Abstract
The Intracellular Na+ concentration in the halotolerant alga Dunaliella salina was measured in intact cells by 23Na-NMR spectroscopy, utilizing the dysprosium tripolyphosphate complex as a sodium shift reagent, and was found to be 88 ± 28 millimolar. Intracellular sodium ion content and intracellular volume were the same, within the experimental error, in cells adapted to grow in media containing between 0.1 and 4.0 molar NaCl. These values assume extracellular and intracellular NMR visibilities of the 23Na nuclei of 100 and 40%, respectively. The relaxation rate of intracellular sodium was enhanced with increasing salinity of the growth medium, in parallel to the intracellular osmosity due to the presence of glycerol, indicating that Na+ ions and glycerol are codistribbuted within the cell volume.
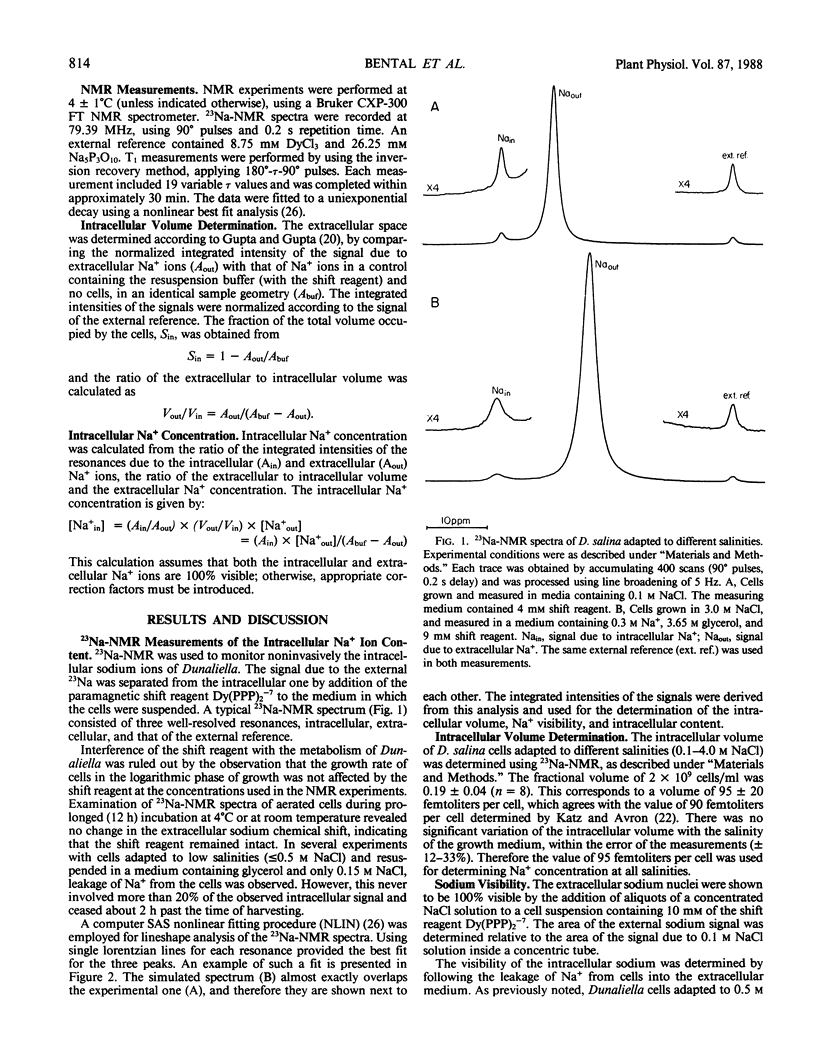
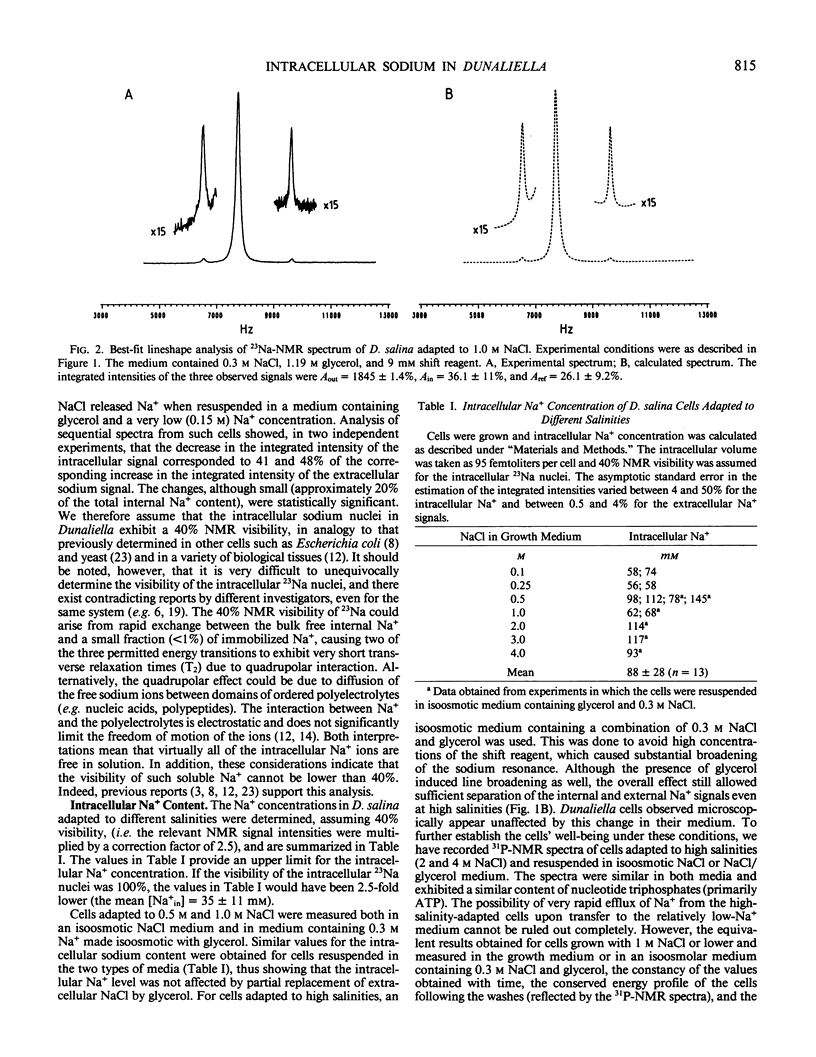
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bental M., Oren-Shamir M., Avron M., Degani H. P and C-NMR Studies of the Phosphorus and Carbon Metabolites in the Halotolerant Alga, Dunaliella salina. Plant Physiol. 1988 Jun;87(2):320–324. doi: 10.1104/pp.87.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle A. M., Macnab R. M., Shulman R. G. Coupling between the sodium and proton gradients in respiring Escherichia coli cells measured by 23Na and 31P nuclear magnetic resonance. J Biol Chem. 1986 Jun 15;261(17):7797–7806. [PubMed] [Google Scholar]
- Castle A. M., Macnab R. M., Shulman R. G. Measurement of intracellular sodium concentration and sodium transport in Escherichia coli by 23Na nuclear magnetic resonance. J Biol Chem. 1986 Mar 5;261(7):3288–3294. [PubMed] [Google Scholar]
- Civan M. M., Degani H., Margalit Y., Shporer M. Observations of 23Na in frog skin by NMR. Am J Physiol. 1983 Sep;245(3):C213–C219. doi: 10.1152/ajpcell.1983.245.3.C213. [DOI] [PubMed] [Google Scholar]
- Forsén S., Lindman B. Ion binding in biological systems as studied by NMR spectroscopy. Methods Biochem Anal. 1981;27:289–486. doi: 10.1002/9780470110478.ch5. [DOI] [PubMed] [Google Scholar]
- Gerasimowicz W. V., Tu S. I., Pfeffer P. E. Energy Facilitated Na Uptake in Excised Corn Roots via P and Na NMR. Plant Physiol. 1986 Jul;81(3):925–928. doi: 10.1104/pp.81.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz A., Avron M. Determination of intracellular osmotic volume and sodium concentration in dunaliella. Plant Physiol. 1985 Aug;78(4):817–820. doi: 10.1104/pp.78.4.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogino T., den Hollander J. A., Shulman R. G. 39K, 23Na, and 31P NMR studies of ion transport in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5185–5189. doi: 10.1073/pnas.80.17.5185. [DOI] [PMC free article] [PubMed] [Google Scholar]


