Abstract
Background:
It remains unclear whether in utero and childhood exposure to air pollution affects pubertal development, particularly age of menarche in girls.
Objective:
The aim of this study was to determine whether residential ambient particulate matter (PM) exposure in utero and during childhood is associated with age of menarche.
Methods:
We studied 5,201 girls in the Growing Up Today Study 2 (2004–present) who were 10–17 y of age at enrollment (47.7% premenarchal; 52.3% postmenarchal). Exposure to three size fractions of PM [fine PM with aerodynamic diameter (), PM with aerodynamic diameters (), and PM with aerodynamic diameter ()] was assigned based on maternal residential address, updated every 2 y, using nationwide spatiotemporal models. We estimated average PM exposure in utero, and time-varying windows: annual average exposure in the prior 1 and 2 y and cumulative average from birth. Age of menarche was self-reported on three surveys administered in 2004, 2006, and 2008. We calculated hazard ratios (HR) for menarche for an interquartile range (IQR) increase in PM exposure using Cox proportional hazard models adjusting for potential confounders.
Results:
Girls attained menarche at 12.3 y of age on average. In the adjusted model, higher residential exposure to ambient during all time windows was associated with earlier age of menarche. The HRs of menarche for each IQR () increase in exposure to during the in utero period, 1 y prior to menarche, and throughout childhood were 1.03 [95% confidence interval (CI): 1.00, 1.06], 1.06 (95% CI: 1.02, 1.10) and 1.06 (95% CI: 1.02, 1.10), respectively. Effect estimates for exposure were similar, albeit attenuated, for all time windows. exposure was not associated with age of menarche.
Discussion:
Among a large, nationwide, prospective cohort of U.S. girls, higher exposure to and in utero and throughout childhood was associated with an earlier age of menarche. Our results suggest that and may have endocrine-disrupting properties that could lead to altered timing of menarche. https://doi.org/10.1289/EHP12110
Introduction
It is estimated that age of menarche in U.S. girls has decreased by over the past century, with more modest declines over the past few decades.1 This decline is concerning because an earlier age of menarche has been associated with increased risk of several adult-onset diseases, including cardiovascular disease,2 diabetes,3 and endometrial4 and ovarian cancer.5 Reasons for the decline in menarche are not fully understood. However, the prevailing hypothesis has linked improved nutritional status and, in more recent decades, childhood obesity with earlier age at menarche.6 Another plausible hypothesis is that environmental exposures, specifically exposures to endocrine-disrupting chemicals (EDCs), are altering age of menarche.1,7 These chemicals could interfere with the endocrine system or may have obesogenic properties. For example, studies have found that exposure to pesticides,8 persistent organic pollutants,9 polybrominated diphenyl ethers,10 phthalates,11 and phenols11,12 all may result in altered pubertal timing. Notably absent from this list, however, is particulate matter (PM) air pollution.
PM is a complex mixture of small particles and gases that may have endocrine-disrupting properties.7 PM is categorized based on aerodynamic diameter into those (), those between 2.5 and (), and the combined metric of or less (). The different diameters are linked with how deeply the particulate may penetrate into the respiratory tract, with being capable of penetrating into the lower respiratory tract and crossing into the blood stream, whereas generally is trapped in the upper respiratory tract. PM is the result of incomplete combustion and major sources include vehicle emissions and the burning of biomass and fossil fuels. Increased exposure to PM has been associated with a variety of adverse reproductive health outcomes including shortened luteal phase length,13 an increased time to cycle regularity,14 and reduced fecundability15 andp fertility.16 These alterations may be due to potential endocrine-disrupting properties of PM.7 In addition to these various reproductive health outcomes, PM has also been associated with puberty timing and development in girls.17–20 However, these studies have been limited by sample size and misclassification of exposures and outcomes, as well as by selection bias because of low follow-up rates. Given that PM may have endocrine-disrupting properties and previous studies have observed alterations in the human reproductive system with PM exposure, it is plausible that PM exposure may alter age of menarche.
Therefore, to address this gap in knowledge, we used a large, prospective, nationwide study of girls in the United States to evaluate the association between residential exposure to ambient PM and timing of menarche. We were specifically interested in investigating the relationship between different PM sizes and different windows of exposure with age of menarche.
Materials and Methods
Study Population
The Growing Up Today Study 2 (GUTS2) is a prospective cohort of 10,917 children of women enrolled in the Nurse’s Health Study II (NHSII).21 NHSII is composed of female nurses who were 25–42 y of age and living in 1 of 14 U.S. states at study initiation in 1989.22 NHSII and GUTS2 participants now live throughout the United States. A baseline questionnaire was sent to GUTS2 participants in 2004 when the children were between 10 and 17 y of age; children were eligible for the cohort if they were born between 1988 and 1996. Follow-up questionnaires, which included a variety of questions on lifestyle and health topics, have been sent annually or biennially ever since. Parents provided written informed consent for their child’s participation, and consent is assumed through completion and return of the follow-up questionnaires. This study protocol was approved by the institutional review boards of the Brigham and Women’s Hospital and the Harvard T.H. Chan School of Public Health. For our analysis, we only included GUTS2 children born on or after January 1989 and who were assigned female at birth. We selected 1989 as the initial time point because air pollution data were not available prior to 1988, enrollment and prospective data collection for NHSII began in 1989, and a key exposure period of interest was in utero. After exclusion of individuals who were not assigned female at birth (), individuals born before 1989 (), and those missing all their exposure data (), 5,201 girls remained in the sample (Figure S1).
Outcome
Participants self-reported their age at menarche on questionnaires in 2004, 2006, and 2008, and we used the earliest measure possible to reduce the chance of recall bias. Menarche is a salient event that is recalled accurately, especially over short intervals of time.23 In this cohort, the mean time between experiencing and reporting menarche was 1.4 y. At enrollment, 52.3% of girls were post menarche (). In each questionnaire, participants were asked “Have you started having menstrual periods?” If participants answered “Yes,” then they were asked “What age periods began?”, “What month periods began?”, and “What year periods began?” Respondents who had not reached menarche by 2008 were right-censored (; age range: 13–19 y). The primary outcome we used was year of menarche.
Exposures
Study participants’ residential history, starting from a year prior to their birth throughout childhood, was assigned based on the addresses of their mothers participating in NHSII. Residential address information for all NHSII participants was updated every 2 y as part of the questionnaire mailing process and was geocoded to obtain latitude and longitude. We predicted monthly ambient exposure to multiple size fractions of PM, including , , and , using nationwide spatiotemporal models from January 1988 through December 2007. Air pollution exposure was predicted using data from the Environmental Protection Agency’s Air Quality System, the Interagency Monitoring of Protected Visual Environments, as well as several geospatial predictors.24 Generalized additive statistical models with smooth terms of space and time were used to create separate PM prediction surfaces for each month. Because monitoring data on was limited prior to 1999, in the period before 1999 was modeled using data on and airport visibility. Information was available on by subtraction of the monthly values of from . The models were evaluated for predictive accuracy using a 10-set cross-validation approach; cross-validation correlation coefficients were high for () and moderate for () and ().24 Using the time-varying monthly PM values, we averaged exposure during several time intervals. We examined multiple time windows because we were interested in the potential impact of acute vs. chronic PM exposure as well as exposure during critical exposure windows. First, we examined a 1-y average exposure prior to birth to capture in utero exposure for each of the three size fractions. Next, we created moving averages, with one capturing the prior year of exposure, another capturing the prior 2 y, and a cumulative moving average to capture exposure to each pollutant from birth.
Covariates
We derived neighborhood socioeconomic status (nSES) at the census tract level based on maternal residential address using data from the closest U.S. Decennial Census (1990, 2000, or 2010). The methodology is based on guidelines outlined by Krieger et al.25 and is similar to other nSES metrics like the neighborhood deprivation index developed by Messer et al.26 We derived the novel metric due to assumed homogeneity in the population.27 Nine census variables (percentage over 25 y of age with college or higher education, median family income, median family home value, percentage of families receiving interest dividends or rent income, percentage of occupied housing units, percentage of population y of age unemployed, percentage White, percentage Black, percentage foreign-born) were transformed into z-scores and summed to create an overall nSES score for this cohort, with increasing values indicating higher neighborhood socioeconomic status. From a previous study, the distribution of the nSES in the NHS was similar to that of the entire United States.28 We also defined region of residence using the U.S. Census Bureau–designated regions: Midwest, Northeast, South, or West. At baseline girls answered questions about their race and ethnicity. Girls were asked to self-report their race and included the following categories: Asian, Black, Native Hawaiian/Pacific Islander, Native American, White, and Other. Due to small sample sizes, we collapsed the categories to White and all Other races (Asian/Black/Native Hawaiian/Pacific Islander/Native American/Other). For ethnicity, girls were asked, “Do you consider yourself to be Spanish/Hispanic/Latino(a)?” Girls were considered Hispanic if they answered yes and non-Hispanic if they answered no. Self-reported race was used as a proxy for structural racism. Maternal surveys provided data on maternal age of menarche and birth weight of the GUTS2 participants.
Statistical Analyses
We used basic descriptive statistics to describe the study sample and across quintiles of in utero exposure. We examined the correlation within and between size fractions of PM using Pearson’s correlation. We used unadjusted and adjusted Cox proportional hazard models to examine the association between exposure to each size fraction of PM in the various time windows and age at menarche. Time to event was measured in person-time from birth, with the event being menarche. Participants were censored if they were lost to follow-up or reached the end of follow-up before menarche. In addition, for the 1- and 2-y models, we used interval censoring for the exposures in our models so that if an individual was missing PM for a specific year, they were censored for that time interval. For the in utero model, individuals who were missing PM data during the in utero period or at birth were excluded from these models. For the cumulative average models, we carried forward the average from the previous year if it was missing. We accounted for clustering by mothers by using a robust sandwich covariance estimate, because mothers could have had more than one child participate in GUTS2. Nonlinear exposure–response relationships were examined using cubic splines for all PM exposure measures restricted to fifth and 95th percentiles of the exposure distribution to reduce the influence of outliers.29 Tests for nonlinearity used the likelihood ratio test, comparing the model with only the linear term to the model with the linear and the cubic spline terms. Hazard ratios (HRs) indicate that the exposure is associated with earlier menarche, and HRs indicate an association with later menarche. We report hazard ratios per interquartile range (IQR) increase in each pollutant, based on the IQR for the cumulative average. We plotted the predicted survival time for girls exposed to PM levels at the 10th and 90th percentiles using the mean value of continuous covariates and most common level for categorical covariates.
We selected covariates for our multivariable models a priori based on previous epidemiological studies and biological plausibility. Covariates included time-varying nSES (quintiles) and region of residence (Midwest, Northeast, South, or West), and time-invariant race (White, Other), ethnicity (Hispanic, non-Hispanic), maternal age at menarche ( y, 12–14 y, y), and birth weight (, , ). Each model included only one PM size fraction exposure at a time. To account for missing covariate data, we used the missing-indicator method. Last, to account for potential time trends and declining PM exposure, we further adjusted our main models (Model 1) for calendar year of birth (1989–1990, 1991–1992, 1993–1995) (Model 2).
Sensitivity Analyses
Because we did not continuously collect address information, it is possible individuals moved during the time windows for address updates (every 2 y), which means some individuals would be assigned the exposure values based on their old addresses. To reduce the risk of exposure misclassification from old address information, we restricted our analyses to include only individuals who did not move during follow-up (; 17.6%). To examine the potential for confounding between the various size fractions, we conducted regression models including two size fractions ( and ) for each exposure period. Lastly, to investigate whether short-term changes in PM exposure above a girl’s cumulative average were associated with risk of menarche, we modeled the difference between 1-y average PM exposure and the cumulative average exposure. We further adjusted these models for the cumulative average exposure.
Results
Sample Characteristics
The majority (90%) of girls in our study had a known age at menarche (mean: 12.3 y; standard deviation: 1.2), whereas 10% of girls were censored prior to reaching menarche. There was little difference in cumulative PM exposure between girls who had a known age at menarche and girls who were right-censored. Overall, GUTS2 participants were predominately White (95%) and non-Hispanic (95%). At birth, 33% resided in the Northeast, 35% in the Midwest, 14% in the West, and 14% in the South. Several participant characteristics varied by in utero exposure to ambient (Table 1). At birth, girls in the highest quintile of in utero exposure were more likely to be born in earlier years (i.e., reflective of declines of PM in the United States) and live in the Midwest and South in comparison with girls in the lowest quintile. Individuals in the lowest quintile of in utero exposure were more likely to live in the lowest nSES quintile (38%) in comparison with the highest exposure group (19%). Birth weight and maternal menarche timing did not vary across quintiles of in utero exposure.
Table 1.
Characteristics of 5,201 girls in the Growing Up Today Study 2 from 1988 to 2008 by quintiles of in utero exposure.
| All subjects | Quintiles of in utero exposurea | |||||
|---|---|---|---|---|---|---|
| () | Q1 () |
Q2 () |
Q3 () |
Q4 () |
Q5 () |
|
| Range of exposureb () | 4.80–32.19 | 4.80–13.09 | 13.10–15.20 | 15.2–16.90 | 16.90–18.90 | 18.90–32.19 |
| Birth year [n (%)] | ||||||
| 1989–1990 | 2,189 (42.1) | 295 (29.9) | 299 (30.1) | 326 (34.2) | 422 (42.2) | 638 (65.7) |
| 1991–1992 | 1,819 (35.0) | 364 (36.8) | 374 (37.6) | 334 (35.0) | 400 (40.0) | 263 (27.1) |
| 1993–1994 | 1,172 (22.5) | 323 (32.7) | 313 (31.5) | 289 (30.3) | 175 (17.5) | 70 (7.2) |
| 21 (0.4) | 6 (0.6) | 8 (0.8) | 4 (0.4) | 3 (0.3) | 0 (0.0) | |
| Raceb [n (%)] | ||||||
| White | 4,913 (94.5) | 944 (95.6) | 929 (93.5) | 903 (94.8) | 958 (95.8) | 899 (92.6) |
| Other | 216 (4.1) | 40 (3.1) | 49 (4.9) | 37 (3.9) | 30 (3.0) | 59 (6.1) |
| Missing | 72 (1.4) | 13 (1.3) | 16 (1.6) | 13 (1.3) | 12 (1.2) | 13 (1.3) |
| Hispanicb [n (%)] | ||||||
| Yes | 121 (2.3) | 28 (2.8) | 16 (1.6) | 13 (1.4) | 15 (1.5) | 38 (3.9) |
| No | 4,937 (94.9) | 933 (94.4) | 947 (95.3) | 917 (96.2) | 955 (95.5) | 909 (93.6) |
| Missing | 143 (2.8) | 27 (2.7) | 31 (3.1) | 23 (2.4) | 30 (3.0) | 24 (2.5) |
| Birth weightb | ||||||
| 240 (4.6) | 49 (5.0) | 44 (4.4) | 44 (4.6) | 42 (4.2) | 50 (5.2) | |
| 3,551 (68.3) | 667 (67.5) | 682 (68.6) | 643 (67.5) | 670 (67.0) | 686 (70.7) | |
| 486 (9.3) | 83 (8.4) | 92 (9.3) | 90 (9.4) | 95 (9.5) | 90 (9.3) | |
| Missing | 924 (17.8) | 189 (19.1) | 176 (17.7) | 176 (18.5) | 193 (19.3) | 145 (14.9) |
| Maternal age at menarcheb (y) | ||||||
| 1,098 (21.1) | 213 (21.6) | 192 (19.3) | 203 (21.3) | 219 (21.9) | 202 (20.8) | |
| 12–14 | 3,621 (69.6) | 680 (68.8) | 709 (71.3) | 656 (68.8) | 690 (69.0) | 683 (70.3) |
| 468 (9.0) | 91 (9.2) | 91 (9.2) | 92 (9.7) | 86 (8.6) | 85 (8.6) | |
| Missing | 14 (0.3) | 4 (0.4) | 2 (0.2) | 2 (0.2) | 5 (0.5) | 1 (0.1) |
| Region of residencec [n (%)] | ||||||
| Northeast | 1,689 (32.5) | 221 (22.4) | 408 (41.1) | 394 (41.3) | 359 (35.9) | 274 (28.2) |
| Midwest | 1,801 (34.6) | 269 (27.2) | 352 (35.4) | 348 (36.5) | 426 (42.6) | 377 (38.8) |
| West | 744 (14.3) | 349 (35.3) | 65 (6.5) | 109 (11.4) | 150 (15.0) | 65 (6.7) |
| South | 707 (13.6) | 143 (14.5) | 158 (15.9) | 91 (9.5) | 58 (5.8) | 243 (25.0) |
| Missing | 260 (5.0) | 6 (0.6) | 11 (1.1) | 11 (1.2) | 7 (0.7) | 12 (1.2) |
| Neighborhood SES quintilec,d,e [n (%)] | ||||||
| 0 (, ) | 1,216 (23.4) | 371 (37.6) | 224 (22.5) | 199 (20.9) | 215 (21.5) | 185 (19.1) |
| 1 (, ) | 1,287 (24.7) | 216 (21.9) | 255 (25.7) | 250 (26.2) | 255 (25.5) | 292 (30.1) |
| 2 (, 0.46) | 1,097 (21.1) | 198 (20.0) | 195 (19.6) | 209 (21.9) | 258 (25.8) | 224 (23.1) |
| 3 (0.46, 2.79) | 868 (16.7) | 127 (12.9) | 201 (20.2) | 196 (20.6) | 167 (16.7) | 161 (16.6) |
| 4 (2.79, 20.74) | 473 (9.1) | 70 (7.1) | 108 (10.9) | 88 (9.2) | 98 (9.8) | 97 (10.0) |
| Missing | 260 (5.0) | 6 (0.6) | 11 (1.1) | 11 (1.2) | 7 (0.7) | 12 (1.2) |
| Cumulative average f () | 13.4 (2.6) | 10.5 (1.7) | 12.3 (1.5) | 13.5 (1.5) | 14.7 (1.4) | 16.2 (2.2) |
| Cumulative average f () | 8.5 (4.0) | 9.0 (3.9) | 7.5 (3.5) | 7.2 (3.0) | 7.9 (2.8) | 10.9 (5.0) |
| Cumulative average f () | 21.9 (5.3) | 19.5 (4.5) | 19.8 (3.9) | 20.7 (3.5) | 22.6 (3.3) | 27.1 (6.2) |
PM, particulate matter; SES, socioeconomic status.
Total number of individuals in the quintiles of in utero exposure do not add to the total sample size due to missing values for in utero exposure ().
This is a non–time-varying covariate measured at birth.
This is a time-varying covariate, but values here reflect values at birth.
Increasing quintile of neighborhood socioeconomic status indicates higher socioeconomic status.
Minimum and maximum values for each of the quintiles of the neighborhood socioeconomic status are provided. Quintiles are mutually exclusive but appear to overlap because of rounding.
This is a cumulative time-varying covariate, so values provided were measured from birth until the last available time point for each subject (i.e., menarche, prior to loss to follow-up, or prior to right-censored). Values are reported as means and standard deviations.
PM Exposure
The median in utero , and exposures were (IQR: 4.6), (IQR: 5.9), and (IQR: 7.8), respectively (Table S1). The median cumulative (, IQR: 4.0), (, IQR: 5.0), and (, IQR: 6.6) exposures were, on average, lower than the in utero exposures, because of the decline of PM in the United States over this time frame. For a given PM size, the highest correlations were observed between the 1-y and 2-y averages (: 0.98; : 0.99; : 0.99), whereas the lowest correlation was between the in utero and 1- average (: 0.69; : 0.78; : 0.71) (Table S1). As expected, across all time windows, and as well as and were all highly correlated.
Using the multivariable Cox proportional hazard models, higher in utero exposure to ambient (; 95% CI: 1.04, 1.09 per ), (; 95% CI: 1.01, 1.07 per ), and (; 95% CI: 1.05, 1.11 per ) were associated with increased risk of earlier age of menarche (Figure 1A; Table S2). Higher exposures in the previous year or 2 y and cumulative exposure from birth to and were also associated with earlier time to menarche. The HRs were slightly stronger for in comparison with and for cumulative exposure in comparison with 2-y or 1-y average exposure. For example, every IQR increase in cumulative exposure to and was associated with a 10% (95% CI: 6%, 15%) and 8% (95% CI: 4%, 12%) higher risk of menarche, respectively. exposure was not consistently associated with age of menarche. The associations between PM exposure and age at menarche were attenuated after adjusting for calendar year of birth but were still statistically significant for exposure (; 95% CI: 1.02, 1.10 for an IQR increase in exposure to cumulative ) (Figure 1B; Table S2).
Figure 1.
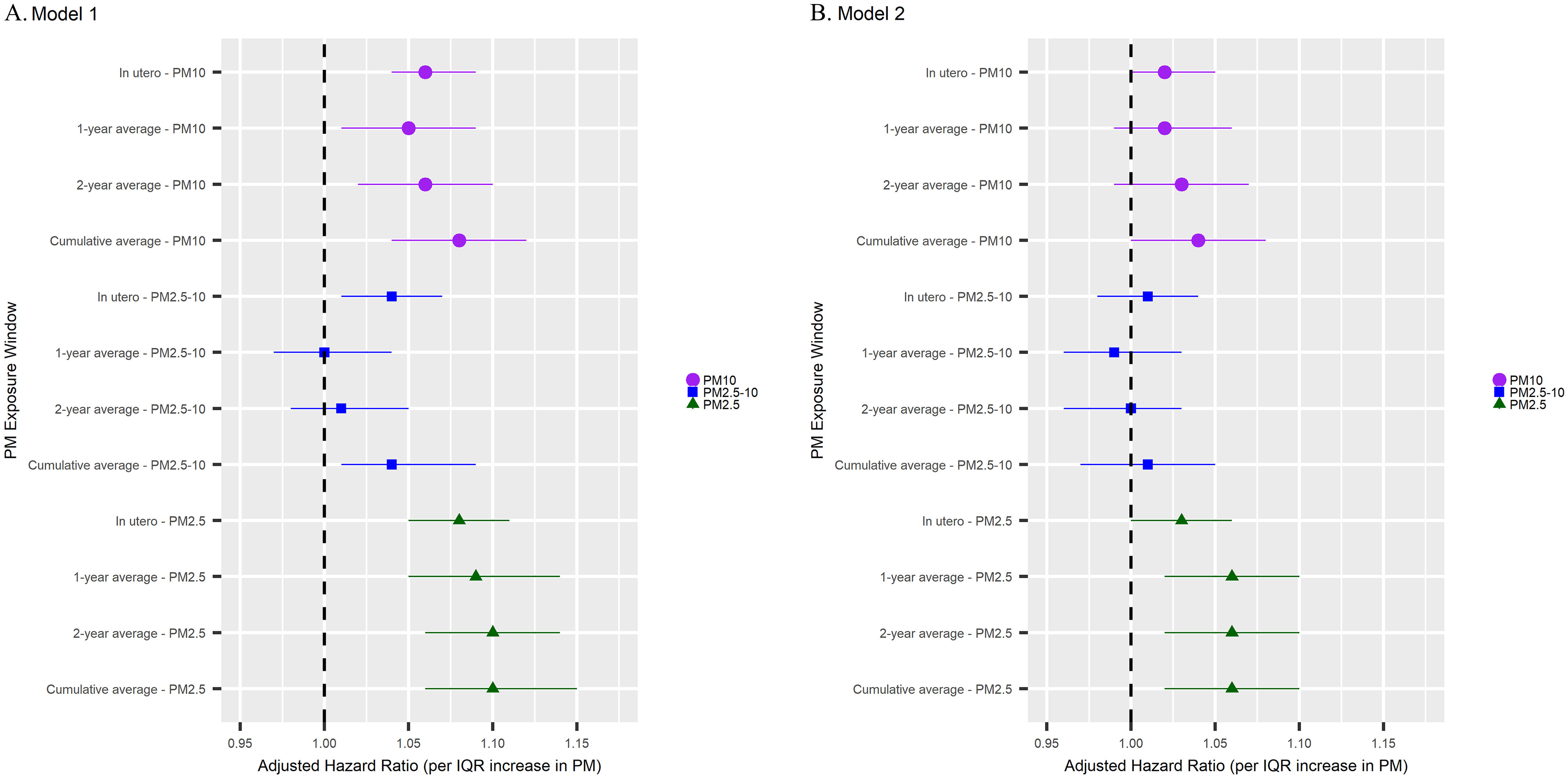
Forest plots of adjusted HRs and 95% CIs for the association between residential , , and exposures and age at menarche in the Growing Up Today Study 2, 1988–2008 () (see Table S2). All Cox proportional hazard models are adjusted for neighborhood SES, region of residence, race, ethnicity, maternal age at menarche, and birth weight (Model 1). Cox proportional hazard models were further adjusted for calendar year (Model 2). Circles with purple lines are for exposure, squares with blue lines are for exposure, and triangles with green lines are for exposure. HRs indicate that the exposure is associated with earlier age at menarche, and HRs indicate an association with later age at menarche. HRs are per increase in IQR. For , the IQR is ; for , the IQR is ; and for , the IQR is . The in utero models exclude individuals who were missing PM during the in utero period or at birth (). Lines indicate width of the 95% CI, and shapes indicates the point estimate. Note: CI, confidence interval; HR, hazard ratio; IQR, interquartile range; PM, particulate matter; SES, socioeconomic status.
There was borderline statistically significant nonlinear association between (at all time points except for in utero) and age at menarche (Figure S2; Table S3). However, the interpretations remained similar to that of the linear models, with higher levels of being associated with earlier age at menarche. There was no evidence of a nonlinear association between or and age at menarche.
To better understand the magnitude of association, we examined the predicted survival time for girls exposed to the 10th and 90th percentiles for each PM exposure and time window (Figure 2; Figures S3–S5; Tables S4–S7). For girls exposed to the 90th percentile of cumulative average exposure, 5.6% and 86.1% had attained menarche by 10 and 14 y of age, respectively. In comparison, 5.2% and 84.1% of girls exposed to the 10th percentile of cumulative average had attained menarche by 10 and 14 y of age. For the cumulative average and exposures, there were few differences in median survival time between the 10th and 90th percentiles of exposure. Similar patterns emerged for in utero, 1-y, and 2-y PM exposure windows.
Figure 2.
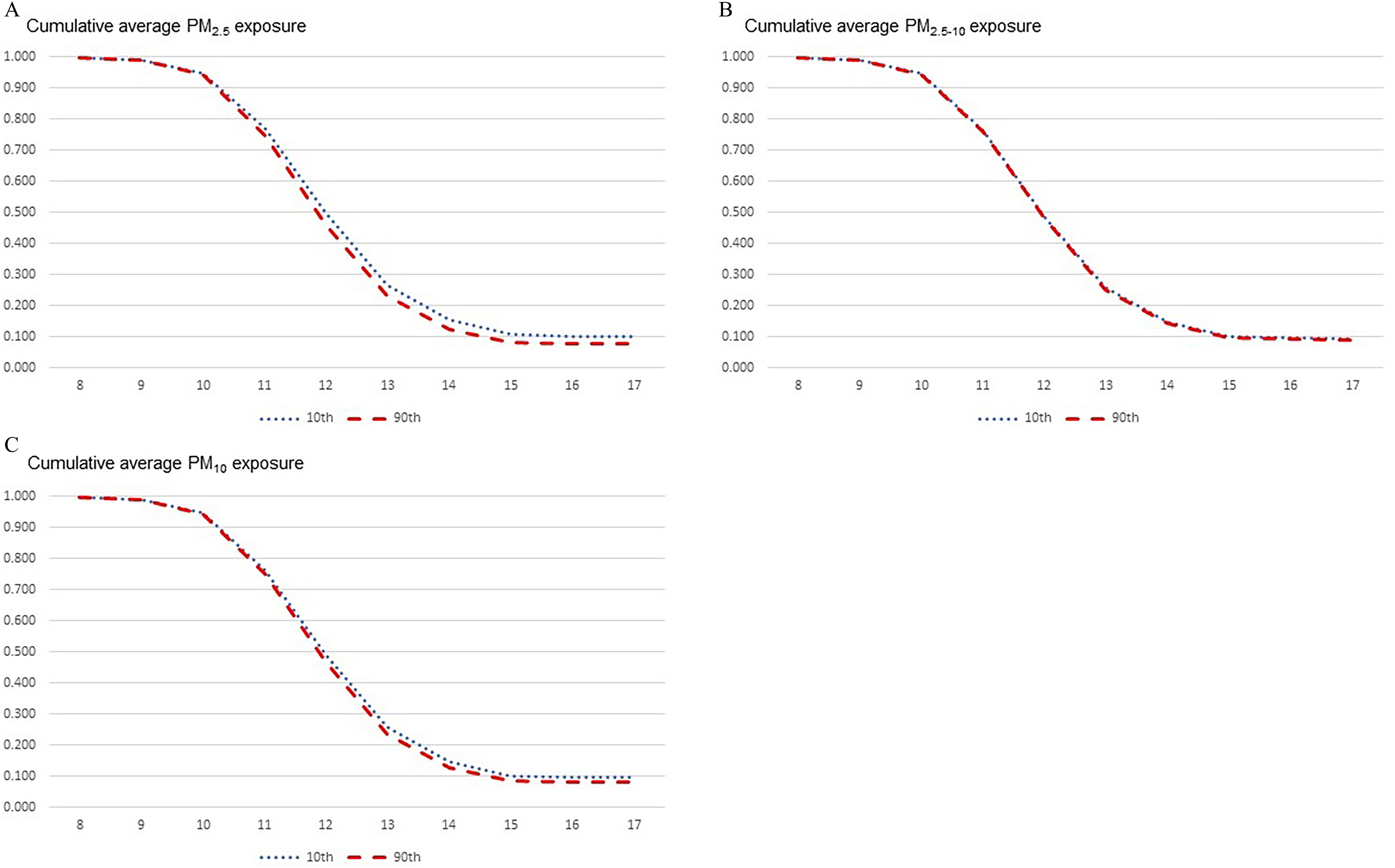
Predicted survival time for girls in the Growing Up Today Study 2 () exposed to the 10th and 90th percentile for the cumulative average PM exposure from 1988 to 2008 using Cox proportional hazard model (see Table S4). Predicted survival curves are modeled from adjusted Cox proportional hazard models using the mean value of continuous covariates and most common level for categorical covariates. These specific curves were estimated for a White, non-Hispanic girl living in the Northeast region in a neighborhood with the lowest SES, who had a birth weight , whose mother had an age of menarche between 12 and 15 y, and who was born between 1989 and 1990. The solid red line is the predicted survival curve for the 90th percentile for cumulative average PM exposure (: ; : ; : ). The dashed blue line is the predicted survival curve for the 10th percentile for the cumulative average PM exposure (: ; : ; : ). Note: PM, particulate matter; SES, socioeconomic status.
Sensitivity Analysis
After restricting the sample to individuals who never moved during the study period (; 18.2%), similar results were observed for all air pollutants and time periods, although the confidence intervals widened (Table S8). When we ran a multipollutant model was the fraction most consistently associated with an increased hazard of earlier menarche, not (Table S9). The associations between 1-y average PM exposures and age at menarche adjusting for the corresponding cumulative exposures were attenuated, suggesting that short-term fluctuations in PM above and beyond a girl’s cumulative average are likely less important than her long-term exposure (Table S10).
Discussion
In this large, prospective cohort of girls residing across the United States, we observed that girls with higher residential exposure to ambient in utero and throughout childhood (from birth to menarche onset) were more likely to have an earlier age at menarche. After further adjustment for calendar year of birth, exposure throughout childhood was still associated with earlier age at menarche but was attenuated slightly. Although higher short-term (e.g., 1- and 2- y moving average) exposure to was associated with early menarche, on further adjustment for cumulative average exposure these results were attenuated, suggesting a more chronic as opposed to short-term effect of on pubertal timing. Across all exposure windows, was associated with earlier age at menarche, whereas there was little evidence of associations with exposures to .
Although some evidence exists for a potential relationship between air pollution exposure and age at menarche, the majority of the literature focuses on pubertal development and menstrual cycle timing. Currently, there are two studies similar to ours. The first, by Jung et al., investigated the association between and age of menarche among 639 girls, 13–17 y of age, who participated in the Korea National Health and Nutrition Examination Survey.19 They observed that 1-, 2-, and 3-y average exposures to prior to menarche were associated with earlier onset of menses.19 The second study is by Wronka and Kliś, which investigated several air pollutants, including and , among 1,257 Polish women, 19–25 y of age, who were attending college.20 They compared women with low, intermediate, and high levels of and during childhood and adolescence and found those in the highest exposure groups had almost three times the odds of earlier onset of menarche (defined as menarche before 11 y of age).20 Although these results concur with our findings, these studies had important limitations. First, because of the cross-sectional design used by Jung et al., the authors had to assume that the address provided by the girl at the time of the survey was the same address they had lived at in the 1, 2, and 3 y prior to menarche (which could have been up to 10 y prior). For Wronka and Kliś, the authors had the young adult women recall their residences during childhood and then averaged air pollution levels across childhood for that given village, town, or city district, which could have introduced some exposure misclassification. Second, Jung et al. were able to evaluate only annual average exposure because they had information only for year of menarche (not month or date). Both studies were also able to use only city-wide average exposures rather than neighborhood- or address-specific estimates. Taken together these all greatly increased the likelihood of exposure misclassification. Third, Wronka and Kliś used different statistical methods and instead used generalized linear models and logistic models and were not able to incorporate time-varying covariates. Given our prospective design, which allowed for the girls’ addresses to update every 2 y, our precise information obtained on age at menarche, and our use of a nationwide spatiotemporal model to predict PM levels at the residence-level, our study was able to greatly improve on this initial research. In addition, we were able to investigate several time-varying exposure windows and multisize fraction models. Regardless of the differences, the two earlier studies offer concurring evidence that PM may be associated with earlier onset of menarche.
In addition to menarche, several studies have investigated related end points regarding puberty onset. In a birth cohort of more than 3,000 adolescents in Hong Kong, Huang et al., investigated pubertal development (defined as the highest Tanner stage for breast and pubic hair development at age 11 y) and air pollution exposure in utero and during childhood.17 Huang et al. observed that higher exposure in utero was associated with delayed pubertal development in girls.17 In contrast, we observed an opposite association, where higher exposure to and was associated with earlier age of menarche. Several factors, besides differences in outcome, could account for this difference. First, we used a cohort with much lower PM exposures in comparison with cohorts from other countries (e.g., ranged from 47.5 to in Hong Kong, China, vs. 8.2 to for cumulative in our study).17 In addition, the specific chemistry and sources of PM are known to vary by location, and this could account for some of these differences. We also used different statistical methods. We used time-to-event data and survival analysis, whereas Huang et al. used cross-sectional data and partial least squares regression (to allow for multiple pollutants).17 There was also a large amount of attrition in the Huang et al. study, with only 68% of the original birth cohort being included in the analysis, which could have led to selection bias. In another study, McGuin et al. observed that higher exposure to traffic-related pollutants and measures of traffic exposure were associated with earlier age of breast and pubic hair development (assessed using Tanner staging) among 437 girls in California.18 Our results are in concordance with these findings, although we did not specifically investigate traffic. In addition to puberty timing, PM exposure has been tentatively linked to menstrual cycle disruption. In an analysis from the NHSII that included more than 34,000 women, a increase in exposure to total suspended PM during adolescence was associated with having a longer time to cycle regularity in high school.14 In addition, in a study of 133 women of reproductive age, and measured during the menstrual cycle were associated with shortened length of the luteal phase,13 and in a different study of 184 women, a higher exposure in the 30 d prior to the observed menstrual cycle was associated with a lengthened follicular phase.30 Although studies of air pollution and puberty or menstrual cycle timing may be relevant to the discussion of air pollution and menarche, these end points are not the same, so direct comparison with our study is not possible. However, taken together, these studies lend credibility to the hypothesis that PM may alter both the timing of first menses as well as characteristics of women’s menstrual cycles.
Of brief mention are several studies that have examined polyaromatic hydrocarbons (PAHs) and puberty timing. PAHs are often found bound to but can also come from other sources. In several studies, urinary PAHs were associated with puberty timing as measured by the Tanner stages.31–33 However, it is not entirely clear whether PAHs are associated with delayed or earlier puberty onset. An interesting aspect is that the association between PAHs exposure and puberty timing may be modified by childhood body mass index (BMI),32,33 with higher PAHs being associated with changes in puberty timing among children with higher BMI in comparison with normal BMI. In one study of 196 girls that examined prenatal exposure to PAHs measured via personal air monitors, PAHs were associated with delayed puberty timing.34 Although we did not measure PAHs directly, it is interesting that in our study in utero exposure was associated with earlier menarche, which is in line with several studies using urinary PAHs but not the study of PAHs measured via air monitoring. It is possible that the differing time windows (1989–1995 vs. 1998–2006) and differing geographic locations (across the United States vs. New York City) of these studies may account for the differences. In general, exposures to PAHs are linked to exposures to air pollution and have important endocrine-disrupting properties that need to be more carefully examined.
The leading biological hypothesis through which ambient PM could affect pubertal timing and specifically age of menarche is that it contains EDCs that interfere with the body’s hormones, which are key regulators of reproductive development.7 PM itself could have endocrine-disrupting properties, and it can provide binding sites for volatile organic compounds (VOCs) and semivolatile organic compounds (SVOCs), which are known to interfere with the endocrine system.7 It is not entirely clear what specifically in PM disrupts the endocrine system. In addition, it is unclear whether PM (and its attached gases) directly act on endocrine glands to disrupt synthesis or whether they mimic hormones and alter the endocrine system that way.7 Another potential mechanism is that PM could have obesogenic properties. Several previous studies have shown that children with higher prenatal and early postnatal exposure to had higher BMI z-scores and higher risk of obesity development in childhood.35,36 Because the accumulation of adipose tissue has long been known to be a trigger of pubertal onset in girls,6 it is therefore possible that increased PM exposure could increase body fat, which in turn could accelerate the timing of menarche. Mechanistic studies will be needed to investigate these questions. There is also biological plausibility behind the in utero findings. When mothers are exposed to during pregnancy, these particles can cross from their bloodstream through the placenta and into the developing fetus,37 thereby affecting the developing reproductive system. Although our study did not have information on the constituents of to directly evaluate this hypothesis further, a more careful examination of the endocrine-disrupting properties of and how these might relate to pubertal timing is warranted.
Although the study findings are interesting, there are several limitations that should be considered when interpreting these results. First, we only assessed ambient air pollution at the girls’ residences throughout childhood and not personal exposure. Lack of information on personal exposures and time spent outdoors could have led to nondifferential exposure misclassification, which means these results could be conservative estimates. Second, it is possible that girls moved between address updates, which would create exposure misclassification. However, as a sensitivity analysis, we explored the association between PM exposure and age at menarche among individuals who did not move, and we observed similar results. Third, some individuals in this study may have experienced menarche before the first survey in 2004, which could have potentially led to recall error. However, self-report of menarche is known to be highly accurate and given that our exposure was based on the subject’s residential address (and not self-report), the likelihood of recall bias is minimal.23,38,39 Fourth, we did not adjust for exposure to other known EDCs or other common air pollutants, such as , because this information was not available to us. In addition, the nSES measure was specific to our cohort and may not fully captured the range of SES across the United States. Therefore, residual confounding by these factors is possible, so our results should be cautiously interpreted. Fifth, PM exposure declined over the course of the study, so it is possible there was some confounding by time. However, when we further adjusted for calendar year of birth, we observed similar but attenuated effect estimates. Sixth, we did not have information on some risk factors for menarche assessed prior to onset of menarche, which prevented us from examining these factors as potential mediators. In particular, childhood BMI would have been interesting to investigate. However, a little over 50% of the sample had already achieved menarche prior to competing the first questionnaire, so we did not have premenarchal BMI data on these individuals. Future analyses should consider the mediating effect of BMI on the relationship between PM and age at menarche. Seventh, in our analysis, we used the missing-indicator method, which may have biased our results if data are not missing at random. Last, there is a concern about a lack of generalizability. Studies have documented earlier age of menarche in Black and Latina girls in comparison with White girls.40,41 The majority of girls in our study were non-Hispanic White. Therefore, we are unable to comment on the association between PM exposure and menarche in the populations with the greatest public health burden. However, we have no reason to believe that being Black or Latina would modify the effect of air pollution on pubertal timing. Rather, it is known that PM exposure tends to be higher in neighborhoods with a higher percentage of Hispanic and Black adults,42–44 and thus it is more likely that PM exposure may mediate some of the observed association between race/ethnicity and early timing of menarche. Future studies are needed to address this study question across more diverse cohorts, particularly in areas with higher PM exposure.
Our study did have several strengths. The first and foremost was that we had updated residential information from girls every 2 y from birth throughout childhood, and we used a validated spatiotemporal model to estimate their monthly ambient PM exposure levels at their address. As evidenced by the low percentage of girls who remained at the same residence during follow-up, this updated information was key to ensuring an accurate exposure assessment. In addition, we used time-varying covariates to better capture changes in exposures and confounders over time, which may more accurately describe an individual’s exposure than simple averages at a single time point. By combining data from the NHSII and GUTS2 cohorts, we were also able to maintain a prospective design and include girls who achieved menarche prior to enrolling in our study, which limited the influence of differential exposure misclassification and selection bias. Although our cohort was racially homogeneous, it covered a wide geographic area, which enhances the generalizability of our findings.
Overall, we observed that higher exposure to throughout childhood (from birth to menarche onset) was associated with earlier age of menarche in a large, prospective cohort of girls in the United States. Although the magnitude of effect was modest, relatively small impacts on individuals could result in noteworthy influences on population health. Although this study does provide valuable insight into the potential role of PM in adolescent reproductive health, questions still remain regarding the mechanisms through which PM may be altering pubertal timing.
Supplementary Material
Acknowledgments
The authors and cohorts are supported by the following National Institutes of Health (NIH) grants U01HL145386, U01CA176726, R00ES026648, R24ES028521, P30ES000002, and T32ES012870. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
The data underlying this article cannot be shared publicly due to privacy of individual participants. Individuals interested in working with the NHSII or GUTS data should follow the procedures provided at https://nurseshealthstudy.org/researchers.
R.B.H. conducted the analysis and wrote and prepared the manuscript for submission. J.H., F.L., B.R., and J.C. designed the study and data collection and edited the manuscript. A.J.G. supervised the analysis and edited the manuscript in preparation for submission.
References
- 1.Euling SY, Selevan SG, Pescovitz OH, Skakkebaek NE. 2008. Role of environmental factors in the timing of puberty. Pediatrics 121 Suppl 3:S167–S171, PMID: , 10.1542/peds.2007-1813C. [DOI] [PubMed] [Google Scholar]
- 2.Chen X, Liu Y, Sun X, Yin Z, Li H, Liu X, et al. 2018. Age at menarche and risk of all-cause and cardiovascular mortality: a systematic review and dose-response meta-analysis. Menopause 26(6):670–676, PMID: , 10.1097/GME.0000000000001289. [DOI] [PubMed] [Google Scholar]
- 3.Janghorbani M, Mansourian M, Hosseini E. 2014. Systematic review and meta-analysis of age at menarche and risk of type 2 diabetes. Acta Diabetol 51(4):519–528, PMID: , 10.1007/s00592-014-0579-x. [DOI] [PubMed] [Google Scholar]
- 4.Braun MM, Overbeek-Wager EA, Grumbo RJ. 2016. Diagnosis and management of endometrial cancer. Am Fam Physician 93(6):468–474, PMID: . [PubMed] [Google Scholar]
- 5.La Vecchia C. 2017. Ovarian cancer: epidemiology and risk factors. Eur J Cancer Prev 26(1):55–62, PMID: , 10.1097/CEJ.0000000000000217. [DOI] [PubMed] [Google Scholar]
- 6.Li W, Liu Q, Deng X, Chen Y, Liu S, Story M. 2017. Association between obesity and puberty timing: a systematic review and meta-analysis. Int J Environ Res Public Health 14(10):1266, PMID: , 10.3390/ijerph14101266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darbre PD. 2018. Overview of air pollution and endocrine disorders. Int J Gen Med 11:191–207, PMID: , 10.2147/IJGM.S102230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ye X, Pan W, Zhao Y, Zhao S, Zhu Y, Liu W, et al. 2017. Association of pyrethroids exposure with onset of puberty in Chinese girls. Environ Pollut 227:606–612, PMID: , 10.1016/j.envpol.2017.04.035. [DOI] [PubMed] [Google Scholar]
- 9.Attfield KR, Pinney SM, Sjödin A, Voss RW, Greenspan LC, Biro FM, et al. 2019. Longitudinal study of age of menarche in association with childhood concentrations of persistent organic pollutants. Environ Res 176:108551, PMID: , 10.1016/j.envres.2019.108551. [DOI] [PubMed] [Google Scholar]
- 10.Chen A, Chung E, DeFranco EA, Pinney SM, Dietrich KN. 2011. Serum PBDEs and age at menarche in adolescent girls: analysis of the National Health and Nutrition Examination Survey 2003–2004. Environ Res 111(6):831–837, PMID: , 10.1016/j.envres.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Binder AM, Corvalan C, Calafat AM, Ye X, Mericq V, Pereira A, et al. 2018. Childhood and adolescent phenol and phthalate exposure and the age of menarche in Latina girls. Environ Health 17(1):32, PMID: , 10.1186/s12940-018-0376-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buttke DE, Sircar K, Martin C. 2012. Exposures to endocrine-disrupting chemicals and age of menarche in adolescent girls in NHANES (2003–2008). Environ Health Perspect 120(11):1613–1618, PMID: , 10.1289/ehp.1104748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merklinger-Gruchala A, Jasienska G, Kapiszewska M. 2017. Effect of air pollution on menstrual cycle length–a prognostic factor of women’s reproductive health. Int J Environ Res Public Health 14(7):816, PMID: , 10.3390/ijerph14070816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahalingaiah S, Missmer SE, Cheng JJ, Chavarro J, Laden F, Hart JE. 2018. Perimenarchal air pollution exposure and menstrual disorders. Hum Reprod 33(3):512–519, PMID: , 10.1093/humrep/dey005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nobles CJ, Schisterman EF, Ha S, Buck Louis GM, Sherman S, Mendola P. 2018. Time-varying cycle average and daily variation in ambient air pollution and fecundability. Hum Reprod 33(1):166–176, PMID: , 10.1093/humrep/dex341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perin PM, Maluf M, Czeresnia CE, Nicolosi Foltran Januário DA, Nascimento Saldiva PH. 2010. Effects of exposure to high levels of particulate air pollution during the follicular phase of the conception cycle on pregnancy outcome in couples undergoing in vitro fertilization and embryo transfer. Fertil Steril 93(1):301–303, PMID: , 10.1016/j.fertnstert.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 17.Huang JV, Leung GM, Schooling CM. 2017. The association of air pollution with pubertal development: evidence from Hong Kong’s “Children of 1997” birth cohort. Am J Epidemiol 185(10):914–923, PMID: , 10.1093/aje/kww200. [DOI] [PubMed] [Google Scholar]
- 18.McGuinn LA, Voss RW, Laurent CA, Greenspan LC, Kushi LH, Windham GC. 2016. Residential proximity to traffic and female pubertal development. Environ Int 94:635–641, PMID: , 10.1016/j.envint.2016.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jung EM, Kim HS, Park H, Ye S, Lee D, Ha EH. 2018. Does exposure to PM10 decrease age at menarche? Environ Int 117:16–21, PMID: , 10.1016/j.envint.2018.04.020. [DOI] [PubMed] [Google Scholar]
- 20.Wronka I, Kliś K. 2022. Effect of air pollution on age at menarche in Polish females, born 1993–1998. Sci Rep 12(1):4820, PMID: , 10.1038/s41598-022-08577-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Z, Laden F, Forman JP, Hart JE. 2016. Long-Term exposure to particulate matter and self-reported hypertension: a prospective analysis in the Nurses’ Health Study. Environ Health Perspect 124(9):1414–1420, PMID: , 10.1289/EHP163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bao Y, Bertoia ML, Lenart EB, Stampfer MJ, Willett WC, Speizer FE, et al. 2016. Origin, methods, and evolution of the three nurses’ health studies. Am J Public Health 106(9):1573–1581, PMID: , 10.2105/AJPH.2016.303338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koo MM, Rohan TE. 1997. Accuracy of short-term recall of age at menarche. Ann Hum Biol 24(1):61–64, PMID: , 10.1080/03014469700004782. [DOI] [PubMed] [Google Scholar]
- 24.Yanosky JD, Paciorek CJ, Laden F, Hart JE, Puett RC, Liao D, et al. 2014. Spatio-temporal modeling of particulate air pollution in the conterminous United States using geographic and meteorological predictors. Environ Health 13:63, PMID: , 10.1186/1476-069X-13-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krieger N, Williams DR, Moss NE. 1997. Measuring social class in US public health research: concepts, methodologies, and guidelines. Annu Rev Public Health 18:341–378, PMID: , 10.1146/annurev.publhealth.18.1.341. [DOI] [PubMed] [Google Scholar]
- 26.Messer LC, Laraia BA, Kaufman JS, Eyster J, Holzman C, Culhane J, et al. 2006. The development of a standardized neighborhood deprivation index. J Urban Health 83(6):1041–1062, PMID: , 10.1007/s11524-006-9094-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeVille NV, Iyer HS, Holland I, Bhupathiraju SN, Chai B, James P, et al. 2023. Neighborhood socioeconomic status and mortality in the Nurses’ Health Study (NHS) and the Nurses’ Health Study II (NHSII). Environ Epidemiol 7(1):e235, PMID: , 10.1097/EE9.0000000000000235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iyer HS, Hart JE, James P, Elliott EG, DeVille NV, Holmes MD, et al. 2022. Impact of neighborhood socioeconomic status, income segregation, and greenness on blood biomarkers of inflammation. Environ Int 162:107164, PMID: , 10.1016/j.envint.2022.107164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Durrleman S, Simon R. 1989. Flexible regression models with cubic splines. Stat Med 8(5):551–561, PMID: , 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 30.Giorgis-Allemand L, Thalabard JC, Rosetta L, Siroux V, Bouyer J, Slama R. 2020. Can atmospheric pollutants influence menstrual cycle function? Environ Pollut 257:113605, PMID: , 10.1016/j.envpol.2019.113605. [DOI] [PubMed] [Google Scholar]
- 31.Fang B, Bravo MA, Wang H, Sheng L, Wu W, Zhou Y, et al. 2022. Polycyclic aromatic hydrocarbons are associated with later puberty in girls: a longitudinal study. Sci Total Environ 846:157497, PMID: , 10.1016/j.scitotenv.2022.157497. [DOI] [PubMed] [Google Scholar]
- 32.John EM, Keegan TH, Terry MB, Koo J, Ingles SA, Nguyen JT, et al. 2022. Urinary biomarkers of polycyclic aromatic hydrocarbons and timing of pubertal development: the California PAH Study. Epidemiology 33(6):777–787, PMID: , 10.1097/EDE.0000000000001535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dobraca D, Laurent CA, Greenspan LC, Hiatt RA, Sjödin A, Kushi LH, et al. 2020. Urinary polycyclic aromatic hydrocarbons in relation to anthropometric measures and pubertal development in a cohort of Northern California girls. Environ Epidemiol 4(4):e0102, PMID: , 10.1097/EE9.0000000000000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kehm RD, Oskar S, Tehranifar P, Zeinomar N, Rundle AG, Herbstman JB, et al. 2021. Associations of prenatal exposure to polycyclic aromatic hydrocarbons with pubertal timing and body composition in adolescent girls: implications for breast cancer risk. Environ Res 196:110369, PMID: , 10.1016/j.envres.2020.110369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin L, Li T, Sun M, Liang Q, Ma Y, Wang F, et al. 2022. Global association between atmospheric particulate matter and obesity: a systematic review and meta-analysis. Environ Res 209:112785, PMID: , 10.1016/j.envres.2022.112785. [DOI] [PubMed] [Google Scholar]
- 36.Gheissari R, Liao J, Garcia E, Pavlovic N, Gilliland FD, Xiang AH, et al. 2022. Health outcomes in children associated with prenatal and early-life exposures to air pollution: a narrative review. Toxics 10(8):458, PMID: , 10.3390/toxics10080458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bové H, Bongaerts E, Slenders E, Bijnens EM, Saenen ND, Gyselaers W, et al. 2019. Ambient black carbon particles reach the fetal side of human placenta. Nat Commun 10(1):3866, PMID: , 10.1038/s41467-019-11654-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moffitt TE, Caspi A, Belsky J, Silva PA. 1992. Childhood experience and the onset of menarche: a test of a sociobiological model. Child Dev 63(1):47–58, PMID: , 10.1111/j.1467-8624.1992.tb03594.x. [DOI] [PubMed] [Google Scholar]
- 39.Petersen AC, Crockett L, Richards M, Boxer A. 1988. A self-report measure of pubertal status: reliability, validity, and initial norms. J Youth Adolesc 17(2):117–133, PMID: , 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- 40.Chumlea WC, Schubert CM, Roche AF, Kulin HE, Lee PA, Himes JH, et al. 2003. Age at menarche and racial comparisons in US girls. Pediatrics 111(1):110–113, PMID: , 10.1542/peds.111.1.110. [DOI] [PubMed] [Google Scholar]
- 41.Kaplowitz P. 2006. Pubertal development in girls: secular trends. Curr Opin Obstet Gynecol 18(5):487–491, PMID: , 10.1097/01.gco.0000242949.02373.09. [DOI] [PubMed] [Google Scholar]
- 42.Miranda ML, Edwards SE, Keating MH, Paul CJ. 2011. Making the environmental justice grade: the relative burden of air pollution exposure in the United States. Int J Environ Res Public Health 8(6):1755–1771, PMID: , 10.3390/ijerph8061755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bell ML, Ebisu K. 2012. Environmental inequality in exposures to airborne particulate matter components in the United States. Environ Health Perspect 120(12):1699–1704, PMID: , 10.1289/ehp.1205201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones MR, Diez-Roux AV, Hajat A, Kershaw KN, O’Neill MS, Guallar E, et al. 2014. Race/ethnicity, residential segregation, and exposure to ambient air pollution: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Public Health 104(11):2130–2137, PMID: , 10.2105/AJPH.2014.302135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


