Abstract
Introduction
Comorbidities are common in patients with chronic obstructive pulmonary disease (COPD). Estimates of prevalence, incidence and prognostic impact of comorbidities provide foundational knowledge of COPD epidemiology. We examined the prevalence, incidence and prognostic impact of 21 comorbidities among patients with COPD compared with the Danish general population.
Methods
We conducted a nationwide, population-based cohort study based on longitudinal Danish registry data, covering all Danish hospitals (2010–2021). The cohorts comprised 142 973 patients with a first-time hospital-based diagnosis of COPD and 428 917 age-matched and sex-matched comparators from the general population. During follow-up, we estimated the 5-year risk and risk difference, using competing risk methods when applicable.
Results
At time of diagnosis, the comorbidities with the highest prevalence were mood, stress-related or anxiety disorders (25.2% for patients with COPD vs 13.1% for comparators), osteoporosis/hip fractures (17.4% vs 9.9%), diabetes (15.6% vs 10.5%), peripheral arterial disease (13.5% vs 4.9%) and heart failure (13.3% vs 4.0%). During follow-up, the risk of most incident comorbidities was markedly elevated among patients with COPD. The five comorbidities associated with the highest 5-year absolute risk difference with respect to the risk in the general population were mood, stress-related or anxiety disorders (5.7%), osteoporosis/hip fractures (5.6%), heart failure (4.2%), smoking-related cancers (2.8%) and peripheral arterial disease (2.7%). The 5-year mortality risk was 43% vs 17.7%. Among patients with COPD, the 5-year mortality risk markedly increased with the number of comorbidities present.
Conclusions
Our population-based findings underscore the importance of considering comorbidities in the management of COPD.
Keywords: COPD epidemiology
WHAT IS ALREADY KNOWN ON THIS TOPIC
Comorbidity is common in patients with chronic obstructive pulmonary disease (COPD), but existing studies assessing the occurrence and prognostic impact of comorbidity in patients with COPD were not population based, lacked longitudinal follow-up, relied on self-reported data and focused on a narrow spectrum of comorbidities.
WHAT THIS STUDY ADDS
This study found that mood, stress-related or anxiety disorders, osteoporosis/hip fractures and heart failure were among the most prevalent and incident comorbidities, and that the presence and increasing number of comorbidities in patients with COPD had a strong influence on mortality, with cancer being the one comorbidity associated with the worst prognosis.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study underscores the importance of comorbidities in the management of COPD and emphasises that COPD is a central disease within the wider issue of multimorbidity. These findings may provide useful information for patients, clinicians and policymakers of effective resource allocation.
Introduction
Chronic obstructive pulmonary disease (COPD), a major global health burden and a leading cause of death,1 is a heterogeneous lung condition characterised by chronic respiratory symptoms due to abnormalities of the airways and/or alveoli, thereby often resulting in progressive airflow obstruction.2 3
Patients with COPD often suffer from other chronic diseases (ie, comorbidities),4–23 which may be linked through shared risk factors (eg, smoking, ageing and environmental exposures) as well as systemic effects, such as systemic inflammation, oxidative stress, tissue hypoxia, impaired functional capacity, skeletal muscle wasting and cachexia.3 16 24 25 With population ageing, the number of patients with COPD with comorbidities is expected to rise. Multimorbidity (ie, the coexistence of two or more chronic conditions26) is a major challenge in current healthcare systems,27 28 and given its substantial global prevalence, COPD is a key disease in this regard. Comorbidity among patients with COPD is a strong predictor of adverse prognosis, impaired quality of life and high healthcare utilisation and costs.3 17 29 Thus, careful consideration of comorbidities is required in treating patients with COPD in clinical practice.
Understanding the prevalence and incidence of comorbidities provides foundational knowledge of COPD epidemiology, and may aid in effective prevention and treatment strategies, healthcare planning and further research. Existing studies describing comorbidities in patients with COPD have not been population based,5 6 8 10 11 13 16 18 20 21 23 have lacked longitudinal follow-up,5 6 9 12 14 have relied on self-reported data5 8 12 16 21 or have focused on a narrow spectrum of comorbidities.7 19 29 In addition, some studies have lacked comparison cohorts, whose inclusion can aid in the contextualisation of risk estimates.8 10 11 13 20
Here, in a nationwide population-based cohort study, we examined the prevalence and incidence of a broad spectrum of comorbidities among patients with COPD compared with a general population cohort matched on age, sex and calendar year. We also assessed the impact of comorbidity on all-cause mortality after COPD.
Methods
Setting and data sources
Denmark has a state-funded healthcare system that provides free access to healthcare for all residents.30 A rich infrastructure of nationwide, population-based clinical and administrative registries has been created in the country.30 All residents are assigned a unique personal identification number that allows unambiguous linkage across registries at an individual level, as well as complete long-term follow-up.30 In this study, we used data from the following sources:
The Danish Civil Registration System is updated daily and contains information on date of birth, sex and vital status for the entire Danish population since 1968.31
The Danish National Patient Registry contains information on all hospital inpatient admissions since 1977.32 Outpatient specialty clinic contacts and emergency department visits were added in 1995. For each inpatient, outpatient clinic and emergency department discharge, one primary diagnosis and optional secondary diagnoses are recorded according to the International Classification of Diseases (ICD, 8th Revision between 1977 and 1993 (ICD-8) and 10th Revision (ICD-10) thereafter).
The Danish National Prescription Registry has recorded all prescriptions filled in community pharmacies since 1995.33 Filled prescriptions are coded according to the Anatomical Therapeutic Chemical Classification System.
The Danish Psychiatric Central Research Registry contains information on all psychiatric hospital admissions since 1969, and all psychiatric outpatient contacts and emergency room visits since 1995.34
Patient and public involvement
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
COPD cohort
We searched the patient registry for inpatient and outpatient clinic diagnoses to identify all people ≥40 years of age with a first-time discharge diagnosis of COPD between 1 January 2010 and 31 December 2021. We did not search for emergency department diagnoses, because these are working diagnoses with a high proportion of misclassification. The positive predictive value of the COPD diagnosis in the patient registry has been found to be high (92%).35 Each patient with COPD was assigned an ‘index date’ corresponding to the discharge date associated with the COPD diagnosis; that is, patients were required to survive the length of the COPD hospitalisation for inclusion in the study.
General population comparison cohort
To contextualise the risks with respect to those expected in the general population, we matched each patient with COPD with up to five individuals from the Danish general population according to birth year, sex and calendar year. Matching was performed with replacement. Comparators were assigned an index date corresponding to the COPD discharge date of the matched patient. To be eligible for matching, comparators were required to have no COPD diagnosis before their index date, and to be alive and living in Denmark.36 Comparators who were diagnosed with COPD during follow-up then contributed risk time in both cohorts to avoid informative censoring.36
Comorbidities
We considered a broad spectrum of comorbidities, which were identified from hospital-based discharge diagnoses recorded in the patient registry and, when applicable, filled prescriptions from the prescription registry. Comorbidities included cancers (smoking-related cancers, non-smoking-related cancers (defined in online supplemental table 1)), circulatory system diseases (acute myocardial infarction, heart failure, peripheral arterial disease, stroke and venous thromboembolism), endocrine system diseases (diabetes mellitus), gastrointestinal system diseases (chronic liver disease, ulcer/chronic gastritis and inflammatory bowel disease), urogenital system diseases (chronic kidney disease and prostate disorders), musculoskeletal system diseases (connective tissue disorders, osteoporosis/hip fractures), neurological system diseases (migraine and dementia) and mental health disorders (mood, stress-related or anxiety disorders, alcohol or substance abuse disorders, bipolar affective disorders and schizophrenia). In the follow-up analysis, we also considered all-cause mortality. The Danish nationwide registries have high data validity,30 and most comorbidities considered in this study have been validated with positive predictive values ranging from 65% to 100% (online supplemental references). Online supplemental table 1 lists all diagnostic codes used in this study and their positive predictive values.
bmjresp-2023-001798supp001.pdf (523.1KB, pdf)
Statistical analysis
Description of patients with COPD
Using medians and IQR for continuous variables and proportions for categorical variables, we described patients and population comparators in terms of the median age at diagnosis, age group (40–49, 50–59, 60–69, 70–79 and 80 years), calendar period of COPD diagnosis (2010–2012, 2013–2015, 2016–2018 and 2019–2021), the prevalence of each pre-existing comorbidity described above (with application of a look-back period from the index date to 1994, when ICD-10 was adopted) and the number of pre-existent comorbidities (0, 1, 2, 3 and ≥4).
Risk of incident comorbidities after COPD
Follow-up began on the index date and continued for 5 years or until the occurrence of an incident outcome, death or emigration, whichever occurred first. For each separate outcome, only patients without a history of that outcome at the time of the index date were included. Because the onset and initial diagnosis of inflammatory bowel disease, migraine, bipolar affective disorders and schizophrenia occur most often during adolescence or early adulthood, we did not include these conditions in the incident risk estimation. We calculated the 5-year absolute risks and risk differences for each outcome, comparing patients with COPD with matched comparators, using the Aalen-Johansen estimator, which accounts for the competing risk of death.37 In addition, as a measure of the relative risk of each outcome associated with COPD, we used the Fine-Gray regression model yielding subdistribution HRs (SHRs) and corresponding 95% CIs. The Aalen-Johansen and Fine-Gray models have a direct one-to-one correspondence. Given the matched cohort design, both absolute and relative contrasts were controlled for differences between patients and comparators regarding the distributions of sex, age and calendar year. This minimal adjustment strategy aligned with the descriptive aim of the study.38 We stratified these analyses according to sex and age group (40–59, 60–69, 70–79 and ≥80 years).
In sensitivity analyses, to account for and assess potential diagnostic bias, we restricted the study population (both patients with COPD and matched comparators) to those with ≥2 visits to a general practitioner within 1 year before the index date.
Impact of pre-existing comorbidities on all-cause mortality after COPD
To examine the impact of pre-existing comorbidities on all-cause mortality, we followed up patients and comparators for 3 years from the index date. We computed the 5-year mortality risk overall, and stratified by the number of pre-existing comorbidities and each individual comorbidity with the complement of the Kaplan-Meier estimator. We also computed the HR of death with Cox proportional hazards regression. The proportionality assumption of the Cox model was inspected using log-log plots and deemed satisfied. In the stratified analyses, we dissolved the matching and adjusted for the matching factors in the Cox regression.
Results
Description of patients with COPD
We identified 142 973 patients with a first-time diagnosis of COPD during 2010–2021 as well as 428 917 matched comparators from the general population (table 1). The median age was 72 years (IQR: 63–79 years), and women composed approximately half (50.3%) of the population.
Table 1.
Characteristics of patients with a first-time diagnosis of chronic obstructive pulmonary disease (COPD), and age-matched, sex-matched and calendar year-matched individuals from the general population, Denmark, 2010–2021
| Patients with COPD | Matched general population comparators | |
| Overall | 142 973 | 428 917 |
| Age (years) | ||
| Median (IQR) | 71 (63–79) | 71 (63–79) |
| 40–49 | 6406 (4.5%) | 19 184 (4.5%) |
| 50–59 | 19 350 (13.5%) | 58 047 (13.5%) |
| 60–69 | 37 662 (26.3%) | 113 151 (26.4%) |
| 70–79 | 46 180 (32.3%) | 138 309 (32.2%) |
| ≥80 | 33 375 (23.3%) | 100 226 (23.4%) |
| Women | 71 928 (50.3%) | 215 784 (50.3%) |
| Calendar period of COPD diagnosis | ||
| 2010–2012 | 38 872 (27.2%) | 116 616 (27.2%) |
| 2013–2015 | 38 725 (27.1%) | 116 175 (27.1%) |
| 2016–2018 | 36 051 (25.2%) | 108 151 (25.2%) |
| 2019–2021 | 29 325 (20.5%) | 87 975 (20.5%) |
| Comorbidities (look-back 1994) | ||
| Smoking-related cancers | 15 861 (11.1%) | 25 304 (5.9%) |
| Non-smoking-related cancers | 13 223 (9.2%) | 36 195 (8.4%) |
| Acute myocardial infarction | 12 938 (9%) | 20 379 (4.8%) |
| Heart failure | 19 051 (13.3%) | 17 062 (4%) |
| Peripheral arterial disease | 19 263 (13.5%) | 21 094 (4.9%) |
| Stroke | 18 006 (12.6%) | 32 060 (7.5%) |
| Venous thromboembolism | 8264 (5.8%) | 11 680 (2.7%) |
| Diabetes mellitus | 22 234 (15.6%) | 45 226 (10.5%) |
| Chronic liver disease | 3767 (2.6%) | 3250 (0.8%) |
| Ulcer/chronic gastritis | 12 029 (8.4%) | 16 453 (3.8%) |
| Inflammatory bowel disease | 2535 (1.8%) | 4826 (1.1%) |
| Chronic kidney disease | 5926 (4.1%) | 6507 (1.5%) |
| Prostate disorders | 13 526 (9.5%) | 35 715 (8.3%) |
| Connective tissue disorders | 8569 (6%) | 15 929 (3.7%) |
| Osteoporosis/hip fractures | 24 947 (17.4%) | 42 521 (9.9%) |
| Migraine | 2525 (1.8%) | 6577 (1.5%) |
| Dementia | 5089 (3.6%) | 13 370 (3.1%) |
| Mood, stress-related or anxiety disorders | 36 024 (25.2%) | 56 286 (13.1%) |
| Alcohol or substance abuse disorders | 14 501 (10.1%) | 9482 (2.2%) |
| Bipolar affective disorders | 1603 (1.1%) | 2066 (0.5%) |
| Schizophrenia | 2143 (1.5%) | 1745 (0.4%) |
| Number of comorbidities | ||
| 0 | 30 559 (21.4%) | 194 583 (45.4%) |
| 1 | 39 010 (27.3%) | 122 951 (28.7%) |
| 2 | 32 263 (22.6%) | 63 265 (14.7%) |
| 3 | 20 692 (14.5%) | 28 693 (6.7%) |
| ≥4 | 20 449 (14.3%) | 19 425 (4.5%) |
The prevalence of virtually all assessed comorbidities was higher in patients with COPD than comparators. The highest comorbidity prevalence was found for mood, stress-related and anxiety disorders (25.2% for patients with COPD vs 13.1% for comparators), osteoporosis/hip fractures (17.4% vs 9.9%), diabetes mellitus (15.6% vs 10.5%), peripheral arterial disease (13.5% vs 4.9%) and heart failure (13.3% vs 4.0%). The prevalence of smoking-related cancers was 11.1% vs 5.9%. Only 21.4% of patients with COPD did not have a history of comorbidities vs 45.4% among comparators; in contrast, the prevalence of a history of one, two, three, and four or more comorbidities was 27.3% vs 28.7%, 22.6% vs 14.7%, 14.5% vs 6.7%, and 14.3% vs 4.5% (table 1).
Risk of incident comorbidities after COPD
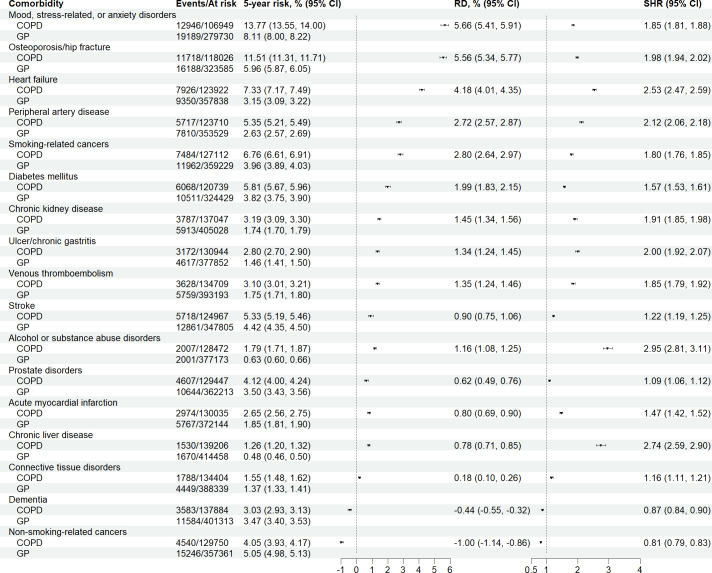
The risk of most incident comorbidities was markedly greater among patients with COPD than general population comparators. Figure 1 illustrates the 5-year risk, risk differences and SHRs for each comorbidity, ranked according to the highest absolute risk difference. The 10 comorbidities associated with the highest absolute risk difference with respect to the risk in the general population were mood, stress-related and anxiety disorders (5-year risk: 13.8% vs 8.1%; risk difference: 5.7% (95% CI: 5.4%, 5.9%)), osteoporosis/hip fractures (11.5% vs 6.0%; risk difference: 5.6% (95% CI: 5.3%, 5.8%)), heart failure (7.3% vs 3.2%; risk difference: 4.2% (95% CI: 4.0%, 4.4%)), smoking-related cancers (6.8% vs 4.0%; risk difference: 2.8% (95% CI: 2.6%, 3.0%)), peripheral arterial disease (5.4% vs 2.6%; risk difference: 2.7% (95% CI: 2.6%, 2.9%)), diabetes mellitus (5.8% vs 3.8%; risk difference: 2.0% (95% CI: 1.8%, 2.2%)), chronic kidney disease (3.2% vs 1.7%; risk difference: 1.5% (95% CI: 1.3%, 1.6%)), venous thromboembolism (3.1% vs 1.8%; risk difference: 1.4% (95% CI: 1.2%, 1.5%)), ulcer/chronic gastritis (2.8% vs 1.5%; risk difference: 1.3% (95% CI: 1.2%, 1.5%)), and alcohol or substance abuse disorders (1.8% vs 0.6%; risk difference: 1.2% (95% CI: 1.1%, 1.3%)). On the relative scale, alcohol or substance abuse disorders (SHR: 3.0 (95% CI: 2.8, 3.1)), chronic liver disease (SHR: 2.7 (95% CI: 2.6, 2.9)) and heart failure (SHR: 2.5 (95% CI: 2.5, 2.6)) showed the strongest associations.
Figure 1.
Numbers of events, numbers at risk, 5-year absolute risks, risk differences (RDs) and subdistribution HRs (SHRs) for a spectrum of comorbidities, ranked according to the largest RD, comparing patients with chronic obstructive pulmonary disease (COPD) with matched individuals from the general population (GP).
These results were similar for men and women for most comorbidities (online supplemental figure 1). However, the 5-year risk of osteoporosis/hip fracture was higher in women (5-year risk: 15.0%; risk difference: 6.9% (95% CI: 6.5%, 7.3%)) than in men (5-year risk: 8.1%; risk difference: 4.2% (95% CI: 4.0%, 4.5%)). The associations tended to decrease with increasing age group (online supplemental figure 2), although this pattern was most clearly observed on the relative scale.
When restricting the study population to those with frequent interaction with primary healthcare, in order to reduce potential diagnostic bias, results remained largely intact (online supplemental figure 3).
Impact of pre-existing comorbidity on all-cause mortality after COPD
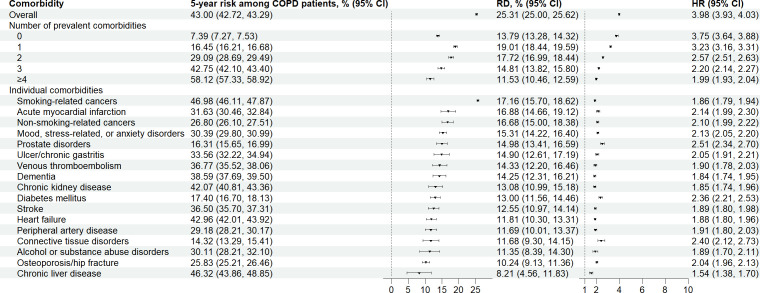
Overall, we observed 54 091 deaths among patients with COPD and 63 032 deaths among individuals from the general population. The 5-year mortality risk was 43% (95% CI: 42.7%, 43.3%) vs 17.7% (17.6%, 17.8%), corresponding to a risk difference of 25.3% (95% CI: 25%, 25.6%) and an HR of 4.0 (95% CI: 3.93, 4.03) (figure 2). Expectedly, the 5-year mortality risk among patients with COPD increased with the number of comorbidities present (eg, 21.2% for those without comorbidities and 69.7% for those with four or more comorbidities). However, the difference in risk with respect to that in the general population did not clearly increase with the number of comorbidities while HRs declined. In the assessment of individual comorbidities, smoking-related cancers showed the greatest difference on the absolute scale with respect to the risk in the general population (risk difference: 17.2% (95% CI: 15.7%, 18.6%)).
Figure 2.
Five-year absolute mortality risks, risk differences (RDs) and HRs overall and stratified by number of prevalent comorbidities, and each individual comorbidity, ranked according to the largest RD, comparing patients with chronic obstructive pulmonary disease (COPD) with matched individuals from the general population.
Discussion
Our study provides key population-based estimates of the prevalence, incidence and prognostic impact of comorbidities in patients with COPD, as compared with individuals of similar age and sex from the Danish general population. First, our study demonstrated a high occurrence of comorbidities among patients with COPD. Mood, stress-related or anxiety disorders and osteoporosis/hip fractures were among the most prevalent and incident comorbidities. Second, we found that 43% of patients with COPD died within 5 years of follow-up, compared with 18% in the general population. Importantly, the presence and increasing number of comorbidities in patients with COPD had a strong influence on mortality, with cancer being the one comorbidity associated with the worst prognosis.
We extend previous research by providing population-based estimates of absolute risks of comorbidities in patients with COPD.5–13 17 Such estimates are important for patients, clinicians and policymakers of effective resource allocation. Here, we note several important points that may aid the interpretation of our findings and those from previous studies.
First, a direct comparison of estimates between studies is challenging. Variations are expected, depending on the setting (primary or hospital care), source population (prevalence of associated risk factors), data sources (population based or not) and disease measures (diagnostic codes or self-reports). In contrast to previous studies,5 6 8 10 our study was population based and relied on diagnostic and prescription codes.
Second, the positive association between COPD and most assessed comorbidities can probably be explained by a combination of shared risk factors (eg, smoking, other unhealthy lifestyle habits, environmental exposures and socioeconomic position3), direct effects of COPD on the heart (eg, cor pulmonale), systematic effects associated with COPD (eg, systemic inflammation, oxidative stress, tissue hypoxia, impaired functional capacity, skeletal muscle wasting and cachexia25) and medication use (eg, glucocorticoids). In many circumstances, separating prevalent and incident comorbidities was difficult. COPD and certain comorbidities can have an insidious onset, thus leading to difficulties in determining temporality. However, our study did not aim to separate COPD-specific effects from underlying risk factors, and, importantly, unadjusted risk estimates are typically more informative for resource allocation and guidance of surveillance and preventive strategies.38 We also cannot rule out the possibility of some degree of diagnostic bias, which might have biased the associations for selected chronic comorbid disorders upward. Compared with individuals from the general population, patients with COPD have greater contact with the healthcare system, and thereby a greater probability of being diagnosed with comorbidities.39 The extent of this issue varies across the assessed comorbidities and is expected to be minor for comorbidities with an acute onset that require hospitalisation (eg, acute myocardial infarction, venous thromboembolism, stroke and hip fractures) and greater for comorbidities with an insidious onset (eg, chronic heart failure, chronic kidney disease and osteoporosis).
Our study has some limitations. First, although diagnostic codes for COPD and most comorbidities assessed in this study have been validated with high positive predictive values in the Danish nationwide registries, the completeness (ie, sensitivity) of these codes remains largely unknown (online supplemental references). For example, patients with COPD were included from hospital-based diagnoses and many patients with less severe COPD were likely not captured. Thus, our findings must be interpreted in the light of the relatively sick population with COPD included. For some comorbidities, this issue is more prominent than for others. For example, virtually all people with acute myocardial infarction or stroke are treated in hospitals and thereby captured. On the other hand, many patients with mild to moderate mental illness are untreated or are seen only by general practitioners, and thereby not given a diagnostic code. However, to alleviate this issue, we included redeemed prescriptions in the definition of certain comorbidities (eg, redemption of at least two antidepressants within 1 year was included in the definition of mood, stress-related or anxiety disorders). We thereby likely captured a large proportion of patients seen only by general practitioners. The 5-year absolute risk of mood, stress-related or anxiety disorders was high (13.8%), which aligns with the fact that comorbid anxiety is known to be common in patients with COPD.40 Second, we lacked detailed clinical data on patients with COPD, for example, information on disease severity such as pulmonary function, smoking history and intensity, and body mass index, all of which are expected to be important prognostic predictors.3 Last, we did not assess several relevant comorbidities, such as hypertension or dyslipidaemia, most of which are treated by general practitioners.
In summary, while several points must be considered when interpreting our findings, our population-based estimates of occurrence and prognostic impact of comorbidities in patients with COPD underscore the importance of comorbidities in the management of COPD and emphasise that COPD is a central disease within the wider issue of multimorbidity. These findings may provide useful information for patients, clinicians and policymakers of effective resource allocation.
Footnotes
Contributors: All authors contributed to the design of the study. HTS acquired the data. All authors directed the analyses, which were carried out by EH-P. NS and KL wrote the initial draft. All authors contributed to the discussion and interpretation of the results, which secured the intellectual content of the manuscript. All authors approved the final version for submission. HTS is the guarantor.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data may be obtained from a third party and are not publicly available. The data used in this study are owned and managed by Statistics Denmark. In accordance with the Danish law and data protection policies, the data used in this study were anonymised, and stored and analysed on a secure server. The data are available to researchers from research environments pre-approved by Statistics Denmark upon project approval by the Danish Data Protection Agency and Statistics Denmark. Researchers can apply for access to data when the request is approved by the Danish Data Protection Agency (https://www.datatilsynet.dk).
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study involves human participants and was registered at Aarhus University (record number 2016-051-000001-810) as mandated by the Danish Data Protection Agency. According to Danish legislation, registry-based studies do not require ethical review board approval or informed consent from the participants.
References
- 1.Safiri S, Carson-Chahhoud K, Noori M, et al. Burden of chronic obstructive pulmonary disease and its attributable risk factors in 204 countries and territories, 1990-2019: results from the global burden of disease study 2019. BMJ 2022;378:e069679. 10.1136/bmj-2021-069679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Celli B, Fabbri L, Criner G, et al. Definition and nomenclature of chronic obstructive pulmonary disease: time for its revision. Am J Respir Crit Care Med 2022;206:1317–25. 10.1164/rccm.202204-0671PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Venkatesan P. GOLD COPD report: 2023 update. Lancet Respir Med 2023;11:18. 10.1016/S2213-2600(22)00494-5 [DOI] [PubMed] [Google Scholar]
- 4.Mapel DW, Hurley JS, Frost FJ, et al. Health care utilization in chronic obstructive pulmonary disease. A case-control study in a health maintenance organization. Arch Intern Med 2000;160:2653–8. 10.1001/archinte.160.17.2653 [DOI] [PubMed] [Google Scholar]
- 5.van Manen JG, Bindels PJ, IJzermans CJ, et al. Prevalence of Comorbidity in patients with a chronic airway obstruction and controls over the age of 40. J Clin Epidemiol 2001;54:287–93. 10.1016/s0895-4356(01)00346-8 [DOI] [PubMed] [Google Scholar]
- 6.Holguin F, Folch E, Redd SC, et al. Comorbidity and mortality in COPD-related hospitalizations in the United States, 1979 to 2001. Chest 2005;128:2005–11. 10.1378/chest.128.4.2005 [DOI] [PubMed] [Google Scholar]
- 7.Sidney S, Sorel M, Quesenberry CP, et al. COPD and incident cardiovascular disease hospitalizations and mortality: Kaiser Permanente medical care program. Chest 2005;128:2068–75. 10.1378/chest.128.4.2068 [DOI] [PubMed] [Google Scholar]
- 8.Crisafulli E, Costi S, Luppi F, et al. Role of Comorbidities in a cohort of patients with COPD undergoing pulmonary rehabilitation. Thorax 2008;63:487–92. 10.1136/thx.2007.086371 [DOI] [PubMed] [Google Scholar]
- 9.Cazzola M, Bettoncelli G, Sessa E, et al. Prevalence of Comorbidities in patients with chronic obstructive pulmonary disease. Respiration 2010;80:112–9. 10.1159/000281880 [DOI] [PubMed] [Google Scholar]
- 10.Terzano C, Conti V, Di Stefano F, et al. Comorbidity, hospitalization, and mortality in COPD: results from a longitudinal study. Lung 2010;188:321–9. 10.1007/s00408-009-9222-y [DOI] [PubMed] [Google Scholar]
- 11.Divo M, Cote C, de Torres JP, et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012;186:155–61. 10.1164/rccm.201201-0034OC [DOI] [PubMed] [Google Scholar]
- 12.Schnell K, Weiss CO, Lee T, et al. The prevalence of clinically-relevant comorbid conditions in patients with physician-diagnosed COPD: a cross-sectional study using data from NHANES 1999-2008. BMC Pulm Med 2012;12:26. 10.1186/1471-2466-12-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vanfleteren LEGW, Spruit MA, Groenen M, et al. Clusters of Comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013;187:728–35. 10.1164/rccm.201209-1665OC [DOI] [PubMed] [Google Scholar]
- 14.Baty F, Putora PM, Isenring B, et al. Comorbidities and burden of COPD: a population based case-control study. PLoS One 2013;8:e63285. 10.1371/journal.pone.0063285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Putcha N, Han M, Martinez C, et al. n.d. Comorbidities of COPD have a major impact on clinical outcomes, particularly in African Americans. J COPD F;1:105–14. 10.15326/jcopdf.1.1.2014.0112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller J, Edwards LD, Agustí A, et al. Comorbidity, systemic inflammation and outcomes in the ECLIPSE cohort. Respir Med 2013;107:1376–84. 10.1016/j.rmed.2013.05.001 [DOI] [PubMed] [Google Scholar]
- 17.Johannesdottir SA, Christiansen CF, Johansen MB, et al. Hospitalization with acute exacerbation of chronic obstructive pulmonary disease and associated health resource utilization: a population-based Danish cohort study. J Med Econ 2013;16:897–906. 10.3111/13696998.2013.800525 [DOI] [PubMed] [Google Scholar]
- 18.Mannino DM, Higuchi K, Yu T-C, et al. Economic burden of COPD in the presence of Comorbidities. Chest 2015;148:138–50. 10.1378/chest.14-2434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liao K-M, Liang F-W, Li C-Y. Risks of all-cause and site-specific fractures among hospitalized patients with COPD. Medicine (Baltimore) 2016;95:e5070. 10.1097/MD.0000000000005070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Westerik JAM, Metting EI, van Boven JFM, et al. Associations between chronic Comorbidity and exacerbation risk in primary care patients with COPD. Respir Res 2017;18:31. 10.1186/s12931-017-0512-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maselli DJ, Bhatt SP, Anzueto A, et al. Clinical epidemiology of COPD: insights from 10 years of the Copdgene study. Chest 2019;156:228–38. 10.1016/j.chest.2019.04.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santos NCD, Miravitlles M, Camelier AA, et al. Prevalence and impact of Comorbidities in individuals with chronic obstructive pulmonary disease: A systematic review. Tuberc Respir Dis (Seoul) 2022;85:205–20. 10.4046/trd.2021.0179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Divo MJ, Marin JM, Casanova C, et al. Comorbidities and mortality risk in adults younger than 50 years of age with chronic obstructive pulmonary disease. Respir Res 2022;23:267. 10.1186/s12931-022-02191-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oudijk EJD, Lammers JWJ, Koenderman L. Systemic inflammation in chronic obstructive pulmonary disease. Europ Resp J 2003;22:5s–13s. 10.1183/09031936.03.00004603a [DOI] [PubMed] [Google Scholar]
- 25.Burke H, Wilkinson TMA. Unravelling the mechanisms driving Multimorbidity in COPD to develop Holistic approaches to patient-centred care. Eur Respir Rev 2021;30:210041. 10.1183/16000617.0041-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ording AG, Sørensen HT. Concepts of Comorbidities, multiple morbidities, complications, and their clinical epidemiologic analogs. Clin Epidemiol 2013;5:199–203. 10.2147/CLEP.S45305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barnett K, Mercer SW, Norbury M, et al. Epidemiology of Multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 2012;380:37–43. 10.1016/S0140-6736(12)60240-2 [DOI] [PubMed] [Google Scholar]
- 28.Skajaa N, Ording AG, Darvalics B, et al. Long-term mortality in young and middle-aged adults hospitalised with chronic disease: a Danish cohort study. BMJ Open 2020;10:e038131. 10.1136/bmjopen-2020-038131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Divo M, Celli BR. Multimorbidity in patients with chronic obstructive pulmonary disease. Clin Chest Med 2020;41:405–19. 10.1016/j.ccm.2020.06.002 [DOI] [PubMed] [Google Scholar]
- 30.Schmidt M, Schmidt SAJ, Adelborg K, et al. The Danish health care system and Epidemiological research: from health care contacts to database records. Clin Epidemiol 2019;11:563–91. 10.2147/CLEP.S179083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt M, Pedersen L, Sørensen HT. The Danish civil registration system as a tool in epidemiology. Eur J Epidemiol 2014;29:541–9. 10.1007/s10654-014-9930-3 [DOI] [PubMed] [Google Scholar]
- 32.Schmidt M, Schmidt SAJ, Sandegaard JL, et al. The Danish national patient Registry: a review of content, data quality, and research potential. Clin Epidemiol 2015;7:449–90. 10.2147/CLEP.S91125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pottegård A, Schmidt SAJ, Wallach-Kildemoes H, et al. Data resource profile: the Danish national prescription Registry. Int J Epidemiol 2017;46:798–798f. 10.1093/ije/dyw213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mors O, Perto GP, Mortensen PB. The Danish psychiatric central research register. Scand J Public Health 2011;39:54–7. 10.1177/1403494810395825 [DOI] [PubMed] [Google Scholar]
- 35.Thomsen RW, Lange P, Hellquist B, et al. Validity and Underrecording of diagnosis of COPD in the Danish national patient Registry. Respir Med 2011;105:1063–8. 10.1016/j.rmed.2011.01.012 [DOI] [PubMed] [Google Scholar]
- 36.Heide-Jørgensen U, Adelborg K, Kahlert J, et al. Sampling strategies for selecting general population comparison cohorts. Clin Epidemiol 2018;10:1325–37. 10.2147/CLEP.S164456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aalen OO, Johansen S. An empirical transition matrix for non-homogeneous Markov chains based on censored observations. Scandinav J Statist 1978;5:141–50. [Google Scholar]
- 38.Fox MP, Murray EJ, Lesko CR, et al. On the need to revitalize descriptive epidemiology. Am J Epidemiol 2022;191:1174–9. 10.1093/aje/kwac056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGee G, Haneuse S, Coull BA, et al. On the nature of informative presence bias in analyses of electronic health records. Epidemiology 2022;33:105–13. 10.1097/EDE.0000000000001432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Willgoss TG, Yohannes AM. Anxiety disorders in patients with COPD: a systematic review. Respir Care 2013;58:858–66. 10.4187/respcare.01862 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjresp-2023-001798supp001.pdf (523.1KB, pdf)
Data Availability Statement
Data may be obtained from a third party and are not publicly available. The data used in this study are owned and managed by Statistics Denmark. In accordance with the Danish law and data protection policies, the data used in this study were anonymised, and stored and analysed on a secure server. The data are available to researchers from research environments pre-approved by Statistics Denmark upon project approval by the Danish Data Protection Agency and Statistics Denmark. Researchers can apply for access to data when the request is approved by the Danish Data Protection Agency (https://www.datatilsynet.dk).