Abstract
Recent evidence revealed important interactions between clonal hematopoiesis (CH) and cellular therapies established for the treatment of hematologic malignancies. The impact of CH on safety, efficacy, and outcome of chimeric antigen receptor (CAR) T-cell therapy is currently under investigation. We analyzed 110 patients with relapsed/refractory B-cell non-Hodgkin lymphoma (n = 105) or acute lymphoblastic leukemia (ALL) (n = 5), treated with Axicabtagene-Ciloleucel (39%), Tisagenlecleucel (51%), or Brexucabtagene autoleucel (10%). Using error-corrected targeted sequencing, a high CH prevalence of 56.4% (variant allele frequency [VAF] ≥1%) at the time of CAR T-cell infusion was detected. The most frequently mutated gene was PPM1D followed by DNMT3A, TET2, ASXL1, and TP53. Variant allele frequencies were significantly lower in B and T cells compared with monocytes and granulocytes. CH did not increase the risk of CAR T-related toxicities. The incidences of cytokine release syndrome and immune effector-cell-associated neurotoxicity syndrome were similar between CHpos and CHneg patients, regardless of clone size, age, or CAR T product. Prolonged cytopenias were not associated with CH. Best overall response rates (ORRs) were numerically but not significantly higher in CHpos patients (ORR 76.7% versus 62.2%; P = 0.13). Furthermore, CH status did not predict progression-free survival or overall survival. Lastly, sequential analysis showed a modest VAF increase of 1.3% and acquisition of novel mutations within 100 days postinfusion. CH was frequent in large B-cell lymphoma/ALL patients receiving CAR T-cells but did not affect toxicity nor treatment response or outcome.
INTRODUCTION
Clonal hematopoiesis (CH) is defined by the acquisition of somatic mutations in hematopoietic stem cells (HSCs) and occurs in 20%–30% of individuals aged >60 years.1–4 Clinically, CH is associated with a proinflammatory phenotype of hematopoietic cells and their progeny, inflammatory conditions, and a poor outcome for patients with hematologic neoplasms and solid tumors.5–7 Well known for the causal relationship between CH, proinflammation, and cardiovascular disease is TET2, one of the most frequently mutated CH genes. Preclinical models have shown an altered function of the NLRP3/IL-1β-inflammasome of mutated monocytes/macrophages leading to accelerated development of atherosclerosis.8–10 Current data indicate pleiotropic effects of mutated clones in CH positive individuals, affecting self-renewal and differentiation, but also inflammatory signaling of mature blood cells.11,12 Until recently, the standard treatment for relapsed or refractory (r/r) aggressive B-cell non-Hodgkin lymphoma (B-NHL) patients consisted of immunochemotherapy and consolidation high-dose chemotherapy with autologous stem cell transplantation (ASCT) in eligible patients. Gibson et al showed that CH occurred in 30% of intensively treated NHL patients and is associated with shorter overall survival (OS). Meanwhile, chimeric antigen receptor (CAR) T-cells have been approved for first or later relapse/refractoriness of large B-cell lymphoma (LBCL).13 Adoptive T-cell transfer therapy with CAR T-cells represents a breakthrough in the treatment of hematologic malignancies.14,15 Although durable responses have been observed in 30%–40% of r/r B-NHL patients treated with CAR T-cell therapy, it is associated with significant systemic inflammatory toxicities, such as cytokine release syndrome (CRS) and immune effector-cell-associated neurotoxicity syndrome (ICANS). Recently, some but not all studies investigating the role of CH in the setting of CAR T-cell therapies reported an increased risk of CRS or ICANS.16–18 These studies suggest that CH can influence CAR T-cell biology and clinical outcome. In line, experimental knockdown of TET2 provided evidence for increased efficacy of CAR T-cells harboring TET2 alterations in a patient with CLL.19
To gain further insights into the importance of CH in the context of CAR T-cell therapies, we investigated CH in a cohort of 110 r/r B-NHL or acute lymphoblastic leukemia (ALL) patients and studied the clonal evolution using serial patient samples up to 2 years after CAR T-cell infusion.
PATIENTS AND METHODS
Patients
In this retrospective cohort study from 5 German university hospitals, patients were included if they were ≥18 years old, received CAR T-cells for the treatment of r/r B-NHL (n = 105) or ALL (n = 5) between 03/2019 and 12/2022, and had peripheral blood (PB) samples available that were obtained at the time of CAR T-cell infusion (d0, ±10 days). Follow-up samples were available for 40 of 110 patients at day 100, for 12 of 110 patients at day 200, for 11 of 110 patients 1 year, and for 6 of 110 patients 2 years after CAR T-cell treatment.
Supportive care, toxicity management, and response assessment followed institutional practices. CRS and ICANS were graded according to the American Society for Transplantation and Cellular Therapy consensus criteria.20,21
Written informed consent was obtained from all patients according to the Declaration of Helsinki. The study was approved by the Institutional Review Board of Hannover Medical School (9098_BO_S_2020) and of Charité – Universitätsmedizin Berlin (EA2_087_16).
Targeted sequencing
DNA was extracted from PB samples and subjected to an error-corrected targeted sequencing workflow, as published previously.22–24 Sequencing libraries were prepped using a commercially available library preparation kit and a customized targeted sequencing panel (Twist BioScience, USA) containing 45 genes recurrently mutated in CH (Suppl. Table S1). Unique molecular identifiers were used for error-correction (xGen UDI-UMI adapters by Integrated DNA Technologies, USA). Libraries were sequenced in paired-end mode on Illumina’s NovaSeq 6000 sequencing platform. Somatic variants with a variant allele frequency (VAF) ≥1% were identified using our in-house variant calling pipeline (Supplemental Methods).22,23 Patients with 1 or more variants ≥1% were defined as CH positive.
Cell Sorting
PB samples from 14 patients were thawed and prepared for cell sorting by staining with anti-human-antibodies CD45-APC-Cy7 (HB-7, CAT 557833), CD3 FITC (SK7, CAT 345763), CD19 APC (HIB19, CAT 555415), CD14-APC-Cy7 (MΦP9, CAT 557831), CD56-PE (B159, CAT 555516) from BD Biosciences (Heidelberg, Germany) and CD66b-PE (G10F5, CAT 305106), CD34-PerCP (581, CAT 343520) from BioLegend (San Diego, USA). The following cell populations were sorted using the respective immunophenotypes: monocytes (CD14+, CD3−, CD19−), B cells (CD19+, CD3−), T cells (CD3+, CD19−), natural killer (NK) cells (CD56+, CD3−, CD14−, CD19−), granulocytes (CD45+, CD66b+), and progenitor cells (CD34+, CD3−). Genomic DNA was extracted using a low cell count DNA extraction kit following the manufacture’s recommendations (NucleoSpin Tissue XS Kit, Macherey-Nagel, Düren, Germany).
Quantification of allelic burden in sorted cell fractions
An amplicon-based error-corrected sequencing and bioinformatics approach established for measurable residual disease was applied to detect mutations in sorted cell populations of 14 patients, as previously described.25 We used the Illumina MiSeq reagent kit version 3 (600 cycles) for sequencing on the MiSeq sequencer, obtaining, on average, 526,161 aligned reads per marker with 251 bases in both forward and reverse sequencing directions. The limit of detection using this approach is a VAF of 0.01%.
Statistical analysis
Median follow-up time for survival and progression-free survival (PFS) was calculated according to the reverse Kaplan-Meier method. PFS end points were disease progression, relapse, or death, measured from the date of CAR T-cell infusion to event date. OS end points, measured from the date of CAR T-cell infusion, were death (failure) and date of last seen (censored). The Kaplan-Meier method and log-rank test were used to estimate PFS and OS and to compare differences between survival curves. For multivariate analysis, a Cox proportional hazards model was constructed for PFS and OS to adjust for potential confounding covariates. Variables with imbalanced distribution between groups (P < 0.2 in Fisher test) or indication for better outcome in univariate analysis were considered. Covariates were tested for satisfying proportional hazards assumption. The OS-model was stratified for “age above versus below 60 years at treatment day.”
The best overall response rate (ORR) was determined in the first 180 days after CAR T-cell treatment, patients with lack of follow-up data or death before detection of progress/response were excluded. Comparisons of variables were performed using Student t test for continuous variables, Fisher exact test for categorical variables, and Mann-Whitney U test to compare independent groups without normal distribution. In every calculation, only patients with complete dataset were considered. The 2-sided level of significance was set at a P-value of <0.05. The statistical analyses were performed with the statistical software package SPSS 27.0 (IBM Corporation, Armonk, NY) and Microsoft excel 2010 (Microsoft Corporation, Redmond, WA).
RESULTS
Clinical and genetic patient characteristics
A total of 110 patients with r/r B-NHL (n = 105; 95.4%) fulfilling SCHOLAR-1 criteria26 or r/r ALL (n = 5; 4.5%) treated with commercially available CAR T-cell products in 5 German institutions were included in this retrospective study (see Table 1 for patient characteristics). In our cohort, 43 patients received Axicabtagene-Ciloleucel (Axi-cel, 39.0%), 56 patients Tisagenlecleucel (Tisa-cel, 50.9%), and 11 patients Brexucabtagene autoleucel (10.0%). Patient characteristics of the cohorts treated with Tisa-cel or Axi-cel were similar to those of the total CAR T-cohort (data not shown). The median age at diagnosis was 60 years and at the day of CAR T-cell infusion 62 years. There was a male predominance (n = 77; 70.0%). LBCL represented the most common histology (80.0%), including diffuse large B-cell lymphoma (DLBCL), not otherwise specified, high-grade B-cell lymphoma with MYC and BCL2 rearrangements, and primary mediastinal large B-cell lymphomas according to the 2022 revised WHO classification.27 Sixty-six percent of the patients had advanced stage disease (III/IV) according to the Ann-Arbor staging classification and were highly pretreated with a median of 4 prior lines of therapy (range, 2–8). Thirty-seven patients (33.6%) underwent prior ASCT and 23.6% of the patients had a central nervous system involvement at any time point before CAR T-cell treatment (Table 1).
Table 1.
Patient Characteristics According to the Presence or Absence of Clonal Hematopoiesis
| Characteristic | A Total Cohort n = 110 |
B CH Negative n = 48 |
C CH Positive n = 62 |
P-value B vs Ca |
|---|---|---|---|---|
| Age at diagnosis, y | ||||
| Median (range) | 60 (3–79) | 54 (3–79) | 61 (17–76) | <0.001b |
| Age at CAR T-cell treatment, y | ||||
| Median (range) | 62 (18–80) | 58 (18–80) | 65 (21–79) | <0.001b |
| Sex | ||||
| Male, no. (%) | 77 (70.0) | 31 (65) | 46 (74) | |
| Female, no. (%) | 33 (30) | 17 (35) | 16 (26) | |
| Diagnosis | ||||
| LBCL, no. (%) | 88 (80) | 40 (83) | 48 (77) | |
| TFL, no. (%) | 6 (6) | 1 (2) | 5 (8) | |
| MCL, no. (%) | 11 (10) | 4 (8) | 7 (11) | |
| ALL, no. (%) | 5 (5) | 3 (6) | 2 (3) | |
| Ann-Arbor stage | 0.51 | |||
| I–II, no. (%) | 28 (25) | 13 (27) | 15 (24) | |
| III–IV, no. (%) | 72 (65.5) | 28 (58) | 44 (71) | |
| Missing data, no. (%) | 10 (9) | 7 (15) | 3 (5) | |
| Prior lines of therapy | ||||
| Median (range) | 4 (2–9) | 4 (2–8) | 4 (2–9) | |
| Autologous HSCT prior CAR T-cell treatment | 0.42 | |||
| Yes, no. (%) | 37 (33.6) | 14 (29) | 23 (37) | |
| No, no. (%) | 73 (66) | 34 (71) | 39 (63) | |
| CNS involvement prior CAR T-cell treatment | 0.26 | |||
| Yes, no. (%) | 26 (23.6) | 14 (29) | 12 (19) | |
| No, no. (%) | 84 (76) | 34 (71) | 50 (81) | |
| Remission prior CAR T-cell treatment (CR/PR vs SD/PD) |
0.02 | |||
| CR, no. (%) | 10 (9) | 4 (8) | 6 (10) | |
| PR, no. (%) | 22 (20) | 4 (8) | 18 (29) | |
| SD, no. (%) | 14 (13) | 4 (8) | 10 (16) | |
| PD, no. (%) | 63 (57) | 35 (73) | 28 (45) | |
| Missing data, no. (%) | 1 (1) | 1 (2) | 0 | |
| CAR T-cell product | ||||
| Axicabtagene-ciloleucel, no. (%) | 43 (39.0) | 19 (40) | 24 (39) | |
| Tisagenlecleucel, no. (%) | 56 (50.9) | 25 (52) | 31 (50) | |
| Brexucabtagene autoleucel, no. (%) | 11 (10) | 4 (8) | 7 (11) |
aCalculated with Fisher exact test, missing data were excluded.
bCalculated with Mann-Whitney U test.
.
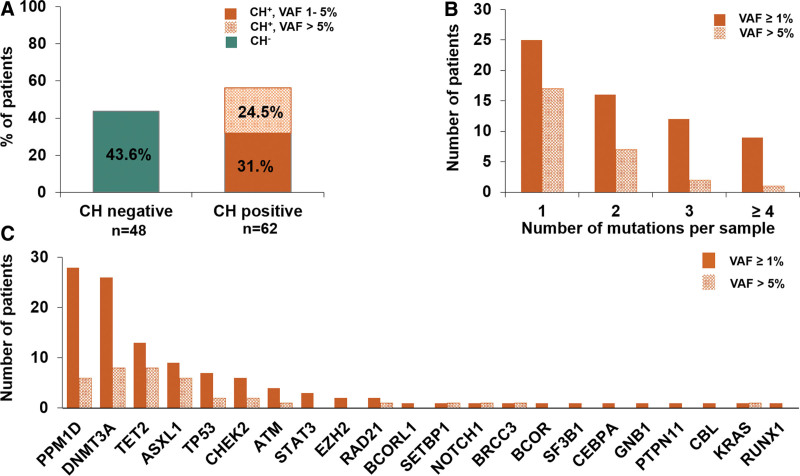
At the time of CAR T-cell treatment (d0, ±10 days from infusion), CH with VAF ≥1% was detected in 62 of 110 patients (56.4%) with a VAF of 1%–5% in 35 of 110 patients (31.8%) and a VAF >5% in 27 of 110 patients (24.5%) (Figure 1A; Suppl. Table S2). Almost 20% (21/110) of our patients harbored at least 3 mutations (Figure 1B). The most frequently mutated gene at the time of CAR T-cell treatment was PPM1D (28 mutated patients with VAF ≥1%, and 6 mutated patients with VAF >5%) followed by DNMT3A (26 mutated patients with VAF ≥1%, and 8 with VAF >5%), TET2, ASXL1, TP53, and CHEK2, respectively (Figure 1C). Patients with CH were significantly older at diagnosis and at infusion of CAR T-cells and more often had partial or complete remission (CR) before CAR T-cell treatment than patients without CH. All other baseline characteristics were similarly distributed (Table 1). No differences between patients with low (1%–5%) or high VAF (>5%) CH with respect to baseline characteristics were observed (Suppl. Table S3). Thirty-six of all CH positive patients (58.1%) showed mutations in at least one DNA Damage Repair Gene (DDR-Group, PPM1D±TP53±CHEK2±ATM) and 39 (62.9%) of CH positive patients were DNMT3A±TET2±ASXL1 (DTA) mutated. The prevalence of CH in DNMT3A, TET2, ASXL1, PPM1D, and TP53 was correlated with prior treatment regimens constituting either high-dose chemotherapy or platinum-based treatment, topoisomerase II inhibitors, and antimetabolites. We found a strong association of PPM1D CH with platinum-based chemotherapy (prevalence 29% with compared to 0% without platinum-based treatment) (Suppl. Tables S4-S7). CH in TET2 and ASXL1 was more frequent in patients undergoing high-dose chemotherapy with autologous transplantation (Suppl. Table S8).
Figure 1.
CH at the time of treatment is very common among patients receiving CAR T-cell therapy. (A) Frequency of CH across the entire cohort (n = 110) as measured by the VAF using a cutoff of 1%. (B) Distribution of the number of mutations found in patients of total cohort. Quantity of mutations stratified by the size of the clone considering low VAF %1–5% and high VAF >5%. (C) Number of mutated patients (y-axis) according to gene information (x-axis) at differing VAF groups (VAF ≥1% and VAF ≥5%). CAR = chimeric antigen receptor; CH = clonal hematopoiesis; VAF = variant allele frequency.
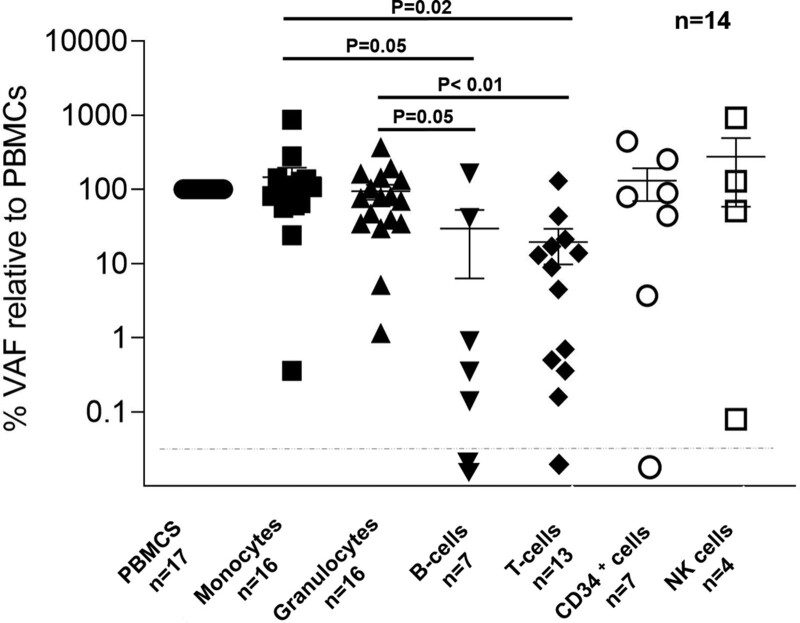
Next, we investigated the allelic burden of 18 different mutations from 14 patients (7 DNMT3A, 3 ASXL1, 3 PPM1D, 2 TET2, 2 CHEK2, and 1 SF3B1 mutations) in flow-sorted cell fractions. VAFs were significantly lower in B and T cells compared with monocytes and granulocytes consistent with previous findings4,28,29 (Figure 2).
Figure 2.
Cellular distribution of clonal hematopoiesis. VAFs of 18 mutations were studied in PBMNCs and flow-sorted peripheral blood cell fractions (monocytes, granulocytes, B cells, T cells, CD34+ cells,and NK cells). PBMNCs = peripheral blood mononuclear cells; VAF = variant allele frequency.
CH and risk of CAR T-related toxicities
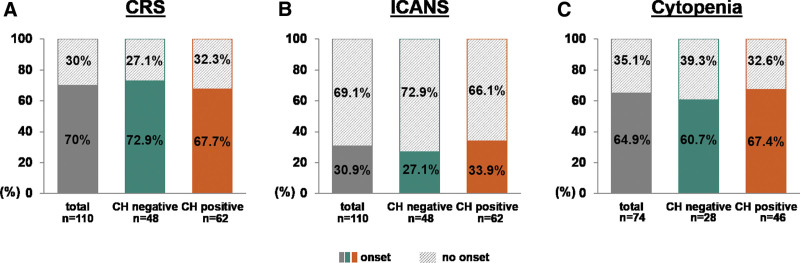
We next analyzed the impact of CH on CAR T-associated toxicities. Seventy-seven of 110 patients (70.0%) developed CRS of any grade (I–IV), while we observed incidence of CRS with up to 67.7% (42/62) in the CH positive versus 72.9% (35/48) in the CH negative group (P = 0.68) (Figure 3A and Suppl. Table S9). ICANS of any grade was diagnosed in 34 of 110 patients (30.9%). Twenty-one of 62 (33.9%) patients developed ICANS in the CH positive cohort and 13 of 48 cases (27.1%) in the CH negative group (P = 0.53) (Figure 3B; and Suppl. Table S9). Eight of 77 (10.4%) and 9 of 34 (26.5%) patients had grade ≥3 CRS and grade ≥3 ICANS. Among CRS-patients, the fraction of severe grade ≥3 was 7.1% (3/42) in the CH positive and 14.3% (5/35) in the CH negative group (P = 0.46). Within the group of patients affected by ICANS, proportions with grade ≥3 were 28.6% (6/21) for CH positive and 23.1% (3/13) for CH negative patients (P = 1.0) (Suppl. Table S9). The incidences of CRS and ICANS were similar between patients with low (1%–5%) and high VAF (>5%) CH (Suppl. Table S10; Suppl. Figure S1A and S1B) and comparison based on mutational status revealed no significant differences if the cohort was stratified by age at d0 (below or above 60 years) or specific CAR T-cell product (Suppl. Tables S11 and S12). Furthermore, we analyzed the toxicity outcomes in patients harboring DTA mutations and found no difference between patients with DTA-CH and without any CH mutations (Suppl. Table S13). Patients with available information (n = 74) were assessed for cytopenia at day 100 after CAR T-infusion as defined by leukopenia (total white blood cell [WBC] count <3000/µL) and/or thrombocytopenia (platelet count <100,000/µL) and/or anemia (hemoglobin <10 g/dL). Cytopenia at day 100 after CAR T-infusion was observed in 48 of 74 patients (64.9%) in the entire cohort. The incidence of cytopenia at day 100 was not different in CH positive patients (67.4%) compared with CH negative (60.7%) (Figure 3C). Also, the CH clone size did not affect the incidence of cytopenias (Suppl. Figure S1C).
Figure 3.
CH and CAR T-therapy toxicity. (A, B) Histogram plots showing prevalence of CRS/ICANS (onset: filled, no onset: hatched) according to the absence (n = 48, green) or presence (n = 62, orange) of clonal hematopoiesis across the total cohort (n = 110, gray). (C) Histogram plots illustrating prevalence of cytopenias at day 100 after CAR T-cell therapy as defined by leukopenia (total WBC count <3000/µL) and/or thrombocytopenia (platelet count <100,000/µL) and/or anemia (hemoglobin <10 g/dL) in patients with available information (n = 74) according to the presence (n = 46) or absence (n = 28) of CH with a VAF cutoff 1%. CAR = chimeric antigen receptor; CH = clonal hematopoiesis; CRS = cytokine release syndrome; ÍCANS = immune effector-cell-associated neurotoxicity syndrome; VAF = variant allele frequency.
CH and response to CAR T-cell therapy
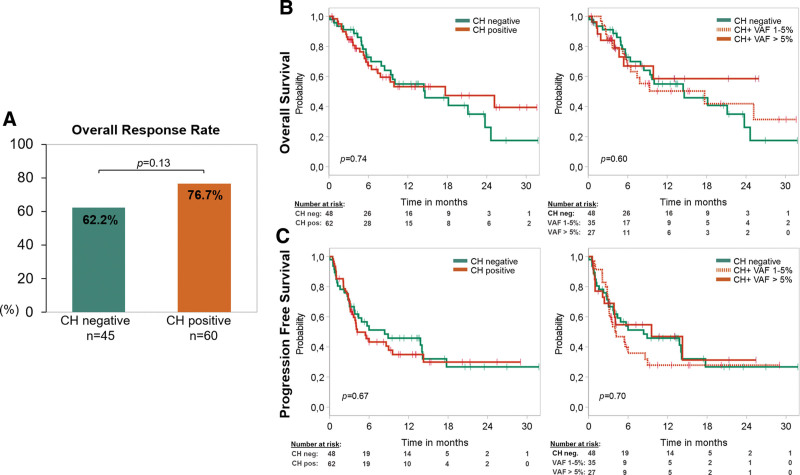
We next investigated the influence of CH on CAR T-cell therapy response. The best ORR in the first 6 months after treatment was 70.5% for the entire cohort of complete cases (n = 105). About 76.7% of all mutated patients (46/60) showed partial or CR, while CH negative patients responded in 62% (28/45), without statistically significant distinction between the 2 groups (P = 0.13) (Figure 4A). Considering specific CAR T-cell products, we did not observe a significant difference of ORR comparing the Axi-cel and Tisa-cel groups (76.2% versus 59.6%; P = 0.13). While small patient numbers impeded thorough statistical analysis of subgroups, the same numerical differences in favor of the CH positive group were noted in both, patients treated with Axi-cel (n = 42, ORR CH+ 87.0% versus ORR CH− 63.2%; P = 0.14) and patients treated with Tisa-cel (n = 52, ORR CH+ 63.3% versus ORR CH− 54.5%; P = 0.58) (Suppl. Figure S2A).
Figure 4.
CH and survival after treatment with CAR T- therapy. (A) Diagram demonstrating the best ORR during the first 180 days after CAR T-cell treatment stratified by absence (green, n = 45) or presence (orange, n = 60) of CH with a VAF cutoff 1%, respectively. P-value was calculated with Fisher exact test. Patients with lack of follow-up data or death before progress/response were excluded (n = 5). (B) Kaplan-Meier curves showing overall survival of 110 patients undergoing CAR T-cell therapy stratified by absence (green, n = 48) or presence (orange, n = 62) of CH with a VAF cutoff 1% and stratified by the size of the clone considering low VAF 1%–5% (orange dashed) and high VAF >5% (orange). (C) Kaplan-Meier curves showing progression-free survival of 110 patients undergoing CAR T-cell therapy stratified by absence (green, n = 48) or presence (orange, n = 62) of CH with a VAF cutoff 1% and stratified by the size of the clone considering low VAF 1%–5% (orange dashed) and high VAF >5% (orange). P-value was calculated for (B) and (C) with log-rank test. CAR = chimeric antigen receptor; CH = clonal hematopoiesis; ORR = overall response rate; VAF = variant allele frequency.
Prognostic effect of CH in patients receiving CAR T-cells
The probability of survival at 1 year (1-y-OS) was 53% for CH positive patients and 55% for CH negative patients (hazard ratio [HR], 0.91; confidence interval [CI], 0.51-1.61; P = 0.74) with a median follow-up of 12.6 months. 1-y-OS with division of CH positive patients in low and high VAF groups was 50% versus 59% (P = 0.60; Figure 4B). PFS at 1 year was 35% versus 46% for CH positive versus CH negative patients (HR, 1.11 [CI, 0.67-1.84]; P = 0.67). 1-y-PFS with CH positive patients divided in low (1%–5%) and high VAF (>5%) CH revealed 28% versus 47%, furthermore (P = 0.70; Figure 4C). OS and PFS was also similar when the prognostic effect of CH was evaluated separately for PPM1D, DNMT3A, and TET2 (Suppl. Figure S3). Furthermore, no differences in PFS and OS outcomes were noted when restricting to specific mutation groups such as DDR (Suppl. Table S13) or DTA-mutated patients (Suppl. Table S14).
PFS and OS in the CH positive and negative groups were similar when stratified by specific CAR T-cell products. After receiving Axi-cel by comparing CH positive versus CH negative patients, 1-y-OS was 65% versus 52% (HR, 0.76 [CI, 0.30-1.92]; P = 0.56) and 1-y-PFS was 33% versus 43% (HR, 1.06 [CI, 0.47-2.40]; P = 0.88). CH positive patients treated with Tisa-cel showed a 1-y-OS of 41% versus 55% compared with negative patients (HR, 1.13 [CI, 0.54-2.36]; P =0.75) and 1-y-PFS was 34% versus 42% for CH positive versus CH negative patients (HR, 1.07 [CI, 0.56-2.06]; P = 0.84) (Suppl. Figure S2B and S2C). OS and PFS outcomes were similar when the impact of CH was evaluated separately in patients younger or older than 60 years (Suppl. Figure S4). For multivariate analysis, 4 variables were found to be potentially predictive for better outcome or showed nonhomogeneous distribution among CH positive and negative groups (age above/below 60 years at d0, CH mutational status, CAR T-cell product, and remission status before CAR T-cell treatment). The model for OS was stratified by “age above versus below 60 years at treatment day.” None of the selected variables were an independent predictor for PFS or OS (Table 2).
Table 2.
Univariate and Multivariate Analysis for OS and PFS
| Univariate Analysisa | Multivariate Analysisa/b n = 98 |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| OS | ||||||
| CH-pos. vs CH-neg. | 0.91 | 0.51-1.61 | 0.74 | 1.09 | 0.55-2.15 | 0.80 |
| Axi-cel vs Tisa-cel | 0.60 | 0.33-1.09 | 0.09 | 0.62 | 0.34-1.14 | 0.12 |
| Remission status prior CAR T-cell treatment SD/PD vs CR/PR |
1.61 | 0.78-3.34 | 0.20 | 1.42 | 0.67-3.00 | 0.36 |
| PFS | ||||||
| Age above vs below 60 y at treatment day | 0.87 | 0.52-1.44 | 0.58 | 0.92 | 0.50-1.67 | 0.78 |
| • CH-pos vs CH-neg | 1.11 | 0.67-1.83 | 0.68 | 1.18 | 0.65-2.12 | 0.59 |
| • Axi-cel vs Tisa-cel | 0.69 | 0.41-1.15 | 0.15 | 0.68 | 0.40-1.15 | 0.15 |
| Remission status prior CAR T-cell treatment SD/PD vs CR/PR |
1.51 | 0.83-2.75 | 0.18 | 1.31 | 0.70-2.46 | 0.40 |
.
aCalculated with univariate/multivariate cox regression analysis, and only complete cases were accepted.
bFor OS-model stratified by “age above vs below 60 years at treatment day.”
To compare the causes of death between patients with and without CH, we grouped them in 4 main categories (relapse/progression, therapy-related, infection in the absence of relapse, and unknown; Suppl. Figure S5A). Causes of death were similarly distributed between patients with and without CH except infections, which were cause of death only in CH positive patients (Suppl. Figure S5B). Cardiovascular events were not recorded in our cohort during the follow-up period after CAR T-cell therapy. One of 110 patients developed a therapy-related myelodysplastic syndrome (MDS) with multilineage dysplasia and ring sideroblasts 723 days after CAR T-cell therapy. The patient carried 1 PPM1D and 1 SF3B1 mutation before CAR T-cell therapy. At the time of diagnosis of MDS, the patient gained a TET2 mutation, lost the PPM1D, and retained the SF3B1 mutation. The patient died 964 days after CAR T-cell therapy due to early DLBCL relapse after undergoing allogeneic HSC transplantation.
For comparability with other studies, we evaluated our dataset using a 2% VAF cutoff for the definition of CHIP, resulting in a frequency of 44.5% (Suppl. Figure S6A). The incidence of CRS and ICANS was similar between CHIP positive and negative patients (Suppl. Figure S6B and S6C). Moreover, response rate, OS, and PFS were also similar in CHIP positive and negative patients (Suppl. Figure S7A-S7C).
Clonal dynamics after CAR T-cell therapy
Lastly, we investigated clonal evolution under CAR T-cell therapy in patients with available samples 100 days (n = 40; 1 patient d75), 200 days (n = 12), 1 year (n = 11), and 2 years (n = 6, 1 patient d700) posttreatment. All patients with follow-up samples (n = 58) were screened at d0. Considering all detected gene variants, we found a mean VAF±SE of 3.6 ± 0.7 at baseline d0 and of 4.9 ± 0.9 at d100 (number of mutated patients n = 30). In comparison to the corresponding baseline-mean at d0, we see at d200 a mean VAF±SE of 2.7 ± 0.7 versus 2.8 ± 0.9 at d0, at 1 year of 3.4 ± 0.9 versus 2.9 ± 0.9 at d0, and at 2 years of 5.4 ± 2.6 versus 5.2 ± 2.1 at d0.
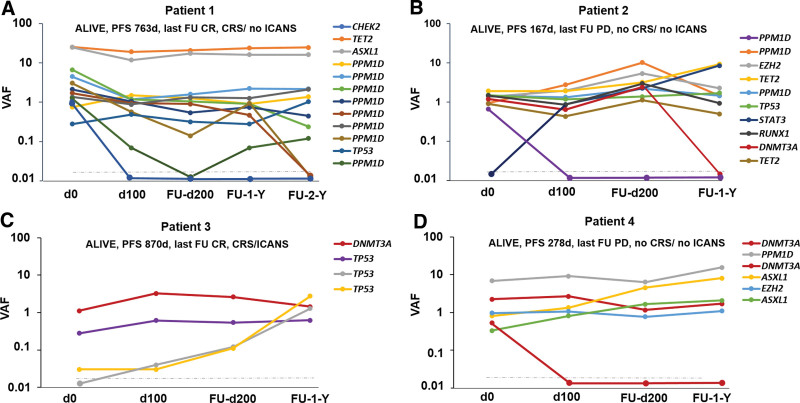
We observed the acquisition of 3 novel mutations in 3 different patients in TP53, STAT3, and GATA2 genes, respectively, within 100 days after CAR T-cell therapy. Loss of 11 mutations (4 PPM1D, 2 TP53, 1 CHEK2, 2 DNMT3A, 1 STAT3, and 1 GATA2) was detected in 6 patients. Specifically, patient 1 who carried 12 mutations in 5 different genes prior CAR T-therapy (8 PPM1D, 1 TP53, 1 CHEK2, 1 TET2, and 1 ASXL1), lost 1 CHEK2 mutation 100 days and 3 of 8 PPM1D mutations 200 days and 2 years after therapy, respectively, developed no ICANS, but CRS grade III and remains alive with a PFS of 763 days (Figure 5A).
Figure 5.
Clonal dynamics after CAR T-cell therapy. (A–D) VAFs at the time of CAR T-cell treatment and individual time points of sampling and corresponding mutational and clinical data. The gray dashed line depicts our VAF detection limit. CAR = chimeric antigen receptor; VAF = variant allele frequency.
Patient 2 carried 9 mutations in 6 different genes prior CAR T-therapy (3 PPM1D, 1 EZH2, 2 TET2, 1 TP53, 1 RUNX1, and 1 DNMT3A), gained a novel STAT3 mutation and lost 1 PPM1D mutation 100 days after therapy. Moreover, the DNMT3A mutation was not detectable 1 year after therapy. The patient developed neither ICANS nor CRS and remains with a PFS of 167 days alive but with progressive disease (Figure 5B). In parallel with an expanding very low TP53 mutation (yellow, VAF <1%), patient 3 (Figure 5C) acquired a novel TP53 mutation (gray) 100 days after CAR T-cell therapy, while a DNMT3A (red) and a third TP53 mutation (purple) remained stable over time. This suggests clonal independence of the expanding and newly acquired TP53 alterations. Patient 3 developed CRS grade II and ICANS grade III and remains with a PFS of 870 days alive in CR. Patient 4 with 6 detectable mutations in 4 different genes prior therapy (2 DNMT3A, 2 ASXL1, 1 PPM1D, and 1 EZH2) lost 1 DNMT3A mutation 100 days posttreatment, while both ASXL1 mutations constantly expanded over time. Specifically, the ASXL1 mutation K618fs (yellow) constantly expanded from VAF 0.81% to 8.12% and the ASXL1 mutation G646fs (green) from VAF 0.33% to 2.09% within 1 year. Patient 4 developed neither ICANS nor CRS and remains alive with a PFS of 287 days after disease progression (Figure 5D).
DISCUSSION
CAR T-cells emerged as an efficacious and highly promising modality for the treatment of hematologic malignancies such as NHL, ALL, and multiple myeloma (MM).15,30–32 However, CAR T-therapy is closely linked to inflammatory side effects, especially CRS and ICANS. Thus, it is conceivable that CH, which has also been associated with various proinflammatory conditions,12 influences the effectiveness, side effects, and long-term outcome of CAR T-cell therapy. In line with this concept, some but not all studies investigating the role of CH in the setting of CAR T-cell therapies reported an increased risk of CRS or ICANS.16–18 In our cohort of 110 patients with r/r B-NHL or ALL, CH had no significant impact on CAR T-cell therapy response, occurrence, and severity of CRS and ICANS, hematologic recovery after CAR T-cell therapy, PFS, or OS. These data might appear conflicting on a first glance; however, all studies were of limited patient size precluding generalization (range, 32–154), included heterogeneous hematologic diseases and/or used different VAF cutoffs to detect CH.16–18 In our heavily pretreated cohort, the CH prevalence of 56% was higher but still similar to that of 48% in a previous study of the Dana-Farber Cancer Institute with a mixed population of 154 patients with B-NLH or MM16 (VAF cutoff 2%). As a consequence of prior exposure to cytotoxic therapies (esp. platinum derivatives), ≈45% of detected clonal events were observed in genes related to DNA damage response. PPM1D was the most frequently mutated gene, predominantly found at modest clone sizes (VAFs 1%–5%) and often affected by multiple mutations. Gibson et al described a negative prognostic effect of CHIP in lymphoma patients undergoing ASCT, which was mostly attributable to PPM1D mutated patients and an increased nonrelapse mortality (mostly therapy-related myeloid neoplasias and cardiovascular events).13 The different prognostic impact of CH in CAR T-cell and ASCT-treated patients may be explained by the selection pressure in ASCT, but not CAR T-cell therapy, due to rapid expansion of hematopoiesis, but also by the shorter median follow-up in our study (12.6 months versus 5 years). In our study, 33.6% of all patients had undergone ASCT before CAR T-cell therapy, while 27% and 22% of all patients in the studies by Miller et al and Saini et al, respectively, had received a prior ASCT.16,17
Little is known about how CAR T-cell therapy and the accompanied inflammatory stress scenario affects HSC expansion. Our serial analyses revealed a certain increase of clone size during the initial 100 days after CAR T-cell therapy, coupled with the acquisition of additional mutations and a stabilization of clone size thereafter. These data support the idea of a self-perpetuating circle between inflammation and preferential expansion of CH-mutated HSCs.33 Comparisons with previous studies investigating CH in the context of CAR T-cell therapies revealed several similarities. In agreement with 2 previous studies, CH had no impact on CAR T-cell therapy response.17,18 Although a numerically higher ORR was observed in the first 6 months posttreatment in CH positive compared with CH negative patients, no significant differences could be found between the groups. In contrast, Miller et al observed a higher complete response rate in CH positive patients ≤60 years.16 In line with Teipel et al,18 we observed no significant differences in terms of onset or severity of CRS or ICANS between CH positive and CH negative patients, which applies also if stratified by clone size, age, or CAR T-cell product. Overall, this finding is in line with previously published reports, in which associations between CH and CAR T-cell-mediated side effects were only found in subgroups. For example, Saini et al found that CH mutations especially DTA mutations were associated with grade ≥3 ICANS,17 and Miller and colleagues found an increased rate of grade ≥2 CRS only in patients with CH who were ≤60 years.16
For the first time, we evaluated whether different CAR T-products influence therapy response regarding CH status but found no differences between CH positive and CH negative patients treated with Tisa-cel or Axi-cel, respectively. This is of particular importance, as a growing body of evidence suggest superior efficacy of Axi-cel when compared with Tisa-cel in a large real-world comparison.34 Furthermore, recent studies of Jain et al have shown that biallelic TET2 disruption enhances T cell-mediated tumor rejection in leukemia and prostate cancer models—illustrating the potential of epigenetic programming to enhance T-cell immunity. However, loss of TET2 also enables antigen-independent CAR T-cell clonal expansions. In our cohort, three patients harbored 2 or more TET2 mutations and all of them developed a CR after CAR T-cell infusion. It will be of great importance to study such cases in much larger patient cohorts.35
Our study is limited by the moderate sample size, heterogeneous patient population, incomplete availability of follow-up samples, and short median follow-up of 12.6 months. A longer follow-up will show whether CH will expand, and whether nonrelapse mortality will eventually increase in the CH cohort.
In conclusion, we here investigated a German multicenter cohort of patients receiving CAR T-cell therapy. Our main findings support that CH (1) is very common in patients treated for r/r B-NHL and ALL, (2) does not predict the risk for toxicity or prolonged cytopenias, (3) does not influence therapy response, and (4) does not impact long-term outcome after CAR T-cell therapy. While CH has been suggested as a poor risk marker in B-NHL patients undergoing autologous transplantation,13 CH currently does not seem to have a prognostic role for CAR T-cell therapy. Based on the currently available data, CAR T-cell therapy seems an equally effective treatment in CH positive and CH negative patients.
ACKNOWLEDGMENTS
We thank all participating patients and their families. We thank Martin Wichmann, Kerstin Görlich, Thomas Fangmann, Tatiana Borodina, and Jeannine Wilde for technical assistance and acknowledge the assistance of the Genomics Core Facility BIH/MDC.
AUTHOR CONTRIBUTIONS
VP, JFK, MH, and FD designed the study. VP, JFK, OP, CMA, CS, AK, PMS, AL, LW, RA, AH, CK, JT, KW, GF, CB, JH, FA, I-KN, GB, LB, GW, NK, VV, MH, and FD contributed patient samples and clinical data. VP, JFK, RA, CMA, RG, MH, and FD analyzed the data. VP, JFK, MH, and FD wrote the article. All authors read and agreed to the final version of the article.
DATA AVAILABILITY
Data underlying this article will be shared upon reasonable request.
DISCLOSURES
MH reports fees for advisory or consultancy services from Abbvie, Agios, BMS, Daiichi Sankyo, Eurocept, Glycostem, Janssen, Jazz Pharmaceuticals, Kura Oncology, Novartis, Pfizer, PinotBio, Roche, Takeda, and Tolremo. FD reports personal fees from Gilead, Incyte, Roche, Novartis, AbbVie, Astra Zeneca outside the submitted work. JFK reports personal fees from Gilead and Janssen outside the submitted work. GW reports honoraria from Gilead, Novartis, Clinigen, and Janssen outside the submitted work. OP has no conflicts of interest directly related to this work. OP has received honoraria or travel support from Gilead, Jazz, MSD, Novartis, Pfizer, and Therakos. He has received research support from Incyte and Priothera. He is member of advisory boards to Equillium Bio, Jazz, Gilead, Novartis, MSD, Omeros, Priothera, Sanofi, Shionogi, and SOBI. All the other authors have no conflicts of interest to disclose.
SOURCES OF FUNDING
This study was supported by grants from the Deutsche Krebshilfe (70113643), the Alfred und Angelika Gutermuth Stiftung (2022/2) both awarded to FD, DJCLS (16 R/2021), and the Deutsche Krebshilfe (70114189) to MH. CMA and AH received support by the Berlin Institute of Health (BIH) junior clinician scientist program. CMS was supported by a stipend from the Berlin School of Integrative Oncology (BSIO). PMS was supported by a Postdoctoral Research Fellowship from Alexander von Humboldt Foundation. OP acknowledges the support of José Carreras Leukämie-Stiftung (3R/2019, 23R/2021), Deutsche Krebshilfe (70113519), Deutsche Forschungsgemeinschaft (PE 1450/7-1, PE 1450/9-1), and Stiftung Charité BIH (BIH_PRO_549, Focus Group Vascular Biomedicine).
Supplementary Material
Footnotes
VP and JFK have contributed equally to this work as first authors.
MH and FD have contributed equally to this work and share senior authorship.
Ethics approval statement: Written informed consent was obtained according to the Declaration of Helsinki. The study was approved by the institutional review board of Hannover Medical School (9098_BO_S_2020) and of Charité – Universitätsmedizin Berlin (EA2_087_16).
Supplemental digital content is available for this article.
REFERENCES
- 1.Abelson S, Collord G, Ng SWK, et al. Prediction of acute myeloid leukaemia risk in healthy individuals. Nature. 2018;559:400–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acuna-Hidalgo R, Sengul H, Steehouwer M, et al. Ultra-sensitive sequencing identifies high prevalence of clonal hematopoiesis-associated mutations throughout adult life. Am J Hum Genet. 2017;101:50–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arends CM, Weiss M, Christen F, et al. Clonal hematopoiesis in patients with anti-neutrophil cytoplasmic antibody-associated vasculitis. Haematologica. 2020;105:e264–e267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buscarlet M, Provost S, Zada YF, et al. DNMT3A and TET2 dominate clonal hematopoiesis and demonstrate benign phenotypes and different genetic predispositions. Blood. 2017;130:753–762. [DOI] [PubMed] [Google Scholar]
- 5.Bolton KL, Ptashkin RN, Gao T, et al. Cancer therapy shapes the fitness landscape of clonal hematopoiesis. Nat Genet. 2020;52:1219–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coombs CC, Zehir A, Devlin SM, et al. Therapy-related clonal hematopoiesis in patients with non-hematologic cancers is common and associated with adverse clinical outcomes. Cell Stem Cell. 2017;21:374–382.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaiswal S, Fontanillas P, Flannick J, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371:2488–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuster JJ, MacLauchlan S, Zuriaga MA, et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science. 2017;355:842–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaiswal S, Natarajan P, Silver AJ, et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. 2017;377:111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sano S, Oshima K, Wang Y, et al. Tet2-mediated clonal hematopoiesis accelerates heart failure through a mechanism involving the IL-1beta/NLRP3 inflammasome. J Am Coll Cardiol. 2018;71:875–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christen F, Hablesreiter R, Hoyer K, et al. Modeling clonal hematopoiesis in umbilical cord blood cells by CRISPR/Cas9. Leukemia. 2022;36:1102–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furer N, Kaushansky N, Shlush LI. The vicious and virtuous circles of clonal hematopoiesis. Nat Med. 2021;27:949–950. [DOI] [PubMed] [Google Scholar]
- 13.Gibson CJ, Lindsley RC, Tchekmedyian V, et al. Clonal hematopoiesis associated with adverse outcomes after autologous stem-cell transplantation for lymphoma. J Clin Oncol. 2017;35:1598–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380:45–56. [DOI] [PubMed] [Google Scholar]
- 16.Miller PG, Sperling AS, Brea EJ, et al. Clonal hematopoiesis in patients receiving chimeric antigen receptor T-cell therapy. Blood Adv. 2021;5:2982–2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saini NY, Swoboda DM, Greenbaum U, et al. Clonal hematopoiesis is associated with increased risk of severe neurotoxicity in axicabtagene ciloleucel therapy of large B-cell lymphoma. Blood Cancer Discov. 2022;3:385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teipel R, Kroschinsky F, Kramer M, et al. Prevalence and variation of CHIP in patients with aggressive lymphomas undergoing CD19-directed CAR T-cell treatment. Blood Adv. 2022;6:1941–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fraietta JA, Nobles CL, Sammons MA, et al. Disruption of TET2 promotes the therapeutic efficacy of CD19-targeted T cells. Nature. 2018;558:307–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25:625–638. [DOI] [PubMed] [Google Scholar]
- 21.Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15:47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arends CM, Dimitriou S, Stahler A, et al. Clonal hematopoiesis is associated with improved survival in patients with metastatic colorectal cancer from the FIRE-3 trial. Blood. 2022;139:1593–1597. [DOI] [PubMed] [Google Scholar]
- 23.Arends CM, Liman TG, Strzelecka PM, et al. Associations of clonal haematopoiesis with recurrent vascular events and death in patients with incident ischemic stroke. Blood. 2023;141:787–799. [DOI] [PubMed] [Google Scholar]
- 24.Frick M, Chan W, Arends CM, et al. Role of donor clonal hematopoiesis in allogeneic hematopoietic stem-cell transplantation. J Clin Oncol. 2019;37:375–385. [DOI] [PubMed] [Google Scholar]
- 25.Heuser M, Heida B, Büttner K, et al. Posttransplantation MRD monitoring in patients with AML by next-generation sequencing using DTA and non-DTA mutations. Blood Adv. 2021;5:2294–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130:1800–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alaggio R, Amador C, Anagnostopoulos I, et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia. 2022;36:1720–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arends CM, Galan-Sousa J, Hoyer K, et al. Hematopoietic lineage distribution and evolutionary dynamics of clonal hematopoiesis. Leukemia. 2018;32:1908–1919. [DOI] [PubMed] [Google Scholar]
- 29.Hartmann L, Hecker JS, Rothenberg-Thurley M, et al. Compartment-specific mutational landscape of clonal hematopoiesis. Leukemia. 2022;36:2647–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20:31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Munshi NC, Anderson LD, Jr, Shah N, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med. 2021;384:705–716. [DOI] [PubMed] [Google Scholar]
- 32.Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020;382:1331–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hormaechea-Agulla D, Matatall KA, Le DT, et al. Chronic infection drives Dnmt3a-loss-of-function clonal hematopoiesis via IFNgamma signaling. Cell Stem Cell. 2021;28:1428–1442.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bachy E, Le Gouill S, Di Blasi R, et al. A real-world comparison of tisagenlecleucel and axicabtagene ciloleucel CAR T cells in relapsed or refractory diffuse large B cell lymphoma. Nat Med. 2022;28:2145–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jain N, Zhao Z, Feucht J, et al. TET2 guards against unchecked BATF3-induced CAR T cell expansion. Nature. 2023;615:315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data underlying this article will be shared upon reasonable request.