Abstract
Gut barrier dysfunction can result in the liver being exposed to an elevated level of gut-derived bacterial products via portal circulation. Growing evidence suggests that systemic exposure to these bacterial products promotes liver diseases including hepatitis, cirrhosis, and hepatocellular carcinoma (HCC). However, prospective studies have not examined the association between biomarkers of gut barrier dysfunction and HCC risk in a population of hepatitis B or C viral (HBV/HCV) carriers. We investigated whether prediagnostic, circulating biomarkers of gut barrier dysfunction were associated with HCC risk, using the Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer (REVEAL)-HBV and REVEAL-HCV cohorts from Taiwan. REVEAL-HBV included 185 cases and 161 matched controls, and REVEAL-HCV 96 cases and 96 matched controls. The biomarkers quantitated were immunoglobulin A (IgA), IgG, and IgM against lipopolysaccharide (LPS) and flagellin, soluble CD14 (an LPS co-receptor), and LPS-binding protein (LBP). Odds ratios (ORs) and 95% confidence intervals (CIs) for associations between biomarker levels and HCC were calculated using multivariable-adjusted logistic regression. A doubling of the circulating levels of anti-flagellin IgA or LBP was associated with a 76–93% increased risk of HBV-related HCC (OR per one unit change in log2 anti-flagellin IgA=1.76, 95%CI: 1.06–2.93; OR for LBP=1.93, 95%CI: 1.10–3.38). None of the other markers were associated with an increased risk of HBV-related or HCV-related HCC. Results were similar when cases diagnosed in the first five years of follow-up were excluded. Our findings contribute to understanding the interplay of gut barrier dysfunction and primary liver cancer etiology.
Keywords: antibodies, cohort study, epidemiology, liver cancer
Graphical Abstract
INTRODUCTION
Primary liver cancer is estimated to be the sixth most frequently occurring cancer in the world and the third most common cause of cancer mortality.1 Globally, hepatocellular carcinoma (HCC) is the dominant histologic type of liver cancer, accounting for approximately 80% of all cases. Factors that contribute to chronic hepatic inflammation are major HCC risk factors, including hepatitis B virus (HBV), hepatitis C virus (HCV), aflatoxin contamination of food, excessive alcohol consumption, smoking, obesity, and diabetes.2
Chronic HBV or HCV infections remain the predominate risk factor for HCC in most of the world. Efforts are underway in many countries to vaccinate newborns against HBV or treat chronic HBV and HCV infections.3 However, the World Health Organization estimates that 296 million people, or approximately 3.8% of world’s population, are chronically infected with HBV,4 while 58 million people (0.8%) are chronically infected with HCV.5 For persons with chronic HBV or HCV, the lifetime risk of developing HCC is between 10% and 25%.6, 7 In 2019, HBV and HCV infections were responsible for 820,000 and 290,000 deaths, respectively, largely due to cirrhosis and HCC.4 Thus, HBV, HCV, and co-factors that further increase risk of HCC in a HBV- or HCV-infected population, such as gut permeability markers, remain a global concern.
Growing evidence implicates the gut-liver axis (i.e., the bidirectional relationship between the gut, and its associated microbiota and metabolome, and the liver)8 in the development of hepatic inflammation, liver disease, cirrhosis, and HCC.9–13 The gut barrier functions to allow permeability of necessary nutrients to pass from the intestinal lumen into circulation and to prevent potentially harmful factors, including bacteria and bacterial products, from moving into circulation.14 The integrity of the gut barrier can be affected by internal and external factors, including diet, medications, alcohol, circadian rhythm disruption, psychological stress, and aging.15 When the gut barrier is damaged, the liver is exposed to an elevated level of gut-derived bacterial products via the portal circulation,16 which provides approximately 70% of the liver’s blood supply.17
Murine studies have reported that exposure to bacterial products causes inflammation and oxidative stress in the liver, which can promote HCC.18–20 Similarly, evidence from human studies suggests that biomarkers of gut barrier dysfunction are positively associated with systemic inflammation21 and chronic liver diseases.22–28 Two epidemiologic studies conducted in European populations have reported significant associations between antibodies to bacterial products, including anti-lipopolysaccharide (LPS) and anti-flagellin, and risk of liver cancer.21, 29 Although over 70% of HCC cases arise in Asia, no studies to date have investigated biomarkers of gut barrier dysfunction in relation to HCC in Asian populations. Chronic infection with HBV or HCV plays a more dominant role in HCC etiology in Asian populations than in European populations; thus, extrapolating results from studies conducted in European populations to Asian populations is not always informative.3 To examine whether biomarkers of gut barrier dysfunction (i.e., anti-LPS, anti-flagellin, soluble CD14, and LPS-binding protein) are related to risk of HCC in chronic HBV and HCV carriers in an Asian population, we analyzed samples from the Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer (REVEAL)-HBV and -HCV cohorts of Taiwan.
METHODS
Study Population.
The REVEAL study was a prospective study designed to examine the characteristics and risk factors for HCC in the general population.30–32 Briefly, between 1991–1992, 23,820 participants aged 30 to 65 years old from 7 townships in Taiwan were enrolled in the study. All study participants donated blood samples, completed study questionnaires and underwent health examinations at study enrollment to confirm that they did not have prevalent HCC. Of the study enrollees, 4,155 (17.4%) were hepatitis B virus surface antigen (HBsAg) seropositive and were followed as part of the REVEAL-HBV study. Similarly, 1,095 (4.6%) persons were anti-HCV seropositive and were followed as part of the REVEAL-HCV study. The seroprevalence of HBV and HCV in parent REVEAL study was similar to the seroprevalence in the general population (12–15% for HBV prior to universal vaccination33 and 1.8–5.5% for HCV34). Regular examinations consisting of blood collection and abdominal ultrasonography occurred every 6–12 months through December 31, 2008.32, 35, 36
Case Ascertainment.
HCC diagnosis was determined by transabdominal ultrasound and α-fetoprotein testing or by data linkage to the Taiwan National Cancer Registry or the National Death Certification profiles.30, 36 The mean duration of follow-up to HCC diagnosis was 13.0 years for the REVEAL-HBV cohort and 14.9 years for the REVEAL-HCV cohort.
For the current investigation, 185 participants of the REVEAL-HBV cohort who developed HCC were frequency matched to 161 controls from REVEAL-HBV cohort based on age (5-year categories), sex, and HBV DNA copy number at baseline (<10,000, 10,000-<1,000,000, ≥1,000,000 copies/mL). Similarly, 96 participants of the REVEAL-HCV cohort who developed HCC were individually matched to 96 controls from REVEAL-HCV cohort on age (5-year categories), sex, cirrhosis, and HCV-RNA seropositivity status (undetectable, <25 IU/mL and detectable, ≥25 IU/mL, matched to a control with the closest HCV RNA status).
Laboratory Methods.
Serum samples were stored at −70°C at the Academia Sinica in Taipei, Taiwan prior to being shipped to the U.S. for analysis. The U.S. laboratories were blinded to sample type (i.e., HCC or control sample). In addition to each laboratory’s internal quality control samples, we included one duplicate of a control in each batch to examine within-batch variation. Each duplicate was compared to its matched control to calculate coefficients of variation (CVs).
Immunoglobulin A (IgA), IgG, and IgM against LPS and flagellin were measured via enzyme-linked immunosorbent assay (ELISA) as previously described.37 Briefly, Costar™ 3590 ELISA plates were coated overnight with laboratory-made flagellin or purified Escherichia coli LPS, and plasma samples diluted 1:200 were applied to the coated wells. After incubation and washing, wells were incubated either with horseradish peroxidase-conjugated anti-IgM, anti-IgA, or anti-IgG. Quantitation of total immunoglobulins was performed using the colorimetric peroxidase substrate tetramethylbenzidine. Optical densities were read at 450 nm and 540 nm and were reported as corrected values by subtracting background and by normalizing to each plate’s control sample. Within-batch CVs were low, ranging from 1.9% to 6.1%.
LBP was measured using R&D Systems DuoSet ELISA kit (Cat# DY870–05 and DY008). Briefly, 96-well microplates were coated overnight with human LBP capture antibody, and plasma samples diluted 1:1000 were applied to the coated wells. After incubation and washing, wells were incubated with streptavidin conjugated to horseradish peroxidase. sCD14 was measured using R&D Systems Quantikine kit (Cat# CD140). Briefly, this kit uses a 96-well polystyrene microplate coated with a monoclonal antibody specific for human CD14. Plasma samples were diluted 1:1000 in R&D Systems recommended diluent and applied to the coated wells. After incubation and washing, wells were incubated with polyclonal antibody specific for human CD14 conjugated to horseradish peroxidase with preservatives. For both LBP and sCD14, optical densities were read at 450 nm and 540 nm. All samples were tested in duplicate and averaged. The final concentration in μg/mL (LBP) or pg/mL (sCD14) was generated using a standard curve. The overall within-batch CVs were 11.4% for LBP and 13.5% for sCD14.
Statistical Analysis.
Participant characteristics were examined by calculating frequencies (for categorical variables) or means and standard deviations (for continuous variables and biomarkers of gut barrier dysfunction). Spearman correlation coefficients were examined for each gut barrier dysfunction biomarker pair, in addition to ALT (Supplemental Table S1). Missing data for the following covariates were imputed using the PROC MI procedure in SAS, version 9.4 (SAS Institute Inc., Cary, NC): body mass index (BMI; n=3), alcohol consumption (n=3), and smoking status (n=3). To further examine and adjust for HBV- and HCV-specific markers, imputation was also performed in the REVEAL-HBV cohort for hepatitis B e-antigen (HBeAg) serostatus (n=3), a marker of active viral replication. In the REVEAL-HCV cohort, imputation was also performed for HCV genotype (n=35), as genotype 1 is associated with a higher likelihood of developing HCC than are other HCV genotypes. For all imputation models, we used case status, age, and education as predictors. Biomarkers were categorized into quartiles, based on the distribution in the control participants. Multivariable-adjusted unconditional (REVEAL-HBV) and conditional (REVEAL-HCV) logistic regression models were used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for the associations between biomarkers of gut barrier dysfunction and HCC risk. Tests of linear trend were performed using quartile-specific biomarker concentration level medians.
All potential covariates were obtained from study baseline. Covariates included a priori were alcohol consumption (defined as having consumed alcohol at least 4 days per week for at least 1 year;38, 39 no, yes), education (illiterate, elementary school, junior high school, high school, college or more), diabetes (yes/no), alanine transaminase (ALT, continuous U/L), and matching factors. Additional potential covariates were examined for evidence of confounding by determining 1) whether each covariate was associated with the exposure in the population of interest (i.e., among the study controls) and 2) whether each covariate was associated with HCC among unexposed individuals (i.e., participants in the 1st quartile of each biomarker). Covariates that met both criteria were removed one at a time from the fully-adjusted model to determine whether the covariate altered the log(OR) by at least 10%.40 Based on this criteria, covariates in the final models included age (5-year categories), sex, smoking status (no, yes), alcohol consumption (no, yes), BMI (continuous), ALT (continuous), baseline cirrhosis status (no, yes), diabetes (no, yes), and education for both cohorts. Additionally, the REVEAL-HBV cohort models included HBV DNA copy number at baseline and HBeAg serostatus (negative, positive). The REVEAL-HCV cohort models also included HCV-RNA seropositive status and HCV genotype (1, non-1). Effect measure modification by sex, smoking status, diabetes, and BMI was assessed. Departures from the null were examined using likelihood ratio tests to compare regression models with and without a multiplicative interaction term.40 There was no evidence of effect measure modification (Ps≥0.05).
Sensitivity analyses included a lag analysis, where any case diagnosed with HCC within 5 years of cohort baseline was excluded from the analysis, and a complete case analysis, where study participants with missing covariate data were not retained in the models. All tests for significance were 2-sided. Analyses were conducted using SAS, version 9.4 (SAS Institute, Cary, NC).
RESULTS
In both the REVEAL-HBV and REVEAL-HCV cohorts, HCC cases had higher BMIs, higher ALT levels, and were less educated than the controls (Table 1). HCC cases in the REVEAL-HBV cohort were also more likely to have cirrhosis at baseline than were their controls, while HCC cases in the REVEAL-HCV cohort were more likely to be smokers than were their controls. Examining the biomarkers of gut barrier dysfunction pairings and ALT, correlations were noted between IgA and IgG against LPS and flagellin (e.g., anti-flagellin IgA and anti-LPS IgA, ρ=0.803, Supplemental Table S1) and ALT with sCD14 and LBP (ρ=0.165 and ρ=0.159, respectively). Circulating levels were higher in the REVEAL-HBV cases, compared to controls, for all biomarkers of gut barrier dysfunction examined (Table 2). However, the difference in levels of anti-flagellin IgM, anti-LPS IgG, and anti-LPS IgM between cases and controls were non-significant. Circulating levels of gut barrier dysfunction biomarkers were similar between the REVEAL-HCV cases and controls with the exception of sCD14, which was higher in controls than cases.
Table 1.
Baseline characteristics of included participants from the REVEAL-HBV and REVEAL-HCV studies.
| REVEAL-HBV | REVEAL-HCV | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Covariate | Controls (N=161) | Cases (N=185) | P-value 1 | Controls (N=96) | Cases (N=96) | P-value 1 |
|
| ||||||
| Mean age (SD), years | 50.7 (9.4) | 51.3 (9.0) | 0.31 | 54.5 (7.3) | 54.4 (6.8) | 0.82 |
| Age, No. (%) | ||||||
| 30 – <40 | 24 (14.9) | 24 (13.0) | 4 (4.2) | 4 (4.2) | ||
| 40 – <50 | 53 (32.9) | 58 (31.4) | 14 (14.6) | 14 (14.6) | ||
| 50 – <60 | 51 (31.7) | 68 (36.8) | 54 (56.2) | 54 (56.2) | ||
| 60+ | 33 (20.5) | 35 (18.9) | 0.79 | 24 (25.0) | 24 (25.0) | 1.0 |
| Sex, No. (%) | ||||||
| Male | 130 (80.8) | 150 (81.1) | 56 (58.3) | 56 (58.3) | ||
| Female | 31 (19.3) | 35 (18.9) | 0.94 | 40 (41.7) | 40 (41.7) | 1.0 |
| Body mass index, No. (%) | ||||||
| <18.5, kg/m2 | 5 (3.1) | 7 (3.8) | 0 (0.0) | 1 (1.0) | ||
| 18.5 – <23, kg/m2 | 68 (42.2) | 52 (28.1) | 45 (46.9) | 28 (29.2) | ||
| 23 – <25 | 42 (26.1) | 45 (24.3) | 19 (19.8) | 19 (19.8) | ||
| ≥25, kg/m2 | 46 (28.6) | 81 (43.8) | 0.014 | 32 (33.3) | 48 (50.0) | 0.043 |
| Alcohol consumption, No. (%) | ||||||
| No | 135 (83.9) | 148 (80.0) | 86 (89.6) | 87 (90.6) | ||
| Yes | 26 (16.1) | 37 (20.0) | 0.35 | 10 (10.4) | 9 (9.4) | 0.81 |
| Smoking status, No. (%) | ||||||
| No | 104 (64.6) | 113 (61.1) | 67 (69.8) | 55 (57.3) | ||
| Yes | 57 (35.4) | 72 (38.9) | 0.50 | 29 (30.2) | 41 (42.7) | 0.072 |
| Education, No. (%) | ||||||
| Illiterate | 20 (12.4) | 24 (13.0) | 35 (36.4) | 39 (40.6) | ||
| Elementary school | 69 (42.9) | 98 (53.0) | 45 (46.9) | 37 (38.6) | ||
| Junior high school | 19 (11.8) | 25 (13.5) | 4 (4.2) | 13 (13.5) | ||
| High school | 32 (19.9) | 25 (13.5) | 7 (7.3) | 5 (5.2) | ||
| College or more | 21 (13.0) | 13 (7.0) | 0.12 | 5 (5.2) | 2 (2.1) | 0.12 |
| ALT, No. (%) | ||||||
| <15 (u/L) | 96 (59.6) | 68 (36.8) | 31 (32.3) | 20 (20.8) | ||
| 15 – <45 (u/L) | 57 (35.4) | 89 (48.1) | 47 (48.9) | 41 (42.7) | ||
| ≥45 (u/L) | 8 (5.0) | 28 (15.1) | <0.0001 | 18 (18.8) | 35 (36.5) | 0.016 |
| Liver cirrhosis at baseline, No. (%) | ||||||
| No | 161 (100.0) | 158 (85.4) | 93 (96.9) | 93 (96.9) | ||
| Yes | 0 (0.0) | 27 (14.6) | <0.0001 | 3 (3.1) | 3 (3.1) | 1.0 |
| Diabetes at baseline, No. (%) | ||||||
| No | 160 (99.4) | 175 (94.6) | 91 (94.8) | 92 (95.8) | ||
| Yes | 1 (0.6) | 10 (5.4) | 0.011 | 5 (5.2) | 4 (4.2) | 0.73 |
| HBeAg | ||||||
| Negative | 142 (88.2) | 107 (57.8) | ||||
| Positive | 19 (11.8) | 78 (42.2) | <0.0001 | |||
| HCV genotype | ||||||
| Genotype 1 | 45 (46.9) | 66 (68.8) | ||||
| Genotype non-1 | 51 (53.1) | 30 (31.2) | 0.0021 | |||
Abbreviations: HBV=hepatitis B virus, HCV=hepatitis C virus, SD=standard deviation, kg=kilogram.
P-values calculated using the chi square test (categorical variables) or the Wilcoxon test (continuous variables).
Table 2.
Baseline mean concentration levels of bacterial translocation markers among cases and controls of the REVEAL-HBV and REVEAL-HCV cohorts.
| REVEAL-HBV | REVEAL-HCV | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Controls Mean (SD) | Cases Mean (SD) | P-value 1 | Controls Mean (SD) | Cases Mean (SD) | P-value 1 | |
|
| ||||||
| anti-Flagellin IgA | 2.74 (1.02) | 3.03 (1.02) | 0.0093 | 2.76 (1.04) | 2.73 (1.02) | 0.83 |
| anti-Flagellin IgG | 2.88 (0.84) | 3.07 (0.89) | 0.042 | 3.04 (0.94) | 3.05 (0.93) | 0.91 |
| anti-Flagellin IgM | 2.56 (0.87) | 2.64 (0.81) | 0.38 | 2.60 (0.85) | 2.68 (0.91) | 0.57 |
| anti-LPS IgA | 2.94 (1.41) | 3.28 (1.52) | 0.034 | 3.14 (1.45) | 2.99 (1.49) | 0.49 |
| anti-LPS IgG | 2.94 (0.84) | 3.11 (0.88) | 0.062 | 3.02 (0.82) | 3.11 (0.86) | 0.45 |
| anti-LPS IgM | 2.24 (0.68) | 2.32 (0.58) | 0.22 | 2.33 (0.72) | 2.34 (0.71) | 0.91 |
| sCD14 (pg/mL) | 1.08 (0.30) | 1.17 (0.37) | 0.013 | 1.31 (0.42) | 1.15 (0.32) | 0.0030 |
| LPS Binding Protein (μg/mL) | 4.00 (1.42) | 4.57 (1.73) | 0.0010 | 5.24 (2.09) | 4.79 (1.58) | 0.093 |
P-values calculated using the analysis of variance F-test.
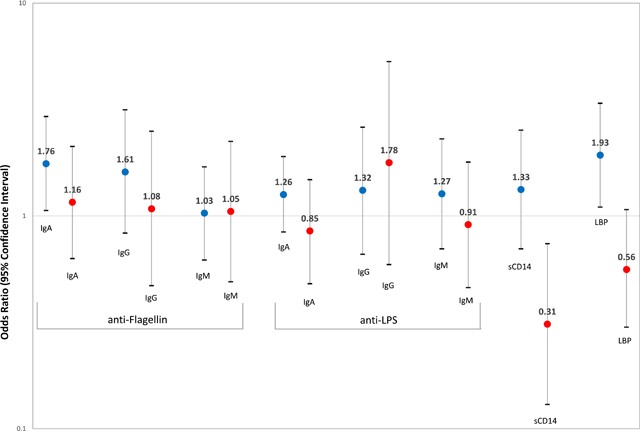
A doubling of the circulating levels of anti-flagellin IgA or LBP was associated with a significantly increased risk of HCC in the REVEAL-HBV cohort (OR per one unit change in log2 anti-flagellin IgA=1.76, 95% CI: 1.06–2.93 and OR for LBP=1.93, 95% CI: 1.10–3.38, Table 3). Similar associations were seen when examining the fourth quartile compared to the first for anti-flagellin IgA or LBP (OR for anti-flagellin IgA=2.02, 95% CI: 0.92–4.43, ptrend=0.13, and OR for LBP=2.17, 95% CI: 1.01–4.65, ptrend=0.027). Little to no association was noted for sCD14 or other antibodies against LPS and flagellin. There was little to no association with HCC for antibodies against LPS and flagellin in the REVEAL-HCV cohort. However, the sCD14 was inversely associated with HCC (OR per one unit change in log2 sCD14=0.31, 95% CI: 0.13–0.74). There was no evidence of effect measure modification by sex, smoking status, diabetes, and BMI in the REVEAL-HBV or REVEAL-HCV cohort (Ps≥0.05).
Table 3.
Multivariate adjusted odds ratios (ORs) and 95% confidence intervals (CIs) for the associations between markers of gut bacterial translocation and hepatocellular carcinoma among participants of the REVEAL-HBV and REVEAL-HCV cohorts.
| REVEAL-HBV | REVEAL-HCV | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Controls | Cases | OR1 | 95% CI | Controls | Cases | OR2 | 95% CI | |
|
| ||||||||
| anti-Flagellin IgA | ||||||||
| Quartile 1 | 41 | 31 | 1.00 | (referent) | 24 | 30 | 1.00 | (referent) |
| Quartile 2 | 41 | 39 | 1.57 | (0.70, 3.55) | 24 | 23 | 0.70 | (0.26, 1.90) |
| Quartile 3 | 40 | 54 | 1.37 | (0.62, 3.04) | 24 | 16 | 0.57 | (0.20, 1.64) |
| Quartile 4 | 39 | 61 | 2.02 | (0.92, 4.43) | 24 | 27 | 1.14 | (0.46, 2.83) |
| P-value for trend3 | 0.13 | 0.94 | ||||||
| Continuous (log2) | 161 | 185 | 1.76 | (1.06, 2.93) | 96 | 96 | 1.16 | (0.63, 2.12) |
| anti-Flagellin IgG | ||||||||
| Quartile 1 | 42 | 33 | 1.00 | (referent) | 24 | 26 | 1.00 | (referent) |
| Quartile 2 | 40 | 40 | 1.30 | (0.58, 2.90) | 24 | 17 | 0.54 | (0.16, 1.75) |
| Quartile 3 | 39 | 49 | 1.66 | (0.75, 3.67) | 24 | 28 | 1.32 | (0.51, 3.40) |
| Quartile 4 | 40 | 63 | 1.78 | (0.82, 3.86) | 24 | 25 | 1.00 | (0.37, 2.70) |
| P-value for trend3 | 0.15 | 0.69 | ||||||
| Continuous (log2) | 161 | 185 | 1.61 | (0.83, 3.15) | 96 | 96 | 1.08 | (0.47, 2.50) |
| anti-Flagellin IgM | ||||||||
| Quartile 1 | 40 | 37 | 1.00 | (referent) | 24 | 24 | 1.00 | (referent) |
| Quartile 2 | 40 | 49 | 1.13 | (0.53, 2.42) | 24 | 25 | 1.05 | (0.38, 2.88) |
| Quartile 3 | 41 | 53 | 0.93 | (0.44, 2.00) | 24 | 18 | 0.52 | (0.18, 1.57) |
| Quartile 4 | 40 | 46 | 0.88 | (0.41, 1.90) | 24 | 29 | 0.98 | (0.37, 2.59) |
| P-value for trend3 | 0.63 | 0.92 | ||||||
| Continuous (log2) | 161 | 185 | 1.03 | (0.62, 1.70) | 96 | 96 | 1.05 | (0.49, 2.24) |
| anti-LPS IgA | ||||||||
| Quartile 1 | 40 | 21 | 1.00 | (referent) | 24 | 30 | 1.00 | (referent) |
| Quartile 2 | 40 | 57 | 2.83 | (1.21, 6.64) | 24 | 25 | 0.71 | (0.30, 1.70) |
| Quartile 3 | 41 | 43 | 1.63 | (0.69, 3.87) | 24 | 18 | 0.47 | (0.18, 1.26) |
| Quartile 4 | 40 | 64 | 2.33 | (1.01, 5.41) | 24 | 23 | 0.97 | (0.37, 2.59) |
| P-value for trend3 | 0.47 | 0.79 | ||||||
| Continuous (log2) | 161 | 185 | 1.26 | (0.84, 1.90) | 96 | 96 | 0.85 | (0.48, 1.48) |
| anti-LPS IgG | ||||||||
| Quartile 1 | 41 | 34 | 1.00 | (referent) | 24 | 22 | 1.00 | (referent) |
| Quartile 2 | 41 | 36 | 0.73 | (0.32, 1.66) | 24 | 23 | 0.92 | (0.32, 2.63) |
| Quartile 3 | 39 | 59 | 1.64 | (0.75, 3.57) | 24 | 24 | 1.40 | (0.47, 4.19) |
| Quartile 4 | 40 | 56 | 1.03 | (0.46, 2.27) | 24 | 27 | 1.58 | (0.53, 4.75) |
| P-value for trend3 | 0.43 | 0.30 | ||||||
| Continuous (log2) | 161 | 185 | 1.32 | (0.66, 2.61) | 96 | 96 | 1.78 | (0.59, 5.31) |
| anti-LPS IgM | ||||||||
| Quartile 1 | 41 | 35 | 1.00 | (referent) | 24 | 22 | 1.00 | (referent) |
| Quartile 2 | 39 | 46 | 1.28 | (0.59, 2.78) | 24 | 31 | 1.42 | (0.58, 3.49) |
| Quartile 3 | 41 | 44 | 0.93 | (0.42, 2.05) | 24 | 17 | 0.69 | (0.26, 1.82) |
| Quartile 4 | 40 | 60 | 1.50 | (0.70, 3.20) | 24 | 26 | 1.00 | (0.38, 2.67) |
| P-value for trend3 | 0.47 | 0.62 | ||||||
| Continuous (log2) | 161 | 185 | 1.27 | (0.70, 2.30) | 96 | 96 | 0.91 | (0.46, 1.79) |
| sCD14 | ||||||||
| Quartile 1 | 40 | 35 | 1.00 | (referent) | 24 | 37 | 1.00 | (referent) |
| Quartile 2 | 41 | 39 | 1.36 | (0.61, 3.07) | 24 | 25 | 0.48 | (0.17, 1.32) |
| Quartile 3 | 40 | 47 | 1.18 | (0.53, 2.64) | 24 | 16 | 0.37 | (0.14, 0.99) |
| Quartile 4 | 40 | 64 | 1.68 | (0.77, 3.68) | 24 | 18 | 0.45 | (0.16, 1.24) |
| P-value for trend3 | 0.24 | 0.049 | ||||||
| Continuous (log2) | 161 | 185 | 1.33 | (0.70, 2.53) | 96 | 96 | 0.31 | (0.13, 0.74) |
| LPS Binding Protein | ||||||||
| Quartile 1 | 42 | 31 | 1.00 | (referent) | 24 | 39 | 1.00 | (referent) |
| Quartile 2 | 39 | 29 | 1.03 | (0.45, 2.38) | 24 | 12 | 0.08 | (0.02, 0.35) |
| Quartile 3 | 40 | 50 | 1.33 | (0.62, 2.86) | 24 | 32 | 0.83 | (0.33, 2.08) |
| Quartile 4 | 40 | 75 | 2.17 | (1.01, 4.65) | 24 | 13 | 0.13 | (0.04, 0.46) |
| P-value for trend3 | 0.027 | 0.086 | ||||||
| Continuous (log2) | 161 | 185 | 1.93 | (1.10, 3.38) | 96 | 96 | 0.56 | (0.30, 1.07) |
Unconditional logistic regression adjusted for age, sex, HBV DNA copies at baseline, smoking status, alcohol consumption, BMI, education status, baseline cirrhosis status, baseline diabetes status, ALT, and HbeAG.
Conditional logistic regression adjusted for matching factors (age, sex, and HCV-RNA-positive rate), smoking status, alcohol consumption, BMI, education status, baseline cirrhosis status, baseline diabetes status, ALT, and HCV genotype.
P-value for trend calculated using the Wald test.
In the lag analysis, cases diagnosed in the first five years of follow-up were excluded (10 HCC cases in the REVEAL-HBV and 3 HCC cases in the REVEAL-HCV). The results were similar (Supplemental Table S2). When we conducted a complete case analysis, the results were not altered (data not shown).
DISCUSSION
In this study leveraging data from two well-characterized prospective cohort studies, we observed significant associations between biomarkers of gut barrier dysfunction and HBV-related HCC risk. We observed that a doubling of the circulating levels of anti-flagellin IgA or LBP was associated with a 76–93% increased risk of HBV-related HCC. None of the biomarkers of gut barrier dysfunction were associated with an increased risk of HCV-related HCC, but sCD14 was inversely associated.
This is the first study to examine the association between prediagnostic levels of gut barrier dysfunction biomarkers and HCC risk in populations of HBV and HCV carriers. Two prior prospective epidemiologic studies have examined the association between biomarkers of gut barrier dysfunction and HCC risk, but both studies were conducted in European populations.21, 29 As European population have lower prevalences of HBV41 and HCV42, the prior studies were unable to examine associations stratified by HBV or HCV status. The European Prospective Investigation into Cancer and Nutrition is a multicenter prospective cohort study, which enrolled approximately 520,000 men and women aged 20–85 years between 1992 and 2000 from 10 European countries.21 From participants with blood samples available, there were 139 incident cases of HCC (16.8% were HBsAg seropositive, 21.8% were anti-HCV seropositive) which were matched to 139 controls (2.0% were HBsAg seropositive, 2.0% were anti-HCV seropositive). Anti-LPS and anti-flagellin IgA and IgG levels were quantitated using ELISA. In comparing the highest versus the lowest quartile, anti-flagellin IgA and IgG and anti-LPS IgA and IgG, were associated with 4 to 8-fold increased risk of HCC in this European population.21 The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study was a randomized controlled trial to test the effects of α-tocopherol and β-carotene on lung cancer incidence. The study enrolled 29,133 men aged 50–69 years, who smoked at least five cigarettes per day, between 1985 and 1988 in Finland.29 All participants provided a blood sample at baseline. The nested case-control study included 224 incident cases of primary liver cancer (<2.0% were HBsAg seropositive, 4.5% were anti-HCV seropositive) which were matched to 224 controls (<1.0% were HBsAg seropositive, 0.4% were anti-HCV seropositive). LBP, sCD14, and anti-LPS and anti-flagellin IgA, IgG, and IgM levels were quantitated using ELISA. In comparing the highest versus the lowest quartile, anti-flagellin IgA and anti-LPS IgA were associated with a 2.5-fold increased risk of liver cancer.29 Similar to the prior European prospective studies,21, 29 we found that anti-flagellin IgA was associated with a 76% increased risk of HBV-related HCC, but there was no association with HCV-related liver cancer. We also found that LBP was associated with a 93% increased risk of HBV-related HCC, which we did not find in our prior study of Finnish male smokers.29 Herein, we noted that LBP had a significant correlation with ALT levels, suggesting that the association between LBP and HBV-related HCC may be through inflammation pathways.
In contrast to the association between LBP and risk of HBV-related HCC, there was little to no association for antibodies against LPS and HBV-related HCC. LPS is challenging to measure directly due to its short half-life and high susceptibility to interfering substances.43 Thus, LBP and anti-LPS serve as proxy measures of LPS. LPS binds to LPB, forming the LPS-LBP complex.44 The LPS-LBP complex subsequently binds to CD14, which results in production of cytokines and other pro-inflammatory mediators.45 Therefore, higher levels of LBP may indicate more systemic inflammation, whereas higher anti-LPS levels may indicate a more robust immune system that has neutralized LPS. Further, we noted a very weak correlation between LBP and anti-LPS (ρ<0.2). IgA antibody against flagellin was associated with an increased risk of HBV-related HCC, while anti-flagellin IgG and IgM were not. Reasons for the lack of an association with anti-flagellin IgG and IgM are unclear. However, IgA is the primary antibody that plays a role in the immune functioning of mucous membranes (e.g., the gut barrier) and protects mucosal surfaces from toxins and bacteria through neutralization or prevention of binding to the mucosal surface.46
Although the current study was the first to examine the relationship between biomarkers of gut barrier dysfunction and HCC in an Asian population prospectively, several studies from China previously reported results from retrospective studies. The first study recruited 433 male and female patients, aged 17–76 years, with a chronic HBV infection from the First Affiliated Hospital of Xi’an Jiaotong University.47 Patients with HCV were excluded. The study included 44 cases of asymptomatic HBV, 72 chronic hepatitis cases, 87 liver cirrhosis cases, and 83 HCC cases. The study also included 59 controls that spontaneously recovered from HBV infection and 89 healthy controls. The study reported that serum levels of sCD14 were significantly elevated in HBV-related HCC.47 Another case-control study from East, Central, and Northwest China collected fecal samples from 190 men and women (average age 48.7 years) to examine the gut microbiome.48 The study included 75 controls, 40 cases of liver cirrhosis cases, and 75 early HCC cases. Information on HBV and HCV infection status was not provided. The study found that LPS-producing genera (i.e., Klebsiella and Haemophilus) were differentially expressed in early HCC compared to controls.48
Mechanisms underlying the association between systemic exposure to bacteria or bacterial products and liver cancer risk are not fully elucidated. However, elevated systemic exposure to bacterial products could be due to gut dysbiosis (i.e., an imbalance in the gut microbiota, the community of microorganisms, including bacteria, fungi, viruses, and archaea that reside in the gut) and gut barrier damage allowing bacterial products into circulation, reaching the liver via the portal vein and eliciting an inflammatory response.49 LPS, also known as endotoxin, is an integral component of the outer membrane of Gram-negative bacteria, contributing to the structural integrity and protecting the bacteria from chemical attack. LPS binds with LBP (an acute-phase protein produced by hepatocytes in response to LPS) and sCD14 (a co-receptor for LPS) to trigger an inflammatory response and activate Toll-like receptor 4 (TLR-4), which contributes to the promotion of HCC in chronically injured murine livers by increasing proliferative and anti-apoptotic signals.18, 49–51 Flagellin is the structural component of bacterial flagella (i.e., the tail-like appendage that protrudes from bacteria, serving locomotion and sensory functions) and is secreted by pathogenic and commensal bacteria. Administration of high-doses of flagellin to mice leads liver injury via over-activation of TLR-5 signaling, which causes inflammation, neutrophil accumulation, and oxidative stress in the liver.19
Cross-sectional studies have indicated that carriers of chronic HBV24, 47, 52 and chronic HCV24, 53, 54 have a higher prevalence and severity of biomarkers of gut barrier dysfunction, including LPS and sCD14, than non-carriers. As chronic HBV progresses, studies have reported that LPS levels and LPS-producing bacterial abundance increase.24, 55 Conversely, in HCV carriers, no differences have been noted between levels of LPS in individuals with early fibrosis versus cirrhosis.53, 56 This suggests a possible role of gut barrier dysfunction in HBV, but not HCV, infection and progression. In this study, with the exception of sCD14, the levels of gut barrier dysfunction biomarkers are similar between HCV-infected HCC cases and controls, whereas HBV-infected HCC cases have elevated levels of all gut barrier dysfunction biomarkers compared to controls. Reasons for the inverse association between sCD14 and HCV-related HCC are unclear, but sCD14 has previously been shown to be down-regulated in more advanced liver disease.57 Compared to serum levels of gut barrier dysfunction biomarkers in controls from our prior study of Finnish male smokers who had a low seroprevalence of HBV or HCV (e.g., mean LBP=0.74 μg/mL), the plasma levels of gut barrier dysfunction biomarkers in controls with HBV or HCV from REVEAL-HBV (e.g., mean LBP=4.00 μg/mL) and REVEAL-HCV (e.g., mean LBP=5.24 μg/mL) are higher.29
The development of chronic HBV is age-dependent: approximately 95% of acute HBV infections in adults are spontaneously cleared, whereas approximately 90% of HBV-exposed neonates develop chronic HBV infection.58 Murine studies have demonstrated that the age-dependent spontaneous clearance of acute HBV infection is reliant on an established microbiota.59, 60 Conversely, HCV infection is usually acquired in adulthood, with approximately 80% of HCV-exposed adults developing chronic HCV infection.6 Thus, our findings of biomarkers of gut barrier dysfunction increasing risk of HBV-related HCC, but not HCV-related HCC, further implicates the microbiota and gut barrier dysfunction in HBV-related HCC.
This study has notable strengths. The REVEAL-HBV and REVEAL-HCV studies are long-standing, population-based cohorts that have been well-characterized for liver disease, including information on the number of HBV DNA copies, HBeAg status, HCV genotype, and cirrhosis. In addition, all serum samples were collected pre-diagnostically, which ensures that the observed associations are not an artifact of the carcinogenic process. Further, we performed a lag analysis, excluding cases diagnosed in the first five years of follow-up to ensure temporality, and report no differences in the results.
A limitation of this study is generalizability of the population, as the REVEAL cohorts are limited to persons who are chronic viral carriers. However, this study is the first to examine biomarkers of gut barrier dysfunction in persons chronically infection with HBV or HCV. In addition, the cohorts were started prior to widespread use of nucleos(t)ide analogues as treatment for HBV infection or direct-acting antivirals as treatment for HCV infection. Thus, it is unclear how these results may translate to populations that are not virally infected or populations where HBV and HCV treatment are more common. Additionally, there was only a single measurement of gut barrier dysfunction biomarkers at study baseline, and the temporal stability of these markers is not well established. However, a previous study demonstrated that LBP is moderately stable up to nine months (intraclass correlation coefficient [ICC]=0.52).61 Another study demonstrated that sCD14 is moderately stable for more than 15 years (ICC=0.60).62 Thus, these studies suggests that gut barrier dysfunction biomarkers are relatively stable, making a one-time blood sample suitable to investigate associations with cancer. Finally, the biomarkers of gut barrier dysfunction cannot distinguish between systemic exposure to bacteria versus bacterial products.
In conclusion, our study found that anti-flagellin IgA and LBP were associated with an increased risk of HBV-related HCC. None of the biomarkers of gut barrier dysfunction were associated with an increased risk of HCV-related HCC, but sCD14 was inversely associated. This study clarifies the role of gut barrier dysfunction in relationship to risk of HCC in chronic HBV and HCV carriers, which is critical as HBV and HCV remain two major risk factors for HCC.
Supplementary Material
Novelty/Impact:
Over 70% of the global burden of hepatocellular carcinoma (HCC) arises in Asia. Chronic infection with hepatitis B or C virus (HBV/HCV) plays a more dominant role in HCC etiology in Asian populations than in European; thus, extrapolating results from prior studies conducted in European populations is not always informative. This study suggests that anti-flagellin IgA and lipopolysaccharide binding protein are associated with an increased HCC risk in individuals with a chronic HBV.
Funding Information:
NIH Intramural Research Program, National Cancer Institute (KA McGlynn), Karin Grunebaum Cancer Research Foundation (JL Petrick), and Boston University Peter Paul Career Development Professorship (JL Petrick).
Abbreviations:
- ALT
alanine transaminase
- BMI
body mass index
- CI
confidence interval
- CV
coefficients of variation
- ELISA
enzyme-linked immunosorbent assay
- HBeAg
hepatitis B e-antigen
- HBsAg
hepatitis B virus surface antigen
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- IgA
immunoglobulin A
- IgG
immunoglobulin G
- IgM
immunoglobulin M
- LBP
lipopolysaccharide-binding protein
- LPS
lipopolysaccharide
- OR
odds ratio
- REVEAL
Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer
- sCD14
soluble CD14
Footnotes
Conflict of Interest:
BY participated in this research during post-doctoral work at the National Cancer Institute and currently is employed by Roche. Roche had no influence or contribution to this project, nor did it have any financial impact on this project. The remaining authors declare no conflict of interest.
Data Availability Statement:
Data are stored at the Genomics Research Center, Academia Sinica, Taipei, Taiwan, and initial requests for data may be directed to Chien-Jen Chen (wt.ude.acinis.etag@jcnehc), Hwai-I Yang (wt.ude.acinis.etag@gnayih), or Mei-Hsuan Lee (wt.ude.utn@eelhiem). Further information is available from the corresponding author upon request.
REFERENCES
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71: 209–49. [DOI] [PubMed] [Google Scholar]
- 2.McGlynn KA, London WT. Epidemiology and natural history of hepatocellular carcinoma. Best practice & research 2005;19: 3–23. [DOI] [PubMed] [Google Scholar]
- 3.Petrick JL, McGlynn KA. The changing epidemiology of primary liver cancer. Curr Epidemiol Rep 2019;6: 104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. WHO Fact Sheet on Hepatitis B. 2021. https://www.who.int/news-room/fact-sheets/detail/hepatitis-b Accessed August 15, 2021. [Google Scholar]
- 5.World Health Organization. WHO Fact Sheet on Hepatitis C. 2021. https://www.who.int/news-room/fact-sheets/detail/hepatitis-c Accessed August 15, 2021. [Google Scholar]
- 6.McGlynn KA, Petrick JL, London WT. Global epidemiology of hepatocellular carcinoma: an emphasis on demographic and regional variability. Clin Liver Dis 2015;19: 223–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang YT, Jen CL, Yang HI, Lee MH, Su J, Lu SN, Iloeje UH, Chen CJ. Lifetime risk and sex difference of hepatocellular carcinoma among patients with chronic hepatitis B and C. J Clin Oncol 2011;29: 3643–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albillos A, de Gottardi A, Rescigno M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J Hepatol 2020;72: 558–77. [DOI] [PubMed] [Google Scholar]
- 9.Almeida J, Galhenage S, Yu J, Kurtovic J, Riordan SM. Gut flora and bacterial translocation in chronic liver disease. World J Gastroenterol 2006;12: 1493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez FJ, Jiang C, Patterson AD. An Intestinal Microbiota-Farnesoid X Receptor Axis Modulates Metabolic Disease. Gastroenterology 2016;151: 845–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jia W, Xie G, Jia W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat Rev Gastroenterol Hepatol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vajro P, Paolella G, Fasano A. Microbiota and gut-liver axis: their influences on obesity and obesity-related liver disease. J Pediatr Gastroenterol Nutr 2013;56: 461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mannisto V, Farkkila M, Pussinen P, Jula A, Mannisto S, Lundqvist A, Valsta L, Salomaa V, Perola M, Aberg F. Serum lipopolysaccharides predict advanced liver disease in the general population. JHEP Rep 2019;1: 345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Assimakopoulos SF, Triantos C, Maroulis I, Gogos C. The Role of the Gut Barrier Function in Health and Disease. Gastroenterology Res 2018;11: 261–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martel J, Chang SH, Ko YF, Hwang TL, Young JD, Ojcius DM. Gut barrier disruption and chronic disease. Trends Endocrinol Metab 2022;33: 247–65. [DOI] [PubMed] [Google Scholar]
- 16.Seki E, Schnabl B. Role of innate immunity and the microbiota in liver fibrosis: crosstalk between the liver and gut. J Physiol 2012;590: 447–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Son G, Kremer M, Hines IN. Contribution of gut bacteria to liver pathobiology. Gastroenterol Res Pract 2010;2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dapito DH, Mencin A, Gwak GY, Pradere JP, Jang MK, Mederacke I, Caviglia JM, Khiabanian H, Adeyemi A, Bataller R, Lefkowitch JH, Bower M, et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell 2012;21: 504–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao Y, Liu F, Yang J, Zhong M, Zhang E, Li Y, Zhou D, Cao Y, Li W, Yu J, Yang Y, Yan H. Over-activation of TLR5 signaling by high-dose flagellin induces liver injury in mice. Cell Mol Immunol 2015;12: 729–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu LX, Yan HX, Liu Q, Yang W, Wu HP, Dong W, Tang L, Lin Y, He YQ, Zou SS, Wang C, Zhang HL, et al. Endotoxin accumulation prevents carcinogen-induced apoptosis and promotes liver tumorigenesis in rodents. Hepatology 2010;52: 1322–33. [DOI] [PubMed] [Google Scholar]
- 21.Fedirko V, Tran HQ, Gewirtz AT, Stepien M, Trichopoulou A, Aleksandrova K, Olsen A, Tjonneland A, Overvad K, Carbonnel F, Boutron-Ruault MC, Severi G, et al. Exposure to bacterial products lipopolysaccharide and flagellin and hepatocellular carcinoma: a nested case-control study. BMC Med 2017;15: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harte AL, da Silva NF, Creely SJ, McGee KC, Billyard T, Youssef-Elabd EM, Tripathi G, Ashour E, Abdalla MS, Sharada HM, Amin AI, Burt AD, et al. Elevated endotoxin levels in non-alcoholic fatty liver disease. J Inflamm (Lond) 2010;7: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Endotoxemia Rao R. and gut barrier dysfunction in alcoholic liver disease. Hepatology 2009;50: 638–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sozinov AS. Systemic endotoxemia during chronic viral hepatitis. Bull Exp Biol Med 2002;133: 153–5. [DOI] [PubMed] [Google Scholar]
- 25.Thuy S, Ladurner R, Volynets V, Wagner S, Strahl S, Konigsrainer A, Maier KP, Bischoff SC, Bergheim I. Nonalcoholic fatty liver disease in humans is associated with increased plasma endotoxin and plasminogen activator inhibitor 1 concentrations and with fructose intake. J Nutr 2008;138: 1452–5. [DOI] [PubMed] [Google Scholar]
- 26.Endotoxemia Rao R. and gut barrier dysfunction in alcoholic liver disease. Hepatology 2009;50: 638–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harte AL, da Silva NF, Creely SJ, McGee KC, Billyard T, Youssef-Elabd EM, Tripathi G, Ashour E, Abdalla MS, Sharada HM. Elevated endotoxin levels in non-alcoholic fatty liver disease. J Inflamm 2010;7: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thuy S, Ladurner R, Volynets V, Wagner S, Strahl S, Königsrainer A, Maier K-P, Bischoff SC, Bergheim I. Nonalcoholic Fatty Liver Disease in Humans Is Associated with Increased Plasma Endotoxin and Plasminogen Activator Inhibitor 1 Concentrations and with Fructose Intake. J Nutr 2008;138: 1452–5. [DOI] [PubMed] [Google Scholar]
- 29.Yang B, Petrick JL, Thistle JE, Pinto LA, Kemp TJ, Tran HQ, Gewirtz AT, Waterboer T, Fedirko V, Jenab M, Graubard BI, Weinstein SJ, et al. Bacterial Translocation and Risk of Liver Cancer in a Finnish Cohort. Cancer Epidemiol Biomarkers Prev 2019;28: 807–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen C-J, Yang H-I, Su J, Jen C-L, You S-L, Lu S-N, Huang G-T, Iloeje UH, REVEAL-HBV Study Group ft. Risk of Hepatocellular Carcinoma Across a Biological Gradient of Serum Hepatitis B Virus DNA Level. JAMA 2006;295: 65–73. [DOI] [PubMed] [Google Scholar]
- 31.Yang H-I, Yuen M-F, Chan HL-Y, Han K-H, Chen P-J, Kim D-Y, Ahn S-H, Chen C-J, Wong VW-S, Seto W-K. Risk estimation for hepatocellular carcinoma in chronic hepatitis B (REACH-B): development and validation of a predictive score. The Lancet Oncology 2011;12: 568–74. [DOI] [PubMed] [Google Scholar]
- 32.Chen C-J, Iloeje UH, Yang H-I. Long-Term Outcomes in Hepatitis B: The REVEAL-HBV Study. Clinics in Liver Disease 2007;11: 797–816. [DOI] [PubMed] [Google Scholar]
- 33.Liu CJ, Chen PJ. Elimination of Hepatitis B in Highly Endemic Settings: Lessons Learned in Taiwan and Challenges Ahead. Viruses 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chien RN, Lu SN, Pwu RF, Wu GH, Yang WW, Liu CL. Taiwan accelerates its efforts to eliminate hepatitis C. Glob Health Med 2021;3: 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang H-I, Tseng T-C, Liu J, Lee M-H, Liu C-J, Su T-H, Batrla-Utermann R, Chan HL-Y, Kao J-H, Chen C-J. Incorporating Serum Level of Hepatitis B Surface Antigen or Omitting Level of Hepatitis B Virus DNA Does not Affect Calculation of Risk for Hepatocellular Carcinoma in Patients Without Cirrhosis. Clinical Gastroenterology and Hepatology 2016;14: 461–8.e2. [DOI] [PubMed] [Google Scholar]
- 36.Mei-Hsuan L, Yang H-I, Lu S-N, Jen C-L, Yeh S-H, Liu C-J, Chen P-J, You S-L, Wang L-Y, Chen WJ, Chen C-J. Hepatitis C Virus Seromarkers and Subsequent Risk of Hepatocellular Carcinoma: Long-Term Predictors From a Community-Based Cohort Study. Journal of Clinical Oncology 2010;28: 4587–93. [DOI] [PubMed] [Google Scholar]
- 37.Ziegler TR, Luo M, Estívariz CF, Moore DA 3rd, Sitaraman SV, Hao L, Bazargan N, Klapproth J-M, Tian J, Galloway JR, Leader LM, Jones DP, et al. Detectable serum flagellin and lipopolysaccharide and upregulated anti-flagellin and lipopolysaccharide immunoglobulins in human short bowel syndrome. American journal of physiology Regulatory, integrative and comparative physiology 2008;294: R402–R10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang LY, You SL, Lu SN, Ho HC, Wu MH, Sun CA, Yang HI, Chien-Jen C. Risk of hepatocellular carcinoma and habits of alcohol drinking, betel quid chewing and cigarette smoking: a cohort of 2416 HBsAg-seropositive and 9421 HBsAg-seronegative male residents in Taiwan. Cancer Causes Control 2003;14: 241–50. [DOI] [PubMed] [Google Scholar]
- 39.Loomba R, Yang HI, Su J, Brenner D, Iloeje U, Chen CJ. Obesity and alcohol synergize to increase the risk of incident hepatocellular carcinoma in men. Clin Gastroenterol Hepatol 2010;8: 891-8, 8 e1–2. [DOI] [PubMed] [Google Scholar]
- 40.Rothman KJ, Greenland S, Lash TL. Modern epidemiology, 3rd ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins, 2008. x, 758 p. [Google Scholar]
- 41.Polaris Observatory C. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol 2018;3: 383–403. [DOI] [PubMed] [Google Scholar]
- 42.Polaris Observatory HCVC. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol 2017;2: 161–76. [DOI] [PubMed] [Google Scholar]
- 43.Novitsky TJ. Limitations of the Limulus amebocyte lysate test in demonstrating circulating lipopolysaccharides. Ann N Y Acad Sci 1998;851: 416–21. [DOI] [PubMed] [Google Scholar]
- 44.Ryu JK, Kim SJ, Rah SH, Kang JI, Jung HE, Lee D, Lee HK, Lee JO, Park BS, Yoon TY, Kim HM. Reconstruction of LPS Transfer Cascade Reveals Structural Determinants within LBP, CD14, and TLR4-MD2 for Efficient LPS Recognition and Transfer. Immunity 2017;46: 38–50. [DOI] [PubMed] [Google Scholar]
- 45.Guha M, Mackman N. LPS induction of gene expression in human monocytes. Cell Signal 2001;13: 85–94. [DOI] [PubMed] [Google Scholar]
- 46.Marshall JS, Warrington R, Watson W, Kim HL. An introduction to immunology and immunopathology. Allergy Asthma Clin Immunol 2018;14: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li N, Zhu Q, Yang C, Li F, Zhou Z, Lv Y, Sang J, Han Q, Liu Z. Elevated serum soluble CD14 levels in chronic HBV infection are significantly associated with HBV-related hepatocellular carcinoma. Tumour Biol 2016;37: 6607–17. [DOI] [PubMed] [Google Scholar]
- 48.Ren Z, Li A, Jiang J, Zhou L, Yu Z, Lu H, Xie H, Chen X, Shao L, Zhang R, Xu S, Zhang H, et al. Gut microbiome analysis as a tool towards targeted non-invasive biomarkers for early hepatocellular carcinoma. Gut 2019;68: 1014–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu LX, Schwabe RF. The gut microbiome and liver cancer: mechanisms and clinical translation. Nat Rev Gastroenterol Hepatol 2017;14: 527–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tobias PS, Soldau K, Gegner JA, Mintz D, Ulevitch RJ. Lipopolysaccharide binding protein-mediated complexation of lipopolysaccharide with soluble CD14. J Biol Chem 1995;270: 10482–8. [DOI] [PubMed] [Google Scholar]
- 51.Shive CL, Jiang W, Anthony DD, Lederman MM. Soluble CD14 is a nonspecific marker of monocyte activation. AIDS 2015;29: 1263–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pan C, Gu Y, Zhang W, Zheng Y, Peng L, Deng H, Chen Y, Chen L, Chen S, Zhang M, Gao Z. Dynamic changes of lipopolysaccharide levels in different phases of acute on chronic hepatitis B liver failure. PLoS One 2012;7: e49460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moon MS, Quinn G, Townsend EC, Ali RO, Zhang GY, Bradshaw A, Hill K, Guan H, Hamilton D, Kleiner DE, Koh C, Heller T. Bacterial Translocation and Host Immune Activation in Chronic Hepatitis C Infection. Open Forum Infect Dis 2019;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sandler NG, Koh C, Roque A, Eccleston JL, Siegel RB, Demino M, Kleiner DE, Deeks SG, Liang TJ, Heller T, Douek DC. Host response to translocated microbial products predicts outcomes of patients with HBV or HCV infection. Gastroenterology 2011;141: 1220-30, 30 e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu LL, Huang TS, Shyu YC, Wang CL, Wang HY, Chen PJ. Gut microbiota in the innate immunity against hepatitis B virus - implication in age-dependent HBV clearance. Curr Opin Virol 2021;49: 194–202. [DOI] [PubMed] [Google Scholar]
- 56.Munteanu D, Negru A, Radulescu M, Mihailescu R, Arama SS, Arama V. Evaluation of bacterial translocation in patients with chronic HCV infection. Rom J Intern Med 2014;52: 91–6. [PubMed] [Google Scholar]
- 57.Guo J, Jing R, Zhong JH, Dong X, Li YX, Liu YK, Huang TR, Zhang CY. Identification of CD14 as a potential biomarker of hepatocellular carcinoma using iTRAQ quantitative proteomics. Oncotarget 2017;8: 62011–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ganem D, Prince AM. Hepatitis B virus infection--natural history and clinical consequences. N Engl J Med 2004;350: 1118–29. [DOI] [PubMed] [Google Scholar]
- 59.Chou HH, Chien WH, Wu LL, Cheng CH, Chung CH, Horng JH, Ni YH, Tseng HT, Wu D, Lu X, Wang HY, Chen PJ, et al. Age-related immune clearance of hepatitis B virus infection requires the establishment of gut microbiota. Proc Natl Acad Sci U S A 2015;112: 2175–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu T, Li F, Chen Y, Wei H, Tian Z, Sun C, Sun R. CD4(+) T Cells Play a Critical Role in Microbiota-Maintained Anti-HBV Immunity in a Mouse Model. Front Immunol 2019;10: 927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Citronberg JS, Wilkens LR, Lim U, Hullar MA, White E, Newcomb PA, Le Marchand L, Lampe JW. Reliability of plasma lipopolysaccharide-binding protein (LBP) from repeated measures in healthy adults. Cancer Causes Control 2016;27: 1163–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McKay HS, Margolick JB, Martinez-Maza O, Lopez J, Phair J, Rappocciolo G, Denny TN, Magpantay LI, Jacobson LP, Bream JH. Multiplex assay reliability and long-term intra-individual variation of serologic inflammatory biomarkers. Cytokine 2017;90: 185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are stored at the Genomics Research Center, Academia Sinica, Taipei, Taiwan, and initial requests for data may be directed to Chien-Jen Chen (wt.ude.acinis.etag@jcnehc), Hwai-I Yang (wt.ude.acinis.etag@gnayih), or Mei-Hsuan Lee (wt.ude.utn@eelhiem). Further information is available from the corresponding author upon request.


