Abstract
Objectives
The survival of motor neuron (SMN) complex has an essential role in the assembly of small nuclear ribonucleoproteins (RNP). Recent reports have described autoantibodies (aAbs) to the SMN complex as novel biomarkers in anti-U1RNP+ myositis patients. The aim of this study was to compare phenotypic features of anti-U1RNP+ mixed connective tissue disease (MCTD) patients with and without anti-SMN aAbs.
Methods
A retrospective MCTD cohort was studied. Addressable laser bead immunoassay was used to detect specific anti-SMN aAbs with <300 mean fluorescence intensity (MFI) as normal reference range, 300–999 MFI as low-titre and ≥1000 MFI as high-titre positivity. Comparison of clinical features between anti-SMN+ and anti-SMN− subgroups used two-tailed Fisher’s exact test, and logistic regression analyses.
Results
Sixty-six patients were included. Median age at MCTD diagnosis was 40.6 years, and duration of follow-up was 12 years. Based on the highest available titre, 39 (59%) were anti-SMN+: 10 (26%) had low titre and 29 (74%) had high titre. Anti-SMN+ patients had a higher frequency of fingertip pitting scars (anti-SMN+ 23% vs anti-SMN− 4%, p=0.04), lower gastrointestinal (GI) involvement (26% vs 4%, p=0.04), and myocarditis (16% vs 0%, p=0.04). The combined outcome of pitting scars and/or lower GI involvement and/or myositis and/or myocarditis was highest among high-titre anti-SMN+ patients: adjusted OR 7.79 (2.33 to 30.45, p=0.002).
Conclusions
Anti-SMN aAbs were present in 59% of our MCTD cohort. Their presence, especially at high-titres, was associated with a severe systemic sclerosis (scleroderma) phenotype including myositis, myocarditis and lower GI involvement.
Keywords: Scleroderma, Systemic; Autoimmunity; Autoimmune Diseases
WHAT IS ALREADY KNOWN ON THIS TOPIC.
Autoantibodies (aAbs) are diagnostic biomarkers in systemic autoimmune diseases that also define phenotypic subsets with distinct patterns of organ involvement.
Detection of coexisting aAbs helps define additional phenotypic differences.
WHAT THIS STUDY ADDS
Anti-survival of motor neuron (anti-SMN) aAbs are frequent in the sera of anti-U1RNP+ mixed connective tissue disease (MCTD) patients.
Anti-SMN aAbs, especially at high-titre, are associated with specific systemic sclerosis (SSc, scleroderma) organ involvement in MCTD, namely fingertip pitting scars, myositis, myocarditis and lower gastrointestinal involvement.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Detection of anti-SMN aAbs in MCTD patients may allow earlier recognition of SSc manifestations and guide clinicians in screening for target organ involvement.
Introduction
The ubiquitous survival of motor neuron (SMN) is part of a complex located in the Cajal body of mammalian cell nuclei with an essential role in the assembly of small nuclear ribonucleoproteins (RNP)1. Previous studies have shown that deficient expression of SMN protein is associated with the pathogenesis of spinal muscular atrophy, a genetic neuromuscular disease2. Recent reports have described autoantibodies (aAbs) to the SMN complex as novel biomarkers in patients with systemic sclerosis (SSc) and myositis3 4. Anti-SMN aAbs positivity detected by addressable laser bead immunoassays (ALBIA) was confirmed by immunoprecipitation (IP)1 4. A study conducted on scurfy mice reported the development of mixed connective tissue disease (MCTD) features, including myositis, with significantly increased levels of anti-SMN along with anti-U1RNP aAbs in the absence of regulatory T cells5. To our knowledge, no previous studies have reported the clinical significance of anti-SMN aAbs in anti-U1RNP+ MCTD patients. The aim of this study was therefore to evaluate the prevalence of anti-SMN aAbs in MCTD patients, and to compare phenotypic features in patients with and without anti-SMN aAbs.
Methods
Patients
Anti-U1RNP+ MCTD patients were from a previous clinically and serologically described retrospective cohort from the Centre hospitalier de l’Université de Montréal (CHUM)4 6 7. Patients fulfilling ≥1 MCTD classification of Alarcón-Segovia (AS), Kasukawa (Ka), Kahn (Kn)8 and/or Tanaka (Ta)9 were included.
Study variables
A retrospective medical record review using a standardised protocol was performed to collect clinical data, laboratory and imaging investigations as described previously in detail4 6 7. Oesophageal dysmotility was defined by either manometry or evidence on chest CT scan of lower oesophageal dilation. Pulmonary arterial hypertension was defined as systolic pulmonary arterial pressure (PAP) ≥35 mmHg on transthoracic echocardiogram (TTE) or mean PAP ≥25 mmHg on right heart catheterisation. Interstitial lung disease was defined on chest CT scan. Abnormal SSc-type capillaroscopy was defined as previously published10. Myositis was defined as proximal (deltoid and/or psoas) muscle weakness on manual muscle testing in association with at least two of the following: (a) elevated serum creatine kinase levels and/or (b) abnormal electromyogram and/or (c) abnormal muscle biopsy on chart review. Dermatomyositis was defined as a myositis associated with a dermatomyositis skin rash on chart review. Myocarditis was defined by signs and symptoms, laboratory findings and/or cardiac imaging on chart review.
Clinical features were assessed for the presence of the American College of Rheumatology (ACR)/(EULAR) and non-ACR/EULAR features of SSc11, systemic lupus erythematosus (SLE)12 and Sjögren syndrome (SS)13.
Serology
Indirect immunofluorescence used HEp-2 cells as a screen for antinuclear aAbs with a positivity threshold value of ≥1:320. An extractable nuclear antigen panel was used to detect aAbs to U1RNP. ALBIA using a full-length recombinant human SMN protein (Enzo Biochem, Farmingdale, New York, USA), as previously described and validated by protein A-assisted IP4 (online supplemental figure 1), was used to detect specific anti-SMN aAbs. The normal reference range was established as <300 mean fluorescence intensity (MFI), 300–999 MFI as low-titre and ≥1000 MFI as high-titre positivity.
rmdopen-2023-003431supp001.pdf (710.4KB, pdf)
Statistical analysis
Frequency comparisons among anti-SMN+ and anti-SMN− subgroups used two-tailed Fisher’s exact test. Logistic regression analyses (univariable and multivariable adjusted for sex, age at diagnosis and disease duration) with 95% CIs were performed on outcomes of interest. P-value of 0.05 or lower was considered statistically significant.
Results
59% of anti-U1RNP+ MCTD patients coexpress anti-SMN aAbs
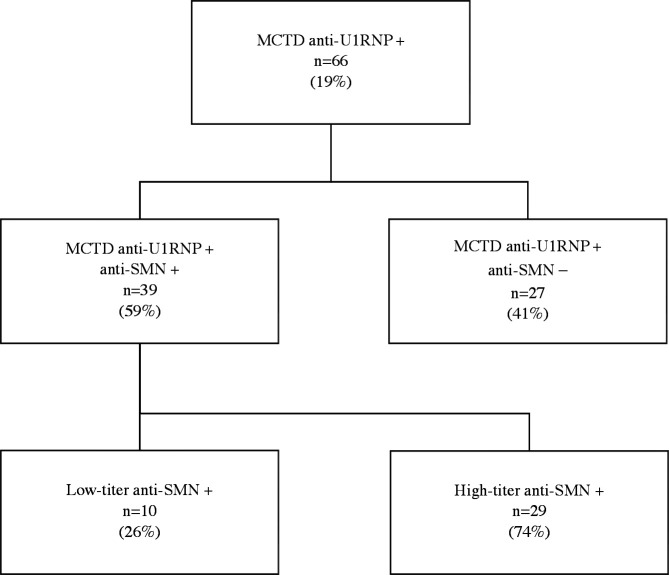
Sixty-six patients were included and the various MCTD criteria were respectively fulfilled in 86% (AS), 73% (Ka), 97% (Kn) and 98% (Ta) of patients (figure 1, table 1). Fifty-eight of the 66 (88%) patients were female and median age at MCTD diagnosis was 40.6 years (range 10.7–70 years). Thirty-nine of 66 (59%) patients were positive for anti-SMN aAbs: 10 (26%) had low-titre and 29 (74%) had high-titre anti-SMN, based on highest available titre during follow-up, with a median value of 3584 MFI (range 320–15 397 MFI) (figure 1). Follow-up duration since diagnosis was similar between anti-SMN+ and anti-SMN− patients (mean 10.2 vs 13.9 years, p=0.54).
Figure 1.
Flowchart. MCTD: mixed connective tissue disease; RNP: ribonucleoprotein; SMN: survival of motor neuron.
Table 1.
Cumulative features of SSc and myositis in anti-SMN+ compared to anti-SMN− MCTD patients
| All patients (n=66) |
MCTD anti- SMN+ (n=39) |
MCTD anti- SMN− (n=27) |
|
| Female sex, n (%) | 58 (88) | 36 (92) | 22 (81) |
| Race, n (%) | |||
| White | 59 (89) | 37 (95) | 22 (81) |
| Black | 4 (6) | 2 (5) | 2 (8) |
| Other | 3 (5) | 0 (0) | 3 (11) |
| Age at diagnosis, median (range), years | 40.6 (10.7–70) | 39.2 (10.7–70) | 42.1 (21.3–61.4) |
| Duration of follow-up, median (range), years | 12 (0–42.4) | 10.2 (0–42.4) | 13.9 (0–39.8) |
| Speckled ANA ≥1:1280, n (%) | 66 (100) | 39 (100) | 27 (100) |
| Median anti-SMN value (range), MFI | 451 (40–15397) | 3584 (320–15397) | 98 (40–281) |
| MCTD classification, n (%) | |||
| Alarcón-Segovia criteria | 57 (86) | 34 (87) | 23 (85) |
| Kahn criteria | 48 (73) | 29 (74) | 19 (70) |
| Kasukawa criteria | 64 (97) | 39 (100) | 25 (93) |
| Tanaka criteria | 65 (98) | 39 (100) | 26 (96) |
| Definite SSc†, n (%) | 47 (71) | 30 (77) | 17 (63) |
| Definite SLE‡, n (%) | 54 (82) | 31 (79) | 23 (85) |
| Definite SS§, n (%) | 8 (12) | 5 (13) | 3 (11) |
| SSc features, n (%) | |||
| Sine scleroderma | 17 (26) | 9 (23) | 8 (30) |
| Skin thickening proximal to MCP | 11 (17) | 9 (23) | 2 (7) |
| Sclerodactyly distal to MCP, proximal to PIP | 49 (74) | 30 (77) | 19 (70) |
| Raynaud’s phenomenon | 65 (98) | 38 (97) | 27 (100) |
| Abnormal nailfold capillaroscopy | 34/46 (74) | 23/28 (82) | 11/18 (61) |
| Puffy fingers | 43/64 (67) | 26/37 (70) | 17 (63) |
| Digital tip ulcers | 21 (32) | 15 (38) | 6 (22) |
| Fingertip pitting scars | 10 (15) | 9 (23)* | 1 (4)* |
| Telangiectasias | 43 (65) | 25 (64) | 18 (67) |
| Calcinosis | 17 (26) | 10 (26) | 7 (26) |
| Pulmonary arterial hypertension¶ | 17/51 (33) | 12/29 (41) | 5/22 (23) |
| Interstitial lung disease†† | 22/56 (39) | 11/30 (37) | 11/26 (42) |
| DLCO <70% | 33/50 (66) | 21/28 (75) | 12/22 (55) |
| Oesophageal dysmotility | 38/63 (60) | 24/37 (65) | 14/26 (54) |
| GERD/Dyspepsia | 54/60 (90) | 33/34 (97) | 21/26 (81) |
| Pneumatosis | 3 (5) | 3 (8) | 0 (0) |
| Pseudo-obstruction | 6 (9) | 5 (13) | 1 (4) |
| Small intestinal bacterial overgrowth | 11 (17) | 10 (26)** | 1 (4)** |
| Scleroderma renal crisis | 1 (2) | 1 (3) | 0 (0) |
| Inflammatory myositis features, n (%) | |||
| Myositis diagnosis | 18/65 (28) | 14/38 (37) | 4 (15) |
| DM diagnosis | 3/65 (5) | 1/38 (3) | 2 (7) |
| Myocarditis | 6/65 (9) | 6/38 (16)*** | 0 (0)*** |
| Elevated serum CK levels (CK >170 UI/L) | 24/65 (37) | 16/38 (42) | 8 (30) |
| Median CK level, if CK >170 UI/L (range) | 938 (185–6000) | 500 (185–6000) | 1152.5 (190–2980) |
Bold values are statistically significant variables.
*p-value 0.04.
**p-value 0.02.
***p-value 0.04.
†Definite SSc criteria: score of ≥9 (ACR/EULAR 2013)11.
‡Definite SS criteria: score of ≥4 (ACR/EULAR 2016)13.
§Definite SLE criteria: score of ≥10 (American College of Rheumatology (ACR)/EULAR 2019)12.
¶Pulmonary arterial hypertension defined as systolic pulmonary arterial pressure (PAP) ≥35 mm Hg on transthoracic echocardiogram or mean PAP ≥25 mm Hg on right heart catheterisation.
††Interstitial lung disease based on thoracic CT scan.
ANA, antinuclear autoantibodies; CK, creatine kinase; DLCO, diffusing lung capacity for carbon monoxide; DM, dermatomyositis; GERD, gastro-oesophageal reflux disease; MCP, metacarpophalangeal; MCTD, mixed connective tissue disease; PIP, proximal interphalangeal; RNP, ribonucleoprotein; SLE, systematic lupus erythematosus; SMN, survival of motor neuron; SS, Sjögren syndrome; SSc, systemic sclerosis.
Anti-SMN aAbs in MCTD are associated with a severe systemic sclerosis phenotype
The presence of anti-SMN aAbs in MCTD patients was associated with a higher frequency of fingertip pitting scars (anti-SMN+ 23% vs anti-SMN− 4%, p=0.04) and small intestinal bacterial overgrowth (26% vs 4%, p=0.02). The risk of having lower gastrointestinal (GI) involvement (defined as pneumatosis and/or pseudo-obstruction and/or small intestinal bacterial overgrowth) was higher among anti-SMN+ patients (26% vs 4%, p=0.04) with an adjusted OR of 10.16 (95% CI 1.63 to 205.89, p=0.04) on multivariable logistic regression (adjusted for age at diagnosis, sex and disease duration at diagnosis). No differences were found for other SSc features (table 1).
The risk of having myositis was numerically higher among anti-SMN+ patients (14/38, 37%) compared to anti-SMN− patients (4/27, 15%), with an adjusted OR of 3.57 (95% CI 1.00 to 15.49, p=0.06). Myocarditis was found in 16% (6/38) of anti-SMN+ patients but in none (0/27) of the patients without anti-SMN aAbs (p=0.04). For the combined outcome of lower GI involvement and/or myositis and/or myocarditis, this combination was significantly more frequent among anti-SMN+ patients (21/38, 55%) compared to anti-SMN− patients (5/27, 19%), with an adjusted OR of 6.23 (95% CI 1.86 to 25.41, p=0.005) (online supplemental figure 1).
High-titre anti-SMN aAbs are associated with an increased risk of severe systemic sclerosis phenotype
High-titre anti-SMN aAbs were more frequently associated with skin thickening proximal to MCP joints (31% vs 7%, p=0.04), fingertip pitting scars (24% vs 4%, p=0.05), small intestinal bacterial overgrowth (28% vs 4%, p=0.03) and myocarditis (21% vs 0%, p=0.02) than patients with low-titre anti-SMN aAbs (table 2). On multivariable logistic regression, the risk of having lower GI involvement was highest among those with high-titre anti-SMN (low-titre adjusted OR 5.98 (95% CI 0.45 to 152.28), p=0.19; high-titre adjusted OR 11.99 (95% CI 1.82 to 248.08), p=0.03). The risk of having myositis was also highest among patients with high-titre anti-SMN (low-titre adjusted OR 2.02 (95% CI 0.27 to 13.91), p=0.47; high-titre adjusted OR 4.30 (95% CI 1.11 to 19.86), p=0.04). The combined outcome of pitting scars and/or lower GI involvement and/or myositis and/or myocarditis was highest among those with high-titre anti-SMN: adjusted OR 7.79 (95% CI 2.33 to 30.45, p=0.002).
Table 2.
Cumulative features of SSc and myositis in high-titre anti-SMN+ compared to anti-SMN− MCTD patients
| All patients (n=66) |
MCTD anti- SMN+ high titre (n=29) |
MCTD anti- SMN− (n=27) |
|
| Female sex, n (%) | 58 (88) | 27 (93) | 22 (81) |
| Race, n (%) | |||
| White | 59 (89) | 27 (93) | 22 (81) |
| Black | 4 (6) | 2 (7) | 2 (7) |
| Other | 3 (5) | 0 (0) | 3 (11) |
| Smoker, n (%) | 30/60 (50) | 15/25 (60) | 13/26 (50) |
| Age at diagnosis, median (range), years | 40.6 (10.7–70) | 39.5 (14.4–70) | 42.1 (21.3–61.4) |
| Duration of follow-up, median (range), years | 12 (0–42.4) | 10.2 (0–42.4) | 13.9 (0–39.8) |
| Speckled ANA ≥1:1280, n (%) | 66 (100) | 29 (100) | 27 (100) |
| Median anti-SMN value (range), MFI | 451 (40–15397) | 7727 (1143–15397) | 98 (40–281) |
| Positive anti-Sm antibody | 13/62 (21) | 7/26 (27) | 2 (7) |
| Positive anti-dsDNA antibody | 19/65 (29) | 7 (24) | 9/26 (35) |
| MCTD classification, n (%) | |||
| Alarcón-Segovia criteria | 57 (86) | 26 (90) | 23 (85) |
| Kahn criteria | 48 (73) | 23 (79) | 19 (70) |
| Kasukawa criteria | 64 (97) | 29 (100) | 25 (93) |
| Tanaka criteria | 65 (98) | 29 (100) | 26 (96) |
| Definite SSc†, n (%) | 47 (71) | 22 (76) | 17 (63) |
| Definite SLE‡, n (%) | 54 (82) | 23 (79) | 23 (85) |
| Definite SS§, n (%) | 8 (12) | 5 (17) | 3 (11) |
| SSc features, n (%) | |||
| Sine scleroderma | 17 (26) | 5 (17) | 8 (30) |
| Skin thickening proximal to MCP | 11 (17) | 9 (31)* | 2 (7)* |
| Sclerodactyly distal to MCP, proximal to PIP | 49 (74) | 24 (83) | 19 (70) |
| Raynaud phenomenon | 65 (98) | 29 (100) | 27 (100) |
| Abnormal nailfold capillaroscopy | 34/46 (74) | 16/20 (80) | 11/18 (61) |
| Puffy fingers | 43/64 (67) | 21/27 (78) | 17 (63) |
| Digital tip ulcers | 21 (32) | 10 (34) | 6 (22) |
| Fingertip pitting scars | 10 (15) | 7 (24)** | 1 (4)** |
| Telangiectasias | 43 (65) | 19 (66) | 18 (67) |
| Calcinosis | 17 (26) | 9 (31) | 7 (26) |
| Pulmonary arterial hypertension¶ | 17/51 (33) | 10/20 (50) | 5/22 (23) |
| Interstitial lung disease†† | 22/56 (39) | 7/22 (32) | 11/26 (42) |
| DLCO <70% | 33/50 (66) | 14/19 (74) | 12/22 (55) |
| Oesophageal dysmotility | 38/63 (60) | 20 (69) | 14/26 (54) |
| GERD/dyspepsia | 54/60 (90) | 24/25 (96) | 21/26 (81) |
| Pneumatosis | 3 (5) | 3 (10) | 0 (0) |
| Pseudo-obstruction | 6 (9) | 5 (17) | 1 (4) |
| Small intestinal bacterial overgrowth | 11 (17) | 8 (28)*** | 1 (4)*** |
| Scleroderma renal crisis | 1 (2) | 0 (0) | 0 (0) |
| Inflammatory myositis features, n (%) | |||
| Myositis diagnosis | 18/65 (28) | 11 (38) | 4 (15) |
| DM diagnosis | 3/65 (5) | 1 (3) | 2 (7) |
| Myocarditis | 6/65 (9) | 6 (21)**** | 0 (0)**** |
| Elevated serum CK levels (CK >170 UI/L) | 24/65 (37) | 12 (41) | 8 (30) |
| Median CK level, if CK >170 UI/L (range) | 938 (185–6000) | 1419 (233–6000) | 1152.5 (190–2980) |
Bold values are statistically significant variables.
*p-value 0.04.
**p-value 0.05.
***p-value 0.03.
****p-value 0.02.
†Definite SSc criteria: score of ≥9 (American College of Rheumatology (ACR)/EULAR 2013)11.
‡Definite SLE criteria: score of ≥10 (ACR/EULAR 2019)12.
§Definite SS criteria: score of ≥4 (ACR/EULAR 2016)13.
¶Pulmonary arterial hypertension defined as systolic pulmonary arterial pressure (PAP) ≥35 mm Hg on transthoracic echocardiogram or mean PAP ≥25 mm Hg on right heart catheterisation.
††Interstitial lung disease based on thoracic CT scan.
ANA, antinuclear autoantibodies; CK, creatine kinase; DLCO, diffusing lung capacity for carbon monoxide; DM, dermatomyositis; dsDNA, double stranded DNA; GERD, gastro-oesophageal reflux disease; MCP, metacarpophalangeal; MCTD, mixed connective tissue disease; PIP, proximal interphalangeal; RNP, ribonucleoprotein; SLE, systematic lupus erythematosus; Sm, Smith; SMN, survival of motor neuron; SS, Sjögren syndrome; SSc, systemic sclerosis.
Anti-SMN aAbs are not associated with distinct SLE or SS features
No significant differences regarding SLE and SS features between anti-SMN+ and anti-SMN− MCTD patients were noted (online supplemental table 1). With a median follow-up duration of 12 years, 71%, 82% and 12% of patients fulfilled the ACR/EULAR criteria for SSc, SLE and SS, respectively.
rmdopen-2023-003431supp002.pdf (106KB, pdf)
Discussion
Anti-SMN aAbs were found in an important proportion of our anti-U1RNP+ MCTD cohort (59%). Their presence, especially at high titre, was associated with a higher risk of having myositis and several SSc features, notably myocarditis and lower GI involvement.
MCTD is a systemic autoimmune disease characterised by the presence of high-titre anti-U1RNP aAbs in combination with Raynaud’s phenomenon and clinical features of SSc, SLE, arthritis and/or myositis9 14. aAbs encountered in patients with autoimmune diseases are useful biomarkers to help or predict diagnosis, clinical phenotype, prognosis and treatment decision-making15. Coexpression of aAbs can lead to additive clinical manifestations, and define phenotypic differences15.
This is the first study assessing the clinical significance of anti-SMN aAbs in an anti-U1RNP+ MCTD population. We previously reported anti-SMN aAbs in SSc patients with features of myositis without anti-U1RNP aAbs4. In these patients, anti-SMN aAbs were associated with calcinosis, trigeminal neuropathy and lower GI involvement. In addition, a case report of high-titre anti-SMN aAbs in a patient who had severe muscle disease and cardiac necrosis that died in intensive care due to severe cardiopulmonary failure supports the current evidence that these aAbs are associated with a severe disease phenotype3. Thus, the present study and our previous data suggest that anti-SMN aAbs are associated with SSc and, in MCTD, are predictive of a more severe disease phenotype4 16.
Interestingly, since 25% of our anti-U1RNP+ MCTD patients with anti-SMN aAbs presented sine scleroderma, early identification of anti-SMN aAbs in MCTD patients may thus serve as a useful diagnostic and prognostic tool to guide clinicians to screen for specific SSc features, especially lower GI involvement, myositis and myocarditis at follow-up. Since myocarditis and lower GI involvement increase morbidity and mortality in SSc patients17–19, anti-SMN aAbs may therefore identify a subset of MCTD patients with a poorer prognosis with important implications for clinical management and therapeutic strategies.
Limitations of this study include its retrospective nature, the small number of patients and the current limited availability of tests for detection of anti-SMN aAbs in routine clinical practice. Nonetheless, we studied a cohort of well-defined MCTD patients with a long follow-up duration and provide novel results that may lead to important changes in clinical care for MCTD patients.
Conclusion
Anti-SMN aAbs were detected in 59% of patients in a criteria-defined MCTD cohort. Their presence, especially with high titres, was associated with an increased frequency of severe SSc features including myocarditis and lower GI involvement. Early detection of anti-SMN aAbs may be useful in defining prognosis in MCTD patients and guiding clinicians in screening for target organ involvement.
Footnotes
Presented at: This study was presented as an abstract at the ACR Convergence 2022. El Kamouni H, Albert A, Hoa S, S. Jalaledin D, Bourré-Tessier J, Rich E, Goulet J, Koenig M, Satoh M, Fritzler M, Choi M, Troyanov Y, Senécal J, Landon-Cardinal O. Anti-SMN Autoantibodies in Mixed Connective Tissue Disease Are Associated with a Scleromyositis Phenotype [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/anti-smn-autoantibodiesin-mixed-connective-tissue-disease-are-associated-with-a-scleromyositis-phenotype/.
Contributors: These authors contributed equally to the manuscript: HEK and DSJ. Conceptualisation and design: HEK, DSJ, AA, SH, YT, J-LS and OL-C. Acquisition of data: HEK, DSJ, AA, SH, CV, JB-T, ER, J-RG, MK, GP, MYC, YT, MS, MJF, J-LS and OL-C. Analysis and interpretation: HEK, DSJ, AA, SH, CV, JB-T, ER, J-RG, MK, GP, MYC, YT, MS, MJF, J-LS and OL-C. Critical revision of the manuscript for important intellectual content: HEK, DSJ, AA, SH, CV, JB-T, ER, J-RG, MK, GP, MYC, YT, MS, MJF, J-LS and OL-C.
Funding: This research was supported in part by a Université de Montréal Department of Medicine Clinician Researcher Award (OL-C) and the Université de Montréal Scleroderma Research Chair (J-LS); and by grants from Myositis Canada (OL-C), Sclérodermie Québec, Scleroderma Society of Ontario, Scleroderma Society of Canada, Scleroderma Manitoba, the Scleroderma Association of Saskatchewan and the Scleroderma Association of British Columbia (J-LS, MK, SH, OL-C); and by generous donations from Mrs Gisèle Sarrazin-Locas (J-LS). JL-S holds the Université de Montréal Scleroderma Research Chair. SH is supported by a Fonds de la recherche du Québec en Santé Clinical Research Scholar Junior 1 award.
Competing interests: HEK, DSJ, AA, SH, CV, JB-T, ER, J-RG, MK, GP, MS and J-LS report no disclosure. MYC reports consulting fees. YT reports consulting fees and stock options. MJF reports consulting fees, payment/honoraria for lectures and support for attending meetings. OL-C reports participation on a data safety/advisory board.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by the CHUM Ethics Committees (reference number 2015-5607-CE14.238). Participants gave informed consent to participate in the study before taking part.
References
- 1.Satoh M, Chan JY, Ross SJ, et al. Autoantibodies to survival of motor neuron complex in patients with polymyositis: Immunoprecipitation of D. Arthritis Rheum 2011;63:1972–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mercuri E, Sumner CJ, Muntoni F, et al. Spinal muscular atrophy. Nat Rev Dis Primers 2022;8:52. 10.1038/s41572-022-00380-8 [DOI] [PubMed] [Google Scholar]
- 3.Amlani A, Hazlewood GS, Hamilton L, et al. Autoantibodies to the survival of motor neuron complex in a patient with necrotizing autoimmune myopathy. Rheumatology (Oxford) 2018;57:199–200. 10.1093/rheumatology/kex392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Landon-Cardinal O, Baril-Dionne A, Hoa S, et al. Recognising the spectrum of scleromyositis: HEp-2 ANA patterns allow identification of a novel clinical subset with anti-SMN autoantibodies. RMD Open 2020;6:e001357. 10.1136/rmdopen-2020-001357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yilmaz OK, Haeberle S, Zhang M, et al. Scurfy mice develop features of connective tissue disease overlap syndrome and mixed connective tissue disease in the absence of regulatory T cells. Front Immunol 2019;10:881. 10.3389/fimmu.2019.00881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Troyanov Y, Targoff IN, Tremblay J-L, et al. Novel classification of idiopathic inflammatory myopathies based on overlap syndrome features and autoantibodies: analysis of 100 French Canadian patients. Medicine 2005;84:231–49. 10.1097/01.md.0000173991.74008.b0 [DOI] [PubMed] [Google Scholar]
- 7.Troyanov Y, Targoff IN, Payette M-P, et al. Redefining dermatomyositis: a description of new diagnostic criteria that differentiate pure dermatomyositis from overlap myositis with dermatomyositis features. Medicine (Baltimore) 2014;93:318–32. 10.1097/MD.0000000000000222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amigues JM, Cantagrel A, Abbal M, et al. Comparative study of 4 diagnosis criteria sets for mixed connective tissue disease in patients with anti-RNP antibodies. J Rheumatol 1996;23:2055–62. [PubMed] [Google Scholar]
- 9.Tanaka Y, Kuwana M, Fujii T, et al. Diagnostic criteria for mixed connective tissue disease (MCTD): from the Japan research committee of the Ministry of health, labor, and welfare for systemic autoimmune diseases. Modern Rheumatology 2021;31:29–33. 10.1080/14397595.2019.1709944 [DOI] [PubMed] [Google Scholar]
- 10.Koenig M, Joyal F, Fritzler MJ, et al. Autoantibodies and microvascular damage are independent predictive factors for the progression of Raynaud’s phenomenon to systemic sclerosis: a twenty-year prospective study of 586 patients, with validation of proposed criteria for early systemic sclerosis. Arthritis Rheum 2008;58:3902–12. 10.1002/art.24038 [DOI] [PubMed] [Google Scholar]
- 11.van den Hoogen F, Khanna D, Fransen J, et al. Classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Ann Rheum Dis 2013;72:1747–55. 10.1136/annrheumdis-2013-204424 [DOI] [PubMed] [Google Scholar]
- 12.Aringer M. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis 2019;78:1151–1159. 10.1136/annrheumdis-2018-214819 [DOI] [PubMed] [Google Scholar]
- 13.Shiboski CH, Shiboski SC, Seror R, et al. American College of Rheumatology/European League against Rheumatism classification criteria for primary Sjögren’s syndrome: A consensus and data-driven methodology involving three international patient cohorts. Arthritis Rheumatol 2017;69:35–45. 10.1002/art.39859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.John KJ, Sadiq M, George T, et al. Clinical and immunological profile of mixed connective tissue disease and a comparison of four diagnostic criteria. Int J Rheumatol 2020;2020:9692030. 10.1155/2020/9692030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang EH, Ha YJ, Lee YJ. Autoantibody biomarkers in rheumatic diseases. Int J Mol Sci 2020;21:1382. 10.3390/ijms21041382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goulet JR, Hurtubise M, Senécal JL. Retropneumoperitoneum and Pneumatosis Intestinalis in 2 patients with mixed connective tissue disease and the overlap syndrome. Clin Exp Rheumatol 1988;6:81–5. [PubMed] [Google Scholar]
- 17.Bruni C, Ross L. Cardiac involvement in systemic sclerosis: getting to the heart of the matter. Best Pract Res Clin Rheumatol 2021;35:101668. 10.1016/j.berh.2021.101668 [DOI] [PubMed] [Google Scholar]
- 18.Theodorou DJ, Theodorou SJ, Rizos D, et al. Myocarditis in scleroderma: an Underrecognized manifestation of the disease. J Clin Rheumatol 2020;26:e146. 10.1097/RHU.0000000000001039 [DOI] [PubMed] [Google Scholar]
- 19.Hughes M, Herrick AL. Systemic sclerosis. Br J Hosp Med (Lond) 2019;80:530–6. 10.12968/hmed.2019.80.9.530 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2023-003431supp001.pdf (710.4KB, pdf)
rmdopen-2023-003431supp002.pdf (106KB, pdf)