Abstract
The collaborative study on the genetics of alcoholism (COGA) is a multi‐site, multidisciplinary project with the goal of identifying how genes are involved in alcohol use disorder and related outcomes, and characterizing how genetic risk unfolds across development and in conjunction with the environment and brain function. COGA is a multi‐generational family‐based study in which probands were recruited through alcohol treatment centers, along with a set of community comparison families. Nearly 18,000 individuals from >2200 families have been assessed over a period of over 30 years with a rich phenotypic battery that includes semi‐structured psychiatric interviews and questionnaire measures, along with DNA collection and electrophysiological data on a large subset. Participants range in age from 7 to 97, with many having longitudinal assessments, providing a valuable opportunity to study alcohol use and problems across the lifespan. Here we provide an overview of data collection methods for the COGA sample, and details about sample characteristics and comorbidity. We also review key research findings that have emerged from analyses of the COGA data. COGA data are available broadly to researchers, and we hope this overview will encourage further collaboration and use of these data to advance the field.
Keywords: alcohol, comorbidity, development, drug, environment, genetics, lifespan
The multi‐generational, family‐based collaborative study on the genetics of alcoholism (COGA) has for over 30 years studied nearly 18,000 individuals from over 2200 families with a rich protocol to better understand alcohol use, problems, and disorder. Participants provide a wide range of information through psychiatric interviews, electrophysiological measures, neuropsychological evaluations, and genomic data. These data are broadly available to other researchers. Here, we provide an overview of COGA's data collection and sample characteristics, along with key research findings, in order to encourage further use of COGA data to help advance the field.
1. INTRODUCTION
The collaborative study on the genetics of alcoholism (COGA) was designed to identify risk and protective genes for alcohol use disorders and related conditions, with additional objectives to study associations with environmental and neurophysiological factors and their interplay with genes. 1 , 2 , 3 COGA has collected a wealth of data on nearly 18,000 individuals over the past 30 years, creating a national resource for studying the onset, offset, and developmental course of alcohol use disorders and related conditions. COGA data and all assessment instruments are made freely available to the scientific community (details at cogastudy.org). In this paper, we describe how data collection has evolved across the COGA project (Part 1), as well as key areas of investigation and findings that have emerged from the COGA project (Part 2), in the hope of inspiring additional scientists to collaborate and work with this rich dataset.
2. PART 1: OVERVIEW OF DATA COLLECTION
Since its inception in 1989, COGA has used a family design, recruiting multigenerational families affected with alcohol use disorder (AUD) through probands in treatment for AUD, and a smaller set of community comparison families. To date, there have been 17,878 individuals in 2246 families who have participated in one or more of the assessment waves. Data collection has focused on different participant subsets over time, depending on the research emphasis. There have been four principal data collection waves that may be broadly described as follows: (a) “Wave 1” initial ascertainment and assessment from 1991 to 1999 (initial AUD case and community families); (b) “Wave 2”: follow‐up of individuals in Wave 1 and other family members not assessed at Wave 1, as well as families from a new research site from 1996 to 2005; (c) the “Prospective wave” from 2004 to 2019 which focused on assessments of youth ages 12–22 (born 1982 or later) in both case and comparison families at 2 year intervals; and (d) the current, “Lifespan” wave, begun in 2019, in which previously assessed participants now in midlife (ages 30–40) and later life (aged 50 or older) are re‐assessed. With each phase of data collection, the core assessment interview (SSAGA) was revised to reflect changes in diagnostic criteria or to expand assessment to address newly arising and age‐specific research questions. We provide here an overview of the different data collection waves, the research question guiding the focus of the data collection, the numbers of participants assessed at each, and tabular summaries of their characteristics.
2.1. Wave 1: Initial ascertainment and assessment
At the study's inception, high‐risk families were ascertained through probands who were undergoing treatment for AUDs at six sites across the United States. These were the University of Connecticut (Farmington, CT), SUNY Downstate (Brooklyn, NY), Indiana University (Indianapolis, IN), University of Iowa (Iowa City, IA), Washington University (St Louis, MO), and University of California San Diego. Probands were recruited from inpatient and outpatient treatment facilities to ensure a high level of AUD severity that would optimize identification of AUD susceptibility genes and AUD‐linked brain dysfunction. Probands were interviewed with a comprehensive assessment created specifically for the project—the SSAGA interview—which was found to be highly reliable and valid. 4 , 5 , 6 Those who met criteria for both DSM‐3R alcohol dependence 7 and Feighner alcoholism at the definite level 8 were enrolled. The assessment covered a wide range of health behaviors, substance use patterns and associated problems, and psychiatric symptoms that may have occurred at any point in participants' lifetimes. All first‐degree relatives aged 7 or older were sought for interview with developmentally appropriate versions of the SSAGA. In addition, for children ages 7–17, parental corroborating interviews were obtained. 9 In addition to the SSAGA interview, individuals completed personality assessments and were administered a family history assessment. 10 This was considered the core COGA protocol. (See Table 1 for assessments conducted at each data collection wave, including neurophysiological and neuropsychological evaluations.)
TABLE 1.
Assessments in each data collection wave.
| Wave 1 | Wave 2 | Prospective | Lifespan (ongoing) | ||
|---|---|---|---|---|---|
| Mid life | Later life | ||||
| AUD and related | |||||
| SSAGA interview | SSAGA‐I a | SSAGA‐II b | SSAGA‐IV c | SSAGA‐V d | SSAGA‐V d |
| Achenbach youth self‐report | X | ||||
| Achenbach adult self‐report | X | ||||
| Achenbach teacher report form | X | ||||
| Iowa Conners Teacher Rating Scale | X | ||||
| Self‐rating of response to ethanol (SRE) | X | X | X | X | |
| Drinking to cope | X | X* | X* | ||
| Alcohol expectancy questionnaire (adult) | X | X | X* | X* | |
| Alcohol expectancy questionnaire (adolescent) | X | X | |||
| Desires for alcohol (DAQ) | X | X | X* | X* | |
| Ethanol Dependence Syndrome Scale | X | X | |||
| Short Form Health Survey (SF‐36) | X | X | |||
| Personality and traits | |||||
| Tri‐dimensional personality questionnaire | X | ||||
| Temperament and character inventory | X | ||||
| Zuckerman Sensation Seeking Scale | X | X | X | ||
| Russo Sensation Seeking Scale—children | X | X | |||
| Dimensions of temperament survey | X | ||||
| Externalizing behaviors | X | ||||
| Harter self‐perception profile (child & adolescent) | X | ||||
| Harter Importance rating (child) | X | ||||
| Harter Importance rating (adolescent) | X | ||||
| Barrett Impulsivity Scale (child) | X | ||||
| Barrett Impulsivity Scale (adolescent) | X | ||||
| UPPS‐P Impulsive Behavior Scale | X | X | |||
| Five Factor Personality Inventory (NEO‐FFI) | X | X | X | X | |
| NIH toolbox—Emotional battery | X | X | |||
| Borderline Personality Disorder Screener | X* | X* | |||
| Environment & family background | |||||
| Family History Assessment Module | X | X | X | ||
| Harter Social Support Scale for children | X | X | |||
| Perceived Social Support—Family | X | ||||
| Perceived social support—Friends | X | ||||
| Important people & activities | X | ||||
| Parental bonding | X | ||||
| McMaster family assessment | X | ||||
| COVID questionnaire | X | X | |||
| Daily hassles & uplifts (adolescent) | X | X | |||
| Daily hassles & uplifts (adult) | X | X | |||
| Stressful life events | X* | X* | |||
| Experiences of discrimination scale | X | X | |||
| Work/employment questionnaire | X* | X* | |||
| Neurophysiologic experiments 1 | |||||
| Resting‐state EEG | X | X | X | X | X |
| Visual oddball task | X | X | X | X | X |
| Auditory oddball | X | X | X | X | X |
| Semantic priming | x | x | x | x | x |
| Go‐NoGo task | X | X | |||
| Monetary gambling task | X | X | |||
| Continuous performance test | X | ||||
| Color/Word Stroop | X | ||||
| Auditory novel stimuli | X | ||||
| Cognitive/affective stroop | X | X | X | ||
| Mismatch negativity | X | X | |||
| Bereitschafts potentials | X | X | |||
| Contingent negative variation | X | X | |||
| Object recognition | X | X | |||
| Intertrial interference | X | X | |||
| Neuropsychological tasks 1 | |||||
| Tower of London b | X | X | |||
| Visual span test | X | X | |||
| NIH toolbox cognitive battery | X | X | |||
| NIH toolbox emotional battery | X | X | |||
| Porteus maze test | X | X | |||
| TRAILS A & B | X | X | |||
| Wechsler adult & child intelligence scales, rev | X | X | |||
| California verbal learning test adult & child | X | X | |||
| Ravens progressive matrices | X | X | |||
| Wide range achievement test revised | X | X | |||
| Discontinued Jan 2021 | |||||
A full list and description of all neurophysiological and neuropsychological measures can be found in the Supplemental Methods section of the companion paper: 3. Brain function.
SSAGA‐I included demographics, medical history, tobacco history, suicidal thoughts and behavior, psychosis screener, and diagnostic information for: somatization, alcohol abuse and dependence, cannabis abuse and dependence, drug abuse and dependence (cocaine, other stimulants, sedatives and opiates), major depression, dysthymia, mania, conduct disorder, antisocial personality disorder, panic disorder, agoraphobia, social phobia, obsessive‐compulsive disorder, and eating disorders.
SSAGA‐II included demographics, home environment ages 6–13 (e.g., household income, relationship with parents, family tension, parental monitoring, punishment, and consistency), medical history, psychosis screener, suicidal thoughts and behavior, and dDSM‐3r, 4 diagnostic information for: somatization disorder, alcohol abuse and dependence, cannabis abuse and dependence, drug abuse and dependence (cocaine, other stimulants, sedatives and opiates), nicotine dependence, and fagerstrom test for nicotine dependence (FTND), major depression, dysthymia, mania, conduct disorder, antisocial personality disorder, attention deficit hyperactivity disorder, panic disorder, agoraphobia, social phobia, obsessive‐compulsive disorder, generalized anxiety disorder, post‐traumatic stress disorder, and eating disorders.
SSAGA‐IV included demographics, home environment ages 12–17 (e.g., household income, relationship with parents, family tension, parental monitoring, punishment, and consistency, sibling and peer substance use), medical history, psychosis screener, suicidal thoughts and behavior, and DSM‐3r, IV diagnostic information for: alcohol abuse and dependence, cannabis abuse and dependence, drug abuse and dependence (cocaine, other stimulants, sedatives and opiates), nicotine dependence, FTND, major depression, dysthymia, mania, conduct disorder, antisocial personality disorder, attention deficit hyperactivity disorder, oppositional defiant disorder, panic disorder, agoraphobia, social phobia, obsessive‐compulsive disorder, post‐traumatic stress disorder, and eating disorders.
SSAGA‐V included demographics, medical history, social relationships (quality of relationship with spouse/partner, drug and alcohol use/problems of parents, partner, peers, siblings, and offspring), Religiosity, suicidal thoughts and behavior, and DSM‐3r and IV diagnostic information for: alcohol abuse and dependence, cannabis abuse and dependence, drug abuse and dependence (cocaine, other stimulants, sedatives and opiates), nicotine dependence; FTND, and DSM‐5 use disorders (alcohol, tobacco, cannabis, cocaine, stimulants, sedatives, and opiates), major depression, mania, conduct disorder, antisocial personality disorder, panic disorder, agoraphobia, social phobia, obsessive‐compulsive disorder, and post‐traumatic stress disorder.
From the initial pool of families, a subset of families with at least two affected first degree adult relatives in addition to the proband was selected for intensive study. In these families, data collection was expanded to individuals in other branches of the family. For all available consenting members of these extended, densely affected pedigrees, the core protocol was supplemented with neurophysiological (EEG/ERP) and neuropsychological evaluations and blood was collected for DNA and cell lines (see 4. Genetics and 3. Brain function) (in Table 1, specific components of the brain function protocol in the initial data collection are displayed; additional details about these tests may be found in 3. Brain function.) This approach thus identified densely affected families that were suitable for the planned genetic linkage and association studies (see 4. Genetics). Probands and their relatives constituted “case” families. In addition to the case families, a set of families, called “comparison” families, were recruited from a variety of community sources (e.g., dental clinic admissions and state drivers' registries) and assessed with the identical expanded protocol. Extensions to other family branches in the comparison families were also allowed as described above, although this was a rare occurrence. For the comparison families, there had to be two living parents and 3 (or more) children aged 14 or older. The presence of AUDs in the comparison families was not used as an exclusion criterion.
In this first data collection wave, 9325 adults aged 18 or older, and 1333 children aged 6–17 in 2246 families were assessed. Interviews occurred from 1991 to 1999 (most from 1991 to 1996). Participants were administered the SSAGA in person, although telephone assessments were conducted when transportation difficulties or distance precluded an in‐person visit. 11 Tables 2 (adults) and 3 (children) summarize the participant characteristics, separately for the probands, their relatives, and participants in comparison families. A few points merit comment here. Probands and their family members are racially/ethnically diverse, middle aged, with an unsurprising predominance of men in the proband sample (74.8%) which derived from treatment programs, but a higher proportion of women among the relatives (59%). By definition, probands were affected with AUD; as expected, the prevalence of Alcohol Dependence among their relatives was much higher than that observed among comparison family participants (34.6% vs. 16.6%). There is considerable addiction comorbidity among the probands, particularly for cannabis and cocaine where 44.9% and 48.4% met dependence criteria respectively. Comorbidity with other substance dependence is less pronounced among their relatives, where 14.2% and 11% met criteria for dependence on cannabis and cocaine, respectively. The prevalence rates in probands and their relatives were markedly higher than those observed among comparison family participants, where only 5.4% met criteria for cannabis dependence, and 1.4% for cocaine, with rates negligible for the other drugs. In contrast to comparison family participants, prevalence of non‐substance disorders comorbidity was elevated among probands and their relatives; for example, prevalence estimates for major depression were 54%, 33.1%, and 22.9% for probands, relatives and comparison participants, respectively, and for conduct disorder 29.6%, 11.4% and 6.2% for proband, relatives and comparison participants, respectively. The children, most of whom were members of case families (i.e., related to probands), were on average about 12 years old and in 6th grade, younger than those in the comparison families, and substance use initiation was not yet a common behavior. The lower prevalence of substance involvement among case family children compared with their comparison family counterparts is probably due to being several years younger, on average. A striking but unsurprising observation is that only 48% of the children in case families were living with their biological father, compared with 96% of children in comparison families. In children from case families, comorbidity with attention deficit‐hyperactivity disorder (ADHD) and oppositional defiant disorder (ODD), anxiety, and depression, reported by either the child or parent, was elevated over that observed in children from comparison families.
TABLE 2.
Characteristics of adults in wave 1 data collection: Initial ascertainment and assessment, by probands, relatives, and comparison participants.
| Probands‐case fam (N = 1247) | Relatives of probands a N = 7019 | Comparison family participants N = 1059 | |
|---|---|---|---|
| Mean age yrs (sd) | 37.7 (10.6) | 40.8 (15.1) | 36.0 (14.3) |
| Range | 17–77 | 15–97 | 17–81 |
| Female (%) | 314 (25.2) | 4149 (59.1) | 548 (51.8) |
| Male (%) | 933 (74.8) | 2870 (40.9) | 511 (48.2) |
| Race/ethnicity (self‐report) | |||
| Black (%) | 262 (21.0) | 1321 (18.8) | 67 (6.3) |
| White (%) | 946 (75.9) | 5513 (78.5) | 938 (88.6) |
| Hispanic ethnicity (%) | 87 (7.0) | 427 (6.1) | 57 (5.4) |
| Highest years of education | |||
| Mean (sd) | 12.5 (2.1) | 12.7 (2.4) | 14.0 (2.2) |
| Substance use lifetime disorder (may have more than one): n (%) | |||
| DSM‐3R dependence | |||
| Alcohol | 1246 (99.9) | 2426 (34.6) | 176 (16.6) |
| Cannabis | 560 (44.9) | 997 (14.2) | 57 (5.4) |
| Cocaine | 603 (48.4) | 773 (11.0) | 15 (1.4) |
| Other stimulants | 302 (24.2) | 421 (6.0) | 7 (0.7) |
| Opioids | 199 (16.0) | 212 (3.0) | 3 (0.3) |
| Sedatives | 200 (16.0) | 205 (2.9) | 4 (0.4) |
| Tobacco—smoked daily for ≥6 mo (%) | 1062 (85.3) | 4209 (60.0) | 357 (33.7) |
| Non substance lifetime diagnoses (DSM‐3R) (n, %) | |||
| Major depression | 673 (54.0) | 2326 (33.1) | 242 (22.9) |
| Panic | 104 (8.3) | 246 (3.5) | 15 (1.4) |
| Agoraphobia | 61 (4.9) | 172 (2.4) | 15 (1.4) |
| Social phobia | 69 (5.5) | 204 (2.9) | 16 (1.5) |
| Conduct (%) b | 369 (29.6) | 804 (11.4) | 66 (6.2%) |
| ASPD (%) b | 335 (26.9) | 470 (6.7) | 24 (2.3%) |
| Somatization (%) | 1 (0) | 2 (<0%) | 0 (0.0) |
| OCD (%) | 49 (3.9) | 94 (1.3) | 8 (0.8) |
| Eating disorders (%) | |||
| Anorexia | 11 (0.9) | 27 (0.4) | 2 (0.2) |
| Bulimia | 30 (2.4) | 136 (1.9) | 6 (0.6) |
| Suicide attempt | 300 (24.1) | 674 (9.6) | 27 (2.6) |
Includes participants in other family types (e.g., Alcohol challenge families n = 315).
Includes alcohol‐ or other substance‐ induced symptoms.
TABLE 3.
Characteristics of children from Wave 1 data collection, by case and comparison families.
| Case families | Comparison families | |
|---|---|---|
| N = 1104 a | N = 229 | |
| Mean age yrs (sd) | 11.9 (3.2) | 14.3 (2.6) |
| Range | 6–17 | 7–17 |
| Female (%) | 564 (51.1) | 113 (49.3) |
| Race/ethnicity (self‐report) | ||
| Black (%) | 270 (24.5) | 20 (8.7) |
| White (%) | 754 (68.3) | 197 (86.0) |
| Hispanic (see note) | 60 (5.4) | 7 (3.1) |
| Education—current grade (SD) Mean (sd) | 6.2 (3.1) | 8.6 (2.7) |
| Rearing status | ||
| Live with bio dad (%) | 530 (48.0) | 220 (96.1) |
| Live with bio mom (%) | 974 (88.2) | 227 (99.1) |
| Substance use | ||
| Ever had a drink (%) | 340 (30.8) | 102 (44.5) |
| Mean age first full drink (sd) | 12.7 (2.5) | 13.1 (2.1) |
| Ever had seven drinks lifetime (n, %) | 186 (16.8) | 61 (26.6) |
| Ever intoxicated b (n, (%) | 146 (13.2) | 42 (18.3) |
| Age of first intoxication b –mean (sd) | 13.9 (1.7) | 14.1 (1.5) |
| Ever used cannabis (n, %) | 167 (15.1) | 35 (15.3) |
| Mean age first cannabis use (sd) | 13.7 (2.2) | 14.4 (2.0) |
| Ever used tobacco (n, %) | 306 (27.8) | 83 (36.2) |
| Mean age first tobacco use (sd) | 11.8 (2.7) | 12.5 (2.6) |
| Diagnoses by either child or parent report: (n, %) | ||
| ADHD diagnosis | 122 (11.1) | 10 (4.4) |
| Oppositional defiant disorder | 139 (12.6) | 16 (7.0) |
| Conduct disorder | 161 (14.6) | 28 (12.2) |
| Separation anxiety disorder | 139 (12.6) | 12 (5.2) |
| Overanxious disorder | 88 (8.0) | 21 (9.2) |
| Depression‐lifetime | 205 (18.6) | 24 (10.5) |
| Suicide attempt (%) | 56 (5.1) | 6 (2.6) |
Note: Hispanic ethnicity was not asked separately but rather as part of overall racial identity.
Includes other family types (e.g., alcohol challenge families) (n = 14).
Only asked of those having seven or more drinks in lifetime.
2.2. Wave 2: Follow‐up
The second data collection wave, which began in 1996 and continued through 2005, targeted Wave 1 probands, members of densely affected families, and comparison family members. 12 In addition, other family members in the Wave 1 families who had not been assessed in the previous wave were assessed in Wave 2. Also in Wave 2, a seventh data collection site, located at Howard University in Washington DC, was added to increase the racial/ethnic diversity of the sample, with an emphasis on Black families. The SSAGA was updated to reflect the change in diagnostic classification system from DSM‐3R to DSM‐IV, 13 adding new items but retaining the old to preserve consistency across assessment waves. The key elements of the protocol in Wave 2, including the interview, questionnaires, and electrophysiological assessments, remained largely unchanged. Additional neuropsychological tests were included to evaluate participants' decision‐making, memory, attention, and problem solving, and, if not previously collected, a blood sample was taken for genetic analyses and cell lines. Children aged 7–17 were also re‐interviewed, and additional children in the families were recruited. Altogether, 8799 adults aged 18 and older, and 2646 children, participated in this second wave. In Tables 4 and 5, the demographic and psychiatric characteristics of Wave 2 participants are displayed for adults and children.
TABLE 4.
Characteristics of adults in Wave 2 data collection, by case and comparison families.
| Case families a | Comparison families | |
|---|---|---|
| Number of participants | N = 7359 (%) | N = 1440 (%) |
| Mean age (sd) | 39.3 (13.3) | 37.0 (14.4) |
| Range | 17–96 | 18–86 |
| Female (%) | 4027 (54.7) | 795 (55.2) |
| Race/ethnicity (self‐report) | ||
| Black (%) | 2003 (27.2) | 225 (15.6) |
| White (%) | 5146 (69.9) | 1122 (77.9) |
| Hispanic ethnicity (%) | 517 (7.0) | 57 (4.0) |
| Education—highest number of years completed (mean, sd) | 12.7 (2.2) | 14.4 (2.1) |
| Interviewed in Wave 1 (%) | 4167 (56.6) | 915 (63.5) |
| Wave 2 only (%) | 3193 (43.4) | 525 (36.5) |
| Substance dependence (DSM‐3R) (may have more than one) (n, %) | ||
| Alcohol | 3236 (44.0) | 226(15.7) |
| Cannabis | 1686 (22.9) | 105 (7.3) |
| Cocaine | 1298 (17.6) | 33 (2.3) |
| Other stimulants | 544 (7.4) | 12 (0.8) |
| Opiates | 362 (4.9) | 7 (0.5) |
| Sedatives | 248 (3.4) | 6 (0.4) |
| Tobacco—DSM‐4 dependence | 2549 (34.6) | 211 (14.7) |
| Non substance dx: (DSM‐3R) (n, %) | ||
| Major depression | 2791 (37.9) | 303 (21.0) |
| GAD b | 13 (0.2) | 2 (0.2%) |
| Panic b | 184 (2.8) | 17 (1.4) |
| Agoraphobia b | 186 (2.9) | 15 (1.3) |
| Social phobia b | 239 (3.7) | 17 (1.4) |
| Conduct c | 1283 (17.4) | 113 (7.9) |
| ASPD c | 922 (12.5) | 60 (4.2) |
| Obsessive‐compulsive disorder b | 83 (1.1) | 3 (0.2) |
| PTSD b | 388 (6.0) | 19 (1.6) |
| Suicide attempt b | 695 (10.7) | 32 (2.7) |
Includes participants from other family types (e.g., alcohol challenge, n = 10).
Percent calculated on reduced n, owing to omission of some sections across sites, n's from 6487 to 6505 (case families); 1179 (comparison families).
Includes substance‐related symptoms.
TABLE 5.
Characteristics of children assessed at Wave 2, by case and comparison families.
| Case families a | Comparison families | |
|---|---|---|
| Participants | N = 2457 | N = 189 |
| Mean age (sd) | 11.9 (3.2) | 12.6 (3.2) |
| Female (%) | 1219 (49.6) | 97 (51.3) |
| Race/ethnicity (self‐report) b | ||
| Black (%) | 706 (31.5) | 29 (16.5) |
| White (%) | 1428 (63.1) | 137 (77.8) |
| Hispanic ethnicity (%) | 242 (10.7) | 15 (8.5) |
| Education—current grade | ||
| Mean (sd) | 6.2 (3.2) | 7.0 (3.2) |
| % with phase 1 child interview | 461 (18.8) | 35 (18.3) |
| Rearing status (ages 12–17) | ||
| Live with bio dad (%) | 1273 (51.8) | 162 (85.7%) |
| Live with bio mom (%) | 2205 (89.7) | 181 (95.8%) |
| Substance use (may include more than 1) | ||
| Ever had a drink (%) | 487 (19.8) | 34 (18.0) |
| Mean age first full drink (sd) | 13.4 (2.1) | 14.2 (1.9) |
| Ever intoxicated (%) | 296 (12.1) | 15 (7.9) |
| Age of first intoxication—mean (SD) | 14.1 (1.7) | 14.9 (1.3) |
| Ever used cannabis (%) | 376 (15.3) | 15 (7.9) |
| Mean age first cannabis use (sd) | 13.4 (1.8) | 13.8 (2.9) |
| Ever used tobacco (%) | 576 (23.4) | 28 (14.8) |
| Mean age first tob (sd) | 11.7 (2.7) | 12.6 (3.0) |
| Diagnosis met by either child or parent report: | ||
| ADHD | 209 (8.5) | 9 (4.7) |
| Oppositional defiant disorder | 147 (6.0) | 5 (2.6) |
| Conduct disorder | 193 (7.9) | 9 (4.7) |
| Separation anxiety disorder | 52 (2.1) | 2 (1.0) |
| Overanxious disorder | 92 (3.7) | 3 (1.6) |
| Depression | 299 (12.2) | 14 (7.4) |
| Social phobia | 41 (1.7) | 7 (3.7) |
| Suicide attempt | 64 (2.6) | 2 (1.0) |
Includes two participants from other family type.
Based on n = 2261 participants in Case, and 176 Comparison, families.
As indicated in Table 4, 43.4% of those in case and 36.5% of those in comparison families were newly assessed in Wave 2. There is considerable alcohol and other substance dependence in case families compared with comparison families, with prevalence estimates ranging from 3.4% to 44% (case), compared with 0.4% to 15.7% (comparison participants). Not unexpectedly, prevalence of other disorders in the externalizing domain, such as conduct disorder and antisocial personality disorder (ASPD), is also elevated among those in case families (17.4% and 12.5%, respectively), compared with comparison families (7.0% and 4.2%). As shown in Table 5, most of the children interviewed in Wave 2 were from case families (2457 vs. 189, 93%), were on average about 12 years old, and in middle school (6th–7th grade). Regardless of family status, most reported modest substance use. Externalizing comorbidity was elevated among the children in case families as compared with comparison families.*
2.3. Wave 3: The prospective study
In late 2004, the prospective study (2004–2019) was launched to evaluate younger members of the original COGA families who were then between the ages of 12 and 22 (adolescents/young adults). Some had been interviewed in earlier data collection waves, and all had at least one parent who was interviewed in one of the earlier waves of data collection. Participants were interviewed every 2 years, to study how alcohol use, problems, and related behaviors developed and changed over time. For 12‐year‐old children, a parent interview about the child was also obtained. Additional children, upon turning age 12, were invited to participate. The assessments administered in this wave were similar to those used in prior waves of data collection, but updated to target the youthful group being studied, such as asking about school behaviors, friendship groups and quality of relationships with parents (see Table 1). The measures also included neurophysiological and neuropsychological tests to assess neurocognitive performance, particularly frontal lobe function across development during this period (detailed in Table 1 under the column heading “prospective”). If not collected previously, blood samples for genotyping were obtained. Many of the participants were one or two generations younger than the original COGA probands (e.g., nephews and grandchildren), but all were drawn from the same family lines of the probands. Youth were also recruited from the original comparison families. Altogether, 3715 individuals participated in the prospective study and were assessed an average of 4 times. In Table 6, attributes of this sample at the baseline assessment are displayed, while lifetime characteristics, as reported at any assessment wave, are shown in Table 7.
TABLE 6.
Baseline characteristics of prospective study participants in third wave of data collection, by case and comparison families.
| Case families | Comparison/other families+ | |
|---|---|---|
| N | 3203 | 512 |
| Mean age (sd) | 16.2 (3.3) | 15.0 (3.2) |
| % female | 1633 (51.0%) | 270 (52.7%) |
| Race/ethnicity (self‐report) | ||
| Black | 978 (30.5) | 41 (8.0%) |
| White | 1936 (60.4) | 440 (85.9) |
| Hispanic | 410 (12.8%) | 35 (6.8%) |
| Education—highest years of education completed (mean, sd) | 9.9 (2.6) | 9.0 (2.7) |
| Still in school | 2626 (82.0%) | 466 (91.0%) |
| Reared by, ages 12–17: (n, %) | ||
| Both biological parents | 1546 (48.5) | 410 (80.2) |
| Bio mom | 1132 (35.5) | 60 (11.7) |
| Bio dad | 133 (4.2) | 13 (2.5) |
| Other rearing circumstances | 374 (11.7) | 28 (5.5) |
| Substance use (n, %) | ||
| Ever had a drink | 1592 (49.7) | 148 (28.9) |
| Mean age first drink (sd) | 15.0 (2.5) | 15.4 (2.6) |
| Ever intoxicated | 1238 (38.7) | 97 (19.0) |
| Mean age first intoxication (sd) | 15.8 (2.2) | 16.4 (2.1) |
| Ever had a cigarette | 915 (28.6) | 66 (12.9) |
| Smoked 100+ cigarettes | 581 (18.1) | 27 (5.3) |
| Ever used cannabis | 1147 (35.8) | 75 (14.6) |
| Mean age first cannabis use (sd) | 14.9 (2.5) | 15.5 (2.6) |
| Ever used cocaine | 218 (6.8) | 12 (2.3) |
| Ever used other stimulants | 146 (4.6) | 8 (1.6) |
| Ever used sedatives | 179 (5.6) | 12 (2.3) |
| Ever used opiates | 208 (6.5) | 9 (1.8) |
| DSM‐4 substance dependence (n, %) | ||
| Alcohol | 133 (4.2) | 7 (1.4) |
| Cannabis | 217 (6.8) | 10 (2.0) |
| Cocaine | 35 (1.1) | 1 (0.2) |
| Other stimulants | 41 (1.3) | 0 |
| Opiates | 20 (0.6) | 0 |
| Sedatives | 12 (0.4) | 1 (0.2) |
| Tobacco | 277 (8.6) | 13 (2.5) |
| Non substance diagnoses: (n, %) (DSM‐4) | ||
| Major depression | 473 (14.8) | 47 (9.1) |
| Panic | 32 (1.0) | 4 (0.8) |
| Agoraphobia | 39 (1.2) | 0 |
| Social phobia | 53 (1.7) | 5 (1.0) |
| Conduct disorder | 225 (7.0) | 17 (3.3) |
| ASPD | 120 (3.7) | 7 (1.4) |
| ADHD | 110 (3.4) | 11 (2.2) |
| ODD | 141 (4.4) | 7 (1.4) |
| OCD | 12 (0.4) | 0 |
| PTSD | 45 (1.4) | 3 (0.6) |
| Suicide attempt | 99 (3.1) | 10 (2.0) |
TABLE 7.
Prospective study participants: Lifetime characteristics based on summary assessment across all data collection waves.
| Case families | Comparison families | |
|---|---|---|
| 3203 | 512 | |
| Most recent age, mean age (sd) | 23.5 (5.5) | 22.5 (5.7) |
| Education—highest # years completed (mean, sd) | 12.8 (2.4) | 13.2 (2.9) |
| Mean # waves | 4.1, median = 4 | 4.4, median = 4 |
| Substance use (n, %) | ||
| Ever had a drink | 2794 (87.2) | 387 (75.6) |
| Mean age first drink (sd) | 15.7 (2.7) | 16.3 (2.6) |
| Ever intoxicated | 2533 (79.1) | 335 (65.4) |
| Mean age first intoxication (sd) | 16.7 (2.6) | 17.5 (2.2) |
| Smoked 100+ cigarettes | 1158 (36.2) | 89 (17.4) |
| Ever use cannabis | 2306 (72.0) | 258 (50.4) |
| Mean age first cannabis use (sd) | 16.0 (3.0) | 17.1 (2.8) |
| DSM‐4 Substance dependence (n, %) | ||
| Alcohol | 601 (18.8) | 45 (8.8) |
| Cannabis | 700 (21.9) | 49 (9.6) |
| Cocaine | 125 (3.9) | 4 (0.8) |
| Other stimulants | 105 (3.3) | 4 (0.8) |
| Opiates | 133 (4.2) | 6 (1.2) |
| Sedatives | 55 (1.7) | 6 (1.2) |
| Tobacco | 756 (23.6) | 46 (9.0) |
| Non substance diagnoses & suicidality (n, %) | ||
| Major depression | 1183 (37.0) | 144 (28.1) |
| Conduct disorder | 544 (17.0) | 34 (6.6) |
| ASPD | 392 (12.2) | 15 (2.9) |
| OCD | 53 (1.6) | 5 (2.9) |
| PTSD | 163 (5.1) | 8 (1.6) |
| Suicide attempt | 272 (8.5) | 26 (5.1) |
At baseline, participants from case families averaged about 16 years of age, with those from comparison families about 1 year younger. Evidence of having initiated substance use was abundant, and more common among those from case versus comparison families, across all types of substances. Alcohol was the most commonly used substance, with about 50% of those in case, compared with about 30% of those in comparison, families reporting ever having a drink. The most common substance use disorders were Alcohol and Cannabis dependence, with higher prevalence of both among participants from case versus comparison families; 4.2% and 6.8% of case family participants, compared with 1.4% and 2% of comparison family members, met diagnostic criteria for alcohol and cannabis dependence, respectively. Likewise, prevalence estimates of non‐substance externalizing disorders (Conduct Disorder, ADHD, and ODD) were higher among participants from case families compared with their counterparts from comparison families.
As shown in Table 7, by the time data collection had ended in 2019, the paths of the participants from case families continued to diverge from those of their counterparts from comparison families in numerous domains. This was observed in the substance use realm, where across all types of substances, use began at much younger ages, and prevalence estimates for disorders were 1.4 to over 4 times higher among those in case compared with comparison families. Excess prevalence in participants in case compared with comparison families was also observed for major depression, conduct disorder, and ASPD.
2.4. Focus on older individuals with AUD: One‐year study of those age 50+ with AUD
In 2016–2017, we conducted a one‐year study of 2174 participants at least 50 years of age (born 1966 or earlier) who met lifetime criteria for DSM‐IV Alcohol Dependence. On average, these individuals had last participated in COGA 22 years earlier. 14 The main goal of the pilot study was to show recruitment feasibility given the long interval between contacts. Participants were interviewed by telephone with a brief, nondiagnostic assessment covering their current living situation, physical health, and current alcohol consumption and problems. In the brief one‐year recruitment, 706 were interviewed, 524 were found to be deceased, 28 were unable to be interviewed, and 55 refused; with 861 remaining to be recruited at a later time. Of those interviewed, 99% agreed to be contacted again in the future, and that encouraging finding led to the design of a more extensive investigation of older COGA individuals, ushering in the lifespan study.
2.5. Wave 4: The lifespan study
In 2019, we began conducting the current lifespan study of COGA, focused on two groups of prior COGA participants. The first consists of individuals from the Prospective Study who would range in age from 30 to 40 by 2023 (born between 1982 and 1993). This sample was chosen due to the lack of studies that have focused on alcohol use and problems in this particular midlife period. The second group is composed of participants who are currently (or will be by the end of 2023) age 50 or older (born before 1974). We are recruiting them now to increase understanding of alcohol use, problems, and disorder during the later life age period which has not been extensively studied. For both groups, the unified protocol includes the SSAGA interview, questionnaires, and in‐person laboratory‐based neuropsychological and neurophysiological assessments (see Table 1; and also details in the companion paper 3. Brain function). As has been true throughout COGA, the assessment procedures, in addition to being updated when appropriate, maintain continuity with prior evaluations.
The COVID‐19 pandemic required changes to the COGA protocol to ensure the health and safety of research participants and staff. These changes included the temporary suspension of all face‐to‐face evaluations in March 2020, with interviews conducted remotely and questionnaires filled out either via mailed paper versions or online via smartphone, computer, or tablet at a secure website. At several sites, in‐person laboratory testing (neurophysiological and computerized neuropsychological assessment batteries of cognition and emotion) resumed during periods when local infection rates were acceptably low. Interviews and questionnaires continued to be administered remotely. Beginning in August 2022, all sites eventually deemed it sufficiently safe, with appropriate screening and safety protocols in place, to resume in‐person assessment.
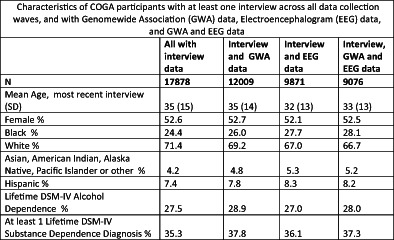
In summary, across all data collection waves and case and comparison families, in the 30+ years of the COGA project, 17,878 individuals have been assessed at least once with a comprehensive protocol including interviews and questionnaires that capture behaviors, psychosocial characteristics, family background, life course experiences and outcomes from both lifetime and current perspectives. A snapshot of their characteristics derived from the deep phenotyping in COGA is provided in Table 8. In addition, the table displays attributes of several key subsets of the entire COGA sample that have been the focus of intense study by COGA. These include the GWAS sample (n = 12,009), discussed in greater detail elsewhere in this issue (see 4. Genetics); the sample of individuals who have completed at least one electrophysiological protocol administered during COGA's course (n = 9871) and who are the focus of the companion paper in this issue (see 3. Brain function); and lastly those participants with genetic, neurophysiologic and comprehensive phenotypic data. The sample is middle‐aged, with slightly more female participants, notably diverse with 25%–28% from Black race/ethnic groups and 7%–8% from participants reporting Hispanic background. Over one third (35%–37%) meet criteria for DSM‐IV substance dependence on one or more substances, and Alcohol Dependence that is comorbid with other drug dependence is the most common pattern. Non‐substance disorders are common, particularly major depression, and both conduct disorder and antisocial personality disorder. The brief overview establishes the COGA sample as well‐positioned for continuing on its original path set over 30 years ago to investigate the multifaceted underpinnings and course of AUD, other addictions and related psychiatric disorders.
TABLE 8.
Overall table: Characteristics of all participants, those with GWAS data, those with EEG data and those with both GWAS and EEG data.
| ALL | GWAS | EEG | GWAS & EEG | |
|---|---|---|---|---|
| N | 17,878 | 12,009 | 9871 | 9076 |
| Mean age, most recent interview (SD) | 35 (15) | 35 (14) | 32 (13) | 33 (33) |
| Female % | 52.6 | 52.7 | 52.1 | 52.5 |
| Race/ethnicity (self‐report) | ||||
| Black % | 24.4 | 26.0 | 27.7 | 28.1 |
| Hispanic % | 7.4 | 7.8 | 8.3 | 8.2 |
| Highest # yrs of education | ||||
| Mean (SD) | 13 (3) | 13 (3) | 13 (3) | 13 (3) |
| Mean number of assessments (SD) | 2.0 (1.5) | 2.3 (1.7) | 2.4 (1.8) | 2.5 (1.8) |
| % with GWAS | 12,009 (67.2) | – | 9076 (91.9) | – |
| % with EEG | 9871 (55.2) | 9076 (75.6) | – | – |
| Lifetime DSM‐IV dependence (%) | ||||
| Alcohol | 27.5 | 28.9 | 27.0 | 28.0 |
| Cannabis | 15.3 | 17.2 | 17.3 | 17.8 |
| Cocaine | 11.1 | 11.7 | 11.0 | 11.4 |
| Other stimulants | 5.2 | 5.4 | 4.6 | 4.8 |
| Opiates | 3.9 | 4.2 | 3.7 | 3.9 |
| Sedatives | 2.6 | 2.6 | 2.2 | 2.3 |
| Tobacco—smoked daily for month or more | 51.7 | 51.6 | 47.2 | 48.8 |
| Comorbidity among DSM‐IV substance dependence disorders | ||||
| Alcohol dependence only | 12.4 | 12.7 | 11.8 | 12.2 |
| Alcohol dependence + Cannabis Dep | 3.4 | 3.8 | 3.9 | 4.0 |
| Alcohol Dependence + other noncannabis drug dependence | 5.3 | 5.4 | 4.8 | 4.9 |
| Alcohol + Cannabis + Other drug dependence | 6.4 | 6.9 | 6.5 | 6.8 |
| Cannabis dependence only | 4.1 | 4.9 | 5.3 | 5.4 |
| Cannabis + Other drug dependence (no alcohol) | 1.4 | 1.6 | 1.6 | 1.6 |
| Other drug dependence only (no alcohol or cannabis dependence) | 2.3 | 2.4 | 2.3 | 2.4 |
| At least 1 dependence disorder | 35.3 | 37.8 | 36.1 | 37.3 |
| No dependence | 64.7 | 62.2 | 63.9 | 62.7 |
| Non substance DSM‐IV disorders (%): | ||||
| Major depression | 32.5 | 34.4 | 32.8 | 33.5 |
| Panic | 3.6 | 3.8 | 3.5 | 3.5 |
| Agoraphobia | 2.8 | 3.0 | 2.6 | 2.6 |
| Social phobia | 4.1 | 4.7 | 4.4 | 4.5 |
| Conduct | 16.4 | 18.3 | 17.6 | 18.1 |
| ASPD | 11.9 | 13.7 | 13.0 | 13.5 |
Abbreviations: EEG, electroencephalogram data; GWAS, genomewide association study data.
3. PART 2: EXAMPLES OF THE QUESTIONS ADDRESSED WITH COGA DATA
With these rich, cross‐sectional and longitudinal data, collected within families over decades, a number of critical questions about the causes, consequences, and life course of alcohol use problems and related disorders have been addressed, and more can be studied. Below we provide illustrative examples of areas where COGA data has made substantive contributions to a deeper understanding of the AUD phenotype, its comorbidity with other addictions and with psychiatric disorders, and of the impact of the interplay of genetic and environmental influences on alcohol and other substance use outcomes. We note that COGA data and instruments are made available to any qualified investigator and can be accessed through a variety of routes, including directly from NIAAA, through direct collaboration with COGA investigators, and through dbGAP; details about each of these options are provided at https://cogastudy.org/resources-for-researchers/ with additional details in the companion paper 1. Overview. We welcome collaborations with outside investigators; indeed, some of COGA's most impactful papers have arisen through collaborations with the global community of researchers, such as the Psychiatric Genomics Consortium, 15 , 16 , 17 , 18 , 19 , 20 Externalizing Consortium, 21 and INIA. 22 A complete list of COGA publications can be found at cogastudy.org. COGA is also invested in supporting the development of early career investigators (see 1. Overview) and we encourage the participation of junior faculty in COGA; many of the studies detailed below have been initiated by junior investigators on the project.
3.1. Familial characterization of AUD and associated comorbidity
The cross‐sectional, family‐based data gathered early in COGA yielded a rich series of investigations exploring in‐depth characterizations of the AUD phenotype (subtypes, withdrawal, and time course), familial aggregation of substance use disorders, comorbidity with other substance dependences and with non‐substance disorders like depression, anxiety, eating disorders, and suicidality, to name a few. Among the array of published findings based on COGA data, we highlight several here in acknowledgement of their contributions to some key research questions. An analysis of the large array of alcohol problems collected in Wave 1 contributed to the nosological debate on dimensional versus categorical representations of substance use disorder, finding strong evidence in the full COGA sample that the disorder was best conceptualized on a severity dimension rather than by categories of specific symptoms. 23 Replicating results from a large Australian twin sample, 24 this finding has also been observed in numerous subsequent studies across general population, collegiate and treatment samples. 25 , 26 , 27 In another COGA study, analysis of the early family data led to observations of both common and specific evidence for familial transmission of alcohol, marijuana, and cocaine dependence and habitual smoking, 28 a finding confirmed in later COGA investigations, 29 as well as in a number of independent twin‐family samples analyzed by other researchers. 30 , 31 In another later analysis of the completed Wave 1 data collected on over 9000 probands, their relatives, and comparison participants, we reported a two‐fold excess risk for AUD in relatives of probands with AUD, and observed strong co‐aggregation with antisocial personality disorder, other drug dependence, and anxiety and mood disorders. 3 Similar co‐aggregation of AUD and other externalizing disorders has been documented in independent twin‐family data by other researchers. 32 COGA also found that the presence of a physiological dependence component to alcohol and other substance use disorders implicated a more severe clinical course, that held for alcohol as well as other substances. 33 Other analyses investigated evidence in COGA of a previously established AUD subtype, Type A‐B clusters, 34 finding that among affected individuals in COGA, 5 items usefully distinguished the two types with positive clinical applications. 35 Altogether, data on in‐depth characterization of a broad range of alcohol and other substance misuse problems, as well as other psychopathology, obtained in the members of the COGA families, have supported numerous analyses in the presentation and familial aggregation of alcohol problems. In this area, COGA not only has provided evidence to support past findings by reproduction of results from prior studies, but also has contributed fresh insights into key issues in AUD nosology and familial transmission that have served as a catalyst for further investigations in other data sources by other researchers.
3.2. Developmental effects
AUD is, by its very nature, a developmental disorder, which unfolds in a series of stages, from initiation, to regular use, to the development of problems, well argued in the literature to date. 36 , 37 , 38 Further, there is often a pattern of risk‐related behavior that precedes the onset of alcohol use. 39 , 40 Each of the stages of alcohol use is influenced by genetic factors, 38 , 41 , 42 , 43 as are the premorbid behaviors that comprise the spectrum of preaddiction risk. 44 , 45 , 46 , 47
With the comprehensive and multimodal longitudinal data available in COGA, we can characterize the pathways by which genetic and environmental influences on alcohol‐related problems unfold. Consistent with earlier studies in the literature, 48 , 49 we found that genetic risk for alcohol problems manifests in adolescence and early adulthood as a constellation of clinical behaviors related to impulsivity and behavioral undercontrol, often called the externalizing spectrum. 21 , 50 , 51 , 52 , 53 , 54 These childhood behavior problems 42 and traits related to impulsivity, such as sensation‐seeking, reflect early manifestations of genetic risk for alcohol and other substance use problems. 55 , 56 , 57 With the greater availability of alcohol as individuals age from later adolescence to emerging adulthood, these same genetic influences begin to impact patterns of alcohol use, 58 the rate of escalation in hazardous use, 59 and the development of problems. 42 Measures of the intensity of response to alcohol are additional early indicators of genetic risk for alcohol problems, and include both a low level of response seen at peak and falling blood alcohol levels and higher stimulation observed at rising blood levels. 60 , 61 , 62 , 63 Beginning in 1989, longitudinal studies in COGA focused on the low response across several generations of participants. 61 , 64 Not only do impulsivity and low level of response to alcohol directly influence risk, they also appear to indirectly amplify risk via environmental pathways and attitudes, through the selection of riskier peers, positive alcohol expectancies, and increased drinking to cope with stress. 64 , 65 , 66 Additional insights into the factors that underlie the transitions in alcohol involvement from use to disorder have been provided by COGA's collection of electrophysiological indicators of brain function across development, as discussed in greater detail in the companion article in this volume (see 3. Brain function).
Other COGA analyses have mapped specific risk factors that impact transitions in alcohol involvement from use, to problems, to disorder. Cannabis use, substance‐using peers, externalizing behaviors, and parental history of AUD all increase the likelihood of each stage of alcohol involvement. 67 Nonassaultive trauma is related to early initiation of use, and internalizing disorders such as depression are associated with later stages. 67 We have showed that, consistent with Koob's model of the progression of the addiction cycle, 68 positive reinforcement motives for drinking (e.g., social enhancement) are related to alcohol consumption earlier in the drinking career, whereas negative reinforcement (e.g., drinking to reduce anxiety) predominates once an individual develops AUD. 69 We also showed that alcohol problems cluster and show reciprocal relationships with other disorders and behaviors related to externalizing and internalizing across the lifespan, including suicide attempts and ideation, 70 , 71 , 72 consistent with other studies in the field. 73 , 74 , 75
Finally, in our latest round of follow‐ups of COGA participants approximately 20 years after they were assessed in midlife, we are studying predictors and consequences of alcohol use in later life. 14 Data on outcomes obtained from the brief assessment of older COGA participants with a history of AUD showed that nearly 60% had good outcomes, including 41% abstinent and problem free in the 5 years preceding the follow‐up, and 15% were low risk, nonproblem drinkers. 14 However, roughly 30% were current problem drinkers, and another 14% reported high risk drinking. We have also characterized changing patterns of treatment service use for alcohol problems across generations. 76 In the full COGA sample, individuals with AUDs were classified into generations based on their birth year (1928–45, 1946–64, 1965–1980, and 1981–1996), and we found that those in the younger generations used services earlier than their counterparts in older generations, and women across all generations were less likely to use services. 76
3.3. Trajectories of use—Initiation, onset, offset, and predictors of patterns across time
The richness of the COGA longitudinal data allows characterization of drinking milestones, transitions across stage of AUD development, and patterns of use overtime. Consistent with earlier studies in the literature, 77 , 78 , 79 we have shown that age of first drink of alcohol is best predicted by multiple factors. In a multivariate model, we found that best friend alcohol use, a positive family history of having any adult family member in treatment for alcohol dependence, conduct disorder symptoms, and youth externalizing behavioral problems were associated with higher likelihood of earlier alcohol initiation, whereas youth social problems (e.g., friendship difficulties and loneliness) were associated with reduced likelihood of earlier alcohol initiation, underscoring the social component of youth alcohol initiation. 80 In addition, leveraging the multigenerational family‐based data, our team has shown that parental AUDs are associated not only with offspring initiation of alcohol use, but also with other risky behaviors, including offspring early involvement with cigarettes and cannabis use, and age at first consensual sexual intercourse. 81 Parental divorce/separation exerted additional effects on the early initiation of alcohol and substance use, with the effect size comparable to that of having two AUD‐affected parents. 81 Using COGA's longitudinal electrophysiological data, we have also found that the age of onset of AUD in adolescents and young adults is associated with both genetic and neurophysiological factors, most strongly among those who used substances before the age of 16 82 (see 3. Brain function).
Other COGA analyses have explored heterogeneity in drinking patterns and predictors across time. For example, using latent class growth analysis, we identified four distinct subgroups of trajectories of alcohol intake, characterized by maximum drinks per occasion, across adolescence and young adulthood in this high‐risk sample: 64 consistent low drinking, low to high drinking; high to low drinking; and consistent high drinking. This pattern of identified subgroups is consistent with the literature of trajectories of alcohol use across adolescence and young adulthood. 83 , 84 Group membership was predicted by environmentally‐influenced factors (demographics and age of first drink) and genetically‐influenced characteristics (sensitivity to alcohol, externalizing behaviors, and personality), that operate together to influence patterns of alcohol use over time. In another study, our team found that after controlling for parent's knowledge of youth's whereabouts and youth's perceived substance use of school peers, close friend substance use was associated with a higher initial heavy drinking status and a greater rate of increase in heavy episodic drinking during the transition between adolescence and young adulthood. 85
3.4. Intergenerational transmission of risk
The genetically‐informative multigenerational COGA sample, which includes direct assessments alongside information about one's home environment while growing up, can illuminate the mechanisms through which risk for AUD and related disorders is transmitted across generations. For example, consistent with the idea of genetic nurture (a parent's genotype shapes the environment they provide for their children) that others in the field have documented, 86 , 87 , 88 we found that even the risk‐increasing alleles for alcohol problems that children did not inherit from their parents (i.e., the nontransmitted alleles) were associated with earlier age at initiation, first intoxication and a greater number of lifetime AUD criteria in offspring. 89 In another example of genetic nurture, we found that parental externalizing polygenic scores (PGS) were associated with adolescent externalizing behavior over and above the effect of offspring's own externalizing polygenic scores, and that these associations were mediated by parental externalizing psychopathology. 90
COGA has also investigated why some individuals from high‐risk family environments are resistant to AUD development. Drawing on the notion of “addiction resistance” proposed by others, 91 , 92 we took advantage of the high risk family design and deep longitudinal assessments which positioned COGA well for exploring this issue. In one study using COGA data from the prospective study, the only factor found to promote resistance to initiation of alcohol use was high quality of the child's paternal relationship. 93 In contrast, other adolescent social relationship factors, including those involving parenting, peer, and romantic partners, were not associated with resistance to initiating drinking or heavy episodic drinking, or developing AUD. 93 This pattern of largely null effects highlights how little is known regarding potential resistance factors and processes among individuals with high familial and genetic risk for AUD. 91 , 92
3.5. Gene–environment interplay
The depth of phenotypic and environmental information collected on participants makes it possible to study environmental as well as genetic influences on alcohol use outcomes. Importantly, this enables us to study the complex ways in which genetic and environmental risk are intertwined via gene–environment interaction and correlation processes. 57 , 94 , 95 It has become clear that genetic influences impact alcohol use outcomes in part by disrupting the family system. For example, genetic risk contributes to hazardous adolescent drinking by adversely impacting parent–child relationships and positive parenting practices, 96 parent–child closeness and parental monitoring, 97 and contributing to lower levels of family support, 98 as has also been found in other samples. 99 , 100 , 101 , 102
Peer influences are also closely intertwined with genetic risk. We have found that individuals with higher externalizing genetic risk 21 show increased affiliation with substance‐using peers, in addition to decreased parental monitoring, which then increases future externalizing behavior. 97 Further, parental monitoring of their adolescent offspring along with perceived peer substance use have been found to moderate the association between polygenic scores and externalizing disorders, whereby risky peer groups and reduced parental monitoring exacerbate genetic risk. 103 Conversely, high friend support has been found to attenuate the association between genetic risk, sensation seeking and alcohol use. 98
Romantic relationships are another important context impacting and affected by alcohol use outcomes. We have found that AUD and other substance use problems are associated with a reduced likelihood of marriage and higher risk of divorce in our COGA families. 104 Further, among those who marry young (average age 21), individuals with higher genetic risk for alcohol consumption report more heavy episodic drinking, suggesting that early marriage may exacerbate risk for those with higher polygenic load. 105 These findings are in line with other studies that have found environments that are more restrictive tend to reduce genetic influences, whereas environments that promote greater availability or acceptance of substance use increase the importance of genetic effects. 94 , 106 , 107
3.6. Remission and recovery
A by‐product of the high‐risk, familial structure of the COGA sample, with its elevated rates of AUD, is an increased number of participants who enter remission from AUD compared with the general population. Thus, the search for the genetics that underpin AUDs has produced a sample that is well‐positioned to examine genetic, environmental, and familial influences on remission, an advancement over self‐selected samples. 108 , 109 The genetic and brain function data available from COGA participants can contribute to the evolving field of recovery science, which to date has been based largely on self‐selected samples of individuals who consider themselves to be in recovery but which lacks objective measures of recovery and biomarkers. 110
Of the COGA participants who develop an AUD, a majority have periods of abstinence from alcohol lasting from 3 months to more than 5 years. 111 Individuals with periods of abstinence tend to have more severe histories of AUD, characterized by a younger age at onset, a greater number of symptoms, and co‐occurring drug use disorders. They are also more likely to have accessed professional treatment and attended alcoholics anonymous. 111 , 112 In contrast to abstainers, individuals who no longer have symptoms of AUD but continue drinking at low‐risk levels have fewer lifetime AUD symptoms and are less likely to access treatment. Overall, among those who have met criteria for AUD, abstainers in COGA resemble clinical samples in their severity of AUD and high rates of treatment, and low‐risk drinkers resemble “natural remitters” from population‐based samples in their lower severity and lack of treatment. 113 , 114
There appears to be a familial influence on abstinent remission among 1st‐degree relatives in COGA. Individuals with AUD who are related to a proband who becomes abstinent are more than 3 times as likely to be abstinent themselves compared with individuals related to a proband with persistent AUD. 115 This suggests that remission might cluster in families, but does not clarify the mechanisms—genetic, environment, or a combination—by which familial influences are transmitted. These findings stand in contrast to a lack of evidence for an association between family history of AUD and probability of remission in a variety of studies. 116 , 117 , 118 , 119 , 120 , 121 The innovation made possible by the high‐risk family‐based COGA sample is the ability to examine how remission in one family member relates to remission in others, rather than testing AUD‐remission associations.
Machine learning models using multiple phenotypes—PGS, neurophysiological information, and behavioral data—have previously been used to identify risk for AUD development. 122 , 123 , 124 When applied to remission and recovery, these models hinted at some improvement in accuracy in predicting remission in COGA participants. 125 Specific patterns in EEG‐derived brain network functional connectivity consistently predicted remission across sex and race/ethnic groups, but the addition of a number of PGS related to personality traits, aggression, depression, and alcohol use, along with marital status, medication use, and employment status, further improved prediction, in particular among sex‐ and race/ethnicity‐defined subgroups. 125 This work highlighted the utility of incorporating a wide array of measured indicators, including polygenic, electrophysiological, and psychosocial domains, in the construction of predictive models of remission from AUD.
Data from young adult participants in the Prospective Study have also been used to construct a measure of social recovery capital that reflects the composition of an individual's social network with respect to their drinking habits and support for drinking or abstinence. 126 Social recovery capital influences the development of alcohol problems and recovery from them. While there are other measures of recovery capital, 127 , 128 , 129 none have been collected in samples that include the extensive genetic, physiological, and behavioral measures that are available in COGA data, making this measure valuable for its ability to examine recovery capital in conjunction with biological and psychological factors.
3.7. Risk prediction and contributions to precision medicine
The promise of genetics is that understanding and characterizing how genetic influences impact alcohol use outcomes will lead to improved prevention, intervention, and treatment. 130 Our efforts to map the pathways by which genetic factors influence alcohol use outcomes via intermediary behaviors and environmental factors have already led to the development of novel intervention approaches aimed at early indicators of risk, such as level of response to alcohol 66 , 131 , 132 and externalizing and internalizing characteristics. 133 These interventions target genetically‐influenced pathways of risk and can be tailored to an individual's risk profile.
As our gene identification efforts continue to evolve, COGA has initiated efforts to combine genetic information with known environmental risk factors to predict risk for substance use disorders in clinical and population‐based samples. 134 , 135 Although these are not yet ready for integration into clinical practice, we have begun to lay the groundwork for the utilization of genetic risk information by reviewing the current status of genetic feedback for psychiatric conditions, 136 studying public interest in receiving personalized genetic risk scores, 137 and examining how we can return complex risk information in ways that will promote healthy behavior. 138
It will be critical to carefully consider the benefits and potential harm that could result from the incorporation of genetic risk information into clinical care. 139 To enhance public understanding of the complex ways in which genes influence risk for alcohol use outcomes, COGA investigators have built out resources for the public, available at cogastudy.org. Through videos, graphics, and text, we explain core concepts necessary to understand complex genetics, such as heritability and genome‐wide association studies. Additionally, we have translated key findings from the field into videos and graphical elements in order to make research findings more accessible and engaging to lay audiences. We believe that these tools for enhancing public understanding of complex genetic concepts will help lay the foundation for precision medicine.
4. SUMMARY AND FUTURE DIRECTIONS
The rich longitudinal family‐based data collected by COGA over the last 30 years provides a national resource for the field. With clinical interviews, surveys, environmental information, electrophysiological phenotypes, and genotypic data collected from >17,000 individuals from multi‐generational families, COGA enables a wealth of important questions about the onset, developmental course, and consequences of AUD. The genomic data has allowed COGA to make substantive, leading contributions to gene identification for AUD and related substance use and psychiatric conditions (see 4. Genetics). Further, the longitudinal electrophysiological assessments enable study of the neural development that both precedes and follows alcohol use and problems (see 3. Brain function). Integrating these data with our lifespan clinical and environmental assessments has allowed us to characterize the pathways and mechanisms by which risk unfolds and remission takes place, and to translate these findings into personalized prevention and intervention studies (see 1. Overview). The evolving nature of the sample allows us to continually address new questions of interest to the field. As COGA participants age into mid‐ and later‐life, it provides a valuable opportunity to study the consequences of alcohol use across the lifespan. In summary, the COGA study has made substantive contributions to understanding why some individuals are more at risk of developing AUD than others, and as the sample continues to age and evolve, its value only continues to grow.
FUNDING INFORMATION
This national collaborative study is supported by NIH Grant U10AA008401 from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) and the National Institute on Drug Abuse (NIDA).
CONFLICT OF INTEREST STATEMENT
Dr. Laura Bierut is listed as an inventor on a patent covering the use of certain SNPs in the diagnosis, prognosis, and treatment of addiction.
ACKNOWLEDGEMENTS
We are indebted to Lingwei Sun for her meticulous assistance with data management and analysis of the COGA samples in the preparation of this article. We would also like to express our deepest gratitude to Dr Michie Hesselbrock for her many contributions to COGA since its inception. She has been a generous and invaluable scientific colleague.
Authors listed by name directly contributed to the writing of this manuscript; however, the COGA project is made possible by a large interdisciplinary team: The Collaborative Study on the Genetics of Alcoholism (COGA), Principal Investigators B. Porjesz, V. Hesselbrock, T. Foroud; Scientific Director, A. Agrawal; Translational Director, D. Dick, includes 10 different centers: University of Connecticut (V. Hesselbrock); Indiana University (H.J. Edenberg, T. Foroud, Y. Liu, M.H. Plawecki); University of Iowa Carver College of Medicine (S. Kuperman, J. Kramer); SUNY Downstate Health Sciences University (B. Porjesz, J. Meyers, C. Kamarajan, A. Pandey); Washington University in St. Louis (L. Bierut, J. Rice, K. Bucholz, A. Agrawal); University of California at San Diego (M. Schuckit); Rutgers University (J. Tischfield, D. Dick, R. Hart, J. Salvatore); The Children's Hospital of Philadelphia, University of Pennsylvania (L. Almasy); Icahn School of Medicine at Mount Sinai (A. Goate, P. Slesinger); and Howard University (D. Scott). Other COGA collaborators include: L. Bauer (University of Connecticut); J. Nurnberger, L. Wetherill, X., Xuei, D. Lai, S. O'Connor, (Indiana University); G. Chan (University of Iowa; University of Connecticut); D.B. Chorlian, J. Zhang, P. Barr, S. Kinreich, G. Pandey (SUNY Downstate); N. Mullins (Icahn School of Medicine at Mount Sinai); A. Anokhin, S. Hartz, E. Johnson, V. McCutcheon, S. Saccone (Washington University); J. Moore, F. Aliev, Z. Pang, S. Kuo (Rutgers University); A. Merikangas (The Children's Hospital of Philadelphia and University of Pennsylvania); H. Chin and A. Parsian are the NIAAA Staff Collaborators. We continue to be inspired by our memories of Henri Begleiter and Theodore Reich, founding PI and Co‐PI of COGA, and also owe a debt of gratitude to other past organizers of COGA, including Ting‐Kai Li, P. Michael Conneally, Raymond Crowe, and Wendy Reich, for their critical contributions.
Dick DM, Balcke E, McCutcheon V, et al. The collaborative study on the genetics of alcoholism: Sample and clinical data. Genes, Brain and Behavior. 2023;22(5):e12860. doi: 10.1111/gbb.12860
This is the second paper a in a linked series of reviews
The other papers in the series are: 1. The Collaborative Study on the Genetics of Alcoholism: Overview. 3. The Collaborative Study on the Genetics of Alcoholism: Brain Function. 4. The Collaborative Study on the Genetics of Alcoholism: Genetics. 5. The Collaborative Study on the Genetics of Alcoholism: Functional Genomics.
Samuel Kuperman, John Kramer, and Kathleen Bucholz shared last authorship.
ENDNOTE
From 2003 to 2005, a small set of individuals from Waves 1 and 2 was followed up approximately 5 years after their second assessment. This included 200 adults, as well as 272 adolescents and children.
Contributor Information
Danielle M. Dick, Email: Danielle.m.dick@rutgers.edu.
Kathleen Bucholz, Email: bucholzkk@wustl.edu.
COGA Collaborators:
B. Porjesz, V. Hesselbrock, T. Foroud, A. Agrawal, D. Dick, V. Hesselbrock, H.J. Edenberg, T. Foroud, Y. Liu, M.H. Plawecki, S. Kuperman, J. Kramer, B. Porjesz, J. Meyers, C. Kamarajan, A. Pandey, L. Bierut, J. Rice, K. Bucholz, A. Agrawal, M. Schuckit, J. Tischfield, D. Dick, R. Hart, J. Salvatore, L. Almasy, A. Goate, P. Slesinger, D. Scott, L. Bauer, J. Nurnberger, L. Wetherill, X. Xuei, D. Lai, S. O'Connor, G. Chan, D.B. Chorlian, J. Zhang, P. Barr, S. Kinreich, G. Pandey, N. Mullins, A. Anokhin, S. Hartz, E. Johnson, V. McCutcheon, S. Saccone, J. Moore, F. Aliev, Z. Pang, S. Kuo, A. Merikangas, H. Chin, Ting‐Kai Li, P. Michael Conneally, Raymond Crowe, and Wendy Reich
DATA AVAILABILITY STATEMENT
COGA data are available in dbGaP (phs000125, phs000763, phs000976, phs001208), or via an application to the National Institute on Alcohol Abuse and Alcoholism (https://www.niaaa.nih.gov/research/major-initiatives/collaborative-studies-genetics-alcoholism-coga-study), or a COGA investigator sponsored secondary analysis proposal.
REFERENCES
- 1. Begleiter H, Reich T, Hesselbrock V, et al. The collaborative study on the genetics of alcoholism. Alcohol Health Res World. 1995;19:228. [PMC free article] [PubMed] [Google Scholar]
- 2. Reich T, Edenberg HJ, Goate A, et al. Genome‐wide search for genes affecting the risk for alcohol dependence. Am J Med Genet. 1998;81(3):207‐215. [PubMed] [Google Scholar]
- 3. Nurnberger JI Jr, Wiegand R, Bucholz K, et al. A family study of alcohol dependence: coaggregation of multiple disorders in relatives of alcohol‐dependent probands. Arch Gen Psychiatry. 2004;61(12):1246‐1256. doi: 10.1001/archpsyc.61.12.1246 [DOI] [PubMed] [Google Scholar]
- 4. Bucholz KK, Nurnberger JI Jr, Kramer JR, Hesselbrock VM, Schuckit MA, Bierut LJ. Comparison of psychiatric diagnoses from interview reports with those from best‐estimate procedures. J Stud Alcohol. 2006;67(1):157‐168. doi: 10.15288/jsa.2006.67.157 [DOI] [PubMed] [Google Scholar]
- 5. Bucholz KK, Cadoret R, Cloninger CR, et al. A new, semi‐structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55(2):149‐158. doi: 10.15288/jsa.1994.55.149 [DOI] [PubMed] [Google Scholar]
- 6. Hesselbrock M, Easton C, Bucholz KK, Schuckit M, Hesselbrock V. A validity study of the SSAGA: a comparison with the SCAN. Addiction. 1999;94(9):1361‐1370. doi: 10.1046/j.1360-0443.1999.94913618.x [DOI] [PubMed] [Google Scholar]
- 7. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 3rd Ed., Revised ed. The American Psychiatric Association; 1987. [Google Scholar]
- 8. Feighner JP, Robins E, Guze SB, Woodruff RA, Winokur G, Munoz R. Diagnostic criteria for use in psychiatric research. Arch Gen Psychiatry. 1972;26(1):57‐63. doi: 10.1001/archpsyc.1972.01750190059011 [DOI] [PubMed] [Google Scholar]
- 9. Kuperman S, Schlosser SS, Lidral J, Reich W. Relationship of child psychopathology to parental alcoholism and antisocial personality disorder. J Am Acad Child Adolesc Psychiatry. 1999;38(6):686‐692. doi: 10.1097/00004583-199906000-00015 [DOI] [PubMed] [Google Scholar]
- 10. Rice JP, Reich T, Bucholz KK, et al. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcohol Clin Exp Res. 1995;19(4):1018‐1023. doi: 10.1111/j.1530-0277.1995.tb00983.x [DOI] [PubMed] [Google Scholar]
- 11. Kramer JR, Chan G, Kuperman S, et al. A comparison of diagnoses obtained from in‐person and telephone interviews, using the semi‐structured assessment for the genetics of alcoholism (SSAGA). J Stud Alcohol Drugs. 2009;70(4):623‐627. doi: 10.15288/jsad.2009.70.623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Culverhouse R, Bucholz KK, Crowe RR, et al. Long‐term stability of alcohol and other substance dependence diagnoses and habitual smoking: an evaluation after 5 years. Arch Gen Psychiatry. 2005;62(7):753‐760. doi: 10.1001/archpsyc.62.7.753 [DOI] [PubMed] [Google Scholar]
- 13. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. The American Psychiatric Association; 1994. [Google Scholar]
- 14. Schuckit MA, Smith TL, Danko G, et al. A 22‐year follow‐up (range 16–23) of original subjects with baseline alcohol use disorders from the collaborative study on genetics of alcoholism. Alcohol Clin Exp Res. 2018;42(9):1704‐1714. doi: 10.1111/acer.13810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Walters RK, Polimanti R, Johnson EC, et al. Transancestral GWAS of alcohol dependence reveals common genetic underpinnings with psychiatric disorders. Nat Neurosci. 2018;21(12):1656‐1669. doi: 10.1038/s41593-018-0275-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Deak JD, Zhou H, Galimberti M, et al. Genome‐wide association study in individuals of European and African ancestry and multi‐trait analysis of opioid use disorder identifies 19 independent genome‐wide significant risk loci. Mol Psychiatry. 2022;27(10):3970‐3979. doi: 10.1038/s41380-022-01709-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gaddis N, Mathur R, Marks J, et al. Multi‐trait genome‐wide association study of opioid addiction: OPRM1 and beyond. Sci Rep. 2022;12(1):16873. doi: 10.1038/s41598-022-21003-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Johnson EC, Demontis D, Thorgeirsson TE, et al. A large‐scale genome‐wide association study meta‐analysis of cannabis use disorder. Lancet Psychiatry. 2020;7(12):1032‐1045. doi: 10.1016/s2215-0366(20)30339-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bountress KE, Brick LA, Sheerin C, et al. Alcohol use and alcohol use disorder differ in their genetic relationships with PTSD: a genomic structural equation modelling approach. Drug Alcohol Depend. 2022;234:109430. doi: 10.1016/j.drugalcdep.2022.109430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bountress KE, Bustamante D, Subbie‐Saenz de Viteri S, et al. Differences in genetic correlations between posttraumatic stress disorder and alcohol‐related problems phenotypes compared to alcohol consumption‐related phenotypes. Psychol Med. 2022;1‐11. Online ahead of print. doi: 10.1017/s0033291722002999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Karlsson Linnér R, Mallard TT, Barr PB, et al. Multivariate analysis of 1.5 million people identifies genetic associations with traits related to self‐regulation and addiction. Nat Neurosci. 2021;24(10):1367‐1376. doi: 10.1038/s41593-021-00908-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kapoor M, Wang JC, Farris SP, et al. Analysis of whole genome‐transcriptomic organization in brain to identify genes associated with alcoholism. Transl Psychiatry. 2019;9(1):89. doi: 10.1038/s41398-019-0384-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bucholz KK, Heath AC, Reich T, et al. Can we subtype alcoholism? A latent class analysis of data from relatives of alcoholics in a multicenter family study of alcoholism. Alcohol Clin Exp Res. 1996;20(8):1462‐1471. doi: 10.1111/j.1530-0277.1996.tb01150.x [DOI] [PubMed] [Google Scholar]
- 24. Heath AC, Bucholz KK, Slutske WS, et al. The assessment of alcoholism in surveys of the general community: what are we measuring? Some insights from the Australian twin panel interview survey. Int Rev Psychiatry. 1994;6(4):295‐307. doi: 10.3109/09540269409023269 [DOI] [Google Scholar]
- 25. Beseler CL, Taylor LA, Kraemer DT, Leeman RF. A latent class analysis of DSM‐IV alcohol use disorder criteria and binge drinking in undergraduates. Alcohol Clin Exp Res. 2012;36(1):153‐161. doi: 10.1111/j.1530-0277.2011.01595.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sacco P, Bucholz KK, Spitznagel EL. Alcohol use among older adults in the National Epidemiologic Survey on alcohol and related conditions: a latent class analysis. J Stud Alcohol Drugs. 2009;70(6):829‐838. doi: 10.15288/jsad.2009.70.829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ko JY, Martins SS, Kuramoto SJ, Chilcoat HD. Patterns of alcohol‐dependence symptoms using a latent empirical approach: associations with treatment usage and other correlates. J Stud Alcohol Drugs. 2010;71(6):870‐878. doi: 10.15288/jsad.2010.71.870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bierut LJ, Dinwiddie SH, Begleiter H, et al. Familial transmission of substance dependence: alcohol, marijuana, cocaine, and habitual smoking: a report from the collaborative study on the genetics of alcoholism. Arch Gen Psychiatry. 1998;55(11):982‐988. doi: 10.1001/archpsyc.55.11.982 [DOI] [PubMed] [Google Scholar]
- 29. Raimo EB, Smith TL, Danko GP, Bucholz KK, Schuckit MA. Clinical characteristics and family histories of alcoholics with stimulant dependence. J Stud Alcohol. 2000;61(5):728‐735. doi: 10.15288/jsa.2000.61.728 [DOI] [PubMed] [Google Scholar]
- 30. Xian H, Scherrer JF, Grant JD, et al. Genetic and environmental contributions to nicotine, alcohol and cannabis dependence in male twins. Addiction. 2008;103(8):1391‐1398. doi: 10.1111/j.1360-0443.2008.02243.x [DOI] [PubMed] [Google Scholar]
- 31. Palmer RH, Button TM, Rhee SH, et al. Genetic etiology of the common liability to drug dependence: evidence of common and specific mechanisms for DSM‐IV dependence symptoms. Drug Alcohol Depend. 2012;123(Suppl 1):S24‐S32. doi: 10.1016/j.drugalcdep.2011.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hicks BM, Krueger RF, Iacono WG, McGue M, Patrick CJ. Family transmission and heritability of externalizing disorders: a twin‐family study. Arch Gen Psychiatry. 2004;61(9):922‐928. [DOI] [PubMed] [Google Scholar]
- 33. Schuckit MA, Daeppen J‐B, Danko GP, et al. Clinical implications for four drugs of the DSM‐IV distinction between substance dependence with and without a physiological component. Am J Psychiatry. 1999;156(1):41‐49. doi: 10.1176/ajp.156.1.41 [DOI] [PubMed] [Google Scholar]
- 34. Babor TF, Dolinsky ZS, Meyer RE, Hesselbrock M, Hofmann M, Tennen H. Types of alcoholics: concurrent and predictive validity of some common classification schemes. Br J Addict. 1992;87(10):1415‐1431. doi: 10.1111/j.1360-0443.1992.tb01921.x [DOI] [PubMed] [Google Scholar]
- 35. Schuckit MA, Tipp JE, Smith TL, et al. An evaluation of type a and B alcoholics. Addiction. 1995;90(9):1189‐1203. doi: 10.1046/j.1360-0443.1995.90911894.x [DOI] [PubMed] [Google Scholar]
- 36. Sher KJ, Grekin ER, Williams NA. The development of alcohol use disorders. Annu Rev Clin Psychol. 2005;1(1):493‐523. [DOI] [PubMed] [Google Scholar]
- 37. Maggs JL, Schulenberg JE. Initiation and course of alcohol consumption among adolescents and Young adults. In: Galanter M, Lowman C, Boyd GM, Faden VB, Witt E, Lagressa D, eds. Recent Developments in Alcoholism: Alcohol Problems in Adolescents and Young Adults. US; 2005:29‐47. [DOI] [PubMed] [Google Scholar]
- 38. Pagan JL, Rose RJ, Viken RJ, Pulkkinen L, Kaprio J, Dick DM. Genetic and environmental influences on stages of alcohol use across adolescence and into young adulthood. Behav Genet. 2006;36(4):483‐497. doi: 10.1007/s10519-006-9062-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kuperman S, Schlosser SS, Kramer JR, et al. Developmental sequence from disruptive behavior diagnosis to adolescent alcohol dependence. Am J Psychiatry. 2001;158(12):2022‐2026. doi: 10.1176/appi.ajp.158.12.2022 [DOI] [PubMed] [Google Scholar]
- 40. Bonomo YA, Bowes G, Coffey C, Carlin JB, Patton GC. Teenage drinking and the onset of alcohol dependence: a cohort study over seven years. Addiction. 2004;99(12):1520‐1528. doi: 10.1111/j.1360-0443.2004.00846.x [DOI] [PubMed] [Google Scholar]
- 41. Kapoor M, Wang JC, Wetherill L, et al. Genome‐wide survival analysis of age at onset of alcohol dependence in extended high‐risk COGA families. Drug Alcohol Dependence. 2014;142:56‐62. doi: 10.1016/j.drugalcdep.2014.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dick DM, Bierut L, Hinrichs A, et al. The role of GABRA2 in risk for conduct disorder and alcohol and drug dependence across developmental stages. Behav Genet. 2006;36(4):577‐590. doi: 10.1007/s10519-005-9041-8 [DOI] [PubMed] [Google Scholar]
- 43. Edwards AC, Kendler KS. Alcohol consumption in men is influenced by qualitatively different genetic factors in adolescence and adulthood. Psychol Med. 2013;43(9):1857‐1868. doi: 10.1017/S0033291712002917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Marel C, Sunderland M, Mills KL, Slade T, Teesson M, Chapman C. Conditional probabilities of substance use disorders and associated risk factors: progression from first use to use disorder on alcohol, cannabis, stimulants, sedatives and opioids. Drug Alcohol Depend. 2019;194:136‐142. doi: 10.1016/j.drugalcdep.2018.10.010 [DOI] [PubMed] [Google Scholar]
- 45. McLellan AT, Koob GF, Volkow ND. Preaddiction—a missing concept for treating substance use disorders. JAMA Psychiatry. 2022;79(8):749‐751. doi: 10.1001/jamapsychiatry.2022.1652 [DOI] [PubMed] [Google Scholar]
- 46. Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior, and personality: modeling the externalizing spectrum. J Abnorm Psychol. 2002;111(3):411‐424. [PubMed] [Google Scholar]
- 47. Dick DM, Viken RJ, Kaprio J, Pulkkinen L, Rose RJ. Understanding the covariation among childhood externalizing symptoms: genetic and environmental influences on conduct disorder, attention deficit hyperactivity disorder, and oppositional defiant disorder symptoms. J Abnorm Child Psychol. 2005;33(2):219‐229. doi: 10.1007/s10802-005-1829-8 [DOI] [PubMed] [Google Scholar]
- 48. Conrod PJ, O'Leary‐Barrett M, Newton N, et al. Effectiveness of a selective, personality‐targeted prevention program for adolescent alcohol use and misuse: a cluster randomized controlled trial. JAMA Psychiatry. 2013;70(3):334‐342. [DOI] [PubMed] [Google Scholar]
- 49. Slutske WS, Heath AC, Dinwiddie SH, et al. Common genetic risk factors for conduct disorder and alcohol dependence. J Abnorm Psychol. 1998;107:363‐374. doi: 10.1037/0021-843X.107.3.363 [DOI] [PubMed] [Google Scholar]
- 50. Zucker RA, Heitzeg MM, Nigg JT. Parsing the undercontrol–disinhibition pathway to substance use disorders: a multilevel developmental problem. Child Dev Perspect. 2011;5(4):248‐255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Achenbach TM. The classification of children's psychiatric symptoms: a factor‐analytic study. Psychol Monogr Gen Appl. 1966;80(7):1‐37. [DOI] [PubMed] [Google Scholar]
- 52. Zucker RA, Donovan JE, Masten AS, Mattson ME, Moss HB. Early developmental processes and the continuity of risk for underage drinking and problem drinking. Pediatrics. 2008;121(Supplement_4):S252‐S272. doi: 10.1542/peds.2007-2243B [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Achenbach TM. Manual for the Young Adult Self‐Report and Young Adult Behavior Checklist. University of Vermont, Department of Psychiatry. 1997.
- 54. Babor TF, Hofmann M, DelBoca FK, et al. Evidence for an empirically derived typology based on indicators of vulnerability and severity. Arch Gen Psychiatry. 1992;49(8):599‐608. doi: 10.1001/archpsyc.1992.01820080007002 [DOI] [PubMed] [Google Scholar]
- 55. Aliev F, Wetherill L, Bierut L, et al. Genes associated with alcohol outcomes show enrichment of effects with broad externalizing and impulsivity phenotypes in an independent sample. J Stud Alcohol Drugs. 2015;76(1):38‐46. [PMC free article] [PubMed] [Google Scholar]
- 56. Dick DM, Aliev F, Latendresse S, et al. How phenotype and developmental stage affect the genes we find: GABRA2 and impulsivity. Twin Res Hum Genet. 2013;16(3):661‐669. doi: 10.1017/thg.2013.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dick DM, Adkins AE, Kuo SI. Genetic influences on adolescent behavior. Neurosci Biobehav Rev. 2016;70:198‐205. doi: 10.1016/j.neubiorev.2016.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nurnberger JI, Wang Y, Zang Y, et al. High polygenic risk scores are associated with age of onset of alcohol use disorder in adolescents and young adults at risk. Biol Psychiatry. 2021;2:379‐388. doi: 10.1016/j.bpsgos.2021.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dick DM, Cho SB, Latendresse SJ, et al. Genetic influences on alcohol use across stages of development: GABRA2 and longitudinal trajectories of drunkenness from adolescence to young adulthood. Addict Biol. 2014;19(6):1055‐1064. doi: 10.1111/adb.12066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lai D, Wetherill L, Kapoor M, et al. Genome‐wide association studies of the self‐rating of effects of ethanol (SRE). Addict Biol. 2020;25(2):e12800. doi: 10.1111/adb.12800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Schuckit MA, Smith TL, Tipp JE. The self‐rating of the effects of alcohol (SRE) form as a retrospective measure of the risk for alcoholism. Addiction. 1997;92(8):979‐988. doi: 10.1111/j.1360-0443.1997.tb02977.x [DOI] [PubMed] [Google Scholar]
- 62. Chung T, Martin CS. Subjective stimulant and sedative effects of alcohol during early drinking experiences predict alcohol involvement in treated adolescents. J Stud Alcohol Drugs. 2009;70(5):660‐667. doi: 10.15288/jsad.2009.70.660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. King A, Vena A, Hasin DS, deWit H, O'Connor SJ, Cao D. Subjective responses to alcohol in the development and maintenance of alcohol use disorder. Am J Psychiatry. 2021;178(6):560‐571. doi: 10.1176/appi.ajp.2020.20030247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Schuckit MA, Smith TL, Danko GP, et al. Predictors of subgroups based on maximum drinks per occasion over six years for 833 adolescents and young adults in COGA. J Stud Alcohol Drugs. 2014;75(1):24‐34. doi: 10.15288/jsad.2014.75.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Schuckit MA, Smith TL, Danko G, et al. A prospective comparison of how the level of response to alcohol and impulsivity relate to future DSM‐IV alcohol problems in the COGA youth panel. Alcohol Clin Exp Res. 2017;41(7):1329‐1339. doi: 10.1111/acer.13407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Savage JE, Neale Z, Cho SB, et al. Level of response to alcohol as a factor for targeted prevention in college students. Alcohol Clin Exp Res. 2015;39(11):2215‐2223. doi: 10.1111/acer.12874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bucholz KK, McCutcheon VV, Agrawal A, et al. Comparison of parent, peer, psychiatric, and cannabis use influences across stages of offspring alcohol involvement: evidence from the COGA prospective study. Alcohol Clin Exp Res. 2017;41(2):359‐368. doi: 10.1111/acer.13293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3(8):760‐773. doi: 10.1016/S2215-0366(16)00104-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cho SB, Su J, Kuo SI, et al. Positive and negative reinforcement are differentially associated with alcohol consumption as a function of alcohol dependence. Psychol Addict Behav. 2019;33(1):58‐68. doi: 10.1037/adb0000436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Dick DM, Meyers J, Aliev F, et al. Evidence for genes on chromosome 2 contributing to alcohol dependence with conduct disorder and suicide attempts. Am J Med Genet B: Neuropsychiatr Genet. 2010;153b(6):1179‐1188. doi: 10.1002/ajmg.b.31089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Agrawal A, Tillman R, Grucza RA, et al. Reciprocal relationships between substance use and disorders and suicidal ideation and suicide attempts in the collaborative study of the genetics of alcoholism. J Affect Disord. 2017;213:96‐104. doi: 10.1016/j.jad.2016.12.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Johnson EC, Aliev F, Meyers JL, et al. Associations between suicidal thoughts and behaviors and genetic liability for cognitive performance, depression, and risk‐taking in a high‐risk sample. Complex Psychiatry. 2021;7(1–2):34‐44. doi: 10.1159/000517169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Colbert SMC, Hatoum AS, Shabalin A, et al. Exploring the genetic overlap of suicide‐related behaviors and substance use disorders. Am J Med Genet B Neuropsychiatr Genet. 2021;186(8):445‐455. doi: 10.1002/ajmg.b.32880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Edwards AC, Ohlsson H, Sundquist J, Sundquist K, Kendler KS. Alcohol use disorder and risk of suicide in a Swedish population‐based cohort. Am J Psychiatry. 2020;177(7):627‐634. doi: 10.1176/appi.ajp.2019.19070673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Mullins N, Kang J, Campos AI, et al. Dissecting the shared genetic architecture of suicide attempt, psychiatric disorders, and known risk factors. Biol Psychiatry. 2022;91(3):313‐327. doi: 10.1016/j.biopsych.2021.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bourdon JL, Tillman R, Francis MW, et al. Characterization of service use for alcohol problems across generations and sex in adults with alcohol use disorder. Alcohol Clin Exp Res. 2020;44(3):746‐757. doi: 10.1111/acer.14290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Donovan JE. Adolescent alcohol initiation: a review of psychosocial risk factors. J Adolesc Health. 2004;35(6):529.e7‐529.e18. doi: 10.1016/j.jadohealth.2004.02.003 [DOI] [PubMed] [Google Scholar]
- 78. Kosterman R, Hawkins JD, Guo J, Catalano RF, Abbott RD. The dynamics of alcohol and marijuana initiation: patterns and predictors of first use in adolescence. Am J Public Health. 2000;90(3):360‐366. doi: 10.2105/ajph.90.3.360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Fisher LB, Miles IW, Austin SB, Camargo CA Jr, Colditz GA. Predictors of initiation of alcohol use among US adolescents: findings from a prospective cohort study. Arch Pediatr Adolesc Med. 2007;161(10):959‐966. doi: 10.1001/archpedi.161.10.959 [DOI] [PubMed] [Google Scholar]
- 80. Kuperman S, Chan G, Kramer JR, et al. A model to determine the likely age of an adolescent's first drink of alcohol. Pediatrics. 2013;131(2):242‐248. doi: 10.1542/peds.2012-0880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. McCutcheon VV, Agrawal A, Kuo SI, et al. Associations of parental alcohol use disorders and parental separation with offspring initiation of alcohol, cigarette and cannabis use and sexual debut in high‐risk families. Addiction. 2018;113(2):336‐345. doi: 10.1111/add.14003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Chorlian DB, Rangaswamy M, Manz N, et al. Genetic and neurophysiological correlates of the age of onset of alcohol use disorders in adolescents and young adults. Behav Genet. 2013;43(5):386‐401. doi: 10.1007/s10519-013-9604-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Casswell S, Pledger M, Pratap S. Trajectories of drinking from 18 to 26 years: Identification and prediction. Addiction. 2002;97(11):1427‐1437. doi: 10.1046/j.1360-0443.2002.00220.x [DOI] [PubMed] [Google Scholar]
- 84. Colder CR, Campbell RT, Ruel E, Richardson JL, Flay BR. A finite mixture model of growth trajectories of adolescent alcohol use: predictors and consequences. J Consult Clin Psychol. 2002;70(4):976‐985. doi: 10.1037//0022-006x.70.4.976 [DOI] [PubMed] [Google Scholar]
- 85. Li JJ, Cho SB, Salvatore JE, et al. The impact of peer substance use and polygenic risk on trajectories of heavy episodic drinking across adolescence and emerging adulthood. Alcohol Clin Exp Res. 2017;41(1):65‐75. doi: 10.1111/acer.13282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kong A, Thorleifsson G, Frigge ML, et al. The nature of nurture: effects of parental genotypes. Science. 2018;359(6374):424‐428. [DOI] [PubMed] [Google Scholar]
- 87. Bates TC, Maher BS, Medland SE, et al. The nature of nurture: using a virtual‐parent design to test parenting effects on children's educational attainment in genotyped families. Twin Res Hum Genet. 2018;21(2):73‐83. doi: 10.1017/thg.2018.11 [DOI] [PubMed] [Google Scholar]
- 88. Willoughby EA, McGue M, Iacono WG, Rustichini A, Lee JJ. The role of parental genotype in predicting offspring years of education: evidence for genetic nurture. Mol Psychiatry. 2019;26:3896‐3904. doi: 10.1038/s41380-019-0494-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Thomas NS, Salvatore JE, Kuo SI, et al. Genetic nurture effects for alcohol use disorder. Mol Psychiatry. 2022;28:759‐766. doi: 10.1038/s41380-022-01816-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kuo SI, Poore HE, Barr PB, et al. The role of parental genotype in the intergenerational transmission of externalizing behavior: evidence for genetic nurturance. Dev Psychopathol. 2022;34(5):1‐11. doi: 10.1017/S0954579422000700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kendler KS, Myers J. Addiction resistance: definition, validation and association with mastery. Drug Alcohol Depend. 2015;154:236‐242. doi: 10.1016/j.drugalcdep.2015.06.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Hoffmeister JR, Cohoon AJ, Sorocco KH, Acheson A, Lovallo WR. Addiction resistance to alcohol: what about heavy drinkers who avoid alcohol problems? Drug Alcohol Depend. 2019;204:107552. doi: 10.1016/j.drugalcdep.2019.107552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Stephenson M, Aliev F, Kuo SIC, et al. The role of adolescent social relationships in promoting alcohol resistance: interrupting the intergenerational transmission of alcohol misuse. Dev Psychopathol. 2022;34:1841‐1855. doi: 10.1017/S0954579422000785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Dick DM, Kendler KS. The impact of gene‐environment interaction on alcohol use disorders. Alcohol Res. 2012;34(3):318‐324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Salvatore JE, Dick DM. Gene‐environment interplay: where we are, where we are going. J Marriage Fam. 2015;77(2):344‐350. doi: 10.1111/jomf.12164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Su J, Kuo SI, Aliev F, et al. Influence of parental alcohol dependence symptoms and parenting on adolescent risky drinking and conduct problems: a family systems perspective. Alcohol Clin Exp Res. 2018;42(9):1783‐1794. doi: 10.1111/acer.13827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kuo SI, Salvatore JE, Barr PB, et al. Mapping pathways by which genetic risk influences adolescent externalizing behavior: the interplay between externalizing polygenic risk scores, parental knowledge, and peer substance use. Behav Genet. 2021;51(5):543‐558. doi: 10.1007/s10519-021-10067-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Su J, Kuo SI, Aliev F, et al. The associations between polygenic risk, sensation seeking, social support, and alcohol use in adulthood. J Abnorm Psychol. 2021;130(5):525‐536. doi: 10.1037/abn0000568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Chassin L, Pillow DR, Curran PJ, Molina BS, Barrera M Jr. Relation of parental alcoholism to early adolescent substance use: a test of three mediating mechanisms. J Abnorm Psychol. 1993;102(1):3‐19. doi: 10.1037//0021-843x.102.1.3 [DOI] [PubMed] [Google Scholar]
- 100. Su J, Supple AJ, Leerkes EM, Kuo SI. Latent trajectories of alcohol use from early adolescence to young adulthood: interaction effects between 5‐HTTLPR and parenting quality and gender differences. Dev Psychopathol. 2019;31(2):457‐469. doi: 10.1017/s095457941800024x [DOI] [PubMed] [Google Scholar]
- 101. Elam KK, Chassin L, Pandika D. Polygenic risk, family cohesion, and adolescent aggression in Mexican American and European American families: developmental pathways to alcohol use. Dev Psychopathol. 2018;30(5):1715‐1728. doi: 10.1017/s0954579418000901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Salvatore JE, Aliev F, Edwards AC, et al. Polygenic scores predict alcohol problems in an independent sample and show moderation by the environment. Genes (Basel). 2014;5(2):330‐346. doi: 10.3390/genes5020330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Salvatore JE, Aliev F, Bucholz K, et al. Polygenic risk for externalizing disorders: gene‐by‐development and gene‐by‐environment effects in adolescents and young adults. Clin Psychol Sci. 2015;3(2):189‐201. doi: 10.1177/2167702614534211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Thomas NS, Kuo SIC, Aliev F, et al. Alcohol use disorder, psychiatric comorbidities, marriage and divorce in a high‐risk sample. Psychol Addict Behav. 2022;36:364‐374. doi: 10.1037/adb0000840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Cho SB, Smith RL, Bucholz K, et al. Using a developmental perspective to examine the moderating effects of marriage on heavy episodic drinking in a young adult sample enriched for risk. Dev Psychopathol. 2021;33(3):1097‐1106. doi: 10.1017/s0954579420000371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Dick DM, Pagan JL, Viken R, et al. Changing environmental influences on substance use across development. Twin Res Hum Genet. 2007;10(2):315‐326. doi: 10.1375/twin.10.2.315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Heath AC, Jardine R, Martin NG. Interactive effects of genotype and social environment on alcohol consumption in female twins. J Stud Alcohol. 1989;50(1):38‐48. doi: 10.15288/jsa.1989.50.38 [DOI] [PubMed] [Google Scholar]
- 108. Kaskutas LA, Borkman TJ, Laudet A, et al. Elements that define recovery: the experiential perspective. J Stud Alcohol Drugs. 2014;75(6):999‐1010. doi: 10.15288/jsad.2014.75.999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Kelly JF, Bergman B, Hoeppner BB, Vilsaint C, White WL. Prevalence and pathways of recovery from drug and alcohol problems in the United States population: implications for practice, research, and policy. Drug Alcohol Depend. 2017;181:162‐169. doi: 10.1016/j.drugalcdep.2017.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. McDaniel JM, Brown AM, Thompson Heller A, et al. Interdisciplinary expansions: applying recovery‐informed theory to interdisciplinary areas of recovery science research. Alcohol Treat Quart. 2020;38(4):457‐469. [Google Scholar]
- 111. Schuckit MA, Tipp JE, Smith TL, Bucholz KK. Periods of abstinence following the onset of alcohol dependence in 1,853 men and women. J Stud Alcohol. 1997;58(6):581‐589. doi: 10.15288/jsa.1997.58.581 [DOI] [PubMed] [Google Scholar]
- 112. McCutcheon VV, Kramer JR, Edenberg HJ, et al. Social contexts of remission from DSM‐5 alcohol use disorder in a high‐risk sample. Alcohol Clin Exp Res. 2014;38(7):2015‐2023. doi: 10.1111/acer.12434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Sobell LC, Cunningham JA, Sobell MB. Recovery from alcohol problems with and without treatment: prevalence in two population surveys. Am J Public Health. 1996;86(7):966‐972. doi: 10.2105/ajph.86.7.966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Tucker JA, Cheong J, James TG, Jung S, Chandler SD. Preresolution drinking problem severity profiles associated with stable moderation outcomes of natural recovery attempts. Alcohol Clin Exp Res. 2020;44(3):738‐745. doi: 10.1111/acer.14287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. McCutcheon VV, Schuckit MA, Kramer JR, et al. Familial association of abstinent remission from alcohol use disorder in first‐degree relatives of alcohol‐dependent treatment‐seeking probands. Addiction. 2017;112(11):1909‐1917. doi: 10.1111/add.13890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Knop J, Penick EC, Nickel EJ, et al. Paternal alcoholism predicts the occurrence but not the remission of alcoholic drinking: a 40‐year follow‐up. Acta Psychiatr Scand. 2007;116(5):386‐393. doi: 10.1111/j.1600-0447.2007.01015.x [DOI] [PubMed] [Google Scholar]
- 117. Penick EC, Knop J, Nickel EJ, et al. Do premorbid predictors of alcohol dependence also predict the failure to recover from alcoholism? J Stud Alcohol Drugs. 2010;71(5):685‐694. doi: 10.15288/jsad.2010.71.685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Gilder DA, Lau P, Corey L, Ehlers CL. Factors associated with remission from alcohol dependence in an American Indian community group. Am J Psychiatry. 2008;165(9):1172‐1178. doi: 10.1176/appi.ajp.2008.07081308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Bottlender M, Soyka M. Outpatient alcoholism treatment: predictors of outcome after 3 years. Drug Alcohol Depend. 2005;80(1):83‐89. doi: 10.1016/j.drugalcdep.2005.03.011 [DOI] [PubMed] [Google Scholar]
- 120. Dawson DA, Goldstein RB, Grant BF. Rates and correlates of relapse among individuals in remission from DSM‐IV alcohol dependence: a 3‐year follow‐up. Alcohol Clin Exp Res. 2007;31(12):2036‐2045. doi: 10.1111/j.1530-0277.2007.00536.x [DOI] [PubMed] [Google Scholar]
- 121. Lopez‐Quintero C, Hasin DS, de Los Cobos JP, et al. Probability and predictors of remission from life‐time nicotine, alcohol, cannabis or cocaine dependence: results from the National Epidemiologic Survey on alcohol and related conditions. Addiction. 2011;106(3):657‐669. doi: 10.1111/j.1360-0443.2010.03194.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Whelan R, Watts R, Orr CA, et al. Neuropsychosocial profiles of current and future adolescent alcohol misusers. Nature. 2014;512(7513):185‐189. doi: 10.1038/nature13402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Bi J, Sun J, Wu Y, Tennen H, Armeli S. A machine learning approach to college drinking prediction and risk factor identification. ACM Trans Intell Syst Technol. 2013;4(4):72. doi: 10.1145/2508037.2508053 [DOI] [Google Scholar]
- 124. Kinreich S, Meyers JL, Maron‐Katz A, et al. Predicting risk for alcohol use disorder using longitudinal data with multimodal biomarkers and family history: a machine learning study. Mol Psychiatry. 2021;26(4):1133‐1141. doi: 10.1038/s41380-019-0534-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Kinreich S, McCutcheon VV, Aliev F, et al. Predicting alcohol use disorder remission: a longitudinal multimodal multi‐featured machine learning approach. Transl Psychiatry. 2021;11(1):166. doi: 10.1038/s41398-021-01281-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Francis MW, Bourdon JL, Chan G, et al. Deriving a measure of social recovery capital from the important people and activities instrument: construction and psychometric properties. Alcohol Alcohol. 2022;57(3):322‐329. doi: 10.1093/alcalc/agac014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Groshkova T, Best D, White W. The assessment of recovery capital: properties and psychometrics of a measure of addiction recovery strengths. Drug Alcohol Rev. 2013;32(2):187‐194. doi: 10.1111/j.1465-3362.2012.00489.x [DOI] [PubMed] [Google Scholar]
- 128. Burns J, Marks D. Can recovery capital predict addiction problem severity? Alcohol Treat Quart. 2013;31(3):303‐320. doi: 10.1080/07347324.2013.800430 [DOI] [Google Scholar]
- 129. Laudet AB, Morgen K, White WL. The role of social supports, spirituality, religiousness, life meaning and affiliation with 12‐step fellowships in quality of life satisfaction among individuals in recovery from alcohol and drug problems. Alcohol Treat Quart. 2006;24(1–2):33‐73. doi: 10.1300/J020v24n01_04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372(9):793‐795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Schuckit MA, Smith TL, Clausen P, et al. The low level of response to alcohol‐based heavy drinking prevention program: one‐year follow‐up. J Stud Alcohol Drugs. 2016;77(1):25‐37. doi: 10.15288/jsad.2016.77.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Schuckit MA, Kalmijn JA, Smith TL, Saunders G, Fromme K. Structuring a college alcohol prevention program on the low level of response to alcohol model: a pilot study. Alcohol Clin Exp Res. 2012;36(7):1244‐1252. [DOI] [PubMed] [Google Scholar]
- 133. Dick DM, Saunders T, Balcke E, et al. Genetically influenced externalizing and internalizing risk pathways as novel prevention targets. Psychol Addict Behav. 2022;36(6):595‐606. doi: 10.1037/adb0000759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Barr PB, Ksinan A, Su J, et al. Using polygenic scores for identifying individuals at increased risk of substance use disorders in clinical and population samples. Transl Psychiat. 2020;10(1):196. doi: 10.1038/s41398-020-00865-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Barr PB, Driver MN, Kuo SIC, et al. Clinical, environmental, and genetic risk factors for substance use disorders: characterizing combined effects across multiple cohorts. Mol Psychiatry. 2022;27:4633‐4641. doi: 10.1038/s41380-022-01801-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Driver MN, Kuo SI, Dick DM. Genetic feedback for psychiatric conditions: where are we now and where are we going. Am J Med Genet B Neuropsychiatr Genet. 2020;183(7):423‐432. doi: 10.1002/ajmg.b.32815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Driver MN, Kuo SI, Dick DM, Spit For Science Working G . Interest in genetic feedback for alcohol use disorder and related substance use and psychiatric outcomes among Young adults. Brain Sci. 2020;10(12):1007. doi: 10.3390/brainsci10121007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Driver MN, Kuo SI‐C, Dick DM. Returning complex genetic risk information to promote better health‐related behaviors: a commentary of the literature and suggested next steps. Transl Behav Med. 2022;13:115‐119. doi: 10.1093/tbm/ibac071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Dick DM. The promise and peril of genetics. Curr Dir Psychol Sci. 2022;31:480‐485. doi: 10.1177/09637214221112041 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
COGA data are available in dbGaP (phs000125, phs000763, phs000976, phs001208), or via an application to the National Institute on Alcohol Abuse and Alcoholism (https://www.niaaa.nih.gov/research/major-initiatives/collaborative-studies-genetics-alcoholism-coga-study), or a COGA investigator sponsored secondary analysis proposal.


