbio.2021.152071. [DOI] [PubMed] [Google Scholar]
- Gantner B. N.; Simmons R. M.; Canavera S. J.; Akira S.; Underhill D. M. Collaborative Induction of Inflammatory Responses by Dectin-1 and Toll-like Receptor 2. Journal of Experimental Medicine 2003, 197 (9), 1107–1117. 10.1084/jem.20021787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weck M. M.; Appel S.; Werth D.; Sinzger C.; Bringmann A.; Grünebach F.; Brossart P. HDectin-1 Is Involved in Uptake and Cross-Presentation of Cellular Antigens. Blood 2008, 111 (8), 4264–4272. 10.1182/blood-2006-10-051375. [DOI] [PubMed] [Google Scholar]
- Carter R. W.; Thompson C.; Reid D. M.; Wong S. Y. C.; Tough D. F. Preferential Induction of CD4+ T Cell Responses through in Vivo Targeting of Antigen to Dendritic Cell-Associated C-Type Lectin-1. J. Immunol 2006, 177 (4), 2276–2284. 10.4049/jimmunol.177.4.2276. [DOI] [PubMed] [Google Scholar]
- Trabbic K. R.; Kleski K. A.; Barchi J. J. Stable Gold-Nanoparticle-Based Vaccine for the Targeted Delivery of Tumor-Associated Glycopeptide Antigens. ACS Bio Med. Chem. Au 2021, 1 (1), 31–43. 10.1021/acsbiomedchemau.1c00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J. W.; Tang S. Q.; Rong M. Z.; Zhang M. Q. Synergistic Effect of Dual Targeting Vaccine Adjuvant with Aminated β-Glucan and CpG-Oligodeoxynucleotides for Both Humoral and Cellular Immune Responses. Acta Biomaterialia 2018, 78, 211–223. 10.1016/j.actbio.2018.08.002. [DOI] [PubMed] [Google Scholar]
- Jing Z.; Wang S.; Xu K.; Tang Q.; Li W.; Zheng W.; Shi H.; Su K.; Liu Y.; Hong Z. A Potent Micron Neoantigen Tumor Vaccine GP-Neoantigen Induces Robust Antitumor Activity in Multiple Tumor Models. Advanced Science 2022, 9 (24), 2201496. 10.1002/advs.202201496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y.; Chu X.; Chen J.; Wang Y.; Gao S.; Jiang Y.; Zhu X.; Tan G.; Zhao W.; Yi H.; Xu H.; Ma X.; Lu Y.; Yi Q.; Wang S. Dectin-1-Activated Dendritic Cells Trigger Potent Antitumour Immunity through the Induction of Th9 Cells. Nat. Commun. 2016, 7, 12368. 10.1038/ncomms12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni L.; Gayet I.; Zurawski S.; Duluc D.; Flamar A.-L.; Li X.-H.; O’Bar A.; Clayton S.; Palucka A. K.; Zurawski G.; Banchereau J.; Oh S. Concomitant Activation and Antigen Uptake via Human Dectin-1 Results in Potent Antigen-Specific CD8+ T Cell Responses. J. Immunol. 2010, 185 (6), 3504–3513. 10.4049/jimmunol.1000999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas F. I.; Mauguen A.; Cheung I. Y.; Kramer K.; Kushner B. H.; Ragupathi G.; Cheung N.-K. V.; Modak S. Phase I Trial of Oral Yeast-Derived β-Glucan to Enhance Anti-GD2 Immunotherapy of Resistant High-Risk Neuroblastoma. Cancers (Basel) 2021, 13 (24), 6265. 10.3390/cancers13246265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duluc D.; Joo H.; Ni L.; Yin W.; Upchurch K.; Li D.; Xue Y.; Klucar P.; Zurawski S.; Zurawski G.; Oh S. Induction and Activation of Human Th17 by Targeting Antigens to Dendritic Cells via Dectin-1. J. Immunol. 2014, 192 (12), 5776–5788. 10.4049/jimmunol.1301661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uto T.; Fukaya T.; Takagi H.; Arimura K.; Nakamura T.; Kojima N.; Malissen B.; Sato K. Clec4A4 Is a Regulatory Receptor for Dendritic Cells That Impairs Inflammation and T-Cell Immunity. Nat. Commun. 2016, 7 (1), 11273. 10.1038/ncomms11273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaifu T.; Yabe R.; Maruhashi T.; Chung S.-H.; Tateno H.; Fujikado N.; Hirabayashi J.; Iwakura Y. DCIR and Its Ligand Asialo-Biantennary N-Glycan Regulate DC Function and Osteoclastogenesis. Journal of Experimental Medicine 2021, 218 (12), e20210435 10.1084/jem.20210435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloem K.; Vuist I. M.; van der Plas A.-J.; Knippels L. M. J.; Garssen J.; Garcia-Vallejo J. J.; van Vliet S. J.; van Kooyk Y. Ligand Binding and Signaling of Dendritic Cell Immunoreceptor (DCIR) Is Modulated by the Glycosylation of the Carbohydrate Recognition Domain. PLoS One 2013, 8 (6), e66266 10.1371/journal.pone.0066266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubert K.; Lehmann C. H. K.; Heger L.; Baranska A.; Staedtler A. M.; Buchholz V. R.; Yamazaki S.; Heidkamp G. F.; Eissing N.; Zebroski H.; Nussenzweig M. C.; Nimmerjahn F.; Dudziak D. Antigen Delivery to CD11c+CD8- Dendritic Cells Induces Protective Immune Responses against Experimental Melanoma in Mice In Vivo. J. Immunol. 2014, 192 (12), 5830–5838. 10.4049/jimmunol.1300975. [DOI] [PubMed] [Google Scholar]
- Henriques H. R.; Rampazo E. V.; Gonçalves A. J. S.; Vicentin E. C. M.; Amorim J. H.; Panatieri R. H.; Amorim K. N. S.; Yamamoto M. M.; Ferreira L. C. S.; Alves A. M. B.; Boscardin S. B. Targeting the Non-Structural Protein 1 from Dengue Virus to a Dendritic Cell Population Confers Protective Immunity to Lethal Virus Challenge. PLOS Neglected Tropical Diseases 2013, 7 (7), e2330 10.1371/journal.pntd.0002330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornefett J.; Krause J.; Klose K.; Fingas F.; Hassert R.; Benga L.; Grunwald T.; Müller U.; Schrödl W.; Baums C. G. Comparative Analysis of Humoral Immune Responses and Pathologies of BALB/c and C57BL/6 Wildtype Mice Experimentally Infected with a Highly Virulent Rodentibacter Pneumotropicus (Pasteurella Pneumotropica) Strain. BMC Microbiology 2018, 18 (1), 45. 10.1186/s12866-018-1186-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trunova G. V.; Makarova O. V.; Diatroptov M. E.; Bogdanova I. M.; Mikchailova L. P.; Abdulaeva S. O. Morphofunctional Characteristic of the Immune System in BALB/c and C57Bl/6 Mice. Bull. Exp. Biol. Med. 2011, 151 (1), 99–102. 10.1007/s10517-011-1268-1. [DOI] [PubMed] [Google Scholar]
- Klechevsky E.; Flamar A.-L.; Cao Y.; Blanck J.-P.; Liu M.; O’Bar A.; Agouna-Deciat O.; Klucar P.; Thompson-Snipes L.; Zurawski S.; Reiter Y.; Palucka A. K.; Zurawski G.; Banchereau J. Cross-Priming CD8+ T Cells by Targeting Antigens to Human Dendritic Cells through DCIR. Blood 2010, 116 (10), 1685–1697. 10.1182/blood-2010-01-264960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Vallejo J. J.; Bloem K.; Knippels L. M. J.; Garssen J.; van Vliet S. J.; van Kooyk Y. The Consequences of Multiple Simultaneous C-Type Lectin-Ligand Interactions: DCIR Alters the Endo-Lysosomal Routing of DC-SIGN. Front. Immunol. 2015, 6, 00087. 10.3389/fimmu.2015.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artigas G.; Monteiro J. T.; Hinou H.; Nishimura S.-I.; Lepenies B.; Garcia-Martin F. Glycopeptides as Targets for Dendritic Cells: Exploring MUC1 Glycopeptides Binding Profile toward Macrophage Galactose-Type Lectin (MGL) Orthologs. J. Med. Chem. 2017, 60 (21), 9012–9021. 10.1021/acs.jmedchem.7b01242. [DOI] [PubMed] [Google Scholar]
- Gibadullin R.; Farnsworth D. W.; Barchi J. J. Jr.; Gildersleeve J. C. GalNAc-Tyrosine Is a Ligand of Plant Lectins, Antibodies, and Human and Murine Macrophage Galactose-Type Lectins. ACS Chem. Biol. 2017, 12 (8), 2172–2182. 10.1021/acschembio.7b00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vliet S. J.; van Liempt E.; Saeland E.; Aarnoudse C. A.; Appelmelk B.; Irimura T.; Geijtenbeek T. B. H.; Blixt O.; Alvarez R.; van Die I.; van Kooyk Y. Carbohydrate Profiling Reveals a Distinctive Role for the C-Type Lectin MGL in the Recognition of Helminth Parasites and Tumor Antigens by Dendritic Cells. Int. Immunol. 2005, 17 (5), 661–669. 10.1093/intimm/dxh246. [DOI] [PubMed] [Google Scholar]
- Gu C.; Wang L.; Zurawski S.; Oh S. Signaling Cascade through DC-ASGPR Induces Transcriptionally Active CREB for IL-10 Induction and Immune Regulation. J. Immunol. 2019, 203 (2), 389–399. 10.4049/jimmunol.1900289. [DOI] [PubMed] [Google Scholar]
- Heger L.; Balk S.; Lühr J. J.; Heidkamp G. F.; Lehmann C. H. K.; Hatscher L.; Purbojo A.; Hartmann A.; Garcia-Martin F.; Nishimura S.-I. CLEC10A Is a Specific Marker for Human CD1c+ Dendritic Cells and Enhances Their Toll-Like Receptor 7/8-Induced Cytokine Secretion. Front. Immunol. 2018, 9, 00744. 10.3389/fimmu.2018.00744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S. K.; Streng-Ouwehand I.; Litjens M.; Weelij D. R.; García-Vallejo J. J.; van Vliet S. J.; Saeland E.; van Kooyk Y. Characterization of Murine MGL1 and MGL2 C-Type Lectins: Distinct Glycan Specificities and Tumor Binding Properties. Molecular Immunology 2009, 46 (6), 1240–1249. 10.1016/j.molimm.2008.11.021. [DOI] [PubMed] [Google Scholar]
- Singh S. K.; Streng-Ouwehand I.; Litjens M.; Kalay H.; Saeland E.; van Kooyk Y. Tumour-Associated Glycan Modifications of Antigen Enhance MGL2 Dependent Uptake and MHC Class I Restricted CD8 T Cell Responses. Int. J. Cancer 2011, 128 (6), 1371–1383. 10.1002/ijc.25458. [DOI] [PubMed] [Google Scholar]
- Kleski K. A.; Trabbic K. R.; Shi M.; Bourgault J.-P.; Andreana P. R. Enhanced Immune Response Against the Thomsen-Friedenreich Tumor Antigen Using a Bivalent Entirely Carbohydrate Conjugate. Molecules 2020, 25 (6), 1319. 10.3390/molecules25061319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu C.; Zhao H.; Wang Y.; Cai H.; Xiao Y.; Zeng Y.; Chen H. Tumor-Associated Antigens: Tn Antigen, STn Antigen, and T Antigen. HLA 2016, 88 (6), 275–286. 10.1111/tan.12900. [DOI] [PubMed] [Google Scholar]
- Rosenbaum P.; Artaud C.; Bay S.; Ganneau C.; Campone M.; Delaloge S.; Gourmelon C.; Loirat D.; Medioni J.; Pein F.; Sablin M.-P.; Tredan O.; Varga A.; Leclerc C. The Fully Synthetic Glycopeptide MAG-Tn3 Therapeutic Vaccine Induces Tumor-Specific Cytotoxic Antibodies in Breast Cancer Patients. Cancer Immunol Immunother 2020, 69 (5), 703–716. 10.1007/s00262-020-02503-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoletano C.; Rughetti A.; Agervig Tarp M. P.; Coleman J.; Bennett E. P.; Picco G.; Sale P.; Denda-Nagai K.; Irimura T.; Mandel U.; Clausen H.; Frati L.; Taylor-Papadimitriou J.; Burchell J.; Nuti M. Tumor-Associated Tn-MUC1 Glycoform Is Internalized through the Macrophage Galactose-Type C-Type Lectin and Delivered to the HLA Class I and II Compartments in Dendritic Cells. Cancer Res. 2007, 67 (17), 8358–8367. 10.1158/0008-5472.CAN-07-1035. [DOI] [PubMed] [Google Scholar]
- Freire T.; Lo-Man R.; Bay S.; Leclerc C. Tn Glycosylation of the MUC6 Protein Modulates Its Immunogenicity and Promotes the Induction of Th17-Biased T Cell Responses *. J. Biol. Chem. 2011, 286 (10), 7797–7811. 10.1074/jbc.M110.209742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid E.; Major P.; Bergeron A.; Finn O. J.; Salter R. D.; Eady R.; Yassine-Diab B.; Favre D.; Peretz Y.; Landry C.; Hotte S.; Mukherjee S. D.; Dekaban G. A.; Fink C.; Foster P. J.; Gaudet J.; Gariepy J.; Sekaly R.-P.; Lacombe L.; Fradet Y.; Foley R. Tn-MUC1 DC Vaccination of Rhesus Macaques and a Phase I/II Trial in Patients with Nonmetastatic Castrate-Resistant Prostate Cancer. Cancer Immunology Research 2016, 4 (10), 881–892. 10.1158/2326-6066.CIR-15-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani N.; Brunner P. M.; Stingl G. Changing Views of the Role of Langerhans Cells. J. Invest. Dermatol. 2012, 132, 872–881. 10.1038/jid.2011.437. [DOI] [PubMed] [Google Scholar]
- Flacher V.; Tripp C. H.; Stoitzner P.; Haid B.; Ebner S.; Del Frari B.; Koch F.; Park C. G.; Steinman R. M.; Idoyaga J.; Romani N. Epidermal Langerhans Cells Rapidly Capture and Present Antigens from C-Type Lectin-Targeting Antibodies Deposited in the Dermis. Journal of Investigative Dermatology 2010, 130 (3), 755–762. 10.1038/jid.2009.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Witte L.; Nabatov A.; Pion M.; Fluitsma D.; de Jong M. A. W. P.; de Gruijl T.; Piguet V.; van Kooyk Y.; Geijtenbeek T. B. H. Langerin Is a Natural Barrier to HIV-1 Transmission by Langerhans Cells. Nat. Med. 2007, 13 (3), 367–371. 10.1038/nm1541. [DOI] [PubMed] [Google Scholar]
- Tateno H.; Ohnishi K.; Yabe R.; Hayatsu N.; Sato T.; Takeya M.; Narimatsu H.; Hirabayashi J. Dual Specificity of Langerin to Sulfated and Mannosylated Glycans via a Single C-Type Carbohydrate Recognition Domain *. J. Biol. Chem. 2010, 285 (9), 6390–6400. 10.1074/jbc.M109.041863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idoyaga J.; Cheong C.; Suda K.; Suda N.; Kim J. Y.; Lee H.; Park C. G.; Steinman R. M. Cutting Edge: Langerin/CD207 Receptor on Dendritic Cells Mediates Efficient Antigen Presentation on MHC I and II Products in Vivo. J. Immunol 2008, 180 (6), 3647–3650. 10.4049/jimmunol.180.6.3647. [DOI] [PubMed] [Google Scholar]
- van der Vlist M.; de Witte L.; de Vries R. D.; Litjens M.; de Jong M. A. W. P.; Fluitsma D.; de Swart R. L.; Geijtenbeek T. B. H. Human Langerhans Cells Capture Measles Virus through Langerin and Present Viral Antigens to CD4+ T Cells but Are Incapable of Cross-Presentation. Eur. J. Immunol. 2011, 41 (9), 2619–2631. 10.1002/eji.201041305. [DOI] [PubMed] [Google Scholar]
- Stary G.; Klein I.; Bauer W.; Koszik F.; Reininger B.; Kohlhofer S.; Gruber K.; Skvara H.; Jung T.; Stingl G. Glucocorticosteroids Modify Langerhans Cells To Produce TGF-β and Expand Regulatory T Cells. J. Immunol. 2011, 186 (1), 103–112. 10.4049/jimmunol.1002485. [DOI] [PubMed] [Google Scholar]
- Schulze J.; Rentzsch M.; Kim D.; Bellmann L.; Stoitzner P.; Rademacher C. A Liposomal Platform for Delivery of a Protein Antigen to Langerin-Expressing Cells. Biochemistry 2019, 58 (21), 2576–2580. 10.1021/acs.biochem.9b00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamhoff E.-C.; Schulze J.; Bellmann L.; Rentzsch M.; Bachem G.; Fuchsberger F. F.; Rademacher J.; Hermann M.; Del Frari B.; van Dalen R.; Hartmann D.; van Sorge N. M.; Seitz O.; Stoitzner P.; Rademacher C. A Specific, Glycomimetic Langerin Ligand for Human Langerhans Cell Targeting. ACS Cent. Sci. 2019, 5 (5), 808–820. 10.1021/acscentsci.9b00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus K.; Wamhoff E.-C.; Freichel T.; Grafmüller A.; Rademacher C.; Hartmann L. Asymmetrically Branched Precision Glycooligomers Targeting Langerin. Biomacromolecules 2019, 20 (11), 4088–4095. 10.1021/acs.biomac.9b00906. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek T. B. H.; Torensma R.; van Vliet S. J.; van Duijnhoven G. C. F.; Adema G. J.; van Kooyk Y.; Figdor C. G. Identification of DC-SIGN, a Novel Dendritic Cell-Specific ICAM-3 Receptor That Supports Primary Immune Responses. Cell 2000, 100 (5), 575–585. 10.1016/S0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- van Liempt E.; Bank C. M. C.; Mehta P.; García-Vallejo J. J.; Kawar Z. S.; Geyer R.; Alvarez R. A.; Cummings R. D.; van Kooyk Y.; van Die I. Specificity of DC-SIGN for Mannose- and Fucose-Containing Glycans. FEBS Lett. 2006, 580 (26), 6123–6131. 10.1016/j.febslet.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Jarvis C. M.; Zwick D. B.; Grim J. C.; Alam M. M.; Prost L. R.; Gardiner J. C.; Park S.; Zimdars L. L.; Sherer N. M.; Kiessling L. L. Antigen Structure Affects Cellular Routing through DC-SIGN. Proc. Natl. Acad. Sci. U.S.A. 2019, 116 (30), 14862–14867. 10.1073/pnas.1820165116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S. K.; Stephani J.; Schaefer M.; Kalay H.; García-Vallejo J. J.; den Haan J.; Saeland E.; Sparwasser T.; van Kooyk Y. Targeting Glycan Modified OVA to Murine DC-SIGN Transgenic Dendritic Cells Enhances MHC Class I and II Presentation. Mol. Immunol 2009, 47 (2–3), 164–174. 10.1016/j.molimm.2009.09.026. [DOI] [PubMed] [Google Scholar]
- Schaefer M.; Reiling N.; Fessler C.; Stephani J.; Taniuchi I.; Hatam F.; Yildirim A. O.; Fehrenbach H.; Walter K.; Ruland J.; Wagner H.; Ehlers S.; Sparwasser T. Decreased Pathology and Prolonged Survival of Human DC-SIGN Transgenic Mice during Mycobacterial Infection. J. Immunol. 2008, 180 (10), 6836–6845. 10.4049/jimmunol.180.10.6836. [DOI] [PubMed] [Google Scholar]
- Gargett T.; Abbas M. N.; Rolan P.; Price J. D.; Gosling K. M.; Ferrante A.; Ruszkiewicz A.; Atmosukarto I. I. C.; Altin J.; Parish C. R.; Brown M. P. Phase I Trial of Lipovaxin-MM, a Novel Dendritic Cell-Targeted Liposomal Vaccine for Malignant Melanoma. Cancer Immunol Immunother 2018, 67 (9), 1461–1472. 10.1007/s00262-018-2207-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schetters S. T. T.; Kruijssen L. J. W.; Crommentuijn M. H. W.; Kalay H.; Ochando J.; den Haan J. M. M.; Garcia-Vallejo J. J.; van Kooyk Y. Mouse DC-SIGN/CD209a as Target for Antigen Delivery and Adaptive Immunity. Front. Immunol. 2018, 9, 00990. 10.3389/fimmu.2018.00990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R.-J. E.; Hogervorst T. P.; Achilli S.; Bruijns S. C.; Arnoldus T.; Vivès C.; Wong C. C.; Thépaut M.; Meeuwenoord N. J.; van den Elst H. Systematic Dual Targeting of Dendritic Cell C-Type Lectin Receptor DC-SIGN and TLR7 Using a Trifunctional Mannosylated Antigen. Front. Chem. 2019, 7, 00650. 10.3389/fchem.2019.00650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacken P. J.; Ginter W.; Berod L.; Cruz L. J.; Joosten B.; Sparwasser T.; Figdor C. G.; Cambi A. Targeting DC-SIGN via Its Neck Region Leads to Prolonged Antigen Residence in Early Endosomes, Delayed Lysosomal Degradation, and Cross-Presentation. Blood 2011, 118 (15), 4111–4119. 10.1182/blood-2011-04-346957. [DOI] [PubMed] [Google Scholar]
- Cella M.; Engering A.; Pinet V.; Pieters J.; Lanzavecchia A. Inflammatory Stimuli Induce Accumulation of MHC Class II Complexes on Dendritic Cells. Nature 1997, 388 (6644), 782–787. 10.1038/42030. [DOI] [PubMed] [Google Scholar]
- Fang T.; Van Elssen C. H. M. J.; Duarte J. N.; Guzman J. S.; Chahal J. S.; Ling J.; Ploegh H. L. Targeted Antigen Delivery by an Anti-Class II MHC VHH Elicits Focused ΑMUC1(Tn) Immunity. Chem. Sci. 2017, 8 (8), 5591–5597. 10.1039/C7SC00446J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte J. N.; Cragnolini J. J.; Swee L. K.; Bilate A. M.; Bader J.; Ingram J. R.; Rashidfarrokhi A.; Fang T.; Schiepers A.; Hanke L.; Ploegh H. L. Generation of Immunity against Pathogens via Single-Domain Antibody-Antigen Constructs. J. Immunol 2016, 197 (12), 4838–4847. 10.4049/jimmunol.1600692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pishesha N.; Harmand T. J.; Rothlauf P. W.; Praest P.; Alexander R. K.; van den Doel R.; Liebeskind M. J.; Vakaki M. A.; McCaul N.; Wijne C.; Verhaar E.; Pinney W.; Heston H.; Bloyet L.-M.; Pontelli M. C.; Ilagan M. X. G.; Jan Lebbink R.; Buchser W. J.; Wiertz E. J. H. J.; Whelan S. P. J.; Ploegh H. L. A Class II MHC-Targeted Vaccine Elicits Immunity against SARS-CoV-2 and Its Variants. Proc. Natl. Acad. Sci. U. S. A. 2021, 118 (44), e2116147118 10.1073/pnas.2116147118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Elssen C. H. M. J.; Rashidian M.; Vrbanac V.; Wucherpfennig K. W.; Habre Z. el; Sticht J.; Freund C.; Jacobsen J. T.; Cragnolini J.; Ingram J.; et al. Noninvasive Imaging of Human Immune Responses in a Human Xenograft Model of Graft-Versus-Host Disease. J. Nucl. Med. 2017, 58 (6), 1003–1008. 10.2967/jnumed.116.186007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingalls R. R.; Golenbock D. T. CD11c/CD18, a Transmembrane Signaling Receptor for Lipopolysaccharide. Journal of Experimental Medicine 1995, 181 (4), 1473–1479. 10.1084/jem.181.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadhu C.; Ting H. J.; Lipsky B.; Hensley K.; Garcia-Martinez L. F.; Simon S. I.; Staunton D. E. CD11c/CD18: Novel Ligands and a Role in Delayed-Type Hypersensitivity. Journal of Leukocyte Biology 2007, 81 (6), 1395–1403. 10.1189/jlb.1106680. [DOI] [PubMed] [Google Scholar]
- Castro F. V. V.; Tutt A. L.; White A. L.; Teeling J. L.; James S.; French R. R.; Glennie M. J. CD11c Provides an Effective Immunotarget for the Generation of Both CD4 and CD8 T Cell Responses. Eur. J. Immunol. 2008, 38 (8), 2263–2273. 10.1002/eji.200838302. [DOI] [PubMed] [Google Scholar]
- White A. L.; Tutt A. L.; James S.; Wilkinson K. A.; Castro F. V. V.; Dixon S. V.; Hitchcock J.; Khan M.; Al-Shamkhani A.; Cunningham A. F.; Glennie M. J. Ligation of CD11c during Vaccination Promotes Germinal Centre Induction and Robust Humoral Responses without Adjuvant. Immunology 2010, 131 (1), 141–151. 10.1111/j.1365-2567.2010.03285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H.; Wang S.; Zhang D.; Hou S.; Qian W.; Li B.; Guo H.; Kou G.; He J.; Wang H.; Guo Y. Targeted Delivery of Tumor Antigens to Activated Dendritic Cells via CD11c Molecules Induces Potent Antitumor Immunity in Mice. Clin. Cancer Res. 2009, 15 (14), 4612–4621. 10.1158/1078-0432.CCR-08-3321. [DOI] [PubMed] [Google Scholar]
- Demangel C.; Zhou J.; Choo A. B. H.; Shoebridge G.; Halliday G. M.; Britton W. J. Single Chain Antibody Fragments for the Selective Targeting of Antigens to Dendritic Cells. Mol. Immunol 2005, 42 (8), 979–985. 10.1016/j.molimm.2004.09.034. [DOI] [PubMed] [Google Scholar]
- Skea D. L.; Barber B. H. Studies of the Adjuvant-Independent Antibody Response to Immunotargeting. Target Structure Dependence, Isotype Distribution, and Induction of Long Term Memory. J. Immunol. 1993, 151 (7), 3557–3568. 10.4049/jimmunol.151.7.3557. [DOI] [PubMed] [Google Scholar]
- van Kooten C.; Banchereau J. CD40-CD40 Ligand. Journal of Leukocyte Biology 2000, 67 (1), 2–17. 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- Bajor D. L.; Mick R.; Riese M. J.; Huang A. C.; Sullivan B.; Richman L. P.; Torigian D. A.; George S. M.; Stelekati E.; Chen F.; Melenhorst J. J.; Lacey S. F.; Xu X.; Wherry E. J.; Gangadhar T. C.; Amaravadi R. K.; Schuchter L. M.; Vonderheide R. H. Long-Term Outcomes of a Phase I Study of Agonist CD40 Antibody and CTLA-4 Blockade in Patients with Metastatic Melanoma. OncoImmunology 2018, 7 (10), e1468956 10.1080/2162402X.2018.1468956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonderheide R. H. CD40 Agonist Antibodies in Cancer Immunotherapy. Annual Review of Medicine 2020, 71 (1), 47–58. 10.1146/annurev-med-062518-045435. [DOI] [PubMed] [Google Scholar]
- Hellman P.; Eriksson H. Early Activation Markers of Human Peripheral Dendritic Cells. Hum. Immunol. 2007, 68 (5), 324–333. 10.1016/j.humimm.2007.01.018. [DOI] [PubMed] [Google Scholar]
- Hollenbaugh D.; Mischel-Petty N.; Edwards C. P.; Simon J. C.; Denfeld R. W.; Kiener P. A.; Aruffo A. Expression of Functional CD40 by Vascular Endothelial Cells. Journal of Experimental Medicine 1995, 182 (1), 33–40. 10.1084/jem.182.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schjetne K. W.; Fredriksen A. B.; Bogen B. Delivery of Antigen to CD40 Induces Protective Immune Responses against Tumors1. J. Immunol. 2007, 178 (7), 4169–4176. 10.4049/jimmunol.178.7.4169. [DOI] [PubMed] [Google Scholar]
- Szekeres Z.; Herbáth M.; Szittner Z.; Papp K.; Erdei A.; Prechl J. Modulation of the Humoral Immune Response by Targeting CD40 and FcγRII/III; Delivery of Soluble but Not Particulate Antigen to CD40 Enhances Antibody Responses with a Th1 Bias. Molecular Immunology 2011, 49 (1), 155–162. 10.1016/j.molimm.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Cruz L. J.; Rosalia R. A.; Kleinovink J. W.; Rueda F.; Löwik C. W. G. M.; Ossendorp F. Targeting Nanoparticles to CD40, DEC-205 or CD11c Molecules on Dendritic Cells for Efficient CD8+ T Cell Response: A Comparative Study. J. Controlled Release 2014, 192, 209–218. 10.1016/j.jconrel.2014.07.040. [DOI] [PubMed] [Google Scholar]
- Rosalia R. A.; Cruz L. J.; van Duikeren S.; Tromp A. T.; Silva A. L.; Jiskoot W.; de Gruijl T.; Löwik C.; Oostendorp J.; van der Burg S. H.; Ossendorp F. CD40-Targeted Dendritic Cell Delivery of PLGA-Nanoparticle Vaccines Induce Potent Anti-Tumor Responses. Biomaterials 2015, 40, 88–97. 10.1016/j.biomaterials.2014.10.053. [DOI] [PubMed] [Google Scholar]
- Graham J. P.; Authie P.; Yu C. I.; Zurawski S. M.; Li X.-H.; Marches F.; Flamar A.-L.; Acharya A.; Banchereau J.; Palucka A. K. Targeting Dendritic Cells in Humanized Mice Receiving Adoptive T Cells via Monoclonal Antibodies Fused to Flu Epitopes. Vaccine 2016, 34 (41), 4857–4865. 10.1016/j.vaccine.2016.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamar A.-L.; Xue Y.; Zurawski S. M.; Montes M.; King B.; Sloan L.; Oh S.; Banchereau J.; Levy Y.; Zurawski G. Targeting Concatenated HIV Antigens to Human CD40 Expands a Broad Repertoire of Multifunctional CD4+ and CD8+ T Cells. AIDS 2013, 27 (13), 2041. 10.1097/QAD.0b013e3283624305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin W.; Duluc D.; Joo H.; Xue Y.; Gu C.; Wang Z.; Wang L.; Ouedraogo R.; Oxford L.; Clark A.; Parikh F.; Kim-Schulze S.; Thompson-Snipes L.; Lee S.-Y.; Beauregard C.; Woo J.-H.; Zurawski S.; Sikora A. G.; Zurawski G.; Oh S. Therapeutic HPV Cancer Vaccine Targeted to CD40 Elicits Effective CD8+ T-Cell Immunity. Cancer Immunology Research 2016, 4 (10), 823–834. 10.1158/2326-6066.CIR-16-0128. [DOI] [PubMed] [Google Scholar]
- Yin W.; Gorvel L.; Zurawski S.; Li D.; Ni L.; Duluc D.; Upchurch K.; Kim J.; Gu C.; Ouedraogo R.; Wang Z.; Xue Y.; Joo H.; Gorvel J.-P.; Zurawski G.; Oh S. Functional Specialty of CD40 and Dendritic Cell Surface Lectins for Exogenous Antigen Presentation to CD8+ and CD4+ T Cells. EBioMedicine 2016, 5, 46–58. 10.1016/j.ebiom.2016.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hägerbrand K.; Varas L.; Deronic A.; Nyesiga B.; Sundstedt A.; Ljung L.; Sakellariou C.; Werchau D.; Thagesson M.; Jimenez D. G.; et al. Bispecific Antibodies Targeting CD40 and Tumor-Associated Antigens Promote Cross-Priming of T Cells Resulting in an Antitumor Response Superior to Monospecific Antibodies. J. Immunother. Cancer 2022, 10 (11), e005018 10.1136/jitc-2022-005018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sum E.; Rapp M.; Dürr H.; Mazumdar A.; Romero P. J.; Trumpfheller C.; Umaña P. The Tumor-Targeted CD40 Agonist CEA-CD40 Promotes T Cell Priming via a Dual Mode of Action by Increasing Antigen Delivery to Dendritic Cells and Enhancing Their Activation. J. Immunother Cancer 2022, 10 (3), e003264 10.1136/jitc-2021-003264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilliams M.; Bruhns P.; Saeys Y.; Hammad H.; Lambrecht B. N. The Function of Fcγ Receptors in Dendritic Cells and Macrophages. Nat. Rev. Immunol 2014, 14 (2), 94–108. 10.1038/nri3582. [DOI] [PubMed] [Google Scholar]
- Bruhns P.; Iannascoli B.; England P.; Mancardi D. A.; Fernandez N.; Jorieux S.; Daëron M. Specificity and Affinity of Human Fcγ Receptors and Their Polymorphic Variants for Human IgG Subclasses. Blood 2009, 113 (16), 3716–3725. 10.1182/blood-2008-09-179754. [DOI] [PubMed] [Google Scholar]
- Bruhns P.; Jönsson F. Mouse and Human FcR Effector Functions. Immunological Reviews 2015, 268 (1), 25–51. 10.1111/imr.12350. [DOI] [PubMed] [Google Scholar]
- Junker F.; Gordon J.; Qureshi O. Fc Gamma Receptors and Their Role in Antigen Uptake, Presentation, and T Cell Activation. Front Immunol 2020, 11, 1393. 10.3389/fimmu.2020.01393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemerding A. M.; Köhl J.; Pandey M. K.; Kuipers A.; Leusen J. H.; Boross P.; Nederend M.; Vidarsson G.; Weersink A. Y. L.; van de Winkel J. G. J.; van Kessel K. P. M.; van Strijp J. A. G. Staphylococcus Aureus Formyl Peptide Receptor-like 1 Inhibitor (FLIPr) and Its Homologue FLIPr-like Are Potent FcγR Antagonists That Inhibit IgG-Mediated Effector Functions. J. Immunol. 2013, 191 (1), 353–362. 10.4049/jimmunol.1203243. [DOI] [PubMed] [Google Scholar]
- Chiang C.-Y.; Wu C.-C.; Chen Y.-J.; Liu S.-J.; Leng C.-H.; Chen H.-W. Delivery of Antigen to CD8+ Dendritic Cells by Fusing Antigen With Formyl Peptide Receptor-Like 1 Inhibitor Protein Induces Antitumor Immunity. Front. Immunol. 2019, 10, 01839. 10.3389/fimmu.2019.01839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C.-C.; Chiang C.-Y.; Liu S.-J.; Chen H.-W. A Novel Recombinant Fcγ Receptor-Targeted Survivin Combines with Chemotherapy for Efficient Cancer Treatment. Biomedicines 2021, 9 (7), 806. 10.3390/biomedicines9070806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flinsenberg T. W. H.; Compeer E. B.; Koning D.; Klein M.; Amelung F. J.; van Baarle D.; Boelens J. J.; Boes M. Fcγ Receptor Antigen Targeting Potentiates Cross-Presentation by Human Blood and Lymphoid Tissue BDCA-3+ Dendritic Cells. Blood 2012, 120 (26), 5163–5172. 10.1182/blood-2012-06-434498. [DOI] [PubMed] [Google Scholar]
- Maurer D.; Fiebiger E.; Reininger B.; Ebner C.; Petzelbauer P.; Shi G. P.; Chapman H. A.; Stingl G. Fc Epsilon Receptor I on Dendritic Cells Delivers IgE-Bound Multivalent Antigens into a Cathepsin S-Dependent Pathway of MHC Class II Presentation. J. Immunol 1998, 161 (6), 2731–2739. 10.4049/jimmunol.161.6.2731. [DOI] [PubMed] [Google Scholar]
- Kim M. Y.; Copland A.; Nayak K.; Chandele A.; Ahmed M. S.; Zhang Q.; Diogo G. R.; Paul M. J.; Hofmann S.; Yang M.-S.; Jang Y.-S.; Ma J. K.-C.; Reljic R. Plant-Expressed Fc-Fusion Protein Tetravalent Dengue Vaccine with Inherent Adjuvant Properties. Plant Biotechnology Journal 2018, 16 (7), 1283–1294. 10.1111/pbi.12869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng P. M. L.; Kaliaperumal N.; Lee C. Y.; Chin W. J.; Tan H. C.; Au V. B.; Goh A. X.-H.; Tan Q. W.; Yeo D. S. G.; Connolly J. E.; Wang C.-I. Enhancing Antigen Cross-Presentation in Human Monocyte-Derived Dendritic Cells by Recruiting the Intracellular Fc Receptor TRIM21. J. Immunol. 2019, 202 (8), 2307–2319. 10.4049/jimmunol.1800462. [DOI] [PubMed] [Google Scholar]
- Spirig R.; Campbell I. K.; Koernig S.; Chen C.-G.; Lewis B. J. B.; Butcher R.; Muir I.; Taylor S.; Chia J.; Leong D.; Simmonds J.; Scotney P.; Schmidt P.; Fabri L.; Hofmann A.; Jordi M.; Spycher M. O.; Cattepoel S.; Brasseit J.; Panousis C.; Rowe T.; Branch D. R.; Baz Morelli A.; Käsermann F.; Zuercher A. W. RIgG1 Fc Hexamer Inhibits Antibody-Mediated Autoimmune Disease via Effects on Complement and FcγRs. J. Immunol. 2018, 200 (8), 2542–2553. 10.4049/jimmunol.1701171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerich L.; Marron T. U.; Upadhyay R.; Svensson-Arvelund J.; Dhainaut M.; Hussein S.; Zhan Y.; Ostrowski D.; Yellin M.; Marsh H.; Salazar A. M.; Rahman A. H.; Brown B. D.; Merad M.; Brody J. D. Systemic Clinical Tumor Regressions and Potentiation of PD1 Blockade with in Situ Vaccination. Nat. Med. 2019, 25 (5), 814–824. 10.1038/s41591-019-0410-x. [DOI] [PubMed] [Google Scholar]
- Toes R. E.; Offringa R.; Blom R. J.; Melief C. J.; Kast W. M. Peptide Vaccination Can. Lead to Enhanced Tumor Growth through Specific T-Cell Tolerance Induction. Proc. Natl. Acad. Sci. U. S. A. 1996, 93 (15), 7855–7860. 10.1073/pnas.93.15.7855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toes R. E.; van der Voort E. I.; Schoenberger S. P.; Drijfhout J. W.; van Bloois L.; Storm G.; Kast W. M.; Offringa R.; Melief C. J. Enhancement of Tumor Outgrowth through CTL Tolerization after Peptide Vaccination Is Avoided by Peptide Presentation on Dendritic Cells. J. Immunol 1998, 160 (9), 4449–4456. 10.4049/jimmunol.160.9.4449. [DOI] [PubMed] [Google Scholar]
- Kreutz M.; Giquel B.; Hu Q.; Abuknesha R.; Uematsu S.; Akira S.; Nestle F. O.; Diebold S. S. Antibody-Antigen-Adjuvant Conjugates Enable Co-Delivery of Antigen and Adjuvant to Dendritic Cells in Cis but Only Have Partial Targeting Specificity. PLoS One 2012, 7 (7), e40208 10.1371/journal.pone.0040208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignacio B. J.; Albin T. J.; Esser-Kahn A. P.; Verdoes M. Toll-like Receptor Agonist Conjugation: A Chemical Perspective. Bioconjugate Chem. 2018, 29 (3), 587–603. 10.1021/acs.bioconjchem.7b00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzio M.; Bosisio D.; Polentarutti N.; D’amico G.; Stoppacciaro A.; Mancinelli R.; van’t Veer C.; Penton-Rol G.; Ruco L. P.; Allavena P.; Mantovani A. Differential Expression and Regulation of Toll-Like Receptors (TLR) in Human Leukocytes: Selective Expression of TLR3 in Dendritic Cells1. J. Immunol. 2000, 164 (11), 5998–6004. 10.4049/jimmunol.164.11.5998. [DOI] [PubMed] [Google Scholar]
- Edwards A. D.; Diebold S. S.; Slack E. M. C.; Tomizawa H.; Hemmi H.; Kaisho T.; Akira S.; Sousa C. R. e. Toll-like Receptor Expression in Murine DC Subsets: Lack of TLR7 Expression by CD8α+ DC Correlates with Unresponsiveness to Imidazoquinolines. Eur. J. Immunol. 2003, 33 (4), 827–833. 10.1002/eji.200323797. [DOI] [PubMed] [Google Scholar]
- Tay R. E.; Richardson E. K.; Toh H. C. Revisiting the Role of CD4+ T Cells in Cancer Immunotherapy—New Insights into Old Paradigms. Cancer Gene Ther. 2021, 28 (1), 5–17. 10.1038/s41417-020-0183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukovic N.; van Elsas A.; Verbeek J. S.; Zaiss D. M. W. Isotype Selection for Antibody-Based Cancer Therapy. Clin. Exp. Immunol. 2021, 203 (3), 351–365. 10.1111/cei.13545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang T. H.; Jung S. T. Boosting Therapeutic Potency of Antibodies by Taming Fc Domain Functions. Exp Mol. Med. 2019, 51 (11), 1–9. 10.1038/s12276-019-0345-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arduin E.; Arora S.; Bamert P. R.; Kuiper T.; Popp S.; Geisse S.; Grau R.; Calzascia T.; Zenke G.; Kovarik J. Highly Reduced Binding to High and Low Affinity Mouse Fc Gamma Receptors by L234A/L235A and N297A Fc Mutations Engineered into Mouse IgG2a. Molecular Immunology 2015, 63 (2), 456–463. 10.1016/j.molimm.2014.09.017. [DOI] [PubMed] [Google Scholar]
- Mackness B. C.; Jaworski J. A.; Boudanova E.; Park A.; Valente D.; Mauriac C.; Pasquier O.; Schmidt T.; Kabiri M.; Kandira A.; Radošević K.; Qiu H. Antibody Fc Engineering for Enhanced Neonatal Fc Receptor Binding and Prolonged Circulation Half-Life. mAbs 2019, 11 (7), 1276–1288. 10.1080/19420862.2019.1633883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz L. J.; Tacken P. J.; Pots J. M.; Torensma R.; Buschow S. I.; Figdor C. G. Comparison of Antibodies and Carbohydrates to Target Vaccines to Human Dendritic Cells via DC-SIGN. Biomaterials 2012, 33 (16), 4229–4239. 10.1016/j.biomaterials.2012.02.036. [DOI] [PubMed] [Google Scholar]
- Manolova V.; Flace A.; Bauer M.; Schwarz K.; Saudan P.; Bachmann M. F. Nanoparticles Target Distinct Dendritic Cell Populations According to Their Size. Eur. J. Immunol. 2008, 38 (5), 1404–1413. 10.1002/eji.200737984. [DOI] [PubMed] [Google Scholar]
- Chang T. Z.; Stadmiller S. S.; Staskevicius E.; Champion J. A. Effects of Ovalbumin Protein Nanoparticle Vaccine Size and Coating on Dendritic Cell Processing. Biomater. Sci. 2017, 5 (2), 223–233. 10.1039/C6BM00500D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz L. J.; Tacken P. J.; Fokkink R.; Figdor C. G. The Influence of PEG Chain Length and Targeting Moiety on Antibody-Mediated Delivery of Nanoparticle Vaccines to Human Dendritic Cells. Biomaterials 2011, 32 (28), 6791–6803. 10.1016/j.biomaterials.2011.04.082. [DOI] [PubMed] [Google Scholar]
- Das A.; Ali N. Nanovaccine: An Emerging Strategy. Expert Review of Vaccines 2021, 20 (10), 1273–1290. 10.1080/14760584.2021.1984890. [DOI] [PubMed] [Google Scholar]
- Riley R. S.; June C. H.; Langer R.; Mitchell M. J. Delivery Technologies for Cancer Immunotherapy. Nat. Rev. Drug Discov 2019, 18 (3), 175–196. 10.1038/s41573-018-0006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth G. A.; Picece V. C. T. M.; Ou B. S.; Luo W.; Pulendran B.; Appel E. A. Designing Spatial and Temporal Control of Vaccine Responses. Nat. Rev. Mater. 2022, 7 (3), 174–195. 10.1038/s41578-021-00372-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. M.; Simon J. K.; Baker Jr J. R. Applications of Nanotechnology for Immunology. Nat. Rev. Immunol 2013, 13 (8), 592–605. 10.1038/nri3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L.; Yang H.; Rideout K.; Cho T.; Joo K. il; Ziegler L.; Elliot A.; Walls A.; Yu D.; Baltimore D.; Wang P. Engineered Lentivector Targeting of Dendritic Cells for in Vivo Immunization. Nat. Biotechnol. 2008, 26 (3), 326–334. 10.1038/nbt1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somaiah N.; Block M. S.; Kim J. W.; Shapiro G. I.; Do K. T.; Hwu P.; Eder J. P.; Jones R. L.; Lu H.; ter Meulen J. H.; Bohac C.; Chen M.; Hsu F. J.; Gnjatic S.; Pollack S. M. First-in-Class, First-in-Human Study Evaluating LV305, a Dendritic-Cell Tropic Lentiviral Vector, in Sarcoma and Other Solid Tumors Expressing NY-ESO-1. Clin. Cancer Res. 2019, 25 (19), 5808–5817. 10.1158/1078-0432.CCR-19-1025. [DOI] [PubMed] [Google Scholar]
- Pollack S. M.; Lu H.; Gnjatic S.; Somaiah N.; O’Malley R. B.; Jones R. L.; Hsu F. J.; ter Meulen J. First-in-Human Treatment With a Dendritic Cell-Targeting Lentiviral Vector-Expressing NY-ESO-1, LV305, Induces Deep, Durable Response in Refractory Metastatic Synovial Sarcoma Patient. Journal of Immunotherapy 2017, 40 (8), 302. 10.1097/CJI.0000000000000183. [DOI] [PMC free article] [PubMed] [Google Scholar]