Abstract
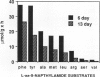
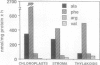
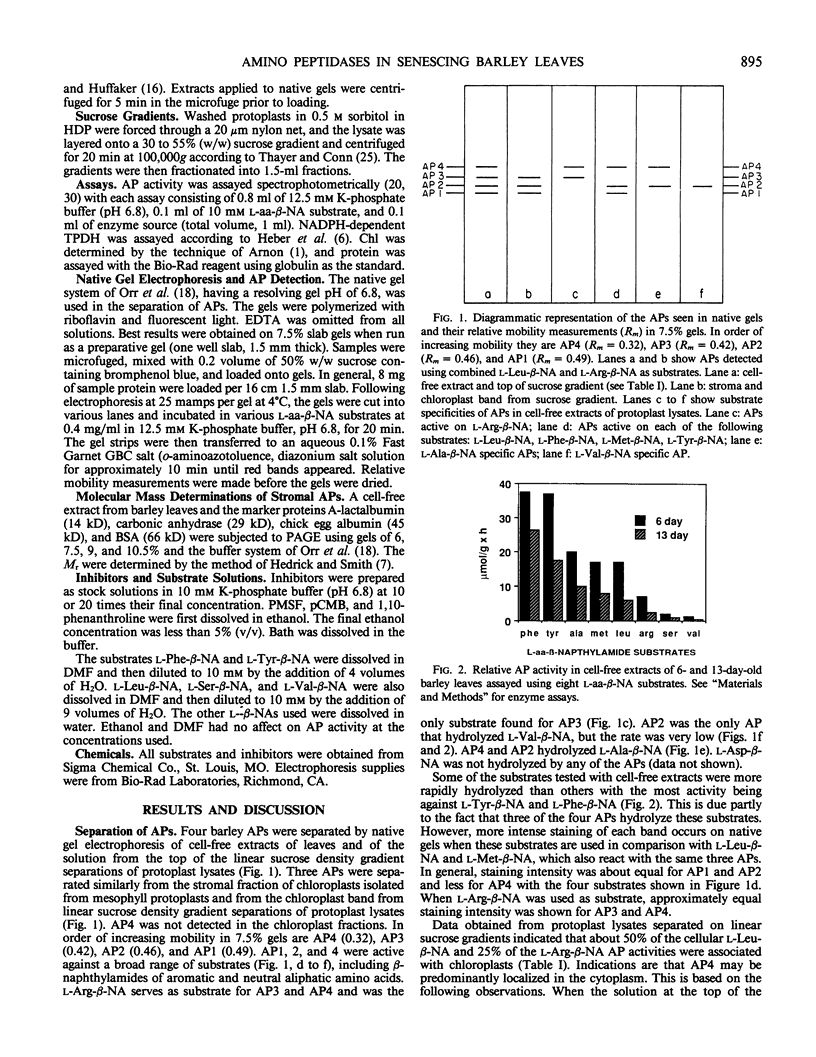
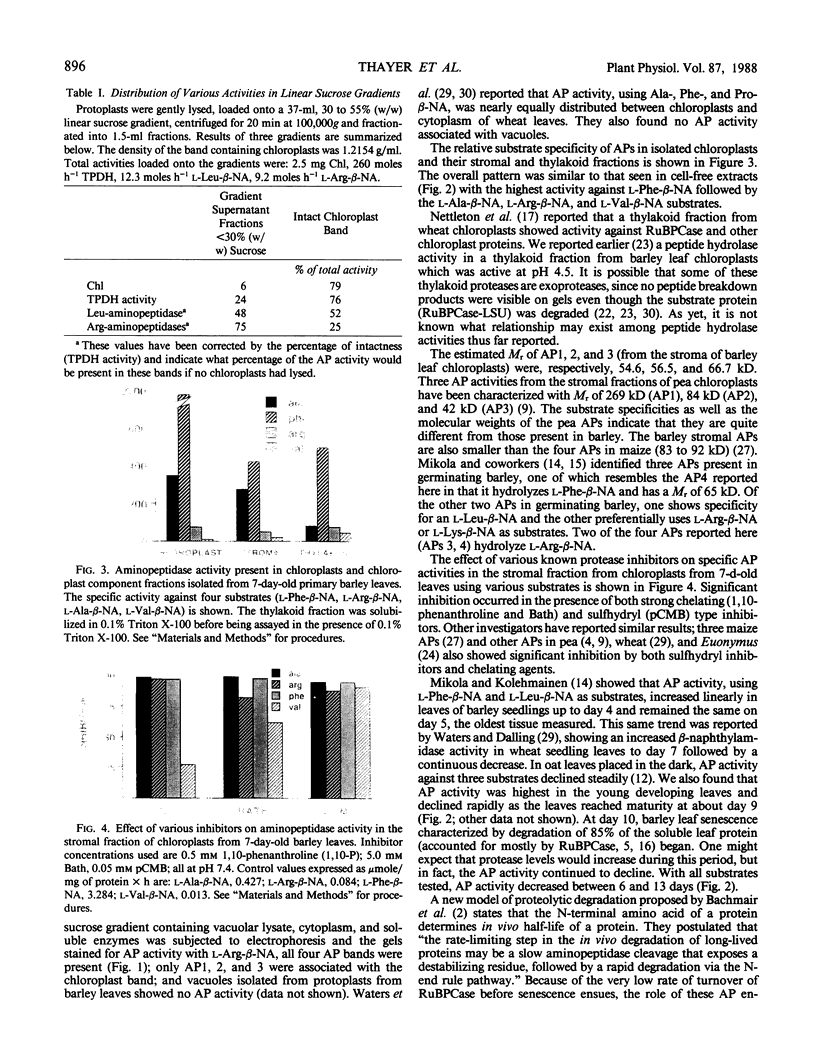
Four aminopeptidases (APs) were separated using native polyacrylamide gel electrophoresis of cell-free extracts and the stromal fractions of isolated chloroplasts prepared from primary barley (Hordeum vulgare L., var Numar) leaves. Activities were identified using a series of aminoacyl-β-naphthylamide derivatives as substrates. AP1, 2, and 3 were found in the stromal fraction of isolated chloroplasts with respective molecular masses of 66.7, 56.5, and 54.6 kilodaltons. AP4 was found only in the cytoplasmic fraction. No AP activity was found in vacuoles of these leaves. It was found that 50% of the l-Leu-β-naphthylamide and 25% of the l-Arg-β-naphthylamide activities were localized in the chloroplasts. Several AP activities were associated with the membranes of the thylakoid fraction of isolated chloroplasts. AP1, 2, and 4 reacted against a broad range of substrates, whereas AP3 hydrolyzed only l-Arg-β-naphthylamide. Only AP2 hydrolyzed l-Val-β-naphthylamide. Since AP2 and AP3 were the only ones reacting against Val-β-naphthylamide and Arg-β-naphthylamide, respectively, several protease inhibitors were tested against these substrates using a stromal fraction from isolated chloroplasts as the source of the two APs. Both APs were sensitive to both metallo and sulfhydryl type inhibitors. Although AP activity decreased as leaves senesced, no new APs appeared on gels during senescence and none disappeared.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmair A., Finley D., Varshavsky A. In vivo half-life of a protein is a function of its amino-terminal residue. Science. 1986 Oct 10;234(4773):179–186. doi: 10.1126/science.3018930. [DOI] [PubMed] [Google Scholar]
- Elleman T. C. Aminopeptides of pea. Biochem J. 1974 Jul;141(1):113–118. doi: 10.1042/bj1410113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich J. W., Huffaker R. C. Photosynthesis, leaf resistances, and ribulose-1,5-bisphosphate carboxylase degradation in senescing barley leaves. Plant Physiol. 1980 Jun;65(6):1103–1107. doi: 10.1104/pp.65.6.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber U., Pon N. G., Heber M. Localization of Carboxydismutase & Triosephosphate Dehydrogenases in Chloroplasts. Plant Physiol. 1963 May;38(3):355–360. doi: 10.1104/pp.38.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- Liu X. Q., Jagendorf A. T. Neutral peptidases in the stroma of pea chloroplasts. Plant Physiol. 1986 Jun;81(2):603–608. doi: 10.1104/pp.81.2.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller B. L., Huffaker R. C. Differential Induction of Endoproteinases during Senescence of Attached and Detached Barley Leaves. Plant Physiol. 1985 Jul;78(3):442–446. doi: 10.1104/pp.78.3.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr M. D., Blakley R. L., Panagou D. Discontinuous buffer systems for analytical and preparative electrophoresis of enzymes on polyacrylamide gel. Anal Biochem. 1972 Jan;45(1):68–85. doi: 10.1016/0003-2697(72)90008-5. [DOI] [PubMed] [Google Scholar]
- Ragster L. E., Chrispeels M. J. Autodigestion in crude extracts of soybean leaves and isolated chloroplasts as a measure of proteolytic activity. Plant Physiol. 1981 Jan;67(1):104–109. doi: 10.1104/pp.67.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starnes W. L., Szechiński J., Behal F. J. Human-liver alanine aminopeptidase. A kinin-converting enzyme sensitive to beta-lactam antibiotics. Eur J Biochem. 1982 May 17;124(2):363–370. [PubMed] [Google Scholar]
- Thayer S. S., Conn E. E. Subcellular Localization of Dhurrin beta-Glucosidase and Hydroxynitrile Lyase in the Mesophyll Cells of Sorghum Leaf Blades. Plant Physiol. 1981 Apr;67(4):617–622. doi: 10.1104/pp.67.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer S. S., Huffaker R. C. Vacuolar Localization of Endoproteinases EP(1) and EP(2) in Barley Mesophyll Cells. Plant Physiol. 1984 May;75(1):70–73. doi: 10.1104/pp.75.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodkin L. O., Scandalios J. G. Comparative properties of genetically defined peptidases in maize. Biochemistry. 1980 Sep 30;19(20):4660–4667. doi: 10.1021/bi00561a019. [DOI] [PubMed] [Google Scholar]
- Wardley T. M., Bhalla P. L., Dalling M. J. Changes in the Number and Composition of Chloroplasts during Senescence of Mesophyll Cells of Attached and Detached Primary Leaves of Wheat (Triticum aestivum L.). Plant Physiol. 1984 Jun;75(2):421–424. doi: 10.1104/pp.75.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters S. P., Noble E. R., Dalling M. J. Intracellular Localization of Peptide Hydrolases in Wheat (Triticum aestivum L.) Leaves. Plant Physiol. 1982 Mar;69(3):575–579. doi: 10.1104/pp.69.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]