Abstract
COVID-19 pandemic has been managed through global vaccination programs. However, the antibody waning in various types of vaccines came to notice. Hereby, PastoCovac Plus as a protein subunit vaccine was investigated in immunized health care workers by COVAXIN (BBV152). The booster vaccine was recommended at least three months post the second dose of COVAXIN. Sera collection was done before and after each injection. SARS-CoV-2 PCR test was done monthly to detect any asymptomatic and symptomatic vaccine breakthrough.
47.9 and 24.3% of the participants were seronegative for anti-N and anti-S antibodies three months after the second dose of COVAXIN, respectively. On average, fold-rises of 70, 93, 8 and mean-rises of 23.32, 892.4, 5.59 were recorded regarding neutralizing antibody, quantitative and semi-quantitative anti-Spike antibody, respectively. Anti-Spike and neutralizing antibodies seroconversion was seen 59.3% and 45.7%, respectively. The vaccine breakthrough assessment showed that all the isolated samples belonged to SARS-CoV-2 Delta variant. PastoCovac Plus boosting is strongly recommended in combination with inactivated vaccine platforms against SARS-CoV-2.
Keywords: PastoCovac plus, SARS-CoV-2, COVID-19, Protein-subunit vaccine, Prime-boost, Iran
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has affected human population for more than three years as the cause of COVID-19 pandemic [[1], [2], [3], [4]]. The emerged infection resulted in an emergency need of vaccination which led to examination different platforms including adenovirus-vectored, nucleic-acid-based, recombinant-protein-based and inactivated virus-based vaccines [[5], [6], [7], [8]]. Inactivated vaccines have been licensed to overcome viral diseases for decades with successful safety profiles [9,10]. It has been shown that inactivated virion-based vaccines can generate neutralizing antibodies against SARS-CoV-2 which has a crucial role in vaccine efficacy. Thus, the development of these vaccines to prevent COVID-19 has been a rational approach based on other successful mix-and-match strategies against other pathogens [11,12]. However, the persistence and level of the generated antibodies in vaccinated individuals and also the cell-mediated immune response are two major elements of a well-established vaccine [13,14].
COVAXIN (BBV152) was developed by Bharat Biotech, India as a COVID-19 vaccine based on SARS-CoV-2 NIV-2020-770 strain which is inactivated with β-propiolactone [15]. This vaccine has been found effective in strong humoral immune response induction in many preclinical and also clinical studies [[16], [17], [18]]. However, some studies showed that protective antibodies are not persistent in some individuals after two doses of COVAXIN or even other vaccine types [19,20]. Therefore, the third dose of a vaccine was strongly suggested mostly 3–6 months after the second dose as the new variants of SARS-CoV-2 appeared [21].
Soberana 02 and Soberana Plus vaccines are developed and produced at the Finlay Vaccine Institute of Cuba and manufactured in Pasteur Institute of Iran as PastoCovac and PastoCovac Plus after a successful technology transfer. PastoCovac (the primary vaccine series) is a recombinant protein vaccine composed of a highly immunogenic region of SARS-CoV-2 Spike (RBD) conjugated to the tetanus toxin which completed clinical trials in Cuba [22]. PastoCovac Plus is the booster vaccine [dimer of RBD (50 μg)] [[22], [23], [24]]. The results of phase III clinical trials in Cuba showed that heterologous combination led to a significant protection against COVID-19 development in which only 15 of 13833 vaccine recipients got infected vs. 155 of 14303 in placebo group. The study proved that the vaccine efficacy against severe form of COVID-19 and the mortality rate as 63.0% and 59.0% for the two-dose schedule, respectively. The mentioned rates reached to a remarkable increase in the heterologous three-dose schedule as 100% in both disease severity and the associated death. In total, Soberana Plus resulted in vaccine immunogenicity enhancement to 92.4% (Clinical Trials IFV/COR/09 number, RPCEC00000354) [22].
Iran conducted a clinical trial phase III on nearly 24000 adults, 18–80 years old, in 8 cities the vaccine efficacy was assessed 76.8% for prevention of severe disease and 77.7% for hospitalization after the 2-dose regimen of PastoCovac, which improved to 96.6% for both factors after the booster dose of PastoCovac Plus [25]. Moreover, PastoCovac Plus has shown to have the potency to reactive the pre-existing immune response after priming with BBIP-CorV [26].
At the early stage of COVID-19 vaccination program, COVAXIN was among the applied vaccines in Iran for the population above 18 years old. The PastoCovac Plus vaccine, got the emergency approval in July 2021 to be produced and applied as the booster for all the vaccine types in Iran [27]. In this study, we studied COVAXIN immunized Iranian health workers to evaluate vaccine immunogenicity as well as the escaped variants determination in a 6–11 months follow-up. Moreover, PastoCovac Plus was applied in volunteers, at least three months after the second dose of COVAXIN, and the specific antibodies were tracked.
2. Materials and methods
2.1. Study design and vaccination
This study was an observational study without randomization, conducted from July 2021 to June 2022 during which 6 months active screening was followed by a 5-month self-reporting. An invitation announcement was sent to health care workers (HCWs) of Pasteur Institute of Iran and about 250 individuals got two doses of COVAXIN. From those, 152 ones agreed to participate the study and complete it. The participants were immunized by COVAXIN according to the manufacturer's instruction in which injections by a 28-day interval was recommended after signing the informed consent form.
Serum samples were collected one month (28 ± 5 days) after the second dose of COVAXIN (this sampling was done after the peak of Alpha variant and before the peak of the Delta variant in Iran). All the enrolled subjects were provided with the written informed consent. Of the HCWs, those who were on treatment with immunosuppressive therapy and also pregnant women were not investigated. The participants were aware of the results through a phone contact.
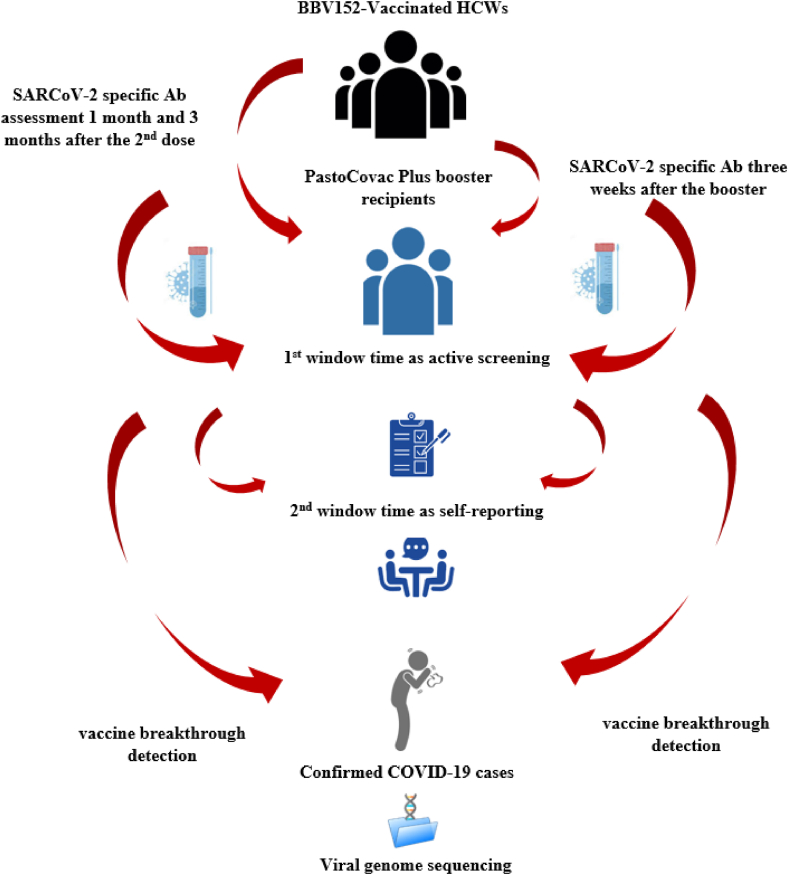
Individuals who didn't show any antibody responses one month following the second dose of COVAXIN were invited to receive PastoCovac Plus vaccine as a booster dose three months after the mentioned time. Moreover, the individuals who lost antibody titer or had remarkable antibody decline were also advised to get the booster dose at least three months after the second dose. In order to evaluate the immunogenicity of the booster dose, sera samples were obtained from individuals before and three weeks after the vaccine shot. The aliquots sera samples were stored frozen at −20 °C for further analyses. Fig. 1 simply illustrates the study process.
Fig. 1.
A summary of study design and follow-up.
The study protocol complied with the Helsinki declaration and was approved by the ethical committee of Pasteur Institute of Iran (IR.PII.REC.1400.043 Ethic code).
2.2. Serologic tests
Evaluation of humoral immune response was carried out on collected sera samples using three different assays including IgG/IgM rapid test cassette [Orient Gene Biotech, Zhejiang, China, (Sensitivity: 95.8%, Specificity: 100%)] and then anti-SARS-CoV-2 ELISA [Anti-Neucleocapcid IgG (Sensitivity: 94.1%, Specificity: 98.3%)] test (Pishtazteb, Iran), anti-SARS-CoV-2 ELISA Anti Spike-1 IgG [EUROIMMUN, Germany (Sensitivity: 94.4%, Specificity: 99.6%)] and SARS-CoV-2 Neutralizing Antibody kit [Pishtazteb, Iran (Sensitivity: 94.1%, Specificity: 98.3%)]. Antibody assessment was done during the six-month follow-up as: one month and 3 months after the second dose of COVAXIN, before and after the PastoCovac Plus booster shot.
During the follow-up, the voluntary vaccinated individuals got a booster of PastoCovac Plus vaccine. The anti-SARS-CoV-2 ELISA (IgG) (Anti Spike-1) [EUROIMMUN, Germany (Sensitivity: 94.4%, Specificity: 99.6%)], SARS-CoV-2 Neutralizing Antibody kit (Pishtazteb, Iran) and anti-SARS-CoV-2 QuantiVac ELISA (IgG) [EUROIMMUN, Germany (Sensitivity: 93.2%, Specificity: 99.8%)] were applied before the booster injection and also three weeks after it to evaluate the booster induction. Two different tests as quantitative and semi-quantitative tests were applied regarding anti-Spike to achieve more accurate results.
Antibodies Cut-off for positive result consideration were as: Anti-N Antibody>1.1, Anti-S Antibody (semi-quantitative) >1.1, Neutralizing Antibody >2.5 and Anti SARS-CoV-2 IgG QuantiVac (quantitative) > 11.
2.3. Neutralization assay
Conventional neutralizing antibody titers (cVNT50) was conducted on randomly selected samples [28]. Briefly, the samples were inactivated for 30 min at 56 °C. Vero cells were seeded in DMEM including 10% FBS. Serial dilution of sera samples was prepared. To achieve that, 10 microtubes were prepared and labeled. 100 μl of the serum sample combined with 100 μl DMEM in the first microtube. In order to make the virus dilution, 10 μl of the main sample (virus stock) was transferred to the 990 μl DMEM to make the working vial. 100 μl of the working and 900 μl DMEM were mixed and 50 μl of this mixture was added to each well. For each well, 100 TCID50 of SARS-CoV-2 was considered. The plate was incubated at room temperature for an hour. The prepared mixture was added into the wells containing monolayers of Vero cells for 60 min incubation at 37 °C. The control wells also were defined as: 1) the wells without the mixture of serum and virus, 2) one well without any serums, and 3) one well without any cells. After the incubation time and supernatant removal, the cells were washed with DMEM. After 72 h of maintaining in DMEM at 37 °C, the CPE (cytopathic effect) was assessed applying an inverted microscope and the titer of neutralizing antibodies was evaluated in accordance to the highest serum dilution in which the virus was neutralized in 50% of the wells [28].
2.4. COVID-19 molecular test
All the subjects were evaluated by SARS-CoV-2 PCR test monthly in order to detect any asymptomatic and symptomatic breakthrough. This was achieved as an active screening during the first 6-month follow-up. After this period, it was also considered according to self-reporting and PCR-documented results by the patients. Nasopharyngeal swabs were taken and viral RNA extraction was performed by the RNJia Virus Kit (ROJE Technologies, Iran) according to the manufacturer's instructions. Real-Time Reverse-Transcription PCR (Real-Time RT-PCR) assay was done using 2019-nCoV Nucleic Acid Diagnostic kit (Sansure biotech, Changsha, China), according to the manufacturer's protocol [2,29]. To determine the mutation pattern and the variant characterization of the detected SARS-CoV-2, whole-genome sequencing of the virus was performed by the GridION NGS system (Oxford Nanopore Technologies, UK), the Midnight RT PCR Expansion (Oxford Nanopore Technologies, UK) and the Rapid Barcoding Kit 96 (Oxford Nanopore Technologies, UK). Finally, the sequence analysis was performed.
2.5. Statistical analysis
All statistical analyses were performed using SPSS statistics software (version 26.0). Descriptive analysis was reported in mean (and standard deviation) and median (and interquartile range) for quantitative variables and frequency and percentage for qualitative variables according to the provided cut-off in the manufacturer's guide. Chi-square test or Fisher's exact were applied to test the unrelated categorical variables and McNemar test for related categorical variables. Wilcoxon signed-rank test was used for nonparametric related titer responses during different sampling. Seroconversion of antibodies was defined as fourfold rise of the antibodies compared to the baseline. All the tests were considered as two-way and a p-value <0.05 was reported statistically significant. The graphs were plotted by GraphPad Prism software (Version 8).
3. Results
152 HCWs from Pasteur Institute of Iran were recruited including 59.2% female (n = 90) and 40.8% male (n = 62) with a mean age of 38.04 ± 8.76 years (min:22, max:59). At least one underlying disease was reported in 12 (7.9%) people, in which Cardio Vascular Disease (CVD) and thyroid dysfunction were the most frequent. Less than half (45.4%) of the participants had a history of SARS-CoV-2 infection before the first dose of COVAXIN and two individuals had COVID-19 positive PCR between two doses of COVAXIN along with 28.3% who had a history of allergy.
All the participants had been immunized against SARS-CoV-2 by two doses of COVAXIN at a 28-day interval. Local pain (23.0%), fever (13.8%) and headache (9.2%) were the most common adverse events after the vaccination.
PastoCovac Plus was injected to 81 individuals (according to the recommendation or their choice) including 48 females and 33 males with a mean age of 38.2 ± 9.0 years (min:22, max:57). Local pain (18.5%) and weakness (11.1%) were the most common events after the booster application. In fact, 60 (39.5%) and 27 (34.8%) of the studied population had at least one adverse event after COVAXIN and PastoCovac Plus, respectively.
4. Antibody assessment
4.1. COVAXIN immunogenicity
The results show that one and three months after the second dose of COVAXIN, 28.3% and 47.9% of the participants were seronegative for anti-N antibody. Moreover, 15.1% of individuals were seronegative for anti-SARS-CoV-2-Spike IgG one month after the second dose of COVAXIN which increased to 24.3% three months after the second dose (Table 1). It is to say that, during the follow-up observation, vaccinated subjects with COAVXIN with a low level of Abs were likely to be seronegative in 3 months. In order to identify the seronegative individuals and subsequently the booster candidates, the individuals were categorized based on the Abs level. The applied category simply provide us to recommend a booster shot at the right time.
Table 1.
Descriptive analysis of immune response after COVAXIN and paired analysis of antibody titer at first and third assessment after full vaccination.
| Antibody Type | 30 days after the second dose of COVAXIN | 90 days after the second dose of COVAXIN | p-value derived from Wilcoxon Rank test |
|---|---|---|---|
| Anti-SARS-CoV-2 Antibody, Rapid test | N = 151 | N = 148 | <0.01 |
| Negative | 82(54.3%) | 97 (65.5%) | |
| Positive | 69 (45.7%) | 51 (34.5%) | |
| Anti-Nucleocapsid Antibody | N = 152 | N = 146 | |
| Median (IQR; 25%-75%) | 2.5 (0.9–4.6) | 1.3 (0.5–3.1) | <0.001 |
| < 1.1 | 43 (28.3%) | 70 (47.9%) | |
| 1.1< Antibody <3.0 | 49 (32.2%) | 36 (24.7%) | |
| 3.0< Antibody<10 | 55 (36.2%) | 36 (24.7%) | |
| >10 | 5(3.3%) | 4 (2.7%) | |
| Anti-Spike Antibody | N = 152 | N = 148 | |
| Median (IQR; 25%-75%) | 3.1 (1.6–5.1) | 2.6 (1.1–4.7) | <0.001 |
| ≤1.1 | 23 (15.1%) | 36 (24.3%) | |
| 1.1<Antibody≤3.0 | 46 (30.3%) | 45 (30.4%) | |
| 3.0<Antibody≤10 | 82(53.9%) | 59 (39.9%) | |
| >10 | 1 (0.7%) | 8 (5.4%) | |
| Neutralizing Antibody | N = 152 | N = 147 | |
| Median (IQR; 25%-75%) | 25.4 (13.1–31.9) | 12.2 (4.6–24.4) | <0.001 |
| < 2.5 | 11 (7.2) | 29 (22.4%) | |
| 2.5<Antibody<10 | 14 (9.2) | 33 (22.4) | |
| >10 | 127 (83.6) | 85 (57.8%) |
The median of anti-N, anti-Spike and neutralizing antibodies decreased significantly 3 months post-vaccination (P < 0.001) in comparison with the results one month after the vaccination (Wilcoxon signed-rank test).
The patients’ history was investigated in which previous infection with SARS-CoV-2, and the antibody rise association were analyzed. The study results showed that there was a significant difference among the two groups in terms of anti-Spike, anti-N and neutralizing antibodies positivity one month and 3 months after the second dose of vaccination. In other words, the prior COVID-19 history led to a better antibody induction in both window times (p < 0.05).
4.2. PastoCovac plus immunogenicity
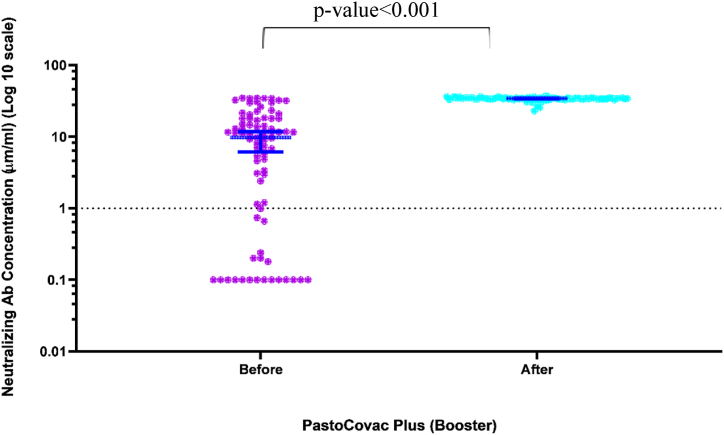
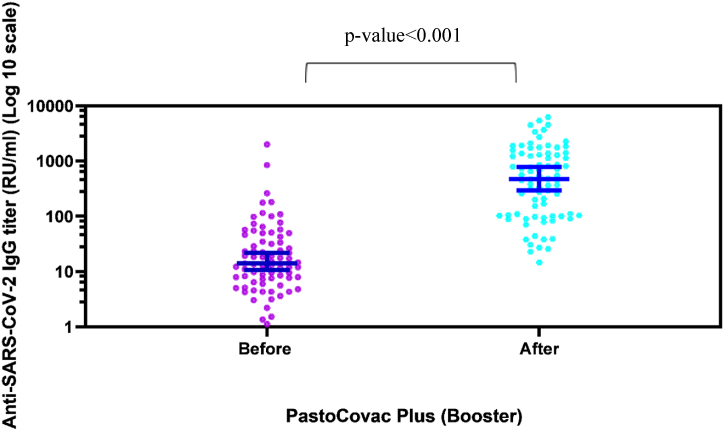
In order to assess anti-Spike antibody more accurately, we applied two different ELISA tests as semi-quantitative and quantitative before and after the booster shot. As Table 2 and Figs. 2 &3 show, the booster shot recipients reached a significant rise in anti-Spike and neutralizing antibody which especially was remarkable regarding neutralizing antibody. What is more, anti-Spike and neutralizing antibody became positive in all the seronegative population (see Fig. 3).
Table 2.
Analysis of immune response before and after PastoCovac Plus booster injection.
| Features | Before booster injection | After booster injection | p-value |
|---|---|---|---|
| Anti-Spike Antibody (semi-quantitative) | N = 81 | N = 81 | |
| Median (IQR; 25%-75%) | 1.6 (0.8–3.2) | 8.9 (7.3–10.3) | <0.001 |
| ≤1.1 | 24 (29.6) | 0 (0.0) | |
| 1.1<Antibody≤3.0 | 34 (42.0) | 0 (0.0) | |
| 3.0< Antibody≤10 | 20 (24.7) | 54 (66.7) | |
| >10 | 3 (3.7) | 27 (33.3) | |
| Neutralizing Antibody | N = 81 | N = 81 | |
| Median (IQR; 25%-75%) | 9.8 (0.9–18.0) | 34.1 (33.6–34.8) | <0.001 |
| < 2.5 | 24 (29.6) | 0 (0) | |
| 2.5< Antibody<10 | 17 (21.0) | 0 (0) | |
| >10 | 40 (49.4) | 81 (100.0) | |
| Anti SARS-CoV-2 IgG QuantiVac (quantitative) | N = 81 | N = 81 | |
| Median (IQR; 25%-75%) | 14.3 (7.5–47.1) | 472.6 (103.1–1372.1) | <0.001 |
| ≤11 | 30 (39.0) | 0 (0.0) | |
| >11.01 | 47 (61) | 81 (100) |
Fig. 2.
Scatter diagram of neutralizing antibody titers before and after injection of PastoCovac Plus as a booster. Median and 95% confidence intervals have been shown on the diagram.
Fig. 3.
Scatter diagram of specific IgG antibody responses against S antigen before and after PastoCovac Plus injection as a booster. Median and 95% confidence intervals have been shown on the diagram.
The mean of anti-SARS-CoV-2 QuantiVac ELISA (IgG) before the booster injection was 67.69 ± 245.95 which raised to 956.74 ± 1254.84 in booster recipients. In addition, the mean of neutralizing antibody before the booster shot was 11.49 ± 10.92 which raised to 33.80 ± 2.14.
On average, fold-rises of 70, 93, 8 and mean-rises of 23.32, 892.4, 5.59 were recorded regarding neutralizing antibody, quantitative and semi-quantitative anti-Spike antibody, respectively.
Anti-Spike antibody seroconversion (four-fold rise over the baseline) was seen in 48 cases (59.3%) who got the booster dose. In addition, four-fold increase of neutralizing antibody was achieved in 37 (45.7%) cases.
4.3. Virus netralization assay
The virus neutralization potency was assessed through cVNT50 test on 6 randomly selected sera samples of immunized individuals after the PastoCovac Plus booster shot against Wuhan variant. The control group was not available according to the fact that no one got COVAXIN as a booster shot. The results showed that all the sera samples included antibodies with neutralization potency against Wuhan variant (Average Ab titer 406.6 AU/ml).
4.4. Vaccine breakthrough detection
According to Real-Time PCR results, SARS-CoV-2 breakthrough infection was obserevd in pariticipants as: Totally 42 individuals of all participants developed COVID-19 after the second dose of COVAXIN, from whom 26 (62%) were captured in the first 6-month (active screening), 16 (38%) were screened in the second window time (based self-reporting), and five people experienced the infection twice in the follow-up (once in each period).
18 individuals developed COVID-19 after PastoCovac Plus booster dose in a long-time follow-up.
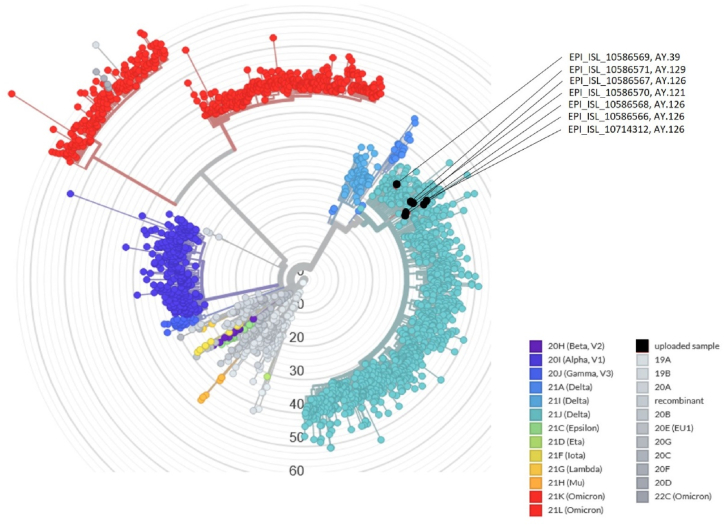
In total, all the vaccine breakthrough cases (COVAXIN and PastoCovac Plus) were symptomatic except one. Nevertheless, the vaccine-breakthrough experienced cases only developed a mild to moderate form of the infection after the booster shot which indicates the importance of an on-time booster injection in prevention of the severe form of the disease. Mild symptoms included headache, low-grade fever, sore throat, runny nose, fatigue and mild body pain which lasted less than 2–3 days. SARS-CoV-2 whole genome sequencing analysis was performed randomly on seven accessible samples with suitable viral load for sequencing (N gene Ct value < 25). The analysis of sequences of SARS-CoV-2 breakthrough infection was performed by the sarscov2Phylo pipeline. The sequence's placement in the phylogenetic tree is performed by Ultrafast Sample placement on Existing tRee (UShER) [30]. The results revealed that all analyzed samples from breakthrough events were characterized as Delta variants of SARS-CoV-2 (Fig. 4). AY.126 was the most common variant (4 samples), one sample was characterized as AY.39, one sample was AY.121 and one sample belonged to AY.129.
Fig. 4.
The phylogenic tree of breakthrough viral samples. The sequence's placement in the phylogenic tree is performed by Ultrafast Sample placement on Existing tRee (UShER).
5. Discussion
Vaccination has been considered as the most effective prophylactic strategy against a variety of infectious diseases due to neutralizing antibody induction and cellular immunity activation. The recent COVID-19 chaotic pandemic has resulted in the development of a multitude of vaccine approaches in which whole inactivated virus type was firstly launched. An optimal vaccine against SARS-CoV-2 should generate specific long-lasting antibodies and would induce immunity to prevent the disease and also the virus transmission [31]. Nevertheless, studies indicated that COVID-19 antibodies might wane after a while in many people post recovery [1,2,32,33] or some months after vaccination [34].
In the present study, of 152 vaccinated individuals by BBV152, 15.1% were seronegative for anti SARS-CoV-2 Spike IgG one month after the second dose which then reached to 24.3% two months later. Moreover, anti-N waned in 28.3% and 47.9% of individuals one and three months after the second dose. In parallel to our observation, a significant decline of anti-S antibody was seen in Shrotri et al. study as a five-fold reduction for ChAdOx1 vaccine (p < 0.001) and a two-fold for BNT162b2 vaccine (p < 0.001) between 21 and 41 days and >70 days post the second dose of both vaccines [34].
Therefore, observational studies on duration of protection and emerging data consistently present a decline in vaccine effectiveness against SARS-CoV-2. In the context of WHO advice in support of booster vaccinations [35] and according to the potentially rapid antibody waning suggested by the published data, heterologous regimens have been considered to elicit stronger antibody and also T-cell responses [36].
Homologous boosters have been shown effective in some studies. BNT162b2 and mRNA-1273 vaccines led to neutralizing antibody titers enhancement against wild-type SARS-CoV-2 and also against the delta variant [37,38]. Nevertheless, it is suggested that heterologous regimens may extend the persistence and longevity of protection provided by primary vaccine doses.
In the other study, we also found that boosting through homologous regimens BBIP-CorV/BBIP-CorV or ChAdOx1/ChAdOx1could be effective, however, a booster dose of a protein subunit vaccine led to a higher antibody generation in individuals (under review data).
Heterologous schedules have the privilage of clinical efficacy and flexibility in supply, which facilitate the application for countries with limited vaccine access or those with different vaccines beside the potential of a stronger response. Furthermore, mixing vaccines migh be necessary with new SARS-CoV-2 variants [39,40].
PastoCovac Plus, a protein subunit vaccine, was investigated as the booster dose in this study which significantly re-induced specific antibodies against COVID-19. Anti-S antibody median before the booster injection was 14.3 which then raised to 472.6 after the application of PastoCovac Plus. In parallel with this significant change, neutralizing antibody showed the median (IQR; 25%–75%) of 34.1 after the booster dose which is dramatically higher than the pre-booster median (IQR; 25%–75%) titration of 9.8.
In a study by Zhang et al., BBIBP-CorV (inactivated vaccine) was applied as a two-dose inactivated vaccine in mice and ZF2001 (protein subunit vaccine) was applied as the booster in parallel with other regimens. The findings showed the highest level of RBD-specific IgG, anti-spike IgG and neutralizing antibodies in BBIBP- BBIBP- ZF2001 immunized group [41]. In the next study, protein subunit vaccine (ZF2001) was administered during a 4–8-month interval in healthy individuals who were primed by two doses of inactivated whole virus vaccines CoronaVac or BBIBP-CorV. The results showed significant increase in neutralizing antibody in addition to high induction of anti-RBD IgG, 14 days post booster injection [42].
In a similar study, ChAdOx1 nCoV-19 booster was evaluated after two shots of CoronaVac in a group of HCWs. The mean anti-spike IgG level one month after the third dose vaccination increased to 2499.89 BAU/mL, which was significantly higher than the baseline IgG level (84.88 BAU/mL; p < 0.001). Nevertheless, anti-spike IgG level decreased to 822.37 BAU/mL, three months post-vaccination. Furthermore, neutralizing antibody was assessed at the same window time in which it increased one month after the booster from the baseline of 20.61%–98.3% (p < 0.001) and was still high three months post vaccination (93.87%, p < 0.001) [43]. Thus, similar to our findings, a different platform of booster significantly boosted specific antibodies after two shots of an inactivated virion vaccine.
From another point of view, the evaluated heterologous regimen in the present data was totally safe and well-tolerated in which the local pain at the injection site and weakness were the most common side effects. Long-term assessment of the vaccine adverse events in a 180-day follow-also proved that were no significant differences between the homologous and heterologous vaccine regimens regarding late adverse events triggered by PastoCovac/PastoCovac Plus booster doses [44].
Overall, the generated vaccines against COVID-19 are presenting well safety profiles among which protein-based type vaccine has shown to have a great potency of anti-Spike and neutralizing antibodies induction [45,46]. PastoCovac Plus, investigated in the present study, has been evaluated notably efficient in specific SARS-CoV-2 antibody enhancement which could be applied as the booster of inactivated-viral vaccines worldwide.
The present study lacks a control group due to the fact that COVAXIN was not applied as a booster dose in Iran. The other limitation could be the platform of booster vaccination as only protein-based type. Other platforms could be evaluated and compared to come up with the most proper type of the booster vaccine. Moreover, there was not a control group in parallel according to the type of the study as an observational one.
6. Conclusions
A different vaccine platform as a booster might provide more durable immune response and a better protection against SARS-CoV-2 variants. Hereby, we showed that PastoCovac Plus as a protein-based vaccine could effectively stimulate specific antibodies against SARS-CoV-2 after two-dose of an inactivated-viral type vaccine, COVAXIN. All the vaccinated subjects in this study who received the booster shot, achieved high titration of the specific antibodies. PastoCovac Plus vaccine could be highly recommended as a safe and effective booster dose in immunized people with inactivated vaccine type. According to the obtained data in this study, it has a high potency to enhance humoral immune responses in terms of anti-S, anti-N and also neutralizing antibodies even in seronegative vaccinated individuals. Furthermore, according to this study, the safety profile of PastoCovac Plus as a booster vaccine in combination with an inactivated vaccine was established.
Funding sources
This study was financially supported by Pasteur Institute of Iran, Tehran, Iran (grant number: 2012).
Author contribution statement
Behrokh Farahmand: Conceived and designed the experiments, Mona Sadat Larijani: Interpreted the data; wrote and revised the manuscript, Fatemeh Fotouhi: Conceptualized the study and administrated the project, Alireza Biglari: Provided technical support, Rahim Sorouri: Provided technical support, Fahimeh Bagheri Amiri: Provided the methodology and data analysis, Ali Eslamifar: Investigated the study, Tahmineh Jalali: Provided the molecular tests analysis, Mostafa Salehi-Vaziri: Provided technical support, Mohammad Banifazl: Contributed to the data curation, Sarah Dahmardeh: Managed and screened the population study; Provided the sampling, Azita Eshratkhah Mohammadnejad: Performed experiments and data, Anahita Bavand: Performed the experiments and managed the data, Mahsa Tavakoli: Contributed to the experiments, Vicente Verez-Bencomo: Project consultant, Ehsan Mostafavi: Contributed to methodology, Hassan Noori Daloii: Contributed to data collection, Fatemeh Ashrafian: Contributed to data curation and writing, Masoumeh Saberpour: Contributed to data collection, Amitis Ramezani: Investigated the study; administrated the project and revised the manuscript.
Data availability
The Data will be available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We do appreciate Pasteur Insttute of Iran for financial support of this study.
Contributor Information
Behrokh Farahmand, Email: b_farahmand@pasteur.ac.ir.
Mona Sadat Larijani, Email: mona.sadat@gmail.com.
Fatemeh Fotouhi, Email: fotouhi@pasteur.ac.ir.
Alireza Biglari, Email: biglari63@hotmail.com.
Rahim Sorouri, Email: rsorouriz@gmail.com.
Fahimeh Bagheri Amiri, Email: fahimehbagheriamiri@gmail.com.
Ali Eslamifar, Email: aeslami@pasteur.ac.ir.
Tahmineh Jalali, Email: jalalitahmineh@gmail.com.
Mostafa Salehi-Vaziri, Email: mostafavaziri1985@gmail.com.
Mohammad Banifazl, Email: mohammadbanifazl@aol.com.
Sarah Dahmardeh, Email: sarahdahmardeh@gmail.com.
Azita Eshratkhah Mohammadnejad, Email: Azi4453@gmail.com.
Anahita Bavand, Email: anahita.bavand@gmail.com.
Mahsa Tavakoli, Email: mahsa.tavakolirad@gmail.com.
Vicente Verez-Bencomo, Email: vicente.verez@finlay.edu.cu.
Ehsan Mostafavi, Email: mostafaviehsan@gmail.com.
Hassan Noori Daloii, Email: hnooridaloii@yahoo.com.
Fatemeh Ashrafian, Email: fatemeh.ashrafian24@gmail.com.
Masoumeh Saberpour, Email: masssabera@gmail.com.
Amitis Ramezani, Email: amitisramezani@hotmail.com.
References
- 1.Salehi-Vaziri M., Pouriayevali M.H., Fotouhi F., Jalali T., Banifazl M., Farahmand B., et al. SARS-CoV-2 re-infection rate in Iranian COVID-19 cases within one-year follow-up. Microb. Pathog. 2021;161 doi: 10.1016/j.micpath.2021.105296. 2021/12/01/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mostafa Salehi-Vaziri T.J., Farahmand Behrokh, Fotouhi Fatemeh, Banifazl Mohammad, Hassan Pouriayevali Mohammad, Sadat Larijani Mona, Neda Afzali, Ramezani Amitis. Clinical characteristics of SARS-CoV-2 by Re-infection vs. Reactivation: a case series from Iran. Eur. J. Clin. Microbiol. Infect. Dis. 2021 doi: 10.1007/s10096-021-04221-6. 2021/02/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sadat Larijani M., Ashrafian F., Bagheri Amiri F., Banifazl M., Bavand A., Karami A., et al. Characterization of long COVID-19 manifestations and its associated factors: a prospective cohort study from Iran. Microb. Pathog. 2022 Aug;169 doi: 10.1016/j.micpath.2022.105618. PubMed PMID: 35690233. Pubmed Central PMCID: PMC9176176. Epub 2022/06/12. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sofian M., Khansarinejad B., Ghaznavi-Rad E., Shokoohi F., Mazaherpour H., Farmani F., et al. SARS-CoV-2 viral shedding and associated factors among COVID-19 inpatients and outpatients. Interdisciplinary Perspectives on Infectious Diseases. 2022;2022 doi: 10.1155/2022/1411106. 2022/06/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sofian M., Velayati A.A., Banifazl M., Fotouhi F., Sadat Larijani M., Afzali N., et al. SARS-CoV-2, a virus with many faces: a series of cases with prolonged persistence of COVID-19 symptoms. Wien Med. Wochenschr. 2020 2020/12/14;171:3–6. doi: 10.1007/s10354-020-00793-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fotouhi F., Salehi-Vaziri M., Farahmand B., Mostafavi E., Pouriayevali M.H., Jalali T., et al. Prolonged viral shedding and antibody persistence in patients with COVID-19. Microb. Infect. 2021 doi: 10.1016/j.micinf.2021.104810. 2021/03/17:104810. Pubmed Central PMCID: PMC7963517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Islam M.F., Cotler J., Jason L.A. Post-viral fatigue and COVID-19: lessons from past epidemics. Fatigue: Biomedicine, Health & Behavior. 2020;8(2):61–69. 2020/04/02. [Google Scholar]
- 8.Sadat S.M., Aghadadeghi M.R., Yousefi M., Khodaei A., Sadat Larijani M., Bahramali G. Bioinformatics analysis of SARS-CoV-2 to approach an effective vaccine candidate against COVID-19. Mol. Biotechnol. 2021;63(5):389–409. doi: 10.1007/s12033-021-00303-0. 2021/05/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sadarangani M., Marchant A., Kollmann T.R. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat. Rev. Immunol. 2021;21(8):475–484. doi: 10.1038/s41577-021-00578-z. 2021/08/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desai D., Khan A.R., Soneja M., Mittal A., Naik S., Kodan P., et al. Effectiveness of an inactivated virus-based SARS-CoV-2 vaccine, BBV152, in India: a test-negative, case-control study. Lancet Infect. Dis. 2022 Mar;22(3):349–356. doi: 10.1016/S1473-3099(21)00674-5. PubMed PMID: 34826383. Pubmed Central PMCID: PMC8610201. Epub 2021/11/27. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larijani M.S., Sadat S.M., Bolhassani A., Khodaie A., Pouriayevali M.H., Ramezani A. HIV-1 p24-nef DNA vaccine plus protein boost expands T-cell responses in BALB/c. Curr. Drug Deliv. 2020 doi: 10.2174/1567201818666210101113601. 2020/12//. PubMed PMID: 33388019. eng. [DOI] [PubMed] [Google Scholar]
- 12.Mona S.L., Mohammad Hassan P., Seyed Mehdi S., Amitis R. Production of recombinant HIV-1 p24-nef protein in two forms as potential candidate vaccines in three vehicles. Curr. Drug Deliv. 2020;17(5):387–395. doi: 10.2174/1567201817666200317121728. [DOI] [PubMed] [Google Scholar]
- 13.Sanders B., Koldijk M., Schuitemaker H. Vaccine analysis: strategies, principles, and control. Vaccine Analysis: Strategies, Principles, and Control. 2015:45–80. [Google Scholar]
- 14.Ashrafian F., Bagheri Amiri F., Bavand A., Zali M., Sadat Larijani M., Ramezani A. A comparative study of immunogenicity, antibody persistence, and safety of three different COVID-19 boosters between individuals with comorbidities and the normal population. Vaccines. 2023;11(8) doi: 10.3390/vaccines11081376. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ganneru B., Jogdand H., Daram V.K., Das D., Molugu N.R., Prasad S.D., et al. Th1 skewed immune response of whole virion inactivated SARS CoV 2 vaccine and its safety evaluation. iScience. 2021 Apr 23;24(4) doi: 10.1016/j.isci.2021.102298. PubMed PMID: 33723528. Pubmed Central PMCID: PMC7944858. Epub 2021/03/17. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang H., Zhang Y., Huang B., Deng W., Quan Y., Wang W., et al. Development of an inactivated vaccine candidate, BBIBP-CorV, with potent protection against SARS-CoV-2. Cell. 2020 Aug 6;182(3):713–721. doi: 10.1016/j.cell.2020.06.008. e9. PubMed PMID: 32778225. Pubmed Central PMCID: PMC7275151. Epub 2020/08/12. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yadav P.D., Ella R., Kumar S., Patil D.R., Mohandas S., Shete A.M., et al. Immunogenicity and protective efficacy of inactivated SARS-CoV-2 vaccine candidate, BBV152 in rhesus macaques. Nat. Commun. 2021 Mar 2;12(1):1386. doi: 10.1038/s41467-021-21639-w. PubMed PMID: 33654090. Pubmed Central PMCID: PMC7925524. Epub 2021/03/04. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ella R., Reddy S., Blackwelder W., Potdar V., Yadav P., Sarangi V., et al. Efficacy, safety, and lot-to-lot immunogenicity of an inactivated SARS-CoV-2 vaccine (BBV152): interim results of a randomised, double-blind, controlled, phase 3 trial. Lancet. 2021;398(10317):2173–2184. doi: 10.1016/S0140-6736(21)02000-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choudhary H.R., Parai D., Chandra Dash G., Kshatri J.S., Mishra N., Choudhary P.K., et al. Persistence of antibodies against spike glycoprotein of SARS-CoV-2 in healthcare workers post double dose of BBV-152 and AZD1222 vaccines. Front. Med. 2021;8 doi: 10.3389/fmed.2021.778129. PubMed PMID: 35004746. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.ICMR Study Shows Significant Drop in Covid Antibodies within 4 Months after Vaccination. India Today; 2021. [Internet] [cited 2020/04/04] [Google Scholar]
- 21.Interim Statement on Booster Doses for COVID-19 Vaccination [Internet]. World Health Organization. [cited 2022/04/11]..
- 22.Toledo-Romani M.E., Garcia-Carmenate M., Silva C.V., Baldoquin-Rodriguez W., Pérez M.M., Rodríguez Gonzalez M.C., et al. Efficacy and safety of SOBERANA 02, a COVID-19 conjugate vaccine in heterologous three-dose combination. medRxiv. 2021:2021. 10.31.21265703. [Google Scholar]
- 23.Gorry C. SOBERANA, Cuba's COVID-19 vaccine candidates: dagmar garcía-rivera PhD. MEDICC review. 2020 Oct;22(4):10–15. doi: 10.37757/MR2020.V22.N4.11. PubMed PMID: 33295312. Epub 2020/12/10. eng. [DOI] [PubMed] [Google Scholar]
- 24.SOBERANA® Plus [Internet]. Instituto Finlay de Vacunas Cuba. [cited 2022/04/11]. Available from: https://www.finlay.edu.cu/blog/wp-content/uploads/2021/12/Commercial-file-SOBERANA-Plus-Eng.pdf..
- 25.Mostafavi E., Eybpoosh S., Karamouzian M., Khalili M., Haji-Maghsoudi S., Salehi-Vaziri M., et al. Efficacy and safety of a protein-based SARS-CoV-2 vaccine: a randomized clinical trial. JAMA Netw. Open. 2023;6(5) doi: 10.1001/jamanetworkopen.2023.10302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramezani A., Sorouri R., Haji Maghsoudi S., Dahmardeh S., Doroud D., Sadat Larijani M., et al. PastoCovac and PastoCovac Plus as protein subunit COVID-19 vaccines led to great humoral immune responses in BBIP-CorV immunized individuals. Sci. Rep. 2023;13(1):8065. doi: 10.1038/s41598-023-35147-y. 2023/05/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Instituto Finlay de Vacunas Cuba: Soberana 02 [Internet]. COVID19 Vaccine Tracker.[cited 2020/04/11]. Available from: https://covid19.trackvaccines.org/vaccines/52/..
- 28.Manenti A., Maggetti M., Casa E., Martinuzzi D., Torelli A., Trombetta C.M., et al. Evaluation of SARS-CoV-2 neutralizing antibodies using a CPE-based colorimetric live virus micro-neutralization assay in human serum samples. J. Med. Virol. 2020 Oct;92(10):2096–2104. doi: 10.1002/jmv.25986. PubMed PMID: 32383254. Pubmed Central PMCID: PMC7267461. Epub 2020/05/10. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freire-Paspuel B., Garcia-Bereguiain M.A. Clinical performance and analytical sensitivity of three SARS-CoV-2 nucleic acid diagnostic tests. Am. J. Trop. Med. Hyg. 2021 Feb 26;104(4):1516–1518. doi: 10.4269/ajtmh.20-1484. PubMed PMID: 33635827. Pubmed Central PMCID: PMC8045661 procedures, and revision and approval of the final version of the manuscript. Byron Freire-Paspuel and Miguel Angel García Bereguiain analyzed the data and wrote the manuscript. Epub 2021/02/27. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turakhia Y., Thornlow B., Hinrichs A.S., De Maio N., Gozashti L., Lanfear R., et al. Ultrafast Sample placement on Existing tRees (UShER) enables real-time phylogenetics for the SARS-CoV-2 pandemic. Nat. Genet. 2021 Jun;53(6):809–816. doi: 10.1038/s41588-021-00862-7. PubMed PMID: 33972780. Epub 2021/05/12. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sette A., Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184(4):861–880. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Self W.H., Tenforde M.W., Stubblefield W.B., Feldstein L.R., Steingrub J.S., Shapiro N.I., et al. Decline in SARS-CoV-2 antibodies after mild infection among frontline health care personnel in a multistate hospital network - 12 states, april-august 2020. MMWR Morbidity and mortality weekly report. 2020 Nov 27;69(47):1762–1766. doi: 10.15585/mmwr.mm6947a2. PubMed PMID: 33237893. Pubmed Central PMCID: PMC7727600 Journal Editors form for disclosure of potential conflicts of interest. Christopher J. Lindsell reports grants from National Institutes of Health, grants from Department of Defense, Marcus Foundation, Endpoint Health, Entegrion, bioMerieux, and Bioscape Digital, outside the submitted work. Daniel J. Henning reports personal fees from CytoVale and grants from Baxter, outside the submitted work. Akram Khan reports grants from United Therapeutics, Actelion Pharmaceuticals, Regeneron, and Reata Pharmaceuticals, outside the submitted work. Samuel M. Brown reports grants from National Institutes of Health, Department of Defense, Intermountain Research and Medical Foundation, and Janssen; consulting fees paid to his employer from Faron and Sedana, all outside the submitted work. Ithan D. Peltan reports grants from the National Institutes of Health and, outside the submitted work, grants from Asahi Kasei Pharma, Immunexpress Inc., Janssen Pharmaceuticals, and Regeneron. Carlos Grijalva reports other from Pfizer, other from Merck, other from Sanofi-Pasteur, grants from Campbell Alliance, the National Institutes of Health, the Food and Drug Administration, the Agency for Health Care Research and Quality, outside the submitted work. Todd W. Rice reports consulting work for Cumberland Pharmaceuticals, Inc, outside the submitted work. H. Keipp Talbot has served on a data safety and monitoring board for Seqirus. Natasha Halasa receives grant support from Quidel and Sanofi, donation of vaccines and hemagglutination inhibition/microneutralization titers performed by Sanofi, and speaker fees by an educational grant supported by Genentech, outside the submitted work. No other potential conflicts of interest were disclosed. Epub 2020/11/26. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salehi-Vaziri M., Omrani M.D., Pouriayevali M.H., Fotouhi F., Banifazl M., Farahmand B., et al. SARS-CoV-2 presented moderately during two episodes of the infection with lack of antibody responses. Virus Res. 2021;299 doi: 10.1016/j.virusres.2021.198421. 2021/07/02/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shrotri M., Navaratnam A.M.D., Nguyen V., Byrne T., Geismar C., Fragaszy E., et al. Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet. 2021;398(10298):385–387. doi: 10.1016/S0140-6736(21)01642-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Interim Statement on Booster Doses for COVID-19 Vaccination. World Health Organization; 2021. [Internet] [cited 2020/04/21] [Google Scholar]
- 36.Mathieu E., Ritchie H., Ortiz-Ospina E., Roser M., Hasell J., Appel C., et al. A global database of COVID-19 vaccinations. Nat. Human Behav. 2021 Jul;5(7):947–953. doi: 10.1038/s41562-021-01122-8. PubMed PMID: 33972767. Epub 2021/05/12. eng. [DOI] [PubMed] [Google Scholar]
- 37.Atmar R.L., Lyke K.E., Deming M.E., Jackson L.A., Branche A.R., El Sahly H.M., et al. Homologous and heterologous covid-19 booster vaccinations. N. Engl. J. Med. 2022 2022/03/17;386(11):1046–1057. doi: 10.1056/NEJMoa2116414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choi A., Koch M., Wu K., Chu L., Ma L., Hill A., et al. Safety and immunogenicity of SARS-CoV-2 variant mRNA vaccine boosters in healthy adults: an interim analysis. Nat. Med. 2021 2021/11/01;27(11):2025–2031. doi: 10.1038/s41591-021-01527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spencer A.J., McKay P.F., Belij-Rammerstorfer S., Ulaszewska M., Bissett C.D., Hu K., et al. Heterologous vaccination regimens with self-amplifying RNA and adenoviral COVID vaccines induce robust immune responses in mice. Nat. Commun. 2021 May 17;12(1):2893. doi: 10.1038/s41467-021-23173-1. PubMed PMID: 34001897. Pubmed Central PMCID: PMC8129084 early development of this vaccine candidate), and is named as an inventor on a patent covering the use of ChAdOx1-vectored vaccines and a patent application covering this SARS-CoV-2 vaccine. T.L. is named as an inventor on a patent application covering this SARS-CoV-2 vaccine and was consultant to Vaccitech. P.M.K. and R.J.S. are co-founders and R.J.S. is a board member of VaxEquity and VacEquity, and are named inventors on a patent application covering the SARS-CoV-2 saRNA vaccine candidate. Epub 2021/05/19. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duarte-Salles T., Prieto-Alhambra D. Heterologous vaccine regimens against COVID-19. Lancet. 2021;398(10295):94–95. doi: 10.1016/S0140-6736(21)01442-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang R., Li D., Xu K., Yang C., Luo T., Zhao X., et al. A protein subunit vaccine booster following two doses of inactivated SARS-CoV-2 vaccine provides high neutralisation of SARS-CoV-2 and its variants in mice. The Lancet Microbe. 2022;3(3):e165–e166. doi: 10.1016/S2666-5247(21)00331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ai J., Zhang H., Zhang Q., Zhang Y., Lin K., Fu Z., et al. Recombinant protein subunit vaccine booster following two-dose inactivated vaccines dramatically enhanced anti-RBD responses and neutralizing titers against SARS-CoV-2 and Variants of Concern. Cell Res. 2022 2022/01/01;32(1):103–106. doi: 10.1038/s41422-021-00590-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prasithsirikul W., Pongpirul K., Nopsopon T., Phutrakool P., Pongpirul W., Samuthpongtorn C., et al. Immunogenicity of ChAdOx1 nCoV-19 booster vaccination following two CoronaVac shots in healthcare workers. Vaccines. 2022;10(2):217. doi: 10.3390/vaccines10020217. PubMed PMID. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sadat Larijani M., Sorouri R., Eybpoosh S., Doroud D., Moradi L., Ahmadinezhad M., et al. Assessment of long-term adverse events regarding different COVID-19 vaccine regimens within an 18-month follow-up study. Pathogens and Disease. 2023 doi: 10.1093/femspd/ftad010. [DOI] [PubMed] [Google Scholar]
- 45.Sadat Larijani Md D., Banifazl M., Karami A., Bavand A., Ashrafian F., Ramezani A. A landscape on disorders following different COVID-19 vaccination: a systematic review of Iranian case reports. Preprintsorg. 2023 doi: 10.1186/s40001-023-01531-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Majeed A., Papaluca M., Molokhia M. Assessing the long-term safety and efficacy of COVID-19 vaccines. J. R. Soc. Med. 2021 Jul;114(7):337–340. doi: 10.1177/01410768211013437. PubMed PMID: 33945346. Pubmed Central PMCID: PMC8276333. Epub 2021/05/05. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The Data will be available on request.