Abstract
Introduction
The COVAX alliance is a novel approach to international partnership in global health intended to achieve the worthy goal of ‘COVID-19 vaccine equity’. This study aimed to identify the implementation challenges and framework gaps of COVAX and to explore the interconnected global health policy and governance gaps in ensuring equity, accessibility and affordability of vaccines.
Methods
A scoping review was conducted to identify the implementation challenges and framework gaps of COVAX and related global health policy and governance gaps. A search was carried out in PubMed, Scopus, Springer Link and Embase databases. Manually searched the grey literature, such as official reports and articles. EndNote V.20 was used to manage the evidence screening, and data extraction was carried out in Microsoft Excel.
Results
Searches of four electronic databases and official UN, GAVI and WHO websites identified 4686 pieces of evidence. The 937 duplicates were removed, and the remaining 3749 articles were screened for the title and abstract. Most articles were eliminated as they do not address global COVAX or COVID-19 vaccine equity. The remaining 53 pieces of evidence were reviewed for full text, and ultimately 40 articles found eligible were included in the scoping review.
Conclusions
The implementation challenges of COVAX were attributed mainly to the phenomenon of vaccine nationalism by rich countries. The future global health policy and governance structure must be re-examined to address the inadequacies of such novel super public-and-private partnership models.
Keywords: Health policy, Health economics, Vaccines, Qualitative study, Review
WHAT IS ALREADY KNOWN ON THIS TOPIC
Global vaccine inequity is not a new phenomenon, and a platform such as COVAX is a novel experimental structure of the global health system where it is supposed to procure, allocate and deliver COVID-19 vaccines to the participant countries equitably.
WHAT THIS STUDY ADDS
The primary implementation challenge faced by the COVAX alliance is vaccine nationalism by high-income countries, which is associated with intellectual property rights.
The functioning of COVAX is explicitly inter-related to global health policy and governance, and the current structure is inadequate to address the challenges the alliance faces.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The implementation and framework challenge the alliance faces as a novel public-and-private partnership model will help create better models for future pandemics.
Global health governance and future policy-making must be reformed to accommodate the challenges identified so that future models can work effectively.
Introduction
The COVAX alliance is a novel approach to international partnership in global health intended to achieve the goal of ‘COVID-19 vaccine equity’.1 The alliance followed the public-and-private partnership (PPP) model between global health actors, regulatory agencies, pharma companies and private philanthropy organisations.2 COVAX is the first such mechanism formed globally to address vaccine nationalism and hoarding by wealthy countries. It primarily focused on the accessibility and affordability issues of LMICs (Lower- Middle Income Countries) and LIC (Low- Income Countries) which were left out of the market in the H1N1 pandemic 2009. Fundamental mechanisms of COVAX—procurement, allocation and delivery—were meant to work interconnected, and the framework of COVAX was meant to support the pandemic needs.1
The initial goal of the alliance was to deliver 2 billion vaccine doses by the end of 2021.3 The COVAX started its first delivery to Ghana on 24 February 2021, less than 3 months after the world’s first COVID-19 vaccine rollout in the UK (United Kingdom).4 COVAX reached 100 countries within 42 days, many entirely dependent on COVAX. But the COVAX official supply forecasts from GAVI (Global Alliance for vaccination and immunization) depicted a decline in expected supply. According to forecast 1, released in January 2021, COVAX is expected to deliver approximately 1.8 billion doses, equivalent to 27% of coverage for 92 advance market commitment (AMC) countries that were available in 2021.5 In supply forecast 2, about 1.5 billion doses (23% of coverage for AMCs) were expected to be available by December 2021.6 But according to supply forecast 3 released in early December, the expected supply was further trimmed to 1.4 billion doses by the end of 2021.7
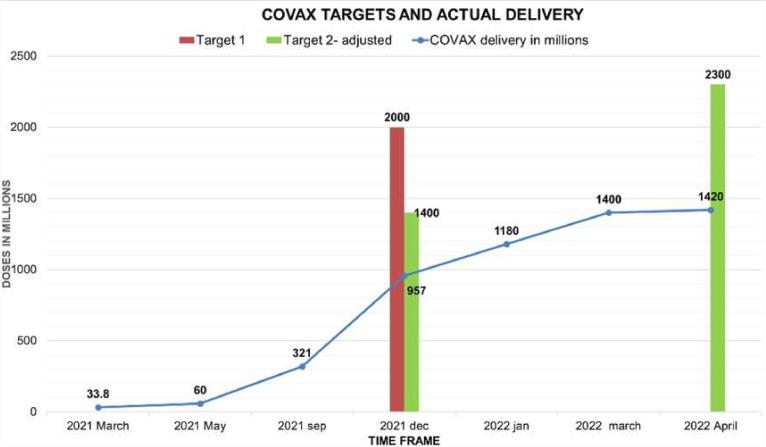
The actual delivery of COVAX was far less than any of these supply forecasts. COVAX delivery data from the UNICEF (United Nations Children’s Fund) vaccine market dashboard (figure 1) clearly showed that COVAX did not meet the original target of 2 billion doses by the end of December 2021. Instead, it delivered less than 50% of what it originally promised.8 9 Even the adjusted delivery targets, that is, 1.4 billion by December 2021 were not achieved. Out of what COVAX delivered, only 819 million doses went to AMC, a fraction of 9.25 billion doses administered in 2021 and less than half of the 1.3 billion pledged to deliver. COVAX aimed at delivering 2.3 billion doses by the first quarter of 2022, and only 1.4 billion were delivered.8 10
Figure 1.
In the primary axis of the graph, the x-axis denotes COVAX-delivered doses in millions, and the y-axis depicts the time frame from March 2021 to April 2022. The data were taken from the UNICEF vaccine market dashboard. COVAX targets for December 2021, both initial and adjusted and that for April 2022 were plotted on the secondary axis.
So, where did COVAX go wrong? The scoping review gave many insights related to the implementation challenges and framework gaps of COVAX, which can be attributed to a great extent to this failure to meet the targets.
Methods
For this study, a scoping review was conducted to identify and map the evidence associated with Global COVID-19 vaccine equity, affordability and accessibility. The objectives of the review were to examine the implementation challenges and framework gaps of the COVAX alliance and to provide insights into the policy gaps in global health and governance regarding the equity and accessibility of pandemic goods. Arksey and O’Malley’s (2020) Methodological Framework11 was used for scoping review along with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines.
Research questions
Since the COVAX alliance is a novel initiative and the first model of fair distribution of vaccines globally, the primary research question focused on the various implementation challenges the alliance faced in a global context. As the challenges identified cannot be read in isolation, examining the gaps in the COVAX Framework was essential. So, combining these aspects, the first research question was framed:
‘What are the implementation challenges and gaps in the framework of the COVAX alliance?’
The literature review showed that international political, economic and governance factors would affect any global health mechanism. So, exploring the global health policy gaps resulting from the elements mentioned above affecting the equitable distribution of COVID-19 vaccines globally was necessary. So, the second research question was:
‘What are the global health policy gaps and global health governance issues that potentially affect the equitable distribution of COVID-19 vaccines?’.
Search strategy
An electronic database search was conducted in PubMed, Scopus, Springer Link and Elsevier, in addition to a manual search for additional evidence from the official websites of GAVI, WHO and the UN. The evidence was in the form of articles, reports, viewpoints, comments and editorials in the English language, published between January 2020 and March 2022. Search key terms focused on COVAX, (COVID-19 vaccine) AND (equity), (COVID-19) AND (vaccine diplomacy), (COVID-19) AND (Vaccine accessibility), (COVID-19) AND (Vaccine affordability), (COVID-19 Vaccine) AND (LMIC), (COVID-19 Vaccine) AND (Nationalism); and official reports published by WHO (World Health Organization), UN (United Nations) and GAVI on the COVAX alliance and global COVID-19 vaccine equity, were included. For the domain of vaccine nationalism, the keywords were restricted to a title search. Studies and documents focused on population subgroups within a country, and not directly addressed the research questions, were excluded.
Charting the data
The primary search results were exported to EndNote V.20, an online reference manager in ‘.ris’ format, and extracted the key features of the evidence such as author, year, title, journal, reference type, abstract and link to the evidence. The duplicates were removed, and the rest of the references were considered for screening.
Screening
The relevant articles selected through title screening were included for abstract screening, and the evidence pieces that did not directly contribute to the research questions were removed. The evidence that qualified through the abstract screening was chosen for full-text screening. On full-text screening, the relevant evidence was chosen for data extraction and further analysis.
Data extraction and analysis
The selected and manually searched evidence from the official websites were read thoroughly and identified the implementation challenges and framework gaps of the COVAX alliance and the policy gaps in global health governance. The extracted data were encoded in Microsoft Excel and further analysed to answer the research questions.
Results
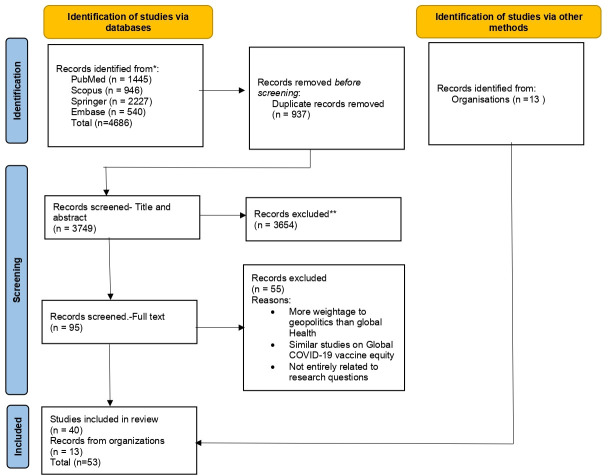
Searches of four electronic databases identified 4686 pieces of evidence (PubMed-1445, Scopus-946, Springer Link- 2227, Embase-540). The evidence was imported to EndNote V.20 and removed 937 duplicates. The remaining 3749 articles were screened for the title and abstract. A vast majority of the evidence, that is, 3654, was eliminated as they have not addressed global COVAX or COVID-19 vaccine equity. The remaining 95 pieces of evidence were reviewed for full text, and ultimately 40 of them were found to be eligible to be included in the scoping review (figure 2). Also, 13 reports have been added to the review from the official websites of the UN, GAVI and WHO, making the total number 53. Some of the evidence has given more weightage to geopolitics rather than global health, while others were not entirely related to the research questions, and many were similar studies with almost the same conclusion. Hence, the most relevant evidence closest to the research questions was included.
Figure 2.
Represents the PRISMA flow chart of the scoping review with the number of evidence taken for identification, screening and final inclusion. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses- 2021 Guidelines was used.* The initial evidence collected from the four databases** First exclusion of evidences
Out of the total 53 pieces of evidence taken for analysis, 13 directly address the first research question, that is, the implementation challenges and framework gaps of the COVAX mechanism.12–24 The rest of the selected evidence comprehensively views global vaccine equity, accessibility, vaccine nationalism, vaccine hoarding and vaccine diplomacy. A significant chunk of evidence mainly focuses on the politics influencing global vaccine production and distribution, such as the IPR TRIPS (Intellectual Property Rights- The Agreement on Trade related aspects of Intellectual property rights), vaccine diplomacy and other international power dynamics.
Findings for research questions
The following implementation challenges for COVAX alliance were identified and compiled from the evidence as per PRISMA protocol for a scoping review. The major implementation challenges identified were as follows:
Vaccine nationalism by wealthy countries.2 12–32
Lack of high-income country (HIC) participation and funding for COVAX.12–14 16 18–20 28 33 34
Lack of transparency in the vaccine deals with pharma companies.15 23 31 35–37
Export restrictions imposed by HICs amidst pandemic.21–23 26 35 38 39
Lack of vaccine production units in the Global South.22 24 40–42
Not enough technology is transferred to other manufacturing partners to speed up production.14 27
Dose sharing and dose donation problems.25 43 44
Dependency on AstraZeneca vaccine.33 39
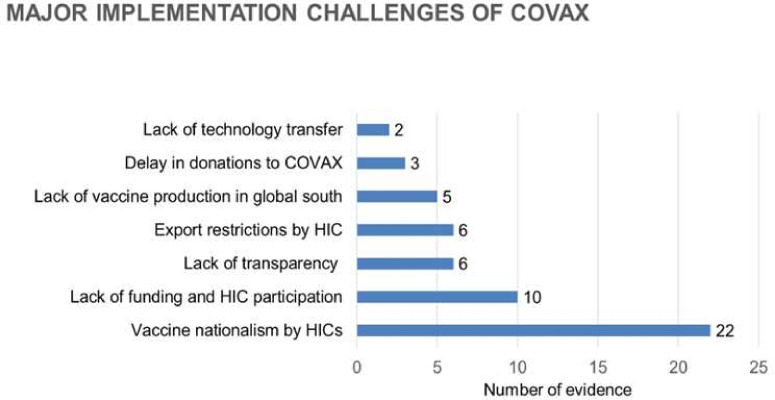
The following bar diagram (figure 3) depicts the major themes of the implementation challenges.
Figure 3.
Graph 1 represents the major implementation challenges identified by the scoping review from the evidence included for the final review. The x-axis represents the number of evidence corresponding to the challenges depicted on the y-axis. HICs, high-income countries.
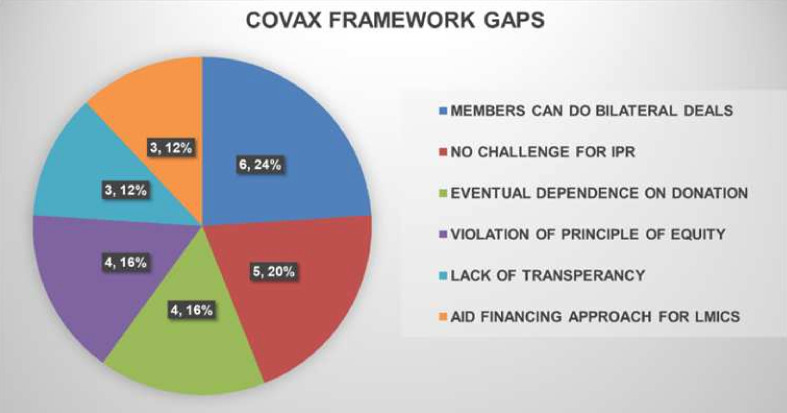
Similar to the implementation challenges, the gaps in framing the COVAX alliance and mechanisms were also identified (figure 4). The main framework gaps of COVAX were as follows:
Figure 4.
Graph 2 represents major framework gaps identified by the scoping review from the evidence included for the final review. The pie diagram depicts the number and the percentage weightage of evidence corresponding to each of the framework gaps identified.
Allowing the members to engage in bilateral deals outside the alliance.13–16 35 45
The COVAX structure did not challenge the existing IPR.16 19 22 25 46 47
COVAX facility was supposed to be a self-procuring mechanism, but due to a lack of supply, the procurement arm was dependent on donations from HICs for the supply.23 38 47 48
COVAX allowed HICs, that is, SFCs (Self Financing Countries), to procure doses up to 50% of their population through the facility through the ‘Optional Purchase Agreement’, whereas, for aided countries, the limit is 20%. This prima facie violates the principle of equity to which the alliance dedicates itself.16 17 19 23
There were not many details about the procurement deals COVAX made with pharma companies.15 35
COVAX was structured as the traditional aid financing for LMICs by the developed world rather than making them self-sufficient.19 25 26 33 47 48
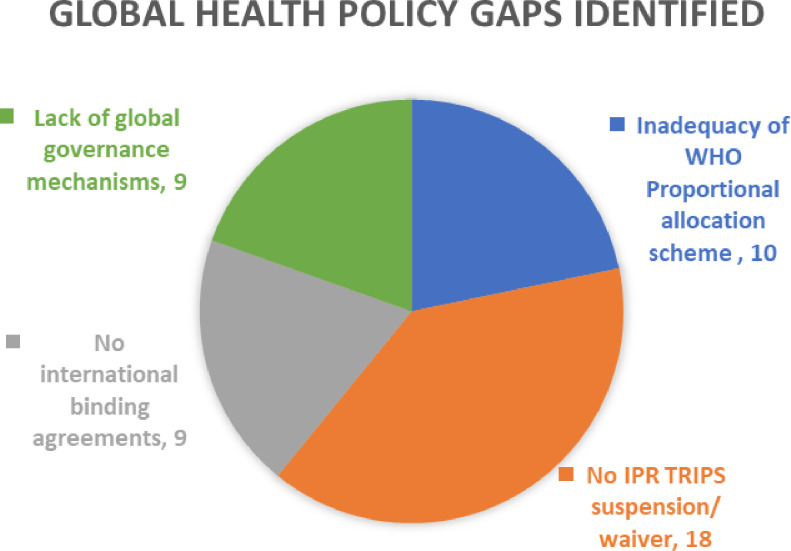
For research question 2, that is, to identify the significant global health policy and governance gaps (figure 5), the results are:
Figure 5.
Graph 3 represents global health and governance gaps identified by the scoping review from the evidence included for the final review. The pie diagram depicts the number of evidence corresponding to each identified gap.
The fair allocation mechanism formulated by the WHO is inadequate to contain the spread of the virus.12 14 15 18 19 24 39 49–51
Lack of consensus to waive/suspend the IPR, especially vaccine patents. The proposal put forward by South Africa and India failed at the WTO (World Trade Organization) session as both the HICs and pharma companies opposed the waiver.14 16 19 22 25–29 32 33 35 46 47 52–55
There is no international binding treaty to cap the bilateral vaccine deals and to manage the allocation and distribution of pandemic goods.14 15 22 35 43 54 56–58
The current global health governance mechanisms were inadequate to control the decision-making power that was entirely vested in giant pharma companies.2 14 15 32 33 43 45 57 59
WHO has released reports on COVAX based on regional deliveries focusing on COVID-19 vaccine equity at each regional level. But this study included evidence strictly restricted to global settings as the research questions revolved around global vaccine equity and COVAX as a global mechanism. The elaborated extraction tables for the research questions and the complete data charting for this scoping review are available.60 online supplemental file 2 online supplemental file 1
bmjgh-2023-012168supp002.pdf (760.6KB, pdf)
bmjgh-2023-012168supp001.pdf (122.6KB, pdf)
Discussion
The primary implementation challenge identified was vaccine nationalism by HICs. Vaccine nationalism means governments with purchasing power sign agreements with vaccine manufacturers to supply their population before it is made available to other countries. COVAX was created to address this accessibility and affordability gap between HICs and other low-income parts of the world.
Consequences of rampant vaccine nationalism by HICs
Because of rampant vaccine nationalism, bilateral deals crowd the global vaccine supply leaving less supply for COVAX and undermining COVAX’s purchasing power. In January 2021, HICs purchased 4.2 billion doses out of the total availability of 7 billion doses, and these countries represent only 15% of the world’s population. And they bought 60% of the entire vaccine pool. COVAX was deprived of supply as wealthy countries went on a bilateral procurement spree. As a result, COVAX was forced to reduce the target, adjusting to the lack of supply availability, thus delaying vaccine deliveries to LMICs and LICs.
Parallel to vaccine nationalism, COVAX faced a lack of funding and participation of HICs. Due to the preference for nationalist aspirations, more funding was allocated to independent national programmes rather than COVAX. For example, Operation Warp Speed by the USA is a PPP model for vaccine development with an initial budget of US$10 billion. Three dozen countries bypassed COVAX and made huge bilateral deals with manufacturers. The inadequacy of self-financing countries participants in COVAX leads to less collective buying power expected. For example, under President Trump’s administration, the USA did not join COVAX, which resulted in an initial lack of funding and participation. Only after the inauguration of the Biden administration the USA started funding COVAX and donated vaccines.61
Countries put several export restrictions to hinder the free flow of vaccines as an extension of national priority. For example, the USA invoked the Defense Production Act, which reduces the active pharma ingredient for AstraZeneca manufacturers. The alliance depended on the AstraZeneca vaccine because it was the first vaccine that got WHO emergency authorisation, cheaper than other vaccines, and was the first private vaccine manufacturing company to join COVAX. However, the second wave in India caused the reallocation of vaccines from the Serum Institute of India (SII) for domestic usage, and COVAX faced a shortfall of 190 million doses in June–July 2021.
IPR and global vaccine inequity
The core issue of inequity and vaccine nationalism lies with IPR TRIPS. The lack of global consensus to tackle IPR, especially vaccine patents, acted as a barrier to global vaccine equity. The framework gap analysis in this review has identified that COVAX Advanced Market Commitment (AMC) countries were used to bypass the IPR, making COVAX a middle-ground solution between the rich and the poor. South Africa and India proposed a waiver or suspension of IPR to WTO. Despite the support from the WHO director-general, the UK, the USA, Canada, Norway and EU (European Union) opposed the TRIPS waiver request.
The phenomenon of ‘pandemic profiteering’ depicts the enormous profits made by pharma companies through vaccine sales. HICs invested public funds in these pharma companies to develop vaccines. The Quid Pro Quo between pharma companies and the HICs is that the respective country will get vaccines on a priority basis, billions of USD as revenue, and increased job opportunities. Similarly, pharma companies can make huge profits from bilateral vaccine deals. In the case of Germany, BioNTech, Pfizer’s vaccine development partner, boosted Germany’s GDP (Gross Domestic Product) by 0.5% in 2021 and paid a revenue of €3.1 billion. So, both HICs and Pharma companies are least likely to let go of these profits, as this arrangement is clearly a win-win situation for them.
Vaccine nationalism and IPR gives a flawed view of Global Health and the Global Economy, where vaccines and medications are treated as market commodities rather than public goods. IPR on COVID-19 vaccines was unfair as taxpayers’ money was granted to pharma companies to develop COVID-19 vaccines. It was expected that these pharma companies distribute and provide access to the vaccines equitably, which has not happened even with the AstraZeneca vaccine. Oxford University developed the vaccine and pledged to donate the rights to any vaccine manufacturer. Ultimately, it was given to SII (Serum Institute of India), which the Bill & Melinda Gates Foundation funded to expand manufacturing and technology transfer. SII pledged to share 300 million vaccines to COVAX at US$3.00 per dose. Despite receiving a grant from CEPI (Coalition for Epidemic Preparedness innovations) in early 2020 for vaccine development to Moderna, it delivered the vaccine to COVAX in the second half of 2021, only after it honoured all its bilateral agreements.
The lack of waiver of IPR obstructs timely access to affordable vaccines. For example, South Africa purchased AstraZeneca for US$5.25 per dose, while the EU purchased the same for US$2.16 around the same time (January 2021). It is important to remember that South Africa hosted clinical trials and still had to pay more than double the price. TRIPS is identified as one of the significant barriers to healthcare equity in most evidence analysed for a scoping review. IPR patenting is the major obstacle for developing countries’ vaccine manufacturers to enter the market. The IPR protection for a pandemic good also constitutes human rights violation. Patent holders, that is, pharma companies, determine the global supply chain of COVID-19 vaccines. Thus, the IPR weakened the COVAX and made it difficult to achieve international cooperation.
COVAX failed to instil a commitment to get vaccines from major vaccine producers regarding the idea of a ‘partnership.’ WHO established the COVID-19 technology Access pool in 2020, and not even a single pharma company shared the technology. Pharma companies exploited their powerful positions as essential medicines suppliers and lobbied to WTO not to waive IPR.19 The People’s vaccine movement argued for a temporary waiver of IPR on COVID-19 tools and vaccines through TRIPS and faced massive opposition from HICs. It is also explicit from the scoping review that COVAX supports the argument that IPR is essential for developing vaccines, and the framework of COVAX never addresses the IPR issue. One of the studies in the review62 points out that even though there is a cosmopolitan view of justice in the construction of COVAX, the relevant actors in COVAX facility are nation—states which always prioritise their needs first.
Weakening of COVAX
This vaccine hoarding undermines the global geopolitical cooperation intended by COVAX by pooling resources. The original intention was that countries must come together in a cooperative mechanism like COVAX and join negotiations with manufacturers to enable fair prices and equitable distribution of vaccines. But in reality, COVAX was left alone without adequate funding and participation and reduced to another competitor in the vaccine market.
COVAX’s slogan is ‘No one is safe until everyone is safe,’ which reflects a vital public health concern and the need for global cooperation. But the COVAX framework did not challenge the IPR, paid pharma companies from public funding, and kept the negotiations secret, demonstrating that COVAX is similar to any other political entity. The optional purchase agreement by COVAX made HICs opt out of commitment and reduced COVAX to a mere insurance policy for HICs from a global buyers’ club. The self-financing countries had requested vaccine doses to vaccinate between 10% and 50% of their populations. This is a prima facie violation of the principle of equity on which the alliance was founded. This arrangement was to improve the participation of HICs but, in turn, acted as a bane to COVAX itself. Contractual obligations to HICs have forced COVAX to supply large quantities and had reserved one-fifth (485 million by 2021) of doses for rich countries, despite running short of doses for its commitment.
Advanced market commitments under COVAX were based on aid and charity, which helped in the increased production and distribution of vaccines across the globe, but restricted COAX’s authority to challenge the IPR issue. AMCs of COVAX were presumed to provide adequate vaccine doses to LMICs free of cost, which was a noble plan to accommodate the affordability factor. But relying on this age-old aid financing approach did not solve the fundamental problems of LMICs, such as equitable and fair access to vaccines, lesser capacity to manufacture and share the patents and technologies. Furthermore, COVAX claimed to represent the LMICs, and 81% of the representation in COVAX governing bodies was from HICs.
Another significant challenge was the limited number of manufacturing facilities globally. They are predominantly located in the Global North than LMICs. This has to be read along with limited knowledge sharing from Vaccine manufacturers. Finally, COVAX has been reduced to a charity-based aid project rather than a global self-procuring mechanism. COVAX’s total delivery includes 60% of donations from HICs. COVAX channelled 70% of all donated doses (776 million) in 2021. The USA was the single largest donor, followed by China and Germany.21 COVAX was mandated to procure vaccines globally to ensure their availability to all countries, the LICs and LMICs, which depended entirely on COVAX. As of May 2021, HICs promised to share 200 million doses, and only a few were delivered. The Biden administration promised to share 500 million Pfizer doses that need UCC facilities in LMICs, while the G7 agreed to share 870 million doses but stated it could only deliver 450 million by 2022.
COVAX claimed it as a ‘global dose sharing hub,’ but more than 30% of donated doses were delivered through bilateral agreements. Also, around 75% of donations to COVAX were earmarked (ie, 42% of total vaccine COVAX delivered in 2021). Dose sharing was sensed as convenient by both the HICs and pharma companies. The HICs could claim themselves as ‘vaccine donors’ in contrast to ‘vaccine hoarders,’ and pharma companies can still make their profit and argue that IPR is not the real problem but the inefficiency of the global redistribution of vaccines. The pharma companies included a non-sharing clause in many bilateral contracts, making the sharing process more complicated, and the contracts must be renegotiated before donating.
Global health governance and policy gaps
The scoping review showed that WHO’s proportional allocation scheme (PAS) has certain flaws. The basic logic of COVAX allocation is that ‘no country should vaccinate over 20% of its population until all nations have vaccinated 20%. Is it possible that HICs will agree on a system that aims to allocate doses for 20% of the population, which is far less to achieve herd immunity?63 With such a proportional allocation system, HICs were not interested in taking part voluntarily in COVAX and stopped procuring vaccines bilaterally for their populations.
Proportional allocation does not meet WHO’s ethical principles for vaccine allocation and ignores needs-based considerations. Different regions and countries have had different vaccination targets. Also, the WHO vaccine coverage target differed from the COVAX supply targets. Under PAS,31 COVAX was obligated to send vaccines to countries less affected by COVID-19, such as New Zealand, South Korea, instead of prioritising those severely affected by the pandemic, such as Mexico and Ecuador. Treating all countries identically, regardless of their circumstances, is equality, not equity. PAS was focused on the equity aspect based on countries’ income status, which became a barrier to achieving global vaccine equity but failed to consider other influencing factors. The PAS ignored the hardest-hit regions of the world that needed priority in vaccine allocation. In February 2021, Ghana got the first shipment from COVAX when Ghana reported 86 000 total cases and 7000 COVID-19 deaths, while Peru, a country with almost the same population, had reported 1.4 million cases and 48 000 deaths. Peru should have given priority to vaccines over Ghana.52 Distributive justice means fairness in the distribution of benefits. In the global COVID-19 vaccine equity context, priorities should be made considering the intensity of the spread of infection, demographic factors, mortality rates, absorptive capacity and income status of the country. It is high time to rethink our popular notions of health equity and global health justice.
Since COVAX is a PPP model with pharma companies as one of the partners, the lack of transparency in negotiation with pharma industries affects the credibility of the COVAX facility. During COVID-19, the situation was worsened by a lack of transparency in the manufacturing order books of Pharma companies and vaccine delivery to COVAX was pushed to the end of their queue. Pharma companies preferred bilateral deal delivery to COVAX because of the profit involved. The primary question here is—as partners of COVAX, do the pharma corporations have public accountability for deciding which countries get vaccines first? COVID-19 vaccine deals were not transparent, and the power rested with a few pharma companies controlling the global supply and distribution of vaccines. These companies decided how to prioritise supply between nations. Current global health architecture and existing regulations were inefficient in safeguarding equity. Also, there were no mechanisms for price controls or regulations that would have capped or set the prices of the vaccines.
COVAX did not share the power of decision-making, as it was governed by unelected officials of GAVI, and CEPI, with influence and support from HICs and private philanthropies. Private enterprises have had direct access to global health policy-making in the PPP model. Even though South Africa was the chair of the ACT-A governing Facilitation council, further representation from the global south was lacking. There was a lack of coordination in accessing and securing supply to COVAX. CEPI could not guarantee the access provisions to ensure an equitable global stockpile of vaccines despite the investment in vaccine development.63 It was GAVI, which later dealt with the procurement of doses. The GAVI and UNICEF procured vaccines at US$1.6625 but no further information was available.
There were no binding international agreements and enforcement of international cooperation by capping the bilateral deals. The international treaties were not sufficient to prevent vaccine hoarding or to specify the rights and obligations of the countries in the context of global public goods. So, under a voluntary scheme like COVAX, it cannot be expected that the HICs will let go of their political priorities and consider the morality of global vaccine equity.
Limitations of the study
The full-text screening involved further filtering of evidence with similar conclusions and recommendations. Also, COVAX has a humanitarian buffer64 specially tailored for the population without state actors, but could not find sufficient evidence according to the inclusion criteria, so the policy and governance gaps related to that could not be taken into consideration. Although this study did not include the most recent understandings of the COVAX initiative after March 2022, it methodologically researched, categorised and presented the existing themes and dilemmas about COVAX scattered over academia.
Conclusions
COVAX demonstrated an improved global cooperation and response level compared with H1N1 influenza in 2009. But still, the framework under which COVAX was formed is a mere middle-ground strategy balancing the opposing concepts of vaccine nationalism and global vaccine equity. COVAX was constructed in a world overpowered by market laws and a lack of decision-making transparency. There is unequal bargaining power between countries, complex geopolitics and a general lack of willingness to cooperate globally. The super PPP model is a remarkable global coordination attempt.22 COVAX is built on a framework around the partnership between governments and corporations as the best way to overcome market failures and embrace the IPR as a driver of innovation. COVAX has shown how hard it is to build a global multilateral institution that enables sharing. In hierarchical global power settings, powerful blocks cannot be coerced into sharing easily. They prefer bilateral deals and donate to emerging countries rather than permit an IPR waiver. HICs have shared corporate risks in developing new vaccines but have not shared the end products as global public goods. COVAX was left alone without adequate funding and participation and was reduced to another competitor in the vaccine market.
COVAX is a novel partnership model in global health but a middle-ground solution without addressing the core issues of inequity, such as patenting of pandemic products, less manufacturing capacity of LMICs, and lack of sharing of technology, knowledge and decision-making power. In a super-PPP structure such as COVAX, accountability and transparency of partners, including the pharma companies, are essential. COVAX does not work on a standalone mechanism and is connected to more significant global health governance issues and policy gaps. But despite these shortcomings, the fact that COVAX has been created as a global cooperative mechanism provides an optimistic approach for future pandemics. COVAX alliance is analogous to making a car while running it.65 COVAX can be a model for future pandemics if the root causes of the gaps in the framework and implementation challenges are addressed adequately. Lessons from COVAX can advise the future design of collaborative platforms for global public goods.
Footnotes
Handling editor: Valery Ridde
Twitter: @vkchattu
Contributors: The authors confirm contribution to the paper as follows: Study conception and design: VKC and PN. Data collection: AP. Analysis and interpretation of results: PN and AP. Draft manuscript preparation: AP. Draft review and corrections: AP, VKC and PN. All authors reviewed the results and approved the final version of the manuscript. PN and VKC act as guarantors.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available in a public, open access repository. All data relevant to the study are available either as supplementary information or uploaded in public, open access repository entitled "Data Charting for Scoping review final". Accessible at doi: 10.13140/RG.2.2.14866.63688.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.COVAX explained. Available: https://www.gavi.org/vaccineswork/covax-explained?gclid=Cj0KCQiAyMKbBhD1ARIsANs7rEGx4i5TYeVbcRoaSOh_T7IlJ9t_d-IdpmbeYhag_RuMc83FT4locZIaAmQeEALw_wcB [Accessed 19 Nov 2022].
- 2.Storeng KT, de Bengy Puyvallée A, Stein F. “COVAX and the rise of the “super public-private partnership” for global health”. Glob Public Health 2021:1–17. 10.1080/17441692.2021.1987502 [DOI] [PubMed] [Google Scholar]
- 3.COVAX Joint Statement: call to action to equip COVAX to deliver 2 billion doses in 2021, Available: https://www.who.int/news/item/27-05-2021-covax-joint-statement-call-to-action-to-equip-covax-to-deliver-2-billion-doses-in-2021
- 4.Ghana becomes recipient of historic first shipment of COVAX vaccine, Available: https://www.unicef.org/press-releases/ghana-becomes-recipient-historic-first-shipment-covax-vaccine [Accessed 24 Dec 2022].
- 5.COVAX global supply forecast, Available: https://www.who.int/publications/m/item/covax-global-supply-forecast [Accessed 19 Nov 2022].
- 6.Joint COVAX statement on supply forecast for 2021 and early 2022, Available: https://www.who.int/news/item/08-09-2021-joint-covax-statement-on-supply-forecast-for-2021-and-early-2022 [Accessed 24 Dec 2022].
- 7.COVAX global supply forecast,. 2021Available: https://www.gavi.org/sites/default/files/covid/covax/COVAX-Supply-Forecast.pdf [Accessed 24 Dec 2022].
- 8.COVID-19 market dashboard. Available: https://www.unicef.org/supply/covid-19-market-dashboard [Accessed 24 Dec 2022].
- 9.Goldhill O. Naively ambitious: how COVAX failed on its promise to vaccinate the world. STAT; 2021. Available: https://www.statnews.com/2021/10/08/how-covax-failed-on-its-promise-to-vaccinate-the-world/ [Accessed 24 Dec 2022]. [Google Scholar]
- 10.COVAX: 1 billion vaccines delivered. Available: https://www.unicef.org/supply/stories/covax-1-billion-vaccines-delivered [Accessed 19 Nov 2022].
- 11.Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol 2005;8:19–32. 10.1080/1364557032000119616 [DOI] [Google Scholar]
- 12.Eccleston-Turner M, Upton H. International collaboration to ensure equitable access to vaccines for COVID‐19: the ACT‐Accelerator and the COVAX facility. Milbank Q 2021;99:426–49. 10.1111/1468-0009.12503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McAdams D, McDade KK, Ogbuoji O, et al. Incentivising wealthy nations to participate in the COVID-19 vaccine global access facility (COVAX): a game theory perspective. BMJ Glob Health 2020;5:e003627. 10.1136/bmjgh-2020-003627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manriquez Roa T, Holzer F, Luna F, et al. Expert views on COVAX and equitable global access to COVID-19 vaccines. Int J Public Health 2021;66:1604236. 10.3389/ijph.2021.1604236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luna F, Holzer F. International cooperation in a non-ideal world: the example of COVAX. Cad Iberoam Direito Sanit 2021;10:199–210. 10.17566/ciads.v10i3.789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stein F. Risky business: COVAX and the financialization of global vaccine equity. Global Health 2021;17:112. 10.1186/s12992-021-00763-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Usher AD. Vaccine shortages prompt changes to COVAX strategy. The Lancet 2021;398:1474. 10.1016/S0140-6736(21)02309-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Access to COVID-19 vaccines: looking beyond COVAX. Lancet 2021;397. 10.1016/S0140-6736(21)00617-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Usher AD. A beautiful idea: how COVAX has fallen short. Lancet 2021;397:2322–5. 10.1016/S0140-6736(21)01367-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Storeng KT, Stein F. Antoine de Bengy Puyvallée. BMJ Glob Health 2021;6:11. 10.1136/bmjgh-2021-007763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Without global vaccination, all COVID immunity is at risk, Available: https://www.gavi.org/vaccineswork/without-global-vaccination-all-covid-immunity-risk?gclid=Cj0KCQjwspKUBhCvARIsAB2IYuueJkhvLVkSzg0IJlLloHICD8b9gB1Aj9-7zBBOG-dtTf38i6ojGRwaAuNMEALw_wcB [Accessed 21 Nov 2022].
- 22.The future of global pandemic security: navigating shifting landscapes – a Gavi white paper, Available: https://www.gavi.org/vaccineswork/future-global-pandemic-security-navigating-shifting-landscapes-gavi-white-paper [Accessed 24 Nov 2022].
- 23.The G20 must recommit to COVAX, Available: https://www.gavi.org/vaccineswork/g20-must-recommit-covax?gclid=Cj0KCQjwspKUBhCvARIsAB2IYutUq6WNd5IbPTAWbrXc2zhSeqA1AQZfGBs1CkKJHUBrrAY2ZtPc6NEaAuTGEALw_wcB [Accessed 24 Nov 2022].
- 24.Wouters OJ, Shadlen KC, Salcher-Konrad M, et al. Challenges in ensuring global access to COVID-19 vaccines: production, affordability, allocation, and deployment. Lancet 2021;397:1023–34. 10.1016/S0140-6736(21)00306-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borowy I. Perspectives on COVID-19 vaccine: the incredible success versus the incredible failure. Hist Soc Res 2021:147–72. 10.12759/hsr.suppl.33.2021.147-172 [DOI] [Google Scholar]
- 26.Massinga Loembé M, Nkengasong JN. COVID-19 vaccine access in Africa: global distribution, vaccine platforms, and challenges ahead. Immunity 2021;54:1353–62. 10.1016/j.immuni.2021.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erfani P, Binagwaho A, Jalloh MJ, et al. 2021. Intellectual property waiver for COVID-19 vaccines will advance global health equity. BMJ:1837. 10.1136/bmj.n1837 [DOI] [PubMed] [Google Scholar]
- 28.Kickbusch I, Holzscheiter A. Can geopolitics derail the pandemic treaty BMJ 2021;375:e069129. 10.1136/bmj-2021-069129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.UN ECOSOC . The united nations economic and social council (ECOSOC) high-level meeting on “a vaccine for all,” . 2021Available: https://www.un.org/ecosoc/sites/www.un.org.ecosoc/files/files/en/2021doc/PresidentialStatement-VaccineForAll.pdf [Accessed 24 Nov 2022].
- 30.Kim JH, Hotez P, Batista C, et al. Operation warp speed: implications for global vaccine security. Lancet Glob Health 2021;9:e1017–21. 10.1016/S2214-109X(21)00140-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.COVAX slot swapping: explained. Available: https://www.gavi.org/vaccineswork/covax-slot-swapping-explained?gclid=Cj0KCQjwyYKUBhDJARIsAMj9lkE7ZJs7UhmADqgheKPgD8iLJozvA37GnLNn4nJ6uk1JstbK_C0c148aAuj5EALw_wcB [Accessed 24 Nov 2022].
- 32.Singh B, Chattu VK, Kaur J, et al. “COVID-19 and global Distributive justice: “health diplomacy” of India and South Africa for the TRIPS waiver”. J Asian Afr Stud 2023;58:747–65. 10.1177/00219096211069652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peacocke EF, Heupink LF, Frønsdal K, et al. Global access to COVID-19 vaccines: a scoping review of factors that may influence equitable access for low and middle-income countries. BMJ Open 2021;11:e049505. 10.1136/bmjopen-2021-049505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fu X, Buckley PJ, Sanchez-Ancochea D, et al. The world has a unique opportunity: accelerating technology transfer and vaccine production through partnerships. J Int Bus Policy 2022;5:406–15. 10.1057/s42214-021-00124-7 [DOI] [Google Scholar]
- 35.Phelan AL, Eccleston-Turner M, Rourke M, et al. Legal agreements: barriers and enablers to global equitable COVID-19 vaccine access. The Lancet 2020;396:800–2. 10.1016/S0140-6736(20)31873-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Usher AD. CEPI criticised for lack of transparency. The Lancet 2021;397:265–6. 10.1016/S0140-6736(21)00143-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Health is a political choice, Available: https://www.gavi.org/vaccineswork/health-political-choice?gclid=Cj0KCQjwspKUBhCvARIsAB2IYuu0oLT8ZfGbCM2I3bQvFXRxbeK5GsaSeac5OR2N_3BReUsNZrChjkIaAvx6EALw_wcB [Accessed 24 Nov 2022].
- 38.Why we need to share vaccine doses now and why COVAX is the right way to do it, Available: https://www.gavi.org/vaccineswork/why-we-need-share-vaccine-doses-now-and-why-covax-right-way-do-it [Accessed 24 Nov 2022].
- 39.Rueda-Barrera EA. The waiver of COVID-19 vaccine patents: a fairness-based approach. J Glob Ethics 2021;17:367–74. 10.1080/17449626.2021.1998191 [DOI] [Google Scholar]
- 40.Du L, Wang M, Raposo VL. International efforts and next steps to advance COVID-19 vaccines research and production in low- and middle-income countries. Vaccines (Basel) 2021;10:42. 10.3390/vaccines10010042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gavi pandemic preparedness Q&A: cold chain experts on keeping vaccines cool. Gavi, the Vaccine Alliance; 2021. Available: https://www.gavi.org/vaccineswork/qa-cold-chain-experts-keeping-vaccines-cool [Google Scholar]
- 42.Mesa-Vieira C, Botero-Rodríguez F, Padilla-Muñoz A, et al. Reprint of: the dark side of the moon: global challenges in the distribution of vaccines and implementation of vaccination plans against COVID-19. Maturitas 2021;150:61–3. 10.1016/j.maturitas.2021.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Bengy Puyvallée A, Storeng KT. COVAX, vaccine donations and the politics of global vaccine inequity. Global Health 2022;18:26. 10.1186/s12992-022-00801-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.COVID-19 boosters: would a third jab really stop the pandemic?, Available: https://www.gavi.org/vaccineswork/covid-19-boosters-would-third-jab-really-stop-pandemic
- 45.Holzer F, Luna F, Manriquez T, et al. A matter of priority: equitable access to COVID-19 vaccines. Swiss Med Wkly 2021;151. 10.4414/smw.2021.20488 [DOI] [PubMed] [Google Scholar]
- 46.Intellectual property and COVID-19 vaccines, Available: https://www.gavi.org/vaccineswork/intellectual-property-and-covid-19-vaccines [Accessed 24 Nov 2022].
- 47.Mendis S. Regional vaccine production is key to ensuring equity. BMJ 2021;374:2354. 10.1136/bmj.n2354 [DOI] [PubMed] [Google Scholar]
- 48.Sekalala S, Forman L, Hodgson T, et al. Decolonising human rights: how intellectual property laws result in unequal access to the COVID-19 vaccine. BMJ Glob Health 2021;6:e006169. 10.1136/bmjgh-2021-006169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herzog LM, Norheim OF, Emanuel EJ, et al. Covax must go beyond proportional allocation of Covid vaccines to ensure fair and equitable access. BMJ 2021;372:m4853. 10.1136/bmj.m4853 [DOI] [PubMed] [Google Scholar]
- 50.Ohchr.org . 2021. Available: https://docstore.ohchr.org/SelfServices/FilesHandler.ashx?enc=4slQ6QSmlBEDzFEovLCuW1AVC1NkPsgUedPlF1vfPMKseJUC1CI6FcIakFK95v85g4Ik7k7QBI8EdfqmClTMrneFvtX1I0IL8hktDGNgWJc7FBC2uG%2b%2fdxsnlN1jrczo [Accessed 24 Nov 2022].
- 51.Sharma S, Kawa N, Gomber A. WHO's allocation framework for COVAX: is it fair? J Med Ethics 2022;48:434–8. 10.1136/medethics-2020-107152 [DOI] [PubMed] [Google Scholar]
- 52.Kalaitzidis G. Equitable global COVID-19 vaccine allocation and distribution: obstacles, contrasting moral perspectives, ethical framework and current standpoints. Ethics & Bioethics 2021;11:163–80. 10.2478/ebce-2021-0015 [DOI] [Google Scholar]
- 53.Okereke M. Towards vaccine equity: should big Pharma waive intellectual property rights for COVID-19 vaccines Public Health Pract (Oxf) 2021;2:100165. 10.1016/j.puhip.2021.100165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hassan F, Yamey G, Abbasi K. Profiteering from vaccine inequity: a crime against humanity? BMJ 2021;374:2027. 10.1136/bmj.n2027 [DOI] [PubMed] [Google Scholar]
- 55.Actions must speak louder than words: five asks to achieve equity in vaccine delivery. UNHCR. Available: https://www.unhcr.org/news/press/2021/10/617ad7ea4/actions-must-speak-louder-words-five-asks-achieve-equity-vaccine-delivery.html [Accessed 14 Oct 2022]. [Google Scholar]
- 56.Karim SA. COVID-19 vaccine affordability and accessibility. The Lancet 2020;396:238. 10.1016/S0140-6736(20)31540-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haugen HM. Does TRIPS (agreement on trade‐related aspects of intellectual property rights) prevent COVID‐19 vaccines as a global public good J World Intellect Prop 2021;24:195–220. 10.1111/jwip.12187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li Z, Lu J, Lv J. The inefficient and unjust global distribution of COVID-19 vaccines: from a perspective of critical global justice. Inquiry 2021;58:004695802110609. 10.1177/00469580211060992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rackimuthu S, Narain K, Lal A, et al. Redressing iCOVID-19 vaccine inequity amidst booster doses: charting a bold path for global health solidarity, together. Global Health 2022;18:23. 10.1186/s12992-022-00817-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chattu VK, Narayanan P. Data charting for Scoping review final. 2023. 10.13140/RG.2.2.14866.63688 [DOI]
- 61.Washington Post . Opinion | Trump’s refusal to join a global vaccine effort epitomizes an America that’s isolated and weak, Available: https://www.washingtonpost.com/opinions/trumps-refusal-to-join-a-global-vaccine-effort-epitomizes-an-america-thats-isolated-and-weak/2020/09/02/f64c23c6-ed44-11ea-99a1-71343d03bc29_story.html [Accessed 24 Dec 2022].
- 62.Ferguson K, Caplan A. Love thy neighbour? allocating vaccines in a world of competing obligations. J Med Ethics 2020. 10.1136/medethics-2020-106887 [DOI] [PubMed] [Google Scholar]
- 63.Lie RK, Miller FG. Allocating a COVID‐19 vaccine: balancing national and international responsibilities. Milbank Q 2021;99:450–66. 10.1111/1468-0009.12494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mukumbang FC. Are asylum seekers, refugees and foreign migrants considered in the COVID-19 vaccine discourse BMJ Glob Health 2020;5:e004085. 10.1136/bmjgh-2020-004085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Katz IT, Weintraub R, Bekker L-G, et al. From vaccine nationalism to vaccine equity — finding a path forward. N Engl J Med 2021;384:1281–3. 10.1056/NEJMp2103614 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2023-012168supp002.pdf (760.6KB, pdf)
bmjgh-2023-012168supp001.pdf (122.6KB, pdf)
Data Availability Statement
Data are available in a public, open access repository. All data relevant to the study are available either as supplementary information or uploaded in public, open access repository entitled "Data Charting for Scoping review final". Accessible at doi: 10.13140/RG.2.2.14866.63688.