Abstract
Zika virus (ZIKV) was identified as a teratogen in 2016 when an increase in severe microcephaly and other brain defects was observed in fetuses and newborns following outbreaks in French Polynesia (2013–2014) and Brazil (2015–2016) and among travelers to other countries experiencing outbreaks. Some have questioned why ZIKV was not recognized as a teratogen before these outbreaks: whether novel genetic changes in ZIKV had increased its teratogenicity or whether its association with birth defects had previously been undetected. Here we examine the evidence for these two possibilities. We describe evidence for specific mutations that arose before the French Polynesia outbreak that might have increased ZIKV teratogenicity. We also present information on children born with findings consistent with congenital Zika syndrome (CZS) as early as 2009 and epidemiological evidence that suggests increases in CZS-type birth defects before 2013. We also explore reasons why a link between ZIKV and birth defects might have been missed, including issues with surveillance of ZIKV infections and of birth defects, challenges to ZIKV diagnostic testing, and the susceptibility of different populations to ZIKV infection at the time of pregnancy. Although it is not possible to prove definitively that ZIKV had teratogenic properties before 2013, several pieces of evidence support the hypothesis that its teratogenicity had been missed in the past. These findings emphasize the need for further investments in global surveillance for emerging infections and for birth defects so that infectious teratogens can be identified more expeditiously in the future.
Keywords: birth defects, congenital Zika syndrome, surveillance, teratogen, Zika virus
1 |. INTRODUCTION
Despite its first identification in Uganda in 1947 (Dick et al., 1952), Zika virus (ZIKV) garnered little attention for many years, with fewer than 20 human infections documented in the second half of the 20th century (Faye et al., 2014). As a single-stranded RNA virus, ZIKV is closely related to other flaviviruses including dengue (DENV), yellow fever, Japanese encephalitis, and West Nile viruses. Phylogenetic analyses have established two primary Zika virus lineages: an African strain and an Asian strain. Based on animal models, the Asian strain demonstrates lower infection rates, less virus production, and poor induction of early cell death, compared to the African strain (Simonin et al., 2017). ZIKV is transmitted via mosquitos, such as Aedes aegypti and Aedes albopictus, sexually, and from mother to fetus during pregnancy or delivery (Gregory et al., 2017). Most persons with ZIKV infection are asymptomatic, while others have mild symptoms, including fever, headache, arthritis or arthralgia, conjunctivitis, and macular or papular rash (Duffy et al., 2009).
Although serologic studies suggest widespread presence of ZIKV in Asia and Africa before 2007, the first documented ZIKV outbreak occurred in 2007, in which 108 cases of confirmed and probable ZIKV disease were reported in Yap State, in the Federated States of Micronesia (Duffy et al., 2009). In 2013–2014, a ZIKV outbreak occurred in French Polynesia, followed by smaller outbreaks in the South Pacific (Baud et al., 2017). In 2015, ZIKV was recognized as the cause of a large outbreak in South America in the Northeast Region of Brazil (Zanluca et al., 2015), although most genomic studies suggest introduction of ZIKV in the Americas as early as 2013 (Faria et al., 2016). After reports of increases in microcephaly during the outbreak in Brazil, a retrospective study identified a similar increase in microcephaly (Cauchemez et al., 2016), as well as an increase in Guillain-Barré syndrome, during the 2013–2014 outbreak in French Polynesia (Dos Santos et al., 2016). Additionally, reports of adverse birth outcomes were noted in early 2016 among travelers to other countries with ZIKV transmission (Driggers et al., 2016; Meaney-Delman et al., 2016). In early 2016, sufficient evidence had accumulated to establish a causal relationship between ZIKV and microcephaly and other serious brain defects (Rasmussen et al., 2016).
Since ZIKV was recognized as a teratogen, numerous studies to characterize congenital Zika syndrome (CZS) have found that ZIKV broadly impacts neurodevelopment by destroying neurological tissue and disrupting future developmental processes. Newborns with CZS can present with structural disease components, which may include severe microcephaly, subcortical calcifications, congenital contractures, and other brain anomalies, as well as functional disease components, which may include neurological impairments, vision and hearing loss, and hypertonia (Moore et al., 2017).
In this review, we examine the evidence, including relevant genetic analyses, case reports, and epidemiological studies, to better understand the emergence of CZS and possible evolution of ZIKV’s teratogenicity (Figure 1). We aim to evaluate whether a novel genetic mutation in ZIKV conferred neurotropism, resulting in teratogenicity, or whether the teratogenic effects of ZIKV went unrecognized for many years.
FIGURE 1.
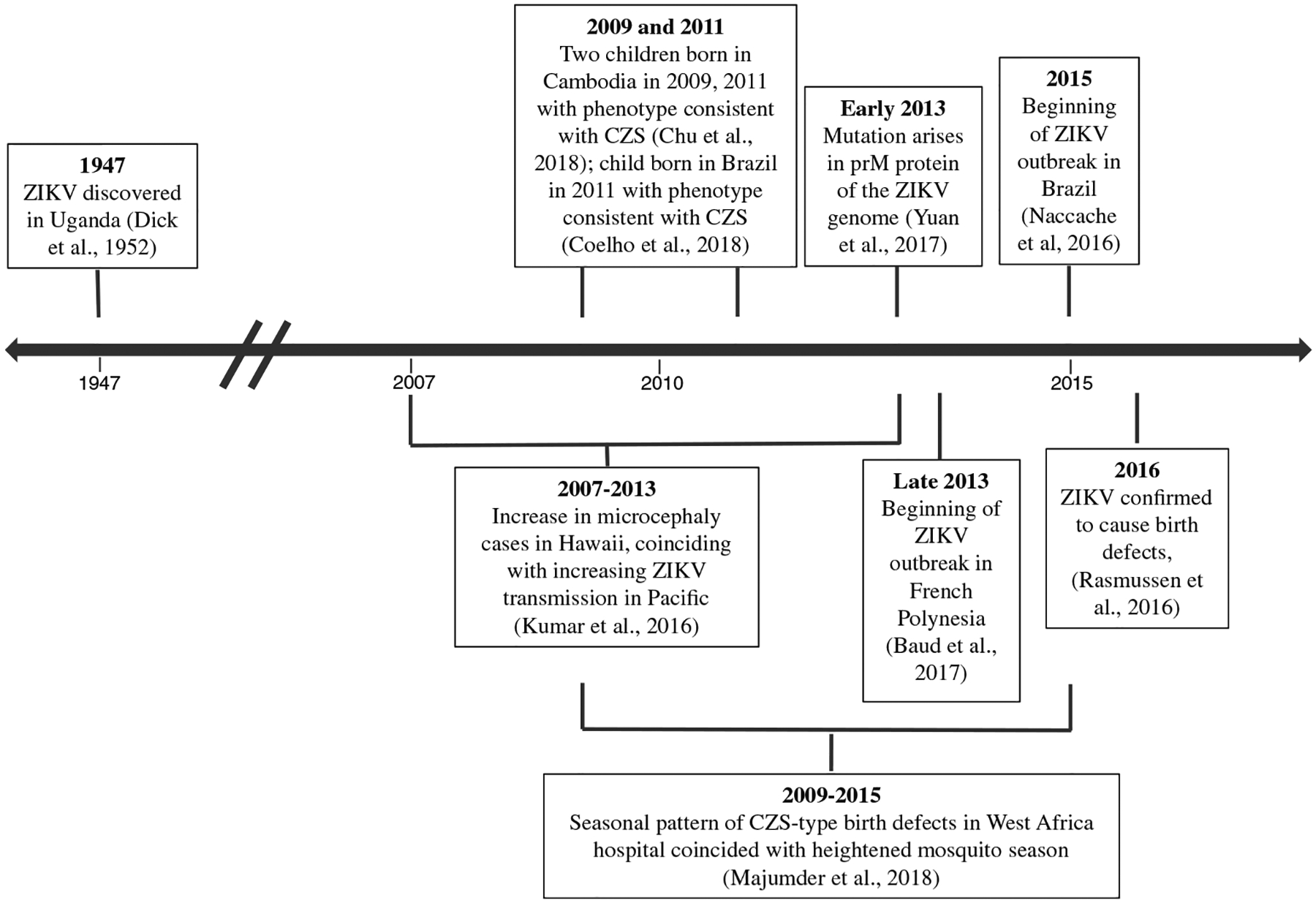
Timeline of key events between discovery of Zika virus (ZIKV) in 1947 and identification of ZIKV as a cause of birth defects in 2016. CZS, congenital Zika syndrome
2 |. EVIDENCE THAT ZIKV EVOLVED TO BECOME MORE TERATOGENIC
After ZIKV was identified as a teratogen, researchers began to examine if genetic changes increased its teratogenicity, leading to the emergence of birth defects. Phylogenetic analyses have demonstrated that multiple mutations occurred in the ancestral Asian strain in recent outbreaks. In one study, investigators identified a single serine-to-asparagine substitution (S139N) present in the prM protein of the Zika virus in the circulating contemporary strains in the Pacific islands and the Americas that likely evolved from a common Asian strain (Yuan et al., 2017). According to this analysis, the mutation appears to have first emerged around May 2013, just before the outbreak in French Polynesia (Pettersson et al., 2016). When studied through reverse genetics and mouse neurovirulence models, researchers found that this mutation conferred increased infectivity of neural progenitor cells, more severe microcephaly in a mouse fetus, and higher mortality rates in newborn mice, compared to an ancestral strain isolated in Cambodia in 2010. These authors proposed that this specific mutation may have enhanced ZIKV teratogenicity, leading to the increased incidence of microcephaly (Yuan et al., 2017).
A spontaneous mutation in the non-structural protein 1 (NS1) gene has also been identified in the Asian lineage of ZIKV that suggests evolutionary enhancement. One study compared the rate of viral transmission to Aedes aegypti feeding on mice infected by different ZIKV strains of Asian lineage (GZ01 isolated in 2016 and FSS13025 in 2010). Researchers found the mice infected with GZ01 had an NS1 mutation (A188V) that resulted in enhanced NS1 antigenemia, subsequently leading to enhanced ZIKV transmission from mice to mosquitos (Liu et al., 2017). Although this mutation appears to facilitate viral transmission rather than increasing teratogenicity, it is possible that the enhanced epidemic potential of the contemporary ZIKV strains led to increased cases and subsequent recognition of a previously unidentified teratogenic association (Table 1).
TABLE 1.
Summary of evidence for Zika virus evolving to produce increased teratogenicity versus Zika virus as a teratogen that had been previously missed
| ZIKV evolution led to increased teratogenicity | ZIKV as a teratogen that had been previously missed |
|---|---|
| prM mutation (S139N) in ZIKV that arose in 2013 led to severe microcephaly in mice model (Yuan et al., 2017) | Case reports of two children born in Cambodia in 2009 and 2011, respectively, with clinical features of CZS (Chu et al., 2018) Case report of a child born in 2011 in Brazil with clinical features of CZS (Coelho et al., 2018) |
| A spontaneous NS1 (A982V) mutation led to increased antigenemia in mouse model and increased infectivity from mouse to mosquito, leading to increased prevalence and recognition of teratogenicity (Liu et al., 2017) | Increase in microcephaly cases in Hawaii during Pacific ZIKV outbreak (2007–2013) (Kumar et al., 2016) |
| E-V473M substitution that emerged between 2010 and 2013 likely increased neurovirulence in neonatal mice and maternal–fetal transmission (Shan et al., 2020) | Seasonal pattern in CZS-type birth defects that coincided with heightened mosquito season in West Africa (Majumder et al., 2018) |
| Numerous factors, including challenges to detection, diagnosis, and surveillance of ZIKV infections and birth defects contributing to a delay in recognizing the teratogenicity of ZIKV |
A single mutation in ZIKV’s envelope protein V473M substitution (E-V473M) also emerged between 2010 and 2013. Researchers engineered the mutation into a pre-epidemic Asian ZIKV strain (isolated in Cambodia in 2010) to further characterize the functional impact in a mouse model (Shan et al., 2020). This study found increased mortality and elevated brain viral titers in mice pups after infection with a ZIKV strain containing E-V473M. Additionally, in a murine pregnancy model, increased viral loads in the placenta and fetal brain were also associated with this mutation. These authors concluded that the introduction of E-V473M in epidemic ZIKV strains increased neurovirulence in neonatal mice and intra-uterine transmission during pregnancy. While some studies indicate that novel genetic mutations may have increased the teratogenicity of ZIKV, others have shown that ZIKV strains without these mutations can still infect brains and possibly even cause birth defects. In one study researchers examined the neurotropic properties of ZIKV isolates tested from 1947 to the present and found that all lineages of ZIKV were neurotropic in organoid models (Rosenfeld et al., 2017). In another study researchers compared a ZIKV strain of Asian lineage (ZIKV-Natal; isolated in 2015) that causes microcephaly, to a ZIKV strain of Asian lineage (ZIK-MY; isolated in 1966) and found that both strains of ZIKV could cause infections in the fetal mouse brain (Setoh et al., 2018). Finally, another study sequenced the complete genome of a ZIKV strain isolated from a patient with microcephaly in Thailand and found that it lacked the S139N mutation (Wongsurawat et al., 2018). Therefore, while the S139N mutation may have conferred increased teratogenicity in the 2015–2016 ZIKV strains, this finding does not negate the possibility of neuroteratogenic effects of earlier ZIKV strains or strains without that mutation.
While most studies mentioned have focused on the Asian-lineage ZIKV, a growing body of literature based on animal models suggests that the African ZIKV strains might cause more severe disease (e.g., higher levels of Zika virus RNA in the placenta, higher level of mortality after intracranial inoculation, and more acute neurological effects and higher lethality after subcutaneous injection) than the Asian strains (Crooks et al., 2021; Duggal et al., 2017; Liu et al., 2019; Tripathi et al., 2017). However, it remains unclear how these findings translate to increased teratogenicity or to humans, largely due to lack of epidemiological research. The literature is also limited with regard to genetic changes or enhanced teratogenicity of contemporary African strains, as many of the aforementioned studies have focused on the historical MR766 strain. Of note, previous research has found that this strain (MR766) is able to infect cultured human neural precursor cells (Tang et al., 2016), but no cases have been reported of associated microcephaly or CZS with the African lineage. Further research is warranted to better understand the evolution of ZIKV and the human neurotropic effects of the African lineage, particularly in regard to fetal outcomes. In addition, integrating phylogenetic and epidemiological methods may also be informative to characterize the impact of the mutations at the population level.
3 |. EVIDENCE TO SUGGEST THAT ZIKV’S TERATOGENIC EFFECTS WERE MISSED
3.1 |. Case reports
One report of two children with clinical features of CZS, born in 2009 and 2011, suggests that ZIKV may have caused birth defects before the outbreaks in French Polynesia and Brazil (Chu et al., 2018). These children were born to mothers who had moved from the United States to Cambodia two and five years before their pregnancies and were pregnant in Cambodia at a time when ZIKV was circulating. Both mothers reported symptoms consistent with ZIKV infection around 13–14 weeks gestation, and at birth, both children had clinical features of CZS, including microcephaly, with neuroimaging showing subcortical calcifications, ventriculomegaly, and corpus callosum abnormalities. Family history, chromosomal analysis, and serologic testing for common congenital infections were unremarkable for both children. Serology obtained from both mothers several years after giving birth was consistent with a previous ZIKV infection, with positive IgG and negative IgM ZIKV antibody titers (both were negative for DENV neutralizing antibodies making cross-reactivity less likely). Of note, both women had returned to the United States after giving birth; thus, subsequent exposure to ZIKV was unlikely. Both children were negative for ZIKV antibodies when tested at six and eight years old; however, ZIKV antibodies are not always present in children who were infected in utero (Adebanjo et al., 2017; Godfred-Cato et al., 2021). Several genetic alterations have been associated with a CZS-like phenotype but the findings in these two cases reported were inconsistent with these disorders (Chu et al., 2018).
Another case report of a child born in Brazil in 2011 raises suspicion that ZIKV may have produced teratogenic effects before 2013 (Coelho et al., 2018). In this case, the mother reported symptoms consistent with ZIKV infection during the second month of pregnancy, and microcephaly was detected on ultrasonography at five months gestation. At birth, the child presented with severe ventriculomegaly with diffuse cortical–subcortical calcifications, a simplified gyral pattern with predominance of pachygyria or polymicrogyria in the frontal lobes, and a hypoplastic corpus callosum on brain imaging. The child was also noted to have occipital prominence and scalp rugae, consistent with CZS. Serologic testing of the mother indicated previous exposure to DENV, which is common among adults in endemic regions. At four months of age, infant serology was positive for DENV IgG, negative for DENV IgM, positive for herpes I/II IgG, and negative for toxoplasmosis, syphilis, rubella, and cytomegalovirus. ZIKV testing was not performed on the mother or child. However, due to the characteristic findings of CZS in this child, the authors proposed that ZIKV might have been circulating in Brazil and causing teratogenic effects more than four years before the 2015–2016 outbreak in South America. A phylogenetic analysis of isolates from Bahia, a state in the northeast region of Brazil where the first autochthonous cases of ZIKV infection were identified and where the largest number of infections in Brazil were reported in 2015, suggests that sustained ZIKV circulation had been occurring as early as mid-2014 in Salvador, the capital city of Bahia (Naccache et al., 2016). While the case report and phylogenetic studies support earlier circulation as a possibility, it seems unlikely that ZIKV could have been circulating at any significant levels without other reports of CZS from this time period.
3.2 |. Retrospective analyses
Testing of archived blood samples collected postpartum from mothers at a single hospital in Hawaii also suggests that ZIKV may have produced teratogenic effects earlier than 2013. Researchers found that the birth prevalence of microcephaly increased from 9.4/10,000 (1986–2005) to 14.7/10,000 (2007–2013), which coincided with ZIKV transmission in the Pacific beginning in 2007 (Kumar et al., 2016). Analyses revealed that mothers who tested positive for ZIKV IgG or IgM were more likely to have babies with microcephaly than mothers who tested negative for antibodies, although their findings were not statistically significant (Kumar et al., 2016). This study is limited by small sample size, with a total of 18 mothers, 6 of whom had infants with microcephaly. Of the babies with microcephaly, 3 of the mothers had positive IgG antibodies. Clinical details and maternal travel and residency history were also not documented. Of note, no locally acquired cases of ZIKV infection have been documented in Hawaii, likely related to limited numbers of Aedes aegypti mosquitos, but travel outside of Hawaii among Hawaii residents as well as deliveries in Hawaii among non-residents are common (Zika Virus, State of Hawaii, Department of Health, Disease Outbreak Control Division, November, 2019).
Retrospective analyses conducted in West Africa provide further evidence that ZIKV might have caused birth defects as early as 2009. One study used available data from a hospital’s birth registry in West Africa between 2009 and 2015. Based on (1) patterns observed in seasonal malaria, which typically peaks a few weeks following the regions “rainy” season from August to October and (2) the temporal relationship observed in Brazil between maternal infection, vertical transmission, and post conception reports of CZS-type birth defects, these authors hypothesized that the number of CZS-type birth defects may follow a seasonal pattern. The study found a peak in potential CZS-associated birth defects from March to July, coinciding with possible maternal infection dating back to the previous August through December (Majumder et al., 2018). The authors concluded that CZS-associated birth defects might follow a seasonal pattern in West Africa, which could be consistent with ZIKV causing birth defects in this area since 2009. Other malaria-endemic countries with birth defects surveillance systems might be able to explore this question further.
4 |. REASONS WHY TERATOGENIC EFFECTS OF ZIKA VIRUS MIGHT HAVE BEEN MISSED UNTIL 2015
Challenges to detection, diagnosis, and surveillance of ZIKV infections and of birth defects might have contributed to the delayed recognition of the teratogenicity of ZIKV. Difficulties with detection likely have led to an underestimation in the global incidence of ZIKV (Musso & Gubler, 2016). ZIKV infection is often clinically silent; studies have shown that up to 80% of individuals with a ZIKV infection are asymptomatic (Nutt & Adams, 2017) and molecular testing is only likely to detect infection within 1–2 weeks of infection, making tracking of maternal infection challenging (Petersen et al., 2016). ZIKV also exhibits serologic cross-reactivity with other flaviviruses, complicating serologic interpretation for tracking (Musso & Gubler, 2016). Although there have been no reported cases of ZIKV-associated microcephaly due to viruses of the African lineage, lab-based findings suggest that African strains exhibit greater pathogenicity in animal models. One study found that recently circulating African strains displayed higher lethality in both adult and fetal mice compared to Asian strains (Aubry et al., 2021). These authors highlighted the possibility that these strains could be more difficult to detect by public health surveillance systems because they might be more likely to cause fetal loss, rather than birth defects in a liveborn infant.
The variation in the existence and quality of birth defects surveillance systems throughout the world also challenges recognition of new teratogens. Most low- and middle-income countries as well as some higher-income countries lack strong birth defects surveillance systems capable of identifying increases in birth defects due to potential teratogenic exposure (Dolk et al., 2021).
For example, in the United States, no national birth defects surveillance system exists; information on the prevalence of major birth defects in the United States depends on a compilation of data across state-based surveillance systems that use varying methods of ascertainment (Mai et al., 2019) and vary in completeness and timeliness (Anderka et al., 2015). Even in longstanding birth defects surveillance systems such as the Metropolitan Atlanta Congenital Defects Program (Metropolitan Atlanta Congenital Defects Program [MACDP], 2021) challenges to the recognition of a teratogenic exposure have been demonstrated (Yang et al., 1997). Although no longer funded, the initial expansion of population-based birth defects surveillance systems across 15 U.S. jurisdictions in response to the 2016 outbreak allowed for greater and more timely detection of ZIKV-related birth defects in areas with local transmission (Delaney et al., 2018).
The identification of adverse neurodevelopmental outcomes associated with a teratogenic exposure presents additional challenges. A recent cohort study found that even children who had been exposed to ZIKV in utero, but did not present with CZS at birth, were at an increased risk for neurodevelopmental consequences within the first 18 months of life (Mulkey et al., 2020). Infants not exhibiting clear findings of CZS are less likely to be recognized. In many countries, lack of access to healthcare and lower proportion of institutional or hospital-based births in rural areas might also impact reporting. For example, Peru’s 2018 Demographic and Family Health Survey (ENDES) revealed that the percentage of institutional births in Loreto, which is the most populous region of the Peruvian Amazon, was the lowest in the country (74.5%), compared to the national average of 92.6% (Instituto Nacional de Estadistica e Informatica [INEI]—Peru: Encuesta Demográfica y de Salud Familiar 2018—Nacional y Regional, 2018) Finally, cultural barriers, stigmatization and limited knowledge particularly in rural communities might lead to significant underreporting of birth defects in some countries, as has been seen in Ecuador (Gonzalez-Andrade & Lopez-Pulles, 2010).
Several reasons might have led to identification of ZIKV as a teratogen in Brazil (and retrospectively in French Polynesia). First, ZIKV circulation and outbreaks had not been previously recognized in South America and thus, most of population was likely to be immunologically naïve. In countries where ZIKV is endemic, such as Africa and Asia, women might have been infected in childhood, providing immunity to ZIKV before their reproductive years. This principle has been seen in other infections such as rubella (Dontigny et al., 2008), in which women acquire immunity from childhood infections and therefore, are not immunologically susceptible to infection during pregnancy. If certain geographic regions had high levels of ZIKV infection in childhood, the teratogenicity of ZIKV might have been missed because low rates of infection during pregnancy minimized the number at risk for adverse pregnancy outcomes caused by ZIKV. Thus, the introduction of ZIKV into an immunologically naïve population likely led initially to a large increase in ZIKV infection during pregnancy and subsequently a large increase in births of babies with severe microcephaly and other birth defects. Some evidence suggests that the sharp decrease in new ZIKV cases in Brazil since 2017 may be due to increased immunity within the population; although the duration of ZIKV immunity is unknown, it is expected to be long-term, based on the experience with absence of repeat infections for similar flaviviruses (Netto et al., 2017; Sampaio et al., 2019).
Additionally, the role of an astute clinician in the identification of an increase in the prevalence of certain birth defects should not be underappreciated (Carey et al., 2009). Dr. Vanessa Van Der Linden, a pediatric neurologist in Brazil, has been recognized for her leadership role in recognizing the potential connection between ZIKV and microcephaly and other serious brain defects and raising the alarm about her concerns (Vanessa Van Der Linden Mota, 2016).
Some have hypothesized that the high numbers of babies with ZIKV-associated birth defects in Brazil, specifically in DENV-endemic areas (Brasil et al., 2016), might be related to antibody-dependent enhancement, a phenomenon in which suboptimal antibodies to a previous infection enhance viral entry into host cells and subsequent viral replication. Several in vitro studies have supported this theory (Rathore et al., 2019), including a recent study that examined how infection with ZIKV in pregnant mice that were pretreated with DENV antibodies altered fetal outcomes and impacted ZIKV replication in placental cells (Brown et al., 2019). This study found a marked increase in infected trophoblasts, cells critical for fetal development, with the introduction of DENV antibodies. These data suggest that DENV antibodies might have dampened host immune response to ZIKV leading to increased severity of viral outcomes. In contrast, another study suggests that previous DENV infection might be protective against ZIKV infection (Pedroso et al., 2019). The case–control study identified ZIKV seropositive mothers with and without children with CZS and found that neutralization of DENV serotypes was associated with reduced risk for CZS. The authors suggested that protection may be due to an immune enhancement, such as DENV-activation of CD4 and CD8 cells, rather than an antibody-mediated immune response (Regla-Nava et al., 2018; Saron et al., 2018). Two other epidemiological studies have suggested that pre-existing DENV immunity was associated with a reduced risk in ZIKV infection and related symptoms (Gordon et al., 2019; Rodriguez-Barraquer et al., 2019). Overall, this epidemiological evidence suggests that DENV immunity does not enhance the severity of ZIKV’s impact clinically. However, more studies are needed to assess whether differences in the levels of DENV antibodies influence immunity or enhancement of ZIKV infection and the generalizability of these findings to other endemic regions (Musso et al., 2019). In addition, while there were high numbers of babies born with Zika-associated birth defects in Brazil, it is important to note that the risk of having a baby with birth defects following a ZIKV-infected pregnancy did not appear to be higher in Brazil compared with French Polynesia and other countries, although differences in case definitions used in different analyses might give a different impression (Honein & Jamieson, 2016). It appears more likely that the large number of babies born with birth defects in Brazil is due to high numbers of ZIKV infections among pregnancies in Brazil, not to a higher risk of birth defects. The higher number of ZIKV-infected pregnancies might be related to socioeconomic conditions (including lower education rate and food insecurity; Nery Jr. et al., 2021), as well as barriers to health services, inadequate sanitation, and poor water supply (Morgan et al., 2021). In addition, countries with transmission starting after recognition of the association between ZIKV and birth defects could better advise pregnant persons on ways to avoid ZIKV transmission.
5 |. CONCLUSIONS
Overall, it is not possible to definitively determine whether ZIKV became more teratogenic or whether the teratogenic effects of ZIKV were simply missed before 2013. Results of genetic studies suggest that ZIKV underwent multiple genetic mutations, such as a S139N substitution, resulting in changes in viral proteins that might have increased its transmission and teratogenicity. Alternatively, populations in non-endemic areas may have been more susceptible to ZIKV infection, leading to an increase in the number of children born with CZS. Finally, it is possible that ZIKV was a teratogen for many years before it was recognized as one. This theory is supported by recent case reports of children who presented with clinical features of CZS prior to the outbreak in French Polynesia in 2013, as well as retrospective analyses that show a rise in birth defects to mothers who were pregnant during periods of heightened mosquito activity. Still today, surveillance of ZIKV-related infections and birth defects is limited in many countries and seroprevalence data are lacking in areas at risk for ZIKV transmission (Kraemer et al., 2019). This pandemic highlights the need for additional investments to improve surveillance of birth defects and of emerging infections including ZIKV. These efforts are vital to prepare for and manage future outbreaks of ZIKV infections and other pathogens as well as to promptly identify other infectious causes of birth defects.
ACKNOWLEDGMENT
The authors acknowledge Ms. Debra McDonald for editorial assistance.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
CONFLICT OF INTEREST
None of the authors have any relevant financial disclosures or conflicts of interest to report.
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- Adebanjo T, Godfred-Cato S, Viens L, Fischer M, Staples JE, Kuhnert-Tallman W, … Moore CA (2017). Update: Interim guidance for the diagnosis, evaluation, and management of infants with possible congenital Zika virus infection - United States, October 2017. MMWR: Morbidity and Mortality Weekly Report, 66(41), 1089–1099. 10.15585/mmwr.mm6641a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderka M, Mai CT, Romitti PA, Copeland G, Isenburg J,Feldkamp ML, … Kirby RS (2015). Development and implementation of the first national data quality standards for population-based birth defects surveillance programs in the United States. BMC Public Health, 15, 925. 10.1186/s12889-015-2223-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubry F, Jacobs S, Darmuzey M, Lequime S, Delang L, Fontaine A, … Lambrechts L (2021). Recent African strains of Zika virus display higher transmissibility and fetal pathogenicity than Asian strains. Nature Communications, 12(1), 916. 10.1038/s41467-021-21199-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud D, Gubler DJ, Schaub B, Lanteri MC, & Musso D (2017). An update on Zika virus infection. Lancet, 390(10107), 2099–2109. 10.1016/s0140-6736(17)31450-2 [DOI] [PubMed] [Google Scholar]
- Brasil P, Pereira JP Jr., Moreira ME, Ribeiro Nogueira RM, Damasceno L, Wakimoto M, … Nielsen-Saines K (2016). Zika virus infection in pregnant women in Rio de Janeiro. New England Journal of Medicine, 375(24), 2321–2334. 10.1056/NEJMoa1602412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JA, Singh G, Acklin JA, Lee S, Duehr JE, Chokola AN, … Lim JK (2019). Dengue virus immunity increases Zika virus-induced damage during pregnancy. Immunity, 50(3), 751–762.e755. 10.1016/j.immuni.2019.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey JC, Martinez L, Balken E, Leen-Mitchell M, & Robertson J (2009). Determination of human teratogenicity by the astute clinician method: Review of illustrative agents and a proposal of guidelines. Birth Defects Research. Part A: Clinical and Molecular Teratology, 85(1), 63–68. 10.1002/bdra.20533 [DOI] [PubMed] [Google Scholar]
- Cauchemez S, Besnard M, Bompard P, Dub T, Guillemette-Artur P, Eyrolle-Guignot D, … Mallet HP (2016). Association between Zika virus and microcephaly in French Polynesia, 2013–15: A retrospective study. Lancet, 387(10033), 2125–2132. 10.1016/s0140-6736(16)00651-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu V, Petersen LR, Moore CA, Meaney-Delman D, Nelson G, Christian Sonne D, … Rasmussen SA (2018). Possible congenital Zika syndrome in older children due to earlier circulation of Zika virus. American Journal of Medical Genetics. Part A, 176(9), 1882–1889. 10.1002/ajmg.a.40378 [DOI] [PubMed] [Google Scholar]
- Coelho K, Silva G, Pinho SF, de Carvalho AL, Petter CM, & Brandi IV (2018). Congenital Zika syndrome phenotype in a child born in Brazil in December 2011. Clinical Case Reports, 6(11), 2053–2056. 10.1002/ccr3.1810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooks CM, Weiler AM, Rybarczyk SL, Bliss M, Jaeger AS, Murphy ME, … Friedrich TC (2021). African-lineage Zika virus replication dynamics and maternal-fetal interface infection in pregnant rhesus macaques. Journal of Virology, 95(16), e0222020. 10.1128/JVI.02220-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney A, Mai C, Smoots A, Cragan J, Ellington S, Langlois P, … Honein MA (2018). Population-based surveillance of birth defects potentially related to Zika virus infection - 15 states and U.S. territories, 2016. MMWR. Morbidity and Mortality Weekly Report, 67(3), 91–96. 10.15585/mmwr.mm6703a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick GW, Kitchen SF, & Haddow AJ (1952). Zika virus. I. Isolations and serological specificity. Transactions of the Royal Society of Tropical Medicine and Hygiene, 46(5), 509–520. 10.1016/0035-9203(52)90042-4 [DOI] [PubMed] [Google Scholar]
- Dolk H, Leke AZ, Whitfield P, Moore R, Karnell K, Barisic I, … Valencia D (2021). Global birth defects app: An innovative tool for describing and coding congenital anomalies at birth in low resource settings. Birth Defects Research, 113(14), 1057–1073. 10.1002/bdr2.1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dontigny L, Arsenault MY, & Martel MJ (2008). Rubella in pregnancy. Journal of Obstetrics and Gynaecology Canada, 30(2), 152–158. 10.1016/s1701-2163(16)32740-2 [DOI] [PubMed] [Google Scholar]
- Dos Santos T, Rodriguez A, Almiron M, Sanhueza A, Ramon P, de Oliveira WK, … Espinal MA (2016). Zika virus and the Guillain-Barre syndrome - Case series from seven countries. New England Journal of Medicine, 375(16), 1598–1601. 10.1056/NEJMc1609015 [DOI] [PubMed] [Google Scholar]
- Driggers RW, Ho CY, Korhonen EM, Kuivanen S, Jaaskelainen AJ, Smura T, … Vapalahti O (2016). Zika virus infection with prolonged maternal viremia and fetal brain abnormalities. The New England Journal of Medicine, 374(22), 2142–2151. 10.1056/NEJMoa1601824 [DOI] [PubMed] [Google Scholar]
- Duffy MR, Chen TH, Hancock WT, Powers AM, Kool JL, Lanciotti RS, … Hayes EB (2009). Zika virus outbreak on Yap Island, Federated States of Micronesia. New England Journal of Medicine, 360(24), 2536–2543. 10.1056/NEJMoa0805715 [DOI] [PubMed] [Google Scholar]
- Duggal NK, Ritter JM, McDonald EM, Romo H, Guirakhoo F, Davis BS, … Brault AC (2017). Differential neurovirulence of African and Asian genotype Zika virus isolates in outbred immunocompetent mice. The American Journal of Tropical Medicine and Hygiene, 97(5), 1410–1417. 10.4269/ajtmh.17-0263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria NR, Azevedo R, Kraemer MUG, Souza R, Cunha MS, Hill SC, … Vasconcelos PFC (2016). Zika virus in the Americas: Early epidemiological and genetic findings. Science, 352(6283), 345–349. 10.1126/science.aaf5036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faye O, Freire CC, Iamarino A, Faye O, de Oliveira JV, Diallo M, … Sall AA (2014). Molecular evolution of Zika virus during its emergence in the 20(th) century. PLoS Neglected Tropical Diseases, 8(1), e2636. 10.1371/journal.pntd.0002636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfred-Cato S, Newton S, Adams L, Valencia-Prado M, Lake-Burger H, Morrison A, … Moore CA (2021). Clinical phenotype in infants with negative Zika virus immunoglobulin M testing born to mothers with confirmed Zika virus infection during pregnancy. Birth Defects Research, 113(17), 1267–1274. 10.1002/bdr2.1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Andrade F, & Lopez-Pulles R (2010). Congenital malformations in Ecuadorian children: Urgent need to create a National Registry of birth defects. The Application of Clinical Genetics, 3, 29–39. 10.2147/tacg.s8794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A, Gresh L, Ojeda S, Katzelnick LC, Sanchez N, Mercado JC, … Harris E (2019). Prior dengue virus infection and risk of Zika: A pediatric cohort in Nicaragua. PLoS Medicine, 16(1), e1002726. 10.1371/journal.pmed.1002726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory CJ, Oduyebo T, Brault AC, Brooks JT, Chung KW, Hills S, … Petersen LR (2017). Modes of transmission of Zika virus. Journal of Infectious Diseases, 216-(suppl_10), S875–s883. 10.1093/infdis/jix396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honein MA, & Jamieson DJ (2016). Monitoring and preventing congenital Zika syndrome. The New England Journal of Medicine, 375(24), 2393–2394. 10.1056/NEJMe1613368 [DOI] [PubMed] [Google Scholar]
- Instituto Nacional de Estadistica e Informatica (INEI)—Peru: Encuesta Demográfica y de Salud Familiar 2018—Nacional y Regional. (2018). Retrieved from https://www.inei.gob.pe/media/MenuRecursivo/publicaciones_digitales/Est/Lib1656/index1.html
- Kraemer MUG, Reiner RC, Brady OJ, Messina JP, Gilbert M, Pigott DM, … Golding N (2019). Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nature Microbiology, 4(5), 854–863. 10.1038/s41564-019-0376-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M, Ching L, Astern J, Lim E, Stokes AJ, Melish M, & Nerurkar VR (2016). Prevalence of antibodies to Zika virus in mothers from Hawaii who delivered babies with and without microcephaly between 2009–2012. PLoS Neglected Tropical Diseases, 10(12), e0005262. 10.1371/journal.pntd.0005262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Liu J, Du S, Shan C, Nie K, Zhang R, … Cheng G (2017). Evolutionary enhancement of Zika virus infectivity in Aedes aegypti mosquitoes. Nature, 545(7655), 482–486. 10.1038/nature22365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZY, Shi WF, & Qin CF (2019). The evolution of Zika virus from Asia to the Americas. Nature Reviews. Microbiology, 17(3), 131–139. 10.1038/s41579-018-0134-9 [DOI] [PubMed] [Google Scholar]
- Mai CT, Isenburg JL, Canfield MA, Meyer RE, Correa A, Alverson CJ, … National Birth Defects Prevention, N. (2019). National population-based estimates for major birth defects, 2010–2014. Birth Defects Research, 111(18), 1420–1435. 10.1002/bdr2.1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder MS, Hess R, Ross R, & Piontkivska H (2018). Seasonality of birth defects in West Africa: Could congenital Zika syndrome be to blame? F1000Research, 7, 159. 10.12688/f1000research.13858.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney-Delman D, Hills SL, Williams C, Galang RR, Iyengar P, Hennenfent AK, … Jamieson DJ (2016). Zika virus infection among U.S. pregnant travelers - August 2015-February 2016. MMWR. Morbidity and Mortality Weekly Report, 65(8), 211–214. 10.15585/mmwr.mm6508e1 [DOI] [PubMed] [Google Scholar]
- Metropolitan Atlanta Congenital Defects Program (MACDP). (2021). CDC, March 12, 2021. https://www.cdc.gov/ncbddd/birthdefects/macdp.html.
- Moore CA, Staples JE, Dobyns WB, Pessoa A, Ventura CV, Fonseca EB, … Rasmussen SA (2017). Characterizing the pattern of anomalies in congenital Zika syndrome for pediatric clinicians. JAMA Pediatrics, 171(3), 288–295. 10.1001/jamapediatrics.2016.3982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J, Strode C, & Salcedo-Sora JE (2021). Climatic and socio-economic factors supporting the co-circulation of dengue, Zika and chikungunya in three different ecosystems in Colombia. PLoS Neglected Tropical Diseases, 15(3), e0009259. 10.1371/journal.pntd.0009259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey SB, Arroyave-Wessel M, Peyton C, Bulas DI, Fourzali Y, Jiang J, … Cure C (2020). Neurodevelopmental abnormalities in children with in utero Zika virus exposure without congenital Zika syndrome. JAMA Pediatrics, 174(3), 269–276. 10.1001/jamapediatrics.2019.5204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso D, & Gubler DJ (2016). Zika virus. Clinical Microbiology Reviews, 29(3), 487–524. 10.1128/CMR.00072-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso D, Ko AI, & Baud D (2019). Zika virus infection — After the pandemic. New England Journal of Medicine, 381(15), 1444–1457. 10.1056/nejmra1808246 [DOI] [PubMed] [Google Scholar]
- Naccache SN, Theze J, Sardi SI, Somasekar S, Greninger AL, Bandeira AC, … Chiu CY (2016). Distinct Zika virus lineage in Salvador, Bahia, Brazil. Emerging Infectious Diseases, 22(10), 1788–1792. 10.3201/eid2210.160663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nery N Jr., Aguilar Ticona JP, Gambrah C, Doss-Gollin S, Aromolaran A, Rastely-Junior V, … Costa F (2021). Social determinants associated with Zika virus infection in pregnant women. PLoS Neglected Tropical Diseases, 15(7), e0009612. 10.1371/journal.pntd.0009612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netto EM, Moreira-Soto A, Pedroso C, Hoser C, Funk S, Kucharski AJ, … Drexler JF (2017). High Zika virus seroprevalence in Salvador, northeastern Brazil limits the potential for further outbreaks. mBio, 8(6), e01390–17. 10.1128/mBio.01390-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt C, & Adams P (2017). Zika in Africa - the invisible epidemic? Lancet, 389(10079), 1595–1596. 10.1016/s0140-6736(17)31051-6 [DOI] [PubMed] [Google Scholar]
- Pedroso C, Fischer C, Feldmann M, Sarno M, Luz E, Moreira-Soto A, … Drexler JF (2019). Cross-protection of dengue virus infection against congenital Zika syndrome, northeastern Brazil. Emerging Infectious Diseases, 25(8), 1485–1493. 10.3201/eid2508.190113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen LR, Jamieson DJ, Powers AM, & Honein MA (2016). Zika virus. New England Journal of Medicine, 374(16), 1552–1563. 10.1056/NEJMra1602113 [DOI] [PubMed] [Google Scholar]
- Pettersson JH-O, Eldholm V, Seligman SJ, Lundkvist A, Falconar AK, Gaunt MW, … de Lamballerie X (2016). How did Zika virus emerge in the Pacific Islands and Latin America? mBio, 7(5), e01239–16. 10.1128/mBio.01239-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen SA, Jamieson DJ, Honein MA, & Petersen LR (2016). Zika virus and birth defects – Reviewing the evidence for causality. New England Journal of Medicine, 374(20), 1981–1987. 10.1056/NEJMsr1604338 [DOI] [PubMed] [Google Scholar]
- Rathore APS, Saron WAA, Lim T, Jahan N, & St John AL (2019). Maternal immunity and antibodies to dengue virus promote infection and Zika virus-induced microcephaly in fetuses. Science Advances, 5(2), eaav3208. 10.1126/sciadv.aav3208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regla-Nava JA, Elong Ngono A, Viramontes KM, Huynh AT, Wang YT, Nguyen AT, … Shresta S (2018). Cross-reactive dengue virus-specific CD8(+) T cells protect against Zika virus during pregnancy. Nature Communications, 9(1), 3042. 10.1038/s41467-018-05458-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Barraquer I, Costa F, Nascimento EJM, Nery NJ, Castanha PMS, Sacramento GA, … Ko AI (2019). Impact of preexisting dengue immunity on Zika virus emergence in a dengue endemic region. Science, 363(6427), 607–610. 10.1126/science.aav6618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld AB, Doobin DJ, Warren AL, Racaniello VR, & Vallee RB (2017). Replication of early and recent Zika virus isolates throughout mouse brain development. Proceedings of the National Academy of Sciences, 114(46), 12273–12278. 10.1073/PNAS.1714624114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaio GS, Brites C, Drexler JF, Moreira-Soto A, Miranda F, & Martins Netto E (2019). Expansion of Zika virus circulation from Africa to the Americas, 1947–2018: A literature review. Epidemiologia e Serviços de Saúde, 28(2), e2018411. 10.5123/s1679-49742019000200022. (Expansao da circulacao do virus Zika da Africa a America, 1947–2018: revisao da literatura.). [DOI] [PubMed] [Google Scholar]
- Saron WAA, Rathore APS, Ting L, Ooi EE, Low J, Abraham SN, & St John AL (2018). Flavivirus serocomplex cross-reactive immunity is protective by activating heterologous memory CD4 T cells. Science Advances, 4(7), eaar4297. 10.1126/sciadv.aar4297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setoh Y, Peng N, Nakayama E, Amarilla A, Prow N, Suhrbier A, & Khromykh A (2018). Fetal brain infection is not a unique characteristic of Brazilian Zika viruses. Viruses, 10(10), 541. 10.3390/v10100541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan C, Xia H, Haller SL, Azar SR, Liu Y, Liu J, … Shi PY (2020). A Zika virus envelope mutation preceding the 2015 epidemic enhances virulence and fitness for transmission. Proceedings of the National Academy of Sciences of the United States of America, 117(33), 20190–20197. 10.1073/pnas.2005722117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonin Y, van Riel D, Van de Perre P, Rockx B, & Salinas S (2017). Differential virulence between Asian and African lineages of Zika virus. PLoS Neglected Tropical Diseases, 11(9), e0005821. 10.1371/journal.pntd.0005821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Hammack C, Ogden SC, Wen Z, Qian X, Li Y, … Ming GL (2016). Zika virus infects human cortical neural progenitors and attenuates their growth. Cell Stem Cell, 18(5), 587–590. 10.1016/j.stem.2016.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi S, Balasubramaniam VR, Brown JA, Mena I, Grant A, Bardina SV, … Garcia-Sastre A (2017). A novel Zika virus mouse model reveals strain specific differences in virus pathogenesis and host inflammatory immune responses. PLoS Pathogens, 13(3), e1006258. 10.1371/journal.ppat.1006258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanessa Van Der Linden Mota. (2016). https://www.thedialogue.org/vanessa-van-der-linden-mota/#:~:text=Van%20Der%20Linden%20Mota%20is,Gala%20on%20November%2016%2C%202016.
- Wongsurawat T, Athipanyasilp N, Jenjaroenpun P, Jun S-R, Kaewnapan B, Wassenaar TM, … Horthongkham N (2018). Case of microcephaly after congenital infection with Asian lineage Zika virus, Thailand. Emerging Infectious Diseases, 24(9), 1758–1761. 10.3201/eid2409.180416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Khoury MJ, James LM, Olney RS, Paulozzi LJ, & Erickson JD (1997). The return of thalidomide: Are birth defects surveillance systems ready? American Journal of Medical Genetics, 73(3), 251–258. [DOI] [PubMed] [Google Scholar]
- Yuan L, Huang XY, Liu ZY, Zhang F, Zhu XL, Yu JY, … Qin CF (2017). A single mutation in the prM protein of Zika virus contributes to fetal microcephaly. Science, 358(6365), 933–936. 10.1126/science.aam7120 [DOI] [PubMed] [Google Scholar]
- Zanluca C, Melo VC, Mosimann AL, Santos GI, Santos CN, & Luz K (2015). First report of autochthonous transmission of Zika virus in Brazil. Memorias Do Instituto Oswaldo Cruz, 110(4), 569–572. 10.1590/0074-02760150192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zika Virus, State of Hawaii, Department of Health, Disease Outbreak Control Division. (November 2019). https://health.hawaii.gov/docd/disease_listing/zika-virus/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


