Abstract
Background
Type 1 diabetes (T1D) results from immune-mediated destruction of insulin-producing beta cells. Prevention efforts have focused on immune modulation and supporting beta cell health before or around diagnosis; however, heterogeneity in disease progression and therapy response has limited translation to clinical practice, highlighting the need for precision medicine approaches to T1D disease modification.
Methods
To understand the state of knowledge in this area, we performed a systematic review of randomized-controlled trials with 50 participants cataloged in PubMed or Embase from the past 25 years testing T1D disease-modifying therapies and/or identifying features linked to treatment response, analyzing bias using a Cochrane-risk-of-bias instrument.
Results
We identify and summarize 75 manuscripts, 15 describing 11 prevention trials for individuals with increased risk for T1D, and 60 describing treatments aimed at preventing beta cell loss at disease onset. Seventeen interventions, mostly immunotherapies, show benefit compared to placebo (only two prior to T1D onset). Fifty-seven studies employ precision analyses to assess features linked to treatment response. Age, beta cell function measures, and immune phenotypes are most frequently tested. However, analyses are typically not prespecified, with inconsistent methods of reporting, and tend to report positive findings.
Conclusions
While the quality of prevention and intervention trials is overall high, the low quality of precision analyses makes it difficult to draw meaningful conclusions that inform clinical practice. To facilitate precision medicine approaches to T1D prevention, considerations for future precision studies include the incorporation of uniform outcome measures, reproducible biomarkers, and prespecified, fully powered precision analyses into future trial design.
Subject terms: Type 1 diabetes, Pre-diabetes, Type 1 diabetes, Randomized controlled trials, Diabetes
Plain language summary
Type 1 diabetes (T1D) is a condition that results from the destruction of a type of cell in the pancreas that produces the hormone insulin, leading to lifelong dependence on insulin injections. T1D prevention remains a challenging goal, largely due to the immense variability in disease processes and progression. Therapies tested to date in medical research settings (clinical trials) work only in a subset of individuals, highlighting the need for more tailored prevention approaches. We reviewed clinical trials of therapies targeting the disease process in T1D. While the overall quality of trials was high, studies testing individual features affecting responses to treatments were low. This review reveals an important need to carefully plan high-quality analyses of features that affect treatment response in T1D, to ensure that tailored approaches may one day be applied to clinical practice.
Felton et al. conducted a systematic review to evaluate studies testing disease-modifying therapies and features linked to treatment response for type 1 diabetes prevention. While the quality of prevention and intervention trials is found to be high, precision analyses on factors associated with treatment response are of poorer quality.
Introduction
Type 1 diabetes (T1D) results from immune-mediated destruction of pancreatic beta cells1. Since the discovery of insulin over a century ago, treatment options for persons with type 1 diabetes (T1D) have shown remarkable advancements, including improved insulin formulations, delivery methods, and tools to monitor glycemia2. Even with these transformative advances, considerable negative impacts remain on health outcomes and quality of life3–5. In contrast, effective disease-modifying therapies aimed at the preservation of endogenous insulin production could not only improve these outcomes but also, if given early enough in the disease course, prevent the need for insulin replacement6–9. Because T1D is an autoimmune disease, many agents tested as potential disease-modifying therapies are immunomodulatory, while others target pathologic contributors such as glucose toxicity and beta cell health and function10. In 2022, the US Food and Drug Administration approved teplizumab, a monoclonal antibody targeting CD3, as the first therapy to delay the onset of clinical T1D in at-risk individuals11.
The Precision Medicine in Diabetes Initiative (PMDI) was established in 2018 by the American Diabetes Association (ADA) in partnership with the European Association for the Study of Diabetes (EASD). The ADA/EASD PMDI includes global thought leaders in precision diabetes medicine who are working to address the burgeoning need for better diabetes prevention and care through precision medicine. This Systematic Review is written on behalf of the ADA/EASD PMDI as part of a comprehensive evidence evaluation of precision prevention in T1D in support of the 2nd International Consensus Report on Precision Diabetes Medicine12. The first ADA/ EASD Precision Medicine in Diabetes Consensus Report defined precision prevention as “using information about a person’s unique biology, environment, and/or context to determine their likely responses to health interventions” and states that “precision prevention should optimize the prescription of health-enhancing interventions”13. Given that agents targeting these pathways may have potential adverse effects, and initial therapies may affect the efficacy and safety of subsequent treatment approaches, precision medicine is uniquely poised to identify which individuals stand to benefit the most from a given intervention and to optimize potential risk-benefit ratios for treated persons. Additionally, once further T1D disease-modifying therapies are approved for clinical use, precision medicine will facilitate the selection of therapies guided by the individual’s disease, including potential combination regimens of disease-modifying therapies14,15.
T1D development occurs along a spectrum of progressive beta cell destruction, beginning with loss of tolerance, reflected by the appearance of islet autoantibodies, and continuing with progressive hyperglycemia, abnormal glucose tolerance, and decline in endogenous insulin production, reflected by a decline in C-peptide8. Based on this, in 2015, the diabetes research community adopted a staging system, with the development of multiple islet autoantibodies now heralding Stage 1 T1D9. At the time of clinical T1D diagnosis, insulin replacement is required but endogenous insulin production, though diminished, can still be detected in most affected individuals. While an ideal goal is clinical T1D prevention, disease-modifying agents aimed throughout the spectrum of T1D progression have the potential to improve long-term outcomes6,7. Furthermore, given widely available participants and shorter total trial durations, agents planning to target earlier stages of the disease are often initially trialed in the new-onset period16. Therefore, we sought to understand the current state of knowledge regarding precision approaches to T1D disease modification, either to prevent the development of early-stage or clinical T1D (referred to as “prevention” studies) or to preserve endogenous insulin function around the time of clinical T1D diagnosis (referred to as “new-onset” studies). Specifically, we asked if individual characteristics have been robustly identified to select persons for therapeutic optimization of T1D disease-modifying therapies before or at the time of diagnosis. We reviewed and summarized existing trials in this area and identified individual characteristics associated with treatment effects.
Methods
Search strategy
We developed a search strategy using an iterative process that involved Medical Subject Headings (MeSH) and text words. This search was refined based on a sensitivity check for key articles identified by members of the group (Supplementary Note 1). This strategy was applied to PubMed and EMBASE databases by librarians from Lund University on 2/22/2022.
Systematic review
The Covidence platform was utilized for stages of systematic review. To qualify for review, studies must have tested a disease-modifying treatment in either initially non-diabetic individuals at risk, or individuals with new-onset type 1 diabetes (within 1 year of diagnosis). Eligible study types included randomized controlled trials (RCTs); systematic reviews or meta-analyses of RCTs, or post hoc analyses of RCTs. Selected primary trials or longitudinal follow-up papers of primary trials had a total sample size ≥50 and were published as a full paper in English in a peer-reviewed journal within 25 years of the search (2/21/1997-2/22/2022). Papers focusing on a precision approach to identify features associated with a treatment response were also included if the total sample size was ≥10. Longitudinal follow-up papers of RCTs were included if they addressed follow-up data on time to diabetes, C-peptide area under the curve (AUC), or included “precision analyses” of specific individual features or measures of treatment response. Studies were excluded if they included mixed participant populations (i.e., type 1 and type 2 diabetes) or populations with inconsistent definitions across papers (i.e., latent autoimmune diabetes in adults). Several additional key articles previously known to the group of experts that also met inclusion criteria but were not included in the search results because of search restrictions designed to improve search feasibility were also included in the analysis. While systematic reviews and meta-analyses were included in the original search strategy to identify any existing meta-analyses aimed at precision approaches, none that met inclusion criteria were identified. All included papers were primary trials or post hoc analyses of primary trials.
Investigators independently screened and reviewed each potentially relevant article according to preliminary eligibility criteria determined by members of the review team. For Level 1 screening two investigators per article screened each title and abstract. Discordant assessments were discussed and resolved by consensus or arbitration after consultation with a member of the review leadership team (JLF, RO, KJG, MR, or EKS). For Level 2 screening of eligible articles, full texts were retrieved and reviewed by two independent reviewers using the inclusion/exclusion criteria. Discordant assessments were similarly discussed and resolved.
Two separate investigators per article extracted data from each article meeting inclusion criteria, with consensus determined by a member of the leadership team. Extracted data included study and publication name and date, if the study was single or multicenter, participant characteristics (age, sex, race, ethnicity, stage of disease), eligibility criteria, intervention details, details of metabolic monitoring, duration of follow-up, primary trial outcome, specific data on outcomes of intervention on time to diabetes (all available results) or C-peptide (at furthest reported timepoint from treatment), and methods and findings surrounding precision analyses focused on treatment response.
The protocol for this review was registered (PROSPERO ID: CRD42022310063) before implementation and amended during review to edit group members and for feasibility, to add further exclusion criteria (populations defined as latent autoimmune diabetes and trials or follow-up studies with <50 participants).
Risk of bias assessments
Investigators also independently performed quality assessments using Covidence’s Cochrane Risk of Bias template in tandem for each eligible study; this included assessments of sequence generation, allocation concealment, masking of participants/personnel, masking of outcome assessment, incomplete outcome data, selective reporting, and any other sources of bias to order to determine the overall risk of bias.
Statistics and reproducibility
Because of the heterogeneity of clinical interventions (e.g., agent tested, study design, analytical methodology, etc.), we were unable to perform a meta-analysis but instead completed summaries of relevant studies. A forest plot was generated using hazard ratios from all included prevention studies. No studies were missing data or required data conversion for this. Reproducibility was ensured by a dual investigator review of each article at each review stage.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Results
Systematic review results
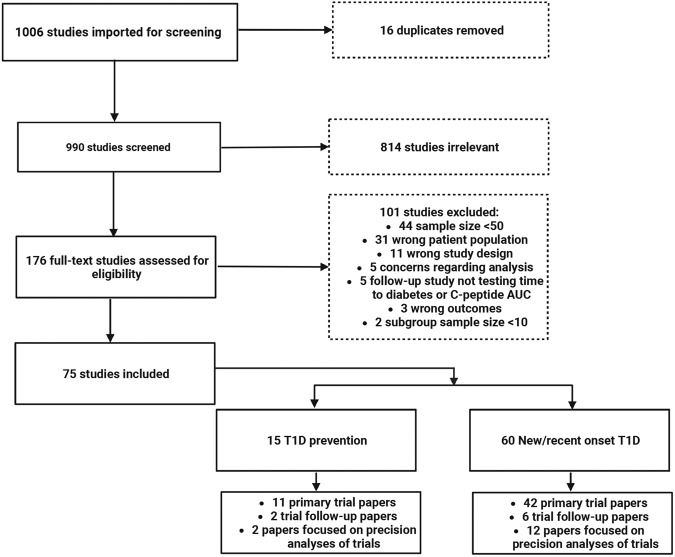
From 1006 studies identified by PubMed and Embase searches, 75 were eligible for extraction (Fig. 1). This included original trial papers, trial longitudinal follow-up papers, and papers focused specifically on a precision analysis surrounding treatment response in prevention trials (15 papers from 11 prevention trial cohorts)17–31 and in individuals with new-onset T1D (60 total papers from 45 new-onset trial cohorts)32–91.
Fig. 1. PRISMA flow diagram.
Flowchart displaying studies screened and excluded as part of abstract screening, then via full text review/eligibility assessment. 75 total papers were included in the extraction. AUC area under the curve; T1D Type 1 diabetes. This image was generated using Biorender.
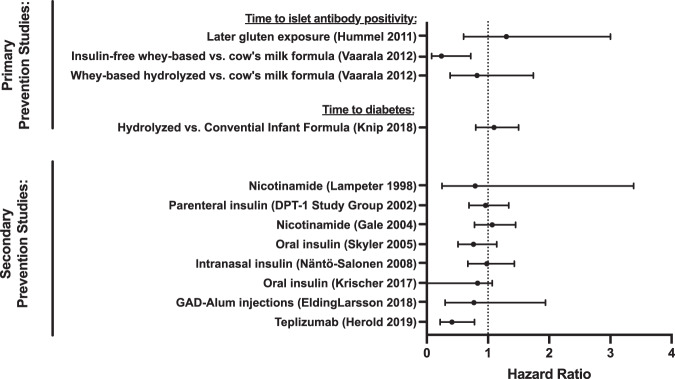
The 15 articles on T1D prevention generated from 11 trials are summarized in Tables 1 and 2. Primary prevention studies, conducted prior to autoantibody seroconversion in genetically at-risk individuals testing development of islet autoantibodies or time to T1D, comprised 27% (3/11) of trials; 63% (7/11) of trials were secondary prevention studies testing effects of interventions after seroconversion in autoantibody-positive individuals on time to T1D; one trial tested both genetically at-risk infants and autoantibody-positive siblings. Further inclusion criteria for trials included measures of beta cell function, with studies testing antigen-based therapies utilizing specific autoantibody positivity criteria. The DPT-1 oral and parental insulin studies and TrialNet oral insulin study identified participants based on insulin autoantibody positivity and first-phase insulin response on intravenous (IV) glucose tolerance testing17,24,28. The TrialNet teplizumab prevention study only enrolled individuals with multiple autoantibody positivity and dysglycemia on oral glucose tolerance testing. Finally, a study testing glutamic acid decarboxylase (GAD) antigen therapy was limited to individuals who were GAD autoantibody positive19. Most prevention trials (9/11; 81%) were multicenter studies; 9/11 (82%) were also double-masked, while 2/11 (18%) had no masking. In addition to these 11 papers, two follow-up papers and two papers focused solely on precision analysis of treatment response were also identified (for a total of 15 papers). Overall, only two prevention studies reported a positive impact on time to islet autoantibody positivity or time to diabetes: the primary prevention study testing whey-based hydrolyzed vs. cow’s milk formula30 and the secondary prevention study testing teplizumab21 (forest plot showing hazard ratios for all prevention studies in Fig. 2).
Table 1.
Primary prevention studies.
| Trial acronym | Population | Intervention | Multi-center? | Blinding | Primary outcome | Follow-up duration | Positive? | Hazard ratio (95%CI) vs. control |
|---|---|---|---|---|---|---|---|---|
| Hummel 201122 (BABYDIET) | 150 infants with a first-degree family history of T1D and high-risk HLA genotypes | Late (12 months) vs. early (6 months) gluten exposure | No | None | Aabs | 3 years (range 3.0–10.0) | No | 1.3 (0.6‚3.0) |
| Vaarala 201230 (FINDIA) | 1113 infants with high-risk HLA genotypes | • Whey-based hydrolyzed vs. cow’s milk formula | Yes | Double | Aabs | n/a | • No | • 0.82 (0.38–1.7) |
| • Insulin-free whey-based vs. cow’s milk formula | • Yes | • 0.24 (0.08–0.72) | ||||||
| Knip 201823 (TRIGR) | 2159 infants with a first-degree family history of T1D and high-risk HLA genotypes | Extensively hydrolyzed casein formula vs. conventional formula | Yes | Double | Time to diabetes | 11.5 years (Q1–Q3, 10.2–12.8 | No | 1.1 (0.8 to 1.5) |
T1D type 1 diabetes, HLA human leukocyte antigen, Aab autoantibody, FINDIA Finnish Dietary Intervention Trial for the Prevention of Type 1 Diabetes, n/a not applicable, TRIGR trial to reduce IDDM in the genetically at risk, Aab+ autoantibody positive.
Table 2.
Secondary prevention studies.
| Trial acronym | Population | Intervention | Multi-center? | Blinding | Primary Outcome | Follow-up duration | Positive? | Hazard ratio (95%CI) vs. control |
|---|---|---|---|---|---|---|---|---|
| Näntö-Salonen 200826 | 264 infants with high-risk HLA genotype and their siblings with high-risk HLA and multiple Aab+ | Intranasal daily recombinant human short-acting insulin vs. placebo | Yes | Double | Time to diabetes |
Insulin: 1.7 years (IQR 0.7–3.0) Placebo: 2.0 years (IQR 0.8–3.2) |
No |
• Infants: 1.2 (0.68–2.0) • Infants + siblings: 0.98 (0.67–1.4) |
| Lampeter 199825 (DENIS) | 55 Islet-cell Aab+ siblings of individuals with T1D | 1.2 g/m2/day Endur-Amide (nicotinamide) vs. placebo | Yes | Double | Time to diabetes | 2.1 years, maximum 3.8 | No | 0.79 (0.25–3.4) |
| Gale 200420 (ENDIT) | 552 Islet-cell Aab+ relatives with nondiabetic OGTT | 1.2 g/m2 po modified release nicotinamide x 5 years vs. placebo | Yes | Double | Time to diabetes | 5 years (intended for all, but only reached by 88%) | No | 1.1 (0.78, 1.5) |
| Skyler 200217 (DPT-1) | 339 Islet-cell Aab+ first-degree relatives with the absence of low-risk HLA and low first-phase insulin response or dysglycemia | 0.25U/kg ultralente + annual 4-day continuous insulin infusion vs. no intervention | Yes | None | Time to diabetes | 1345 days, IQR 784–1737 | No | 0.96 (0.69–1.3) |
| Skyler 200528 (DPT-1) | 372 Islet-cell and insulin Aab + relatives with the absence of low-risk HLA, higher first-phase insulin response, and normal OGTT | Oral insulin (7.5 mg/day) vs. placebo | Yes | Double | Time to diabetes | 4.3 years (IQR: 928–1988 days) | No | 0.76 (0.51‚1.1) |
| • Vehik 201131 (F/u) | • 303/372 | • 9.1 years | ||||||
| • Butty 200818 (Precision) | • 638 from parenteral and oral insulin trials | • n/a | ||||||
| Krischer 201724 (TN07) | 560 Multiple Aab+ relatives with insulin Aab + and high or low first-phase insulin response | 7.5 mg daily po recombinant human insulin vs. placebo | Yes | Double | Time to diabetes | 2.7 years (IQR 1.5–4.7 years) | No | 0.83 (0–1.07) |
| • Sosenko 202029 (Precision: DPT-1 and TN07) | • 208 with high DPTRS | • n/a | • DPT-1: 0.494 (0.26, 0.96) TN07: 0.70 (0.43, 1.2) | |||||
| EldingLarsson 201819 (DiAPREV-IT) | 50 Multiple Aab+ children with GAD Ab+ | 20 ug sc injections of GAD-Alum monthly x 2 vs. placebo | No | Double | Other: safety | 4.92 years (range: 0.47–5.0) | n/a | 0.77 (0.30, 1.9) |
| Herold 201921 (TN10) | 76 Multiple Aab+ relatives with dysglycemia | 14-day course of IV Teplizumab vs. placebo | Yes | Double | Time to diabetes | 745 days (range 74–2683) | Yes | 0.41(0.22–0.78) |
| • Sims 202127 (F/u) | • 923 days | • 0.457 |
Follow-up or precision studies describing a randomized trial that is already included in the table are listed as bulleted subheadings.
T1D type 1 diabetes, HLA human leukocyte antigen, Aab autoantibody, n/a not applicable, Aab+ autoantibody-positive, DENIS The Dutch Nicotinamide Intervention Study, ENDIT European Nicotinamide Diabetes Intervention Trial, OGTT oral glucose tolerance test, Po per oral/orally, DPT-1 Diabetes Prevention Trial Type 1 Diabetes, F/u follow-up, TN07 TrialNet 07 trial, DPTRS diabetes prevention trial-type 1 risk score, DiAPREV-IT diabetes prevention–immune tolerance trial, GAD glutamic acid decarboxylase, Sc subcutaneous, TN10 TrialNet 10 trial, IV intravenous.
Fig. 2. Relative effect of prevention therapies in individuals at risk for T1D.
Forest plot showing hazard ratio with 95% confidence intervals for primary prevention studies in genetically at-risk individuals and secondary prevention studies in individuals with elevated islet autoantibody titers. Primary prevention studies are divided by outcome—either time to islet autoantibody positivity or time to diabetes. All secondary prevention studies used time to diabetes as a primary outcome. DPT-1 Diabetes Prevention Trial Type 1 ; GAD glutamic acid decarboxylase.
The 60 manuscripts generated from 45 trials in the new-onset T1D population included 42 primary trial papers, 6 trial longitudinal follow-up papers and 12 papers focused solely on precision analyses of treatment response (Fig. 1). Additional characteristics of these 60 papers are summarized in Supplementary Table 1. Here, except for variable age criteria, inclusion criteria were more homogeneous than in prevention studies, typically including participants with a clinical diagnosis of T1D (usually with islet autoantibody positivity) and C-peptide above a certain cutoff. Of the 43 trials, 30 (70%) included both adults and children, 9 (21%) tested only children, and 4 (9%) were performed solely in adults. Five trials had inclusion criteria that included positivity for a specific islet autoantibody. Trials described were typically multicenter studies (39/43; 91%) and double-masked (35/43; 81%). Two studies were single-masked, two described only masked outcomes testing, three had no masking, and masking was not described in one study.
A measure of beta cell function was by far the most common primary outcome specified amongst new-onset trials (single primary outcome in 33/43 (77%), co-primary outcome in 2/43; 5%), although other studies used HbA1c and/or insulin dose and one study used T1D remission. Primary outcome was not specified in 5 trials. All follow-up studies focused on a measure of beta cell function. Trials reporting a measure of beta cell function as the primary outcome most commonly utilized mean C-peptide AUC from a mixed meal tolerance test; values for these data were available for 32/35 primary trials and 5/6 follow-up studies and are summarized in Supplementary Table 2. Of trial manuscripts reporting these data, less than a fourth identified a positive effect of the intervention on mean C-peptide AUC. These included trials testing imatinib mesylate, low-dose anti-thymocyte globulin, teplizumab (anti-CD3 antibody), otelixizumab (anti-CD3 antibody), abatacept (CTLA4-Ig), rituximab (anti-CD20 antibody), golimumab (anti-TNF-alpha), recombinant IFN-alpha, and combination of anti-IL-21 antibody with liraglutide.
Precision analyses focused on features associated with disease-modifying treatment response
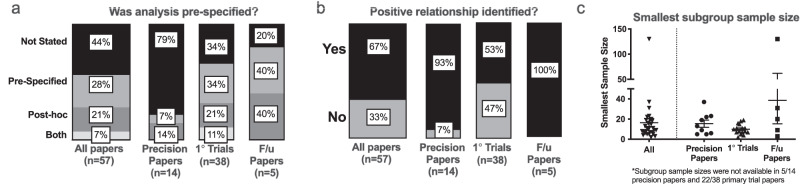
To determine whether there were specific individual features that impacted response to treatment (genetic, metabolic, immune), we assessed papers that included this type of precision analysis. Two papers from prevention and 12 papers from new onset studies focused solely on precision analyses of treatment response (i.e., no analysis of primary trial or longitudinal follow-up analysis of primary trial). An additional 43 papers also included some aspect of precision analysis (summarized in Supplementary Table 3). Of 57 total papers identified, most (38/57; 67%) were primary trial papers with a section focused on features of treatment response. Just over half (5/8) of the primary trial follow-up papers included precision analyses of treatment response; these represented only 8.8% of the 57 papers including a precision analysis.
While precision analysis of treatment response was commonly reported, this was rarely pre-specified, occurring in just 16/57 (28%) of papers studied (Fig. 3b). Prespecified precision analyses were more common in primary trial or primary trial follow-up papers. For primary trials, 34% (13/38) of precision analyses were prespecified, and 10.5% (4/38) had both pre-specified and post hoc analyses. For follow-up papers, 40% (2/5) were pre-specified. In contrast, only 7% (1/14) of papers focused specifically on precision analyses described a prespecified analysis plan. Analyses tended to identify a positive relationship with treatment effect (Fig. 3c), with 37/57 (67%) studies identifying a significant relationship between a feature and treatment response. This was more prevalent for precision analyses in primary trial follow-up papers (5/5; 100%) and in precision analysis-only papers (13/14; 93%).
Fig. 3. Precision analyses focused on treatment response were mostly part of primary trial papers, tended to be post hoc, and were biased toward positive findings.
a Stacked bar graphs showing relative frequencies and percentages of papers with precision analyses that were defined as prespecified, post hoc, or included both prespecified and post hoc analyses in the manuscript text. b Stacked bar graph displaying relative frequencies and percentages of papers reporting positive findings related to associations with treatment effects. c For papers that listed sample sizes of subgroups tested for differential treatment effects (only 53% of all papers with precision analyses), the smallest sample size reported is displayed, with mean and SEM indicated. F/u follow-up; n = 9 for precision papers; n = 16 for primary trials; n = 5 for f/u papers.
Because sample sizes inevitably decrease as groups are subdivided for precision analyses, we next looked at sample sizes for the precision subgroups. Only slightly over half (30/57) of papers reported sample sizes for all subgroups defined by precision features. Within these 30 manuscripts, we observed wide variability in the sample sizes of the subgroups analyzed. Figure 3d displays reported values for the smallest subgroup sample size described. Overall median values were 11 (interquartile range of 7-19) participants, ranging from 2 to 128 participants.
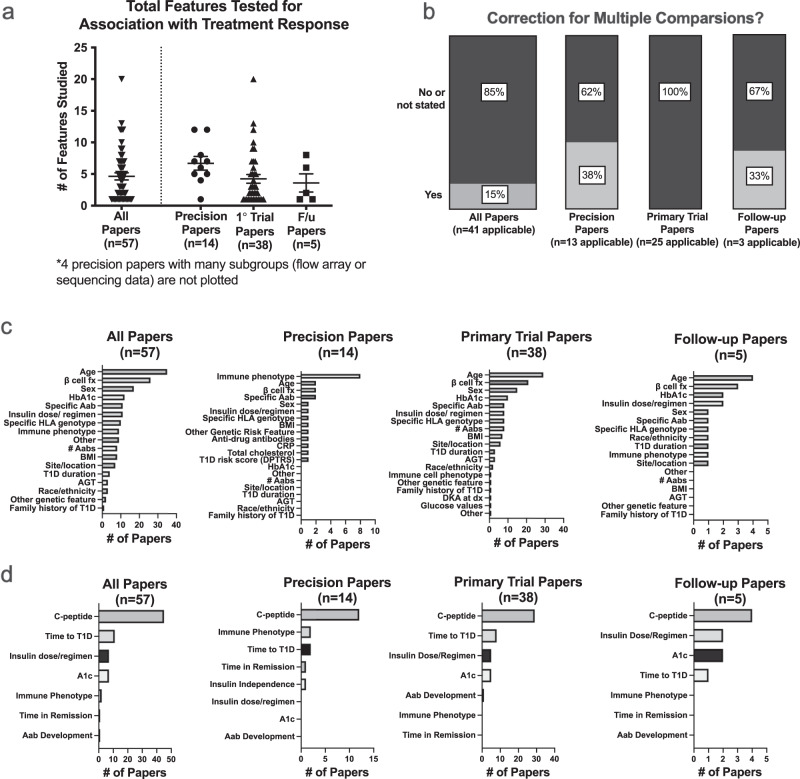
Figure 4a displays the number of precision features tested for each paper. For all papers, the median number of features tested was 3 (interquartile range of 1–7). This tended to be higher in papers focused solely on precision analyses (median of 6.5 with several papers testing numerous subgroups as part of sequencing, array, or flow cytometry analysis). Forty-one papers analyzed multiple precision features. Of these applicable analyses, corrections for multiple comparisons were either not mentioned or not performed in 35/41 (85%) of papers, particularly for trials (100% of applicable papers with multiple comparisons not described or not performed) (Fig. 4b).
Fig. 4. Precision analyses tested many features, most commonly age and beta cell function, infrequently corrected for multiple comparisons, and typically tested for differential impacts on a C-peptide-based measure.
a Total number of features tested for association with each treatment response, with mean and SEM indicated, for all papers with precision analyses. b Stacked bar graph showing relative frequencies and percentages of papers that did or did not correct for multiple comparisons. c Frequencies of individual features tested for associations with treatment response. d Frequencies of outcomes utilized to assess for the presence of any features associated with differential treatment response. The C-peptide measure category was inclusive of any measure of beta cell function, including mixed meal area under the curve, stimulated C-peptide values, fasting C-peptide values, etc. F/u follow-up, fx function, Hba1c hemoglobin A1c, Aab autoantibody, HLA human leukocyte antigen, BMI body mass index, T1D type 1 diabetes, AGT abnormal glucose tolerance, CRP C-reactive Protein, DPTRS diabetes prevention trial-type 1 risk score, DKA diabetes ketoacidosis, Dx diagnosis.
We next examined the types of features tested for relationships with treatment response (Fig. 4c). In trial papers and follow-up papers, age was most commonly tested (>75% of analyses), followed by a measure of beta cell function (>50% of analyses). Only 9/36 (25%) studies testing age identified a significant relationship with treatment response; these were all in the new onset period32,41,46,48,54,58,61,84,87. Here, younger age groups showed improved treatment responses to teplizumab, ChAglyCD3, and Vitamin E. In contrast, older age was linked to a beneficial treatment response vs. placebo with high-dose antithymocyte globulin (ATG) and oral insulin (both studies with negative findings overall)46,48. One study showed that younger age was linked to a more rapid decline of C-peptide compared to placebo in Bacillus Calmette-Guerin (BCG) vaccine-treated individuals32. Baseline measures of beta cell function were linked to differences in treatment response in 10/26 (38%) of analyses where this relationship was tested21,24,40,47,54,60,61,73,88,89. In two papers focused on prevention studies, measures linked to worsened beta cell function were associated with an improved response to treatment (with oral insulin or teplizumab)21,24. Analyses testing trials in the new-onset period had split results: teplizumab, ChAglyCD3, linomide, and atorvastatin performed better compared to placebo in groups with better baseline beta cell function measures40,54,60,61,88. In contrast, canakinumab, imatinib mesylate, and the anti-IL-21/liraglutide combination showed stronger treatment effects in individuals with lower baseline beta cell function measures47,73,89. Taken in aggregate these results highlight evidence that baseline beta cell function may impact treatment response, but the direction of impact likely varies by treatment used and stage of disease.
Interestingly, in contrast to primary trial papers, precision papers most commonly tested relationships of an immune cell phenotype with treatment response (57%). Because only two papers identified included a favorable response to time to type 1 diabetes diagnosis, treatment response was assessed using a range of alternative outcomes (Fig. 4d). For all types of papers, a measure of C-peptide was most commonly used as an alternative outcome to gauge treatment response (range of 44-68%).
Risk of bias/quality assessments
A finding impacting studies in all categories was a lack of racial and ethnic diversity in participant populations. Data on participant race were available in less than a third (23/75) of total papers; for reported papers, participants self-reporting as white race comprised a median of 92% of the total study population (interquartile range of 88-96%). Ethnicity was reported in 20 papers; within these manuscripts, participants self-reporting as identifying with a Hispanic ethnicity comprised a median of 5% of study participants (interquartile range of 3–9%).
When assessing additional risks of bias, we found that many papers did not include details sufficient to assess these risks (Fig. 5). Although over half of primary trial papers were considered to utilize high-quality methods for sequence generation and allocation concealment, 32–37% did not describe methods adequately for assessment. Follow-up and precision papers infrequently described these methods, commonly citing a primary trial paper instead (75–100%). Blinding was described more frequently, with at least double blinding in 63–74% of follow-up and primary trial papers, although 23–25% had single or no blinding. In contrast, blinding of outcome assessments was either not described or did not occur in 79% of primary trial papers. Most precision papers referenced primary papers and so blinding was challenging to assess. Completeness of outcome data reporting was assessed by considering reasons and numbers for attrition or exclusion in studies. Reporting of outcomes was overall high quality for trials and follow-up studies (75–79%). This was less frequently the case for precision papers, only half of which reported on reasons for incomplete outcome data. While the large majority (87%) of trial papers described a prespecified primary endpoint, only 75% of follow-up papers and 21% of precision papers solely included analyses that were noted to be prespecified. Additional sources of bias were identified in 33/75 total papers (44%), and these biases were also acknowledged by study authors. These were most frequently acknowledged funding or support by a pharmaceutical company. However, another source of bias that was not addressed as a limitation by the authors was identified in 3 papers (all primary trial papers). No concerns for other unacknowledged sources of bias were identified in follow-up studies and precision studies.
Fig. 5. Risk of bias assessments for each paper category. Bias was assessed using Covidence’s Cochrane risk of bias tool.
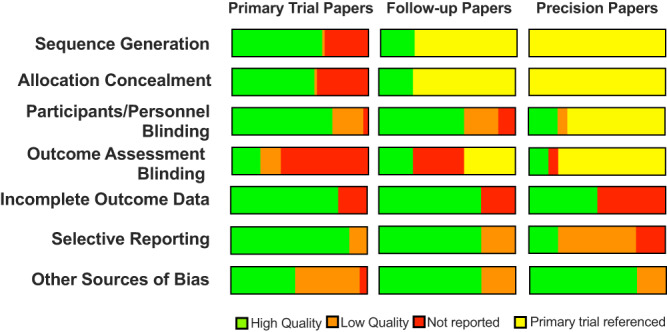
For sequence generation, allocation concealment, and blinding categories, raters had the option of selecting high quality (green), low quality (orange), not reported (red), or that a decision could not be made because of primary trial was referenced in methods (yellow). For incomplete outcome data, raters only had the option to choose high quality/data provided (green) or low quality/data not provided (red). For selective reporting, raters had the option to select high-quality/primary endpoint predefined (green), low-quality/primary endpoint not defined (orange), or low-quality/not reported (red). For other sources of bias, raters had the option to select high quality/none (green), low quality/bias present but identified and considered (orange), or low quality/obvious bias present and not addressed (red). Data are shown as absolute frequencies.
Discussion
We filled a gap in the T1D literature by systematically reviewing 25 years of large randomized controlled trials focused on T1D disease-modifying therapies, as well as precision analyses focused on identifying features of treatment response. Several themes in the literature were identified. Immunotherapies were the most common disease-modifying agents tested, and a resounding majority of these agents were tested in “new-onset” trials after a clinical diagnosis of T1D. Of the 17 interventions that showed benefit in slowing T1D progression or preserving endogenous insulin secretion, only two were tested prior to clinical disease onset. Primary trial outcome papers most commonly included precision analyses testing the impacts of baseline age and beta cell function on treatment response, while post hoc precision analysis papers primarily focused on immune phenotypes.
Based on clinical heterogeneity observed in phenotypes of T1D progression and severity, a precision-based concept that has gained popularity is the idea of the T1D “endotype”, a T1D subtype “defined by a distinct functional or pathobiological mechanism (that is also tractable therapeutically)”14. Along these lines, trials designed to limit participant heterogeneity based on features associated with treatment response could theoretically allow for clearer determinations of effect and a greater number of positive trials. While trials were overall of high quality, a key take-home message is that the current review did not identify high-quality clinical trial data supporting the existence of individual features consistently linked to therapeutic response and justifying translation to clinical care. Below, we highlight important considerations identified by this analysis for the future applicability of precision medicine to T1D disease modification.
Standardization of approaches to outcomes for precision analyses
Time to T1D was the most consistent primary outcome of T1D prevention studies, while the vast majority of new-onset studies used mixed meal-stimulated C-peptide AUC, consistent with the consensus recommendations by Palmer and colleagues92. However, precision endpoints were much more variable and would benefit from a similar consensus definition of “responders” to disease-modifying agents within larger trial populations. Strategies applied have included time to diabetes, insulin use, stratification based on changes in C-peptide, and identification of individuals exhibiting less C-peptide loss compared to placebo21,29,61,67. Although C-peptide was by far the most frequent outcome measure used to identify differential treatment responses, approaches to stratify based on C-peptide were highly variable. Consistent approaches, such as a quantifiable metric based on expected values93 will allow better comparison of features associated with treatment response across trials.
A recurring role for age and measures of beta cell function
Age and measures of beta cell function were most frequently identified as factors associated with differential treatment response in primary trial and primary trial follow-up papers. For example, younger age was linked to improved treatment response in several new-onset trials using CD3-based agents54,58,61,87. The association of age with treatment response is in keeping with the strong associations of age to features of T1D in many observational and natural history studies, before and after clinical diagnosis14,94–96. Differences in pancreas histology have been identified in donors with younger age of diagnosis97,98. However, it is unclear whether differences in treatment response linked to age are associated with differences in underlying disease pathophysiology vs. differences in severity or progression of T1D at the time of treatment. The observation that age differentially impacts outcomes in different trials, in addition to stratification of both immune phenotypes and beta cell function by age, supports the idea that the underlying biological reasons for age associations could be linked to mechanisms and are important to consider in future trial designs and potentially in future precision therapy.
Thirty-eight percent of studies testing the impacts of baseline beta cell function showed a significant link to treatment response, consistent with the substantial body of literature identifying an ongoing dialog between autoimmunity and the beta cell in T1D94,99–106. Interestingly, findings somewhat differed depending on the stage of intervention. Here, two unique prevention studies testing oral insulin and teplizumab showed that worse beta cell function was associated with improved treatment outcomes compared to placebo21,24. In contrast, CD3-based therapy trials after disease onset showed an association between higher baseline insulin secretion and improved outcomes54,60,61. These differences highlight the importance of considering the disease stage in the design and interpretation of intervention efforts107. Especially at earlier stages in the disease process, abnormalities in beta cell function could allow insight into a therapeutic window during active disease or immune attack, and optimal timing of therapy108. In contrast, in more advanced diseases after diagnosis, associations with differences in beta cell function could reflect differences in the degree of disease progression, and so amenability to prolonged preservation of a larger residual beta cell mass. Differences in the relationships between beta cell function measures and outcomes for different agents in the new onset period also highlight agent mechanism of action as a critical consideration for designs incorporating beta cell function into the stratification of trial populations and precision approaches to disease-modifying therapy.
Reproducible biomarkers linked to underlying disease pathology
Specific autoantibodies and immune cell phenotypes were also linked to treatment response for multiple agents. An important consideration in these types of assays is reproducibility. The T1D field has been strengthened by an international standardization program for autoantibody measurement that underpinned the development of T1D staging criteria109. If novel mechanistic markers (immune, metabolic, or other) can be used to predict treatment response, then similar scrutiny and standardization of these markers will be needed for cross-study comparisons and successful implementation.
A need for pre-specified, appropriately powered precision analyses
Our review identified important methodologic considerations with many precision analyses. While there were multiple notable and interesting results, most trial manuscripts (primary or follow-up) included precision analyses that were not prespecified, which decreased the quality ranking of these studies. Corrections for multiple comparisons were rare. Additionally, subgroup sizes were infrequently reported, but when available, these group sizes were highly variable and as small as n = 2 participants. Papers also tended to show positive results, raising concern for publication bias.
While these issues are a known limitation of hypothesis-generating exploratory analyses, follow-up studies focusing on testing positive findings a priori will be critical to the application of clinically meaningful precision medicine. An example of the necessity of hypothesis testing was the TrialNet oral insulin prevention study, which was prospectively designed to test a responder subgroup identified in the Diabetes Prevention Trial Type 1 (DPT-1) trial with high insulin autoantibody titers, and ultimately found no significant impact of treatment within this group24. Interestingly, within this trial, a significant protective effect of oral insulin was identified as part of a prespecified precision analysis of individuals with lower first-phase insulin response. Testing in future studies will be needed to understand the reproducibility of this finding. Another example of the application of this approach moving forward is the DIAGNODE 3 study (NCT05018585), which did not meet inclusion criteria for the current review but will prospectively test for a preferential benefit of GAD-alum injections in the HLA DR3-DQ2 population that was identified in post hoc analyses71,110. Based on frequent testing and existing studies suggesting impacts of baseline age and beta cell function as potential features associated with treatment response, pre-specified analyses for appropriately powered studies testing the impact of these precision features should be considered in trials moving forward.
Impacts of the T1D staging system
Time to T1D was the most consistent primary outcome of T1D prevention studies, but inclusion criteria for these studies varied widely across trials, including combinations of genetic risk, presence of islet autoantibodies, and changes in glycemia and/or beta cell function. Recent progress in understanding the natural history of T1D, particularly the high lifetime risk associated with progression from multiple autoantibodies to clinical T1D111, led to a revision of the definition of T1D to include early stages of disease9,112. Stage 1 and stage 2 T1D are now defined by the presence of multiple autoantibodies without or with dysglycemia, whereas clinical disease is now considered as stage 3 T1D9,113. Because these stages were developed concurrently with many of the trials included in this review, these definitions were not applicable at the time of many of these trials, limiting our ability to apply staging categories to this review. As noted above, the timing of T1D stages impacts study feasibility (rate of progression, participant availability) and may be critical to intervention efficacy, highlighting the importance of considering the disease stage in the design and interpretation of intervention efforts. Moving forward, widespread adoption of the T1D staging system combined with increased screening efforts spurred by the recent positive teplizumab trial in stage 2 T1D may allow for increased numbers of trials in earlier stages of the disease.
Limitations
This study has several limitations. The heterogeneity of included papers limited our ability to perform metanalysis. For feasibility, we restricted our review of primary trials to those enrolling a minimum of 50 total participants. Because of this, some trials were not reviewed, including positive trials testing alefacept114,115 and verapamil116. A large pediatric follow-up trial testing verapamil (positive outcome) and tight metabolic control with a hybrid closed loop (negative outcome) was published after the conclusion of our systematic review117,118. In addition, most studies reviewed did not report data on race or ethnicity. For those that did report these data, populations studied largely identified as non-Hispanic white. Barriers to screening of traditionally underrepresented populations is a recognized issue amongst T1D natural history and intervention studies93,119. This is especially important to address moving forward given the rising incidence of T1D in these populations120.
In summary, our review identified noteworthy progress towards defining effective disease-modifying therapies for T1D but a need for better quality data to support the existence of individual features consistently linked to differences in treatment response. Our findings specifically highlight the need for standardization of precision outcome measures, reproducible biomarkers of disease pathology, and prespecified, adequately powered precision analyses. Reports of future trials would benefit from including adequate details to assess potential risks of bias.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
We thank Krister Aronsson and Maria Bjorklund from Lund University for assistance with database searches and Russell de Souza from McMaster University for advice on critical appraisal. The ADA/EASD Precision Diabetes Medicine Initiative, within which this work was conducted, has received the following support: The Covidence license was funded by Lund University (Sweden) for which technical support was provided by Maria Björklund and Krister Aronsson (Faculty of Medicine Library, Lund University, Sweden). Administrative support was provided by Lund University (Malmö, Sweden), the University of Chicago (IL, USA), and the American Diabetes Association (Washington D.C., USA). The Novo Nordisk Foundation (Hellerup, Denmark) provided grant support for in-person writing group meetings (P.I.: L Phillipson, University of Chicago, IL). J.F.: DiabDocs K12 program 1K12DK133995-01 (DiMeglio, Maahs PIs), The Leona M. & Harry B. Helmsley CharitableTrust Grant #2307-06126 (Felton PI). KG: The Leona M. and Harry B. Helmsley Charitable Trust and Sanford Health. R.A.O.: RAO had a UK MRC confidence in concept award to develop a type 1 diabetes GRS biochip with Randox R&D and has ongoing research funding from Randox; and has research funding from a Diabetes UK Harry Keen Fellowship (16/0005529), National Institute of Diabetes and Digestive and Kidney Diseases grants (NIH R01 DK121843–01 and U01DK127382–01), JDRF (3-SRA-2019–827-S-B, 2-SRA-2022–1261-S-B, 2-SRA-2002–1259-S-B, 3-SRA-2022–1241-S-B, and 2-SRA-2022–1258-M-B), and The Larry M and Leona B Helmsley Charitable Trust; and is supported by the National Institute for Health and Care Research Exeter Biomedical Research Centre. The views expressed are those of the author(s) and not necessarily those of the National Institutes for Health Research or the Department of Health and Social Care. L.A.D.: NIH for TrialNet U01DK106993/6163-1082-00-BO, DiabDocs K12 program 1K12DK133995-01, CTSI UL1TR001108-01, C.E.M.: R01DK093954, R01DK127236, U01DK127786, R01DK127308, and UC4DK104166, U54DK118638, P30 P30 DK097512), a US Department of Veterans Affairs Merit Award (I01BX001733), grants from the JDRF (3-IND-2022-1235-I-X) and Helmsley Charitable Trust (2207-05392), and gifts from the Sigma Beta Sorority, the Ball Brothers Foundation, and the George and Frances Ball Foundation. HI: K23DK129799; RJ: NIH R03-DK127472 and The Leona M. and Harry B. Helmsley Charitable Trust (2103-05094); S.A.L.: NIH NIAID R01 AI141952 (PI), NIH NCI R01 CA231226 (Other support), NIH NIAID 1 R01HL149676 (Other support), NIH NIDDK 1UC4DK117483 (subaward), JDRF 3-SRA-2019-851-M-B; S.O.G.: NIH R01 DK121843–01; S.R.: R01 DK122586, THE LEONA M AND HARRY B HELMSLEY CHARITABLE TRUST 2204-05134; J.W.: JDRF 2-SRA-2022-1282-M-X, 3-SRA-2022-1095-M-B, 4-SRA-2022-1246-M-N, 3-SRA-2023-1374-M-N.; M.R.: NIH NIDDK R01DK124395 and R01DK121843; R01DK121929A1, R01DK133881, U01DK127786, U01 DK127382 (E.K.S.). Effort from this grant (to E.K.S., H.I., J.F.) is also supported by Grant 2021258 from the Doris Duke Charitable Foundation through the COVID-19 Fund to Retain Clinical Scientists collaborative grant program and was made possible through the support of Grant 62288 from the John Templeton Foundation. No funders played any role in the design, implementation, or writing of this review.
Author contributions
J.L.F., K.J.G., R.A.O., M.J.R., and E.K.S. designed the project, performed a systematic review, interpreted results, and wrote and edited the manuscript. C.S., S.A.L., S.O.G., S.S.R., G.S.F.M., C.E.M., L.A.D., H.M.I., A.K.S., D.D., R.K.J., M.U., S.G., and J.M.W. contributed to the design of the project, performed a systematic review, and edited the manuscript.
Peer review
Peer review information
Communications Medicine thanks Zdeněk Šumník, Xiaoling Cai and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
All studies reviewed were identified via publicly available databases (PubMed and Embase). All included articles are outlined in Supplementary Information and Supplementary Data 1. Source data for the figures are included in Supplementary Data 2. Article review data supporting the findings of this study are available upon reasonable request from the corresponding author.
Competing interests
E.K.S. has received compensation for educational lectures from Medscape, ADA, and MJH Life Sciences and as a consultant for DRI Healthcare. C.E.M. reported serving on advisory boards for Provention Bio, Isla Technologies, MaiCell Technologies, Avotres, DiogenyX, and Neurodon; receiving in-kind research support from Bristol Myers Squibb and Nimbus Pharmaceuticals; and receiving investigator-initiated grants from Lilly Pharmaceuticals and Astellas Pharmaceuticals. L.A.D. reports research support to institutions from Dompe, Lilly, Mannkind, Provention, Zealand, and consulting relationships with Abata and Vertex. R.A.O. had a UK MRC Confidence in concept grant to develop a T1D GRS biochip with Randox Ltd and has ongoing research funding from Randox R & D. No other authors report any relevant conflicts of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors jointly supervised this work: Maria J. Redondo, Emily K. Sims.
A list of authors and their affiliations appears at the end of the paper.
Contributor Information
Emily K. Sims, Email: eksims@iu.edu
ADA/EASD PMDI:
Deirdre K. Tobias, Jordi Merino, Abrar Ahmad, Catherine Aiken, Jamie L. Benham, Dhanasekaran Bodhini, Amy L. Clark, Kevin Colclough, Rosa Corcoy, Sara J. Cromer, Daisy Duan, Jamie L. Felton, Ellen C. Francis, Pieter Gillard, Véronique Gingras, Romy Gaillard, Eram Haider, Alice Hughes, Jennifer M. Ikle, Laura M. Jacobsen, Anna R. Kahkoska, Jarno L. T. Kettunen, Raymond J. Kreienkamp, Lee-Ling Lim, Jonna M. E. Männistö, Robert Massey, Niamh-Maire Mclennan, Rachel G. Miller, Mario Luca Morieri, Jasper Most, Rochelle N. Naylor, Bige Ozkan, Kashyap Amratlal Patel, Scott J. Pilla, Katsiaryna Prystupa, Sridharan Raghavan, Mary R. Rooney, Martin Schön, Zhila Semnani-Azad, Magdalena Sevilla-Gonzalez, Pernille Svalastoga, Wubet Worku Takele, Claudia Ha-ting Tam, Anne Cathrine B. Thuesen, Mustafa Tosur, Amelia S. Wallace, Caroline C. Wang, Jessie J. Wong, Jennifer M. Yamamoto, Katherine Young, Chloé Amouyal, Mette K. Andersen, Maxine P. Bonham, Mingling Chen, Feifei Cheng, Tinashe Chikowore, Sian C. Chivers, Christoffer Clemmensen, Dana Dabelea, Adem Y. Dawed, Aaron J. Deutsch, Laura T. Dickens, Linda A. DiMeglio, Monika Dudenhöffer-Pfeifer, Carmella Evans-Molina, María Mercè Fernández-Balsells, Hugo Fitipaldi, Stephanie L. Fitzpatrick, Stephen E. Gitelman, Mark O. Goodarzi, Jessica A. Grieger, Marta Guasch-Ferré, Nahal Habibi, Torben Hansen, Chuiguo Huang, Arianna Harris-Kawano, Heba M. Ismail, Benjamin Hoag, Angus G. Jones, Robert W. Koivula, Aaron Leong, Gloria K. W. Leung, Ingrid M. Libman, Kai Liu, William L. Lowe, Jr., Robert W. Morton, Ayesha A. Motala, Suna Onengut-Gumuscu, James S. Pankow, Maleesa Pathirana, Sofia Pazmino, Dianna Perez, John R. Petrie, Camille E. Powe, Alejandra Quinteros, Rashmi Jain, Debashree Ray, Mathias Ried-Larsen, Zeb Saeed, Vanessa Santhakumar, Sarah Kanbour, Sudipa Sarkar, Gabriela S. F. Monaco, Denise M. Scholtens, Elizabeth Selvin, Wayne Huey-Herng Sheu, Maggie A. Stanislawski, Nele Steenackers, Andrea K. Steck, Norbert Stefan, Julie Støy, Rachael Taylor, Sok Cin Tye, Gebresilasea Gendisha Ukke, Marzhan Urazbayeva, Bart Van der Schueren, Camille Vatier, John M. Wentworth, Wesley Hannah, Sara L. White, Gechang Yu, Yingchai Zhang, Shao J. Zhou, Jacques Beltrand, Michel Polak, Ingvild Aukrust, Elisa de Franco, Sarah E. Flanagan, Kristin A. Maloney, Andrew McGovern, Janne Molnes, Mariam Nakabuye, Pål Rasmus Njølstad, Hugo Pomares-Millan, Michele Provenzano, Cécile Saint-Martin, Cuilin Zhang, Yeyi Zhu, Sungyoung Auh, Russell de Souza, Andrea J. Fawcett, Chandra Gruber, Eskedar Getie Mekonnen, Emily Mixter, Diana Sherifali, Robert H. Eckel, John J. Nolan, Louis H. Philipson, Rebecca J. Brown, Liana K. Billings, Kristen Boyle, Tina Costacou, John M. Dennis, Jose C. Florez, Anna L. Gloyn, Maria F. Gomez, Peter A. Gottlieb, Siri Atma W. Greeley, Kurt Griffin, Andrew T. Hattersley, Irl B. Hirsch, Marie-France Hivert, Korey K. Hood, Jami L. Josefson, Soo Heon Kwak, Lori M. Laffel, Siew S. Lim, Ruth J. F. Loos, Ronald C. W. Ma, Chantal Mathieu, Nestoras Mathioudakis, James B. Meigs, Shivani Misra, Viswanathan Mohan, Rinki Murphy, Richard Oram, Katharine R. Owen, Susan E. Ozanne, Ewan R. Pearson, Wei Perng, Toni I. Pollin, Rodica Pop-Busui, Richard E. Pratley, Leanne M. Redman, Rebecca M. Reynolds, Robert K. Semple, Jennifer L. Sherr, Emily K. Sims, Arianne Sweeting, Tiinamaija Tuomi, Miriam S. Udler, Kimberly K. Vesco, Tina Vilsbøll, Robert Wagner, Stephen S. Rich, and Paul W. Franks
Supplementary information
The online version contains supplementary material available at 10.1038/s43856-023-00357-y.
References
- 1.DiMeglio LA, Evans-Molina C, Oram RA. Type 1 diabetes. Lancet. 2018;391:2449–2462. doi: 10.1016/S0140-6736(18)31320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sims EK, Carr ALJ, Oram RA, DiMeglio LA, Evans-Molina C. 100 years of insulin: celebrating the past, present and future of diabetes therapy. Nat. Med. 2021;27:1154–1164. doi: 10.1038/s41591-021-01418-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Livingstone SJ, et al. Estimated life expectancy in a Scottish cohort with type 1 diabetes, 2008-2010. JAMA. 2015;313:37–44. doi: 10.1001/jama.2014.16425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pambianco G, et al. The 30-year natural history of type 1 diabetes complications: the Pittsburgh Epidemiology of Diabetes Complications Study experience. Diabetes. 2006;55:1463–1469. doi: 10.2337/db05-1423. [DOI] [PubMed] [Google Scholar]
- 5.Sussman M, Benner J, Haller MJ, Rewers M, Griffiths R. Estimated lifetime economic burden of type 1 diabetes. Diabetes Technol. Ther. 2020;22:121–130. doi: 10.1089/dia.2019.0398. [DOI] [PubMed] [Google Scholar]
- 6.Gubitosi-Klug, R. A. et al. Residual β cell function in long-term type 1 diabetes associates with reduced incidence of hypoglycemia. J. Clin. Invest. 131, 10.1172/jci143011 (2021). [DOI] [PMC free article] [PubMed]
- 7.Lachin JM, McGee P, Palmer JP. Impact of C-peptide preservation on metabolic and clinical outcomes in the Diabetes Control and Complications Trial. Diabetes. 2014;63:739–748. doi: 10.2337/db13-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bogun MM, Bundy BN, Goland RS, Greenbaum CJ. C-peptide levels in subjects followed longitudinally before and after type 1 Diabetes Diagnosis in TrialNet. Diabetes Care. 2020;43:1836–1842. doi: 10.2337/dc19-2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Insel RA, et al. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care. 2015;38:1964–1974. doi: 10.2337/dc15-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Besser, R. E. J. et al. ISPAD clinical practice consensus guidelines 2022: stages of type 1 diabetes in children and adolescents. Pediatr. Diabetes10.1111/pedi.13410 (2022). [DOI] [PubMed]
- 11.American Academy of Pediatrics. FDA Approves First Drug That Can Delay Onset of Type 1 Diabetes, https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-can-delay-onset-type-1-diabetes (2023).
- 12.Tobias, D. K. et al. Second international consensus report on gaps and opportunities for the clinical translation of precision diabetes medicine. Nat. Med. (in press), 10.1038/s41591-023-02502-5. [DOI] [PMC free article] [PubMed]
- 13.Chung WK, et al. Precision medicine in diabetes: a Consensus Report from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2020;63:1671–1693. doi: 10.1007/s00125-020-05181-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Battaglia M, et al. Introducing the endotype concept to address the challenge of disease heterogeneity in type 1 diabetes. Diabetes Care. 2020;43:5–12. doi: 10.2337/dc19-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson RL, et al. Innovative designs and logistical considerations for expedited clinical development of combination disease-modifying treatments for type 1 diabetes. Diabetes Care. 2022;45:2189–2201. doi: 10.2337/dc22-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simmons, K. M. & Sims, E. K. Screening and prevention of type 1 diabetes: where are we? J. Clin. Endocrinol. Metab.10.1210/clinem/dgad328 (2023). [DOI] [PMC free article] [PubMed]
- 17.Diabetes Prevention Trial—Type 1 Diabetes Study Group. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N. Engl. J. Med.346, 1685–1691 (2002). [DOI] [PubMed]
- 18.Butty V, Campbell C, Mathis D, Benoist C. Impact of diabetes susceptibility loci on progression from pre-diabetes to diabetes in at-risk individuals of the diabetes prevention trial-type 1 (DPT-1) Diabetes. 2008;57:2348–2359. doi: 10.2337/db07-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elding Larsson H, Lundgren M, Jonsdottir B, Cuthbertson D, Krischer J. Safety and efficacy of autoantigen-specific therapy with 2 doses of alum-formulated glutamate decarboxylase in children with multiple islet autoantibodies and risk for type 1 diabetes: a randomized clinical trial. Pediatr. Diabetes. 2018;19:410–419. doi: 10.1111/pedi.12611. [DOI] [PubMed] [Google Scholar]
- 20.Gale EA, Bingley PJ, Emmett CL, Collier T. European Nicotinamide Diabetes Intervention Trial (ENDIT): a randomised controlled trial of intervention before the onset of type 1 diabetes. Lancet. 2004;363:925–931. doi: 10.1016/S0140-6736(04)15786-3. [DOI] [PubMed] [Google Scholar]
- 21.Herold KC, et al. An anti-CD3 antibody, teplizumab, in relatives at risk for type 1 diabetes. N. Engl. J. Med. 2019;381:603–613. doi: 10.1056/NEJMoa1902226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hummel S, Pflüger M, Hummel M, Bonifacio E, Ziegler AG. Primary dietary intervention study to reduce the risk of islet autoimmunity in children at increased risk for type 1 diabetes: the BABYDIET study. Diabetes Care. 2011;34:1301–1305. doi: 10.2337/dc10-2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knip M, et al. Effect of hydrolyzed infant formula vs conventional formula on risk of type 1 diabetes. JAMA. 2018;319:38. doi: 10.1001/jama.2017.19826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krischer JP, Schatz DA, Bundy B, Skyler JS, Greenbaum CJ. Effect of oral insulin on prevention of diabetes in relatives of patients with type 1 diabetes: a randomized clinical trial. JAMA. 2017;318:1891–1902. doi: 10.1001/jama.2017.17070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lampeter EF, et al. The Deutsche Nicotinamide Intervention Study: an attempt to prevent type 1 diabetes. DENIS group. Diabetes. 1998;47:980–984. doi: 10.2337/diabetes.47.6.980. [DOI] [PubMed] [Google Scholar]
- 26.Näntö-Salonen K, et al. Nasal insulin to prevent type 1 diabetes in children with HLA genotypes and autoantibodies conferring increased risk of disease: a double-blind, randomised controlled trial. Lancet. 2008;372:1746–1755. doi: 10.1016/S0140-6736(08)61309-4. [DOI] [PubMed] [Google Scholar]
- 27.Sims, E. K. et al. Teplizumab improves and stabilizes beta cell function in antibody-positive high-risk individuals. Sci. Transl. Med.13, 10.1126/scitranslmed.abc8980 (2021). [DOI] [PMC free article] [PubMed]
- 28.Skyler JS, et al. Effects of oral insulin in relatives of patients with type 1 diabetes: The Diabetes Prevention Trial–Type 1. Diabetes Care. 2005;28:1068–1076. doi: 10.2337/diacare.28.5.1068. [DOI] [PubMed] [Google Scholar]
- 29.Sosenko JM, et al. Slowed metabolic decline after 1 year of oral insulin treatment among individuals at high risk for type 1 diabetes in the Diabetes Prevention Trial-Type 1 (DPT-1) and TrialNet Oral Insulin Prevention Trials. Diabetes. 2020;69:1827–1832. doi: 10.2337/db20-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vaarala O, et al. Removal of bovine insulin from cow’s milk formula and early initiation of beta-cell autoimmunity in the FINDIA pilot study. Arch. Pediatr. Adolesc. Med. 2012;166:608–614. doi: 10.1001/archpediatrics.2011.1559. [DOI] [PubMed] [Google Scholar]
- 31.Vehik K, et al. Long-term outcome of individuals treated with oral insulin: diabetes prevention trial-type 1 (DPT-1) oral insulin trial. Diabetes Care. 2011;34:1585–1590. doi: 10.2337/dc11-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allen HF, et al. Effect of Bacillus Calmette-Guerin vaccination on new-onset type 1 diabetes. A randomized clinical study. Diabetes Care. 1999;22:1703–1707. doi: 10.2337/diacare.22.10.1703. [DOI] [PubMed] [Google Scholar]
- 33.Ambery P, et al. Efficacy and safety of low-dose otelixizumab anti-CD3 monoclonal antibody in preserving C-peptide secretion in adolescent type 1 diabetes: DEFEND-2, a randomized, placebo-controlled, double-blind, multi-centre study. Diabet Med. 2014;31:399–402. doi: 10.1111/dme.12361. [DOI] [PubMed] [Google Scholar]
- 34.Aronson R, et al. Low-dose otelixizumab anti-CD3 monoclonal antibody DEFEND-1 study: results of the randomized phase III study in recent-onset human type 1 diabetes. Diabetes Care. 2014;37:2746–2754. doi: 10.2337/dc13-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ataie-Jafari A, et al. A randomized placebo-controlled trial of alphacalcidol on the preservation of beta cell function in children with recent onset type 1 diabetes. Clin. Nutr. 2013;32:911–917. doi: 10.1016/j.clnu.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 36.Buckingham B, et al. Effectiveness of early intensive therapy on β-cell preservation in type 1 diabetes. Diabetes Care. 2013;36:4030–4035. doi: 10.2337/dc13-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cabrera SM, et al. Innate immune activity as a predictor of persistent insulin secretion and association with responsiveness to CTLA4-Ig treatment in recent-onset type 1 diabetes. Diabetologia. 2018;61:2356–2370. doi: 10.1007/s00125-018-4708-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chaillous L, et al. Oral insulin administration and residual beta-cell function in recent-onset type 1 diabetes: a multicentre randomised controlled trial. Diabète Insuline Orale group. Lancet. 2000;356:545–549. doi: 10.1016/s0140-6736(00)02579-4. [DOI] [PubMed] [Google Scholar]
- 39.Christie MR, Mølvig J, Hawkes CJ, Carstensen B, Mandrup-Poulsen T. IA-2 antibody-negative status predicts remission and recovery of C-peptide levels in type 1 diabetic patients treated with cyclosporin. Diabetes Care. 2002;25:1192–1197. doi: 10.2337/diacare.25.7.1192. [DOI] [PubMed] [Google Scholar]
- 40.Coutant R, et al. Low dose linomide in Type I juvenile diabetes of recent onset: a randomised placebo-controlled double blind trial. Diabetologia. 1998;41:1040–1046. doi: 10.1007/s001250051028. [DOI] [PubMed] [Google Scholar]
- 41.Crinò A, et al. A randomized trial of nicotinamide and vitamin E in children with recent onset type 1 diabetes (IMDIAB IX) Eur. J. Endocrinol. 2004;150:719–724. doi: 10.1530/eje.0.1500719. [DOI] [PubMed] [Google Scholar]
- 42.Demeester S, et al. Preexisting insulin autoantibodies predict efficacy of otelixizumab in preserving residual β-cell function in recent-onset type 1 diabetes. Diabetes Care. 2015;38:644–651. doi: 10.2337/dc14-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diggins, K. E. et al. Exhausted-like CD8+ T cell phenotypes linked to C-peptide preservation in alefacept-treated T1D subjects. JCI Insight6, 10.1172/jci.insight.142680 (2021). [DOI] [PMC free article] [PubMed]
- 44.Eichmann M, et al. Costimulation blockade disrupts cD4(+) T cell memory pathways and uncouples their link to decline in beta-cell function in type 1 diabetes. J. Immunol. 2020;204:3129–3138. doi: 10.4049/jimmunol.1901439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Enander R, et al. Beta cell function after intensive subcutaneous insulin therapy or intravenous insulin infusion at onset of type 1 diabetes in children without ketoacidosis. Pediatr. Diabetes. 2018;19:1079–1085. doi: 10.1111/pedi.12657. [DOI] [PubMed] [Google Scholar]
- 46.Ergun-Longmire B, et al. Oral insulin therapy to prevent progression of immune-mediated (type 1) diabetes. Ann. N. Y. Acad. Sci. 2004;1029:260–277. doi: 10.1196/annals.1309.057. [DOI] [PubMed] [Google Scholar]
- 47.Gitelman SE, et al. Imatinib therapy for patients with recent-onset type 1 diabetes: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Diabetes Endocrinol. 2021;9:502–514. doi: 10.1016/S2213-8587(21)00139-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gitelman SE, et al. Antithymocyte globulin therapy for patients with recent-onset type 1 diabetes: 2 year results of a randomised trial. Diabetologia. 2016;59:1153–1161. doi: 10.1007/s00125-016-3917-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gitelman SE, et al. Antithymocyte globulin treatment for patients with recent-onset type 1 diabetes: 12-month results of a randomised, placebo-controlled, phase 2 trial. Lancet Diabetes Endocrinol. 2013;1:306–316. doi: 10.1016/S2213-8587(13)70065-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gottlieb PA, et al. Failure to preserve beta-cell function with mycophenolate mofetil and daclizumab combined therapy in patients with new-onset type 1 diabetes. Diabetes Care. 2010;33:826–832. doi: 10.2337/dc09-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Greenbaum, C. J. et al. IL-6 receptor blockade does not slow beta cell loss in new-onset type 1 diabetes. JCI Insight6, 10.1172/jci.insight.150074 (2021). [DOI] [PMC free article] [PubMed]
- 52.Griffin KJ, Thompson PA, Gottschalk M, Kyllo JH, Rabinovitch A. Combination therapy with sitagliptin and lansoprazole in patients with recent-onset type 1 diabetes (REPAIR-T1D): 12-month results of a multicentre, randomised, placebo-controlled, phase 2 trial. Lancet Diabetes Endocrinol. 2014;2:710–718. doi: 10.1016/S2213-8587(14)70115-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Groele, L. et al. Lack of effect of Lactobacillus rhamnosus GG and Bifidobacterium lactis Bb12 on beta-cell function in children with newly diagnosed type 1 diabetes: a randomised controlled trial. BMJ Open Diabetes Res. Care9, 10.1136/bmjdrc-2020-001523 (2021). [DOI] [PMC free article] [PubMed]
- 54.Hagopian W, et al. Teplizumab preserves C-peptide in recent-onset type 1 diabetes: two-year results from the randomized, placebo-controlled Protégé trial. Diabetes. 2013;62:3901–3908. doi: 10.2337/db13-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haller MJ, et al. Low-dose anti-thymocyte globulin preserves c-peptide, reduces HbA(1c), and increases regulatory to conventional t-cell ratios in new-onset type 1 diabetes: two-year clinical trial data. Diabetes. 2019;68:1267–1276. doi: 10.2337/db19-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haller MJ, et al. Low-dose anti-thymocyte globulin (ATG) preserves β-cell function and improves HbA(1c) in new-onset type 1 diabetes. Diabetes Care. 2018;41:1917–1925. doi: 10.2337/dc18-0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Herold KC, et al. Teplizumab (anti-CD3 mAb) treatment preserves C-peptide responses in patients with new-onset type 1 diabetes in a randomized controlled trial: metabolic and immunologic features at baseline identify a subgroup of responders. Diabetes. 2013;62:3766–3774. doi: 10.2337/db13-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Herold KC, et al. Teplizumab treatment may improve C-peptide responses in participants with type 1 diabetes after the new-onset period: a randomised controlled trial. Diabetologia. 2013;56:391–400. doi: 10.1007/s00125-012-2753-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Herold KC, et al. Increased T cell proliferative responses to islet antigens identify clinical responders to anti-CD20 monoclonal antibody (rituximab) therapy in type 1 diabetes. J. Immunol. 2011;187:1998–2005. doi: 10.4049/jimmunol.1100539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Keymeulen B, et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N. Engl. J. Med. 2005;352:2598–2608. doi: 10.1056/NEJMoa043980. [DOI] [PubMed] [Google Scholar]
- 61.Keymeulen B, et al. Four-year metabolic outcome of a randomised controlled CD3-antibody trial in recent-onset type 1 diabetic patients depends on their age and baseline residual beta cell mass. Diabetologia. 2010;53:614–623. doi: 10.1007/s00125-009-1644-9. [DOI] [PubMed] [Google Scholar]
- 62.Kumar S, et al. A high potency multi-strain probiotic improves glycemic control in children with new-onset type 1 diabetes mellitus: a randomized, double-blind, and placebo-controlled pilot study. Pediatr. Diabetes. 2021;22:1014–1022. doi: 10.1111/pedi.13244. [DOI] [PubMed] [Google Scholar]
- 63.Lagarde WH, et al. Human plasma-derived alpha(1)-proteinase inhibitor in patients with new-onset type 1 diabetes mellitus: a randomized, placebo-controlled proof-of-concept study. Pediatr. Diabetes. 2021;22:192–201. doi: 10.1111/pedi.13162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lebenthal, Y. et al. A phase II, double-blind, randomized, placebo-controlled, multicenter study evaluating the efficacy and safety of alpha-1 antitrypsin (AAT) (Glassia(®)) in the treatment of recent-onset type 1 diabetes. Int. J. Mol. Sci. 20, 10.3390/ijms20236032 (2019). [DOI] [PMC free article] [PubMed]
- 65.Linsley PS, et al. Elevated T cell levels in peripheral blood predict poor clinical response following rituximab treatment in new-onset type 1 diabetes. Genes Immun. 2019;20:293–307. doi: 10.1038/s41435-018-0032-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Linsley, P. S., Greenbaum, C. J., Speake, C., Long, S. A. & Dufort, M. J. B lymphocyte alterations accompany abatacept resistance in new-onset type 1 diabetes. JCI Insight4, 10.1172/jci.insight.126136 (2019). [DOI] [PMC free article] [PubMed]
- 67.Long, S. A. et al. Partial exhaustion of CD8 T cells and clinical response to teplizumab in new-onset type 1 diabetes. Sci. Immunol. 1, 10.1126/sciimmunol.aai7793 (2016). [DOI] [PMC free article] [PubMed]
- 68.Long SA, et al. Remodeling T cell compartments during anti-CD3 immunotherapy of type 1 diabetes. Cell Immunol. 2017;319:3–9. doi: 10.1016/j.cellimm.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ludvigsson J, et al. GAD65 antigen therapy in recently diagnosed type 1 diabetes mellitus. N. Engl. J. Med. 2012;366:433–442. doi: 10.1056/NEJMoa1107096. [DOI] [PubMed] [Google Scholar]
- 70.Ludvigsson, J. et al. Combined vitamin D, ibuprofen and glutamic acid decarboxylase-alum treatment in recent onset Type I diabetes: Lessons from the DIABGAD randomized pilot trial. Fut. Sci. OA6, 10.2144/fsoa-2020-0078 (2020). [DOI] [PMC free article] [PubMed]
- 71.Ludvigsson J, et al. Intralymphatic glutamic acid decarboxylase with vitamin d supplementation in recent-onset type 1 diabetes: a double-blind, randomized, placebo-controlled phase IIb trial. Diabetes Care. 2021;44:1604–1612. doi: 10.2337/dc21-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Martin S, et al. Residual beta cell function in newly diagnosed type 1 diabetes after treatment with atorvastatin: the Randomized DIATOR Trial. PLoS ONE. 2011;6:e17554. doi: 10.1371/journal.pone.0017554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moran A, et al. Interleukin-1 antagonism in type 1 diabetes of recent onset: two multicentre, randomised, double-blind, placebo-controlled trials. Lancet. 2013;381:1905–1915. doi: 10.1016/S0140-6736(13)60023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nafei LT, Kadhim KA, Said AM, Ghani SH. Evaluation the effect of vitamin D3 on glycemic indices on Iraqi children with type 1 DM. Int. J. Pharmaceut. Sci. Rev. Res. 2017;42:134–143. [Google Scholar]
- 75.Orban T, et al. Reduction in CD4 central memory T-cell subset in costimulation modulator abatacept-treated patients with recent-onset type 1 diabetes is associated with slower C-peptide decline. Diabetes. 2014;63:3449–3457. doi: 10.2337/db14-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Orban T, et al. Costimulation modulation with abatacept in patients with recent-onset type 1 diabetes: follow-up 1 year after cessation of treatment. Diabetes Care. 2014;37:1069–1075. doi: 10.2337/dc13-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Orban T, et al. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;378:412–419. doi: 10.1016/S0140-6736(11)60886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ortqvist E, et al. Temporary preservation of beta-cell function by diazoxide treatment in childhood type 1 diabetes. Diabetes Care. 2004;27:2191–2197. doi: 10.2337/diacare.27.9.2191. [DOI] [PubMed] [Google Scholar]
- 79.Pescovitz MD, et al. B-lymphocyte depletion with rituximab and β-cell function: two-year results. Diabetes Care. 2014;37:453–459. doi: 10.2337/dc13-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pescovitz MD, et al. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N. Engl. J. Med. 2009;361:2143–2152. doi: 10.1056/NEJMoa0904452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pitocco D, et al. The effects of calcitriol and nicotinamide on residual pancreatic beta-cell function in patients with recent-onset Type 1 diabetes (IMDIAB XI) Diabet. Med. 2006;23:920–923. doi: 10.1111/j.1464-5491.2006.01921.x. [DOI] [PubMed] [Google Scholar]
- 82.Pozzilli, P. et al. Randomized 52-week phase 2 trial of albiglutide versus placebo in adult PAtients with Newly Diagnosed Type 1 diabetes. J. Clin. Endocrinol. Metab.105, 10.1210/clinem/dgaa149 (2020). [DOI] [PubMed]
- 83.Pozzilli P, et al. No effect of oral insulin on residual beta-cell function in recent-onset type I diabetes (the IMDIAB VII). IMDIAB Group. Diabetologia. 2000;43:1000–1004. doi: 10.1007/s001250051482. [DOI] [PubMed] [Google Scholar]
- 84.Pozzilli P, et al. Vitamin E and nicotinamide have similar effects in maintaining residual beta cell function in recent onset insulin-dependent diabetes (the IMDIAB IV study) Eur. J. Endocrinol. 1997;137:234–239. doi: 10.1530/eje.0.1370234. [DOI] [PubMed] [Google Scholar]
- 85.Quattrin T, et al. Golimumab and beta-cell function in youth with new-onset type 1 diabetes. N. Engl. J. Med. 2020;383:2007–2017. doi: 10.1056/NEJMoa2006136. [DOI] [PubMed] [Google Scholar]
- 86.Rother KI, et al. Effect of ingested interferon-alpha on beta-cell function in children with new-onset type 1 diabetes. Diabetes Care. 2009;32:1250–1255. doi: 10.2337/dc08-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sherry N, et al. Teplizumab for treatment of type 1 diabetes (Protégé study): 1-year results from a randomised, placebo-controlled trial. Lancet. 2011;378:487–497. doi: 10.1016/S0140-6736(11)60931-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Strom A, et al. Improved preservation of residual beta cell function by atorvastatin in patients with recent onset type 1 diabetes and high CRP levels (DIATOR trial) PLoS ONE. 2012;7:e33108. doi: 10.1371/journal.pone.0033108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.von Herrath M, et al. Anti-interleukin-21 antibody and liraglutide for the preservation of β-cell function in adults with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Diabetes Endocrinol. 2021;9:212–224. doi: 10.1016/S2213-8587(21)00019-X. [DOI] [PubMed] [Google Scholar]
- 90.Walter M, Philotheou A, Bonnici F, Ziegler AG, Jimenez R. No effect of the altered peptide ligand NBI-6024 on beta-cell residual function and insulin needs in new-onset type 1 diabetes. Diabetes Care. 2009;32:2036–2040. doi: 10.2337/dc09-0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wherrett DK, et al. Antigen-based therapy with glutamic acid decarboxylase (GAD) vaccine in patients with recent-onset type 1 diabetes: a randomised double-blind trial. Lancet. 2011;378:319–327. doi: 10.1016/S0140-6736(11)60895-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Palmer JP, et al. C-peptide is the appropriate outcome measure for type 1 diabetes clinical trials to preserve beta-cell function: report of an ADA workshop, 21-22 October 2001. Diabetes. 2004;53:250–264. doi: 10.2337/diabetes.53.1.250. [DOI] [PubMed] [Google Scholar]
- 93.Bundy BN, Krischer JP, Type 1 Diabetes TrialNet Study, G. A quantitative measure of treatment response in recent-onset type 1 diabetes. Endocrinol. Diabetes Metab. 2020;3:e00143. doi: 10.1002/edm2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sims EK, et al. Elevations in the fasting serum proinsulin-to-c-peptide ratio precede the onset of type 1 diabetes. Diabetes Care. 2016;39:1519–1526. doi: 10.2337/dc15-2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Krischer JP, et al. Genetic and environmental interactions modify the risk of diabetes-related autoimmunity by 6 years of age: the TEDDY Study. Diabetes Care. 2017;40:1194–1202. doi: 10.2337/dc17-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.So M, et al. Characterising the age-dependent effects of risk factors on type 1 diabetes progression. Diabetologia. 2022;65:684–694. doi: 10.1007/s00125-021-05647-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Leete P, et al. Studies of insulin and proinsulin in pancreas and serum support the existence of aetiopathological endotypes of type 1 diabetes associated with age at diagnosis. Diabetologia. 2020;63:1258–1267. doi: 10.1007/s00125-020-05115-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Carr ALJ, et al. Circulating C-peptide levels in living children and young people and pancreatic beta-cell loss in pancreas donors across type 1 diabetes disease duration. Diabetes. 2022;71:1591–1596. doi: 10.2337/db22-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Eizirik DL, et al. The human pancreatic islet transcriptome: expression of candidate genes for type 1 diabetes and the impact of pro-inflammatory cytokines. PLoS Genet. 2012;8:e1002552. doi: 10.1371/journal.pgen.1002552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ramos-Rodriguez M, et al. The impact of proinflammatory cytokines on the beta-cell regulatory landscape provides insights into the genetics of type 1 diabetes. Nat. Genet. 2019;51:1588–1595. doi: 10.1038/s41588-019-0524-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Juan-Mateu J, Villate O, Eizirik DL. Mechanisms in endocrinology: alternative splicing: the new frontier in diabetes research. Eur. J. Endocrinol. 2016;174:R225–R238. doi: 10.1530/EJE-15-0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gonzalez-Duque, S. et al. Conventional and neo-antigenic peptides presented by beta cells are targeted by circulating naive CD8+ T Cells in type 1 diabetic and healthy donors. Cell Metab. 10.1016/j.cmet.2018.07.007 (2018). [DOI] [PubMed]
- 103.Kracht MJ, et al. Autoimmunity against a defective ribosomal insulin gene product in type 1 diabetes. Nat. Med. 2017;23:501–507. doi: 10.1038/nm.4289. [DOI] [PubMed] [Google Scholar]
- 104.Marre ML, et al. Modifying enzymes are elicited by er stress, generating epitopes that are selectively recognized by CD4(+) T cells in patients with type 1 diabetes. Diabetes. 2018;67:1356–1368. doi: 10.2337/db17-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Thompson PJ, et al. Targeted elimination of senescent beta cells prevents type 1 diabetes. Cell Metab. 2019;29:1045–1060.e1010. doi: 10.1016/j.cmet.2019.01.021. [DOI] [PubMed] [Google Scholar]
- 106.Sims EK, et al. Proinsulin secretion is a persistent feature of type 1 diabetes. Diabetes Care. 2019;42:258–264. doi: 10.2337/dc17-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Habib T, et al. Dynamic immune phenotypes of b and t helper cells mark distinct stages of T1D progression. Diabetes. 2019;68:1240–1250. doi: 10.2337/db18-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chatenoud L, Primo J, Bach JF. CD3 antibody-induced dominant self tolerance in overtly diabetic NOD mice. J. Immunol. 1997;158:2947–2954. [PubMed] [Google Scholar]
- 109.Marzinotto I, et al. Islet Autoantibody Standardization Program: interlaboratory comparison of insulin autoantibody assay performance in 2018 and 2020 workshops. Diabetologia. 2023;66:897–912. doi: 10.1007/s00125-023-05877-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hannelius U, Beam CA, Ludvigsson J. Efficacy of GAD-alum immunotherapy associated with HLA-DR3-DQ2 in recently diagnosed type 1 diabetes. Diabetologia. 2020;63:2177–2181. doi: 10.1007/s00125-020-05227-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ziegler AG, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA. 2013;309:2473–2479. doi: 10.1001/jama.2013.6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.ElSayed NA, et al. 2. Classification and diagnosis of diabetes: standards of care in diabetes-2023. Diabetes Care. 2023;46:S19–S40. doi: 10.2337/dc23-S002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Greenbaum CJ, et al. Strength in numbers: opportunities for enhancing the development of effective treatments for type 1 diabetes-The TrialNet Experience. Diabetes. 2018;67:1216–1225. doi: 10.2337/db18-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rigby MR, et al. Alefacept provides sustained clinical and immunological effects in new-onset type 1 diabetes patients. J. Clin. Invest. 2015;125:3285–3296. doi: 10.1172/JCI81722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rigby MR, et al. Targeting of memory T cells with alefacept in new-onset type 1 diabetes (T1DAL study): 12 month results of a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Diabetes Endocrinol. 2013;1:284–294. doi: 10.1016/S2213-8587(13)70111-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ovalle F, et al. Verapamil and beta cell function in adults with recent-onset type 1 diabetes. Nat. Med. 2018;24:1108–1112. doi: 10.1038/s41591-018-0089-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Forlenza, G. P. et al. Effect of verapamil on pancreatic beta cell function in newly diagnosed pediatric type 1 diabetes: a randomized clinical trial. JAMA10.1001/jama.2023.2064 (2023). [DOI] [PMC free article] [PubMed]
- 118.McVean, J. et al. Effect of tight glycemic control on pancreatic beta cell function in newly diagnosed pediatric type 1 diabetes: a randomized clinical trial. JAMA10.1001/jama.2023.2063 (2023). [DOI] [PMC free article] [PubMed]
- 119.Sims EK, et al. Who Is Enrolling? The path to monitoring in type 1 diabetes TrialNet’s pathway to prevention. Diabetes Care. 2019;42:2228–2236. doi: 10.2337/dc19-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Divers JM-D, et al. Trends in incidence of type 1 and type 2 diabetes among youths—selected counties and indian reservations, United States, 2002–2015. MMWR Morb. Mortal. Wkly. Rep. 2020;69:161–165. doi: 10.15585/mmwr.mm6906a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
All studies reviewed were identified via publicly available databases (PubMed and Embase). All included articles are outlined in Supplementary Information and Supplementary Data 1. Source data for the figures are included in Supplementary Data 2. Article review data supporting the findings of this study are available upon reasonable request from the corresponding author.