Abstract
Background
Mobile phones are potential reservoirs for pathogens and sources of healthcare-associated infections. More microbes can be found on a mobile phone than on a man's lavatory seat, the sole of a shoe, or a door handle. When examining patients, frequent handling of mobile phones can spread bacteria. Nevertheless, evidence of bacterial contamination of mobile phones used by healthcare workers in Africa was inconclusive. Thus, this meta-analysis and systematic review was conducted to estimate the pooled prevalence of bacterial contamination of mobile phones used by healthcare workers and the most frequent bacterial isolates in Africa.
Methods
We systematically retrieved relevant studies using PubMed/MEDLINE, POPLINE, HINARI, Science Direct, Cochrane Library databases, and Google Scholar from July 1, 2023 to August 08, 2023. We included observational studies that reported the prevalence of bacterial contamination of mobile phones among healthcare workers. The DerSimonian–random Laird's effect model was used to calculate effect estimates for the pooled prevalence of bacterial contamination in mobile phones and a 95% confidence interval (CI).
Results
Among 4544 retrieved studies, 26 eligible articles with a total sample size of 2,887 study participants were included in the meta-analysis. The pooled prevalence of mobile phone bacterial contamination among healthcare workers was 84.5% (95% CI 81.7, 87.4%; I2 = 97.9%, p value < 0.001). The most dominant type of bacteria isolated in this review was coagulase-negative staphylococci (CONS) which accounted for 44.0% of the pooled contamination rate of mobile phones used by healthcare workers, followed by Staphylococcus aureus (31.3%), and Escherichia coli (10.7%).
Conclusions
In this review, the contamination of mobile phones used by HCWs with various bacterial isolates was shown to be considerable. The most prevalent bacteria isolates were coagulase-negative staphylococci, Staphylococcus aurous, and Escherichia coli. The prevalence of bacterial contamination in mobile phones varies by country and sub-region. Hence, healthcare planners and policymakers should establish norms to manage healthcare workers' hand hygiene and disinfection after using mobile phones.
Supplementary Information
The online version contains supplementary material available at 10.1186/s41182-023-00547-3.
Keywords: Healthcare, Mobile, Phones, Prevalence, Workers
Introduction
Mobile phones have become essential accessories for healthcare workers and social life [1, 2]. Mobile phones have become an important part of the healthcare delivery system, because they improve the quality of care and communication [1, 3]. It also makes interdepartmental communication easier, allowing for faster interactions within healthcare institutions and more efficient access to information for patient care [4, 5]. Despite the potential benefits, mobile phones play a critical role in becoming potential germ reservoirs and are known to induce healthcare-associated diseases [6–9].
Various bacteria, including skin flora and pathogenic bacteria, have been identified on the surface of mobile phones [3, 10]. In high-income countries, 75–96% of healthcare workers' mobile phones were found to be colonized with bacteria [11–18]. Coagulase-negative staphylococci (CoNS) and Micrococcus were the most commonly recovered bacteria, followed by methicillin-sensitive and methicillin-resistant Staphylococcus aureus (MRSA), Acinetobacter, and Pseudomonas species [11–18]. In low- and middle-income countries' healthcare settings, bacterial contamination rates of mobile phones used by healthcare workers ranged from 42% to 100%. The most prevalent bacteria isolated were coagulase-negative staphylococci, Escherichia coli, Acinetobacter species, Pseudomonas species, and MRSA bacteria [19–25]. Several infectious illnesses, including diarrhea, food poisoning, and wound infections, are caused by these bacteria [3, 26, 27].
The global burden of healthcare-associated infections (HAIs) is increasing, resulting in increased patient morbidity and mortality and significant challenges for healthcare systems [7, 28, 29]. The cumulative incidence of HAIs ranges from 5.7% to 48.5% within African countries [30]. Contamination of inanimate gadgets used by healthcare workers, such as mobile phones, is one of the sources of healthcare-acquired infections [29, 31]. More bacteria can be found on a mobile phone than on a man's lavatory seat, the sole of a shoe, or a door handle [30, 32–35]. Drug-resistant organisms such as MRSA and vancomycin-resistant enterococci (VRE) have also been found on mobile phones used in healthcare settings [15]. The drug-resistant bacterium that can cause HAIs is responsible for 40–70% of healthcare workers’ mobile phone contamination [13, 32].
Although there has been some small-scale research on the bacterial contamination of mobile phones among healthcare workers, a comprehensive review and meta-analysis was not conducted in Africa. Therefore, this systematic review and meta-analysis aimed to estimate the pooled prevalence of bacterial contamination of mobile phones used by healthcare workers and the most common bacterial isolates in Africa. Besides, we anticipated summarizing bacterial isolates' antimicrobial susceptibility and multidrug resistance patterns descriptively.
Methods
Patient and public involvement
There was no direct patient or public involvement in this study.
Registration and protocol
This systematic review and meta-analysis (SRMA) was conducted to estimate the pooled prevalence of bacterial contamination of mobile phones among HCWs in Africa. To ensure the usefulness of this SRMA to the readers, we developed a transparent, complete, and accurate report of the purpose of this review, using the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) criteria (Additional file 1). The systematic review was carried out following the Joanna Briggs Institute (JBI) methodology for systematic reviews of a proportion of evidence [36]. The systematic review and meta-analysis were prospectively registered in PROSPERO (record ID: CRD42022306250, February 22, 2022).
Search strategy
We systematically retrieved relevant studies using PubMed/MEDLINE, POPLINE, HINARI, Science Direct, Cochrane Library databases, and Google Scholar from January 20,2022 to February 20, 2022 (first round), February 20, 2023, to March 25, 2023 (second round), July 1, 2023 to August 08, 2023 (third round). All the databases were comprehensively searched to find potentially relevant papers published and unpublished between July 2009 and October 2022. All searches were limited to papers published in English-language. In addition to the electronic database search, Google was used to find for grey literature. We also looked for related studies in the reference lists of included studies. For the PubMed/MEDLINE search, the following phrases and keywords were used: [“Bacterial Contamination” OR “microbial contamination” AND "Cell Phones" OR "Mobile Phone" OR "Mobile Phones" OR "Smart Phones" AND “Health Personnel” OR “HealthCare Providers” OR “Health Care Provider” OR “Healthcare Provider” OR “Healthcare Workers” OR “Healthcare Worker” OR “Health Care Professionals” OR “Health Care Professional”]. We used database-specific subject headings linked with the above terms and keywords used in PubMed for the other electronic databases (Additional file 2).
Eligibility criteria
Inclusion criteria
The review process included all studies that met the following criteria: (1) studies that reported the magnitude of bacterial contamination from healthcare workers' mobile phones surfaces, (2) studies published in English but conducted only in Africa at any given time, and (3) studies conducted using standard bacteriological techniques (i.e., swab method or settle plate sampling method) [31, 37, 38]. (4) Studies that accurately reported the swab culture growth rate for bacterial isolates, (5) all relevant free-of-charge full-text original research articles, and (6) all observational study designs, including published and unpublished studies, were all taken into account.
Exclusion criteria
The study was excluded for the following reasons: inaccessible or irretrievable full-text articles after contacting the corresponding authors via email at least two times; reviews, commentaries, letters to the editor, conference proceedings, and abstracts; studies with unclear methods; reports from inanimate objects other than mobile phones (such as Stethoscopes, BP apparatus, and patient beds); studies conducted on non-healthcare workers; and studies that did not report the outcome of interest.
Assessment of outcome variables
The primary outcome variable was the prevalence of bacterial contamination of mobile phones used by healthcare workers, as defined by the included studies' operational definition. The prevalence of mobile phone bacterial contamination was calculated by dividing the total number of swabs with bacterial isolates by the total number of swabs taken from healthcare workers' mobile phones and multiplying by 100. This study's second objective was to characterize the most common types of bacteria isolated from healthcare workers' mobile phones and their drug sensitivity and resistance patterns, utilizing studies that were included.
Operational definitions
Non-selective bacteria isolation method
Culture mediums such as blood agar and nutrient agar can grow a wide variety of bacteria [38].
Selective bacteria isolation method
A culture medium such as MacConkey agar is more selective to isolate ‘bile tolerant’ bacteria in the large intestine [38].
Study selection and data extraction
All the retrieved citations were imported into EndNote version X8 and duplicates were removed. The JBI data extraction format was used to extract the data [39]. Based on the established inclusion criteria, two authors (DZ and BS) independently assessed and identified papers by their titles, abstracts, and full texts. Any disagreements that arose were resolved by consensus or with the additional author/s. The data extraction format included the primary author, publication year, country, study area, bacteria isolation method, optimum temperature, incubation period, the most prevalent types of bacteria isolated, isolated bacteria drug sensitivity, isolated bacteria drug resistance, sample size, and prevalence of mobile phone bacterial contamination.
Assessment of risk of bias
The quality of the appended studies was assessed using the JBI meta-analysis of statistics assessment and review instrument (MAStARI) quality rating tool [39, 40]. An appropriate sampling frame, proper sampling technique, study subject and setting description, sufficient data analysis, the use of valid methods for the identified conditions, a valid measurement for all participants, using appropriate statistical analysis in a valid and reliable outcome measure with a 50% or higher overall score considered low risk of bias as per the JBI parameters. As a result, bias risks were classified as low (total score of 2), moderate (total score of 3–4), or high (total score of > 5) [40]. Two independent authors rated the quality of the included studies (DZ and BS). Any disagreements that arose were addressed through consensus. Finally, papers with a score of 5 or higher were ruled out as having a significant risk of bias (Additional file 3).
Data synthesis
Before being evaluated, the data were extracted into a Microsoft Excel file. The data were analyzed using STATA software version 16. The standard errors of the included studies were determined using the formula (SE = p (1p)/n). The I2 statistics and p values of the Cochrane Q test were utilized to investigate heterogeneity in the stated proportion. The Cochrane Q test p values are less than 0.1 and are deemed to indicate the presence of heterogeneity among studies. To assess the percentage of total variance owing to heterogeneity across trials, we used the Higgins I2 test statistics [40]. Although no specific criterion exists for when heterogeneity becomes substantial, some researchers suggest low heterogeneity when I2 values are between (25–50%), moderate (50–75%), and high (> 75%) [40], because the test statistic revealed significant heterogeneity among the research (I2 = 98%, p value 0.001), the DerSimonian–influence Laird's was analyzed using a random-effects model. The effect sizes were calculated as a percentage with a 95% confidence interval (CI). There was a lot of variation in the included studies in this review according to the I2 category. We used subgroup analysis by sub-region, study area, bacteria isolation method, sample size, and publication year to find the source of variation. The meta-analysis findings were displayed using a forest plot. A funnel plot was employed in conjunction with meta-regression to assess publication bias. The plot resembles an asymmetrical, huge, inverted funnel in the absence of publication bias. Egger's weighted regression and Begg's rank correlation tests (p value < 0.05) were used to objectively assess publication bias; however, only Egger's test was shown to be statistically significant (p value = 0.001). To test the robustness of our findings, we conducted a leave-one-out sensitivity analysis.
Results
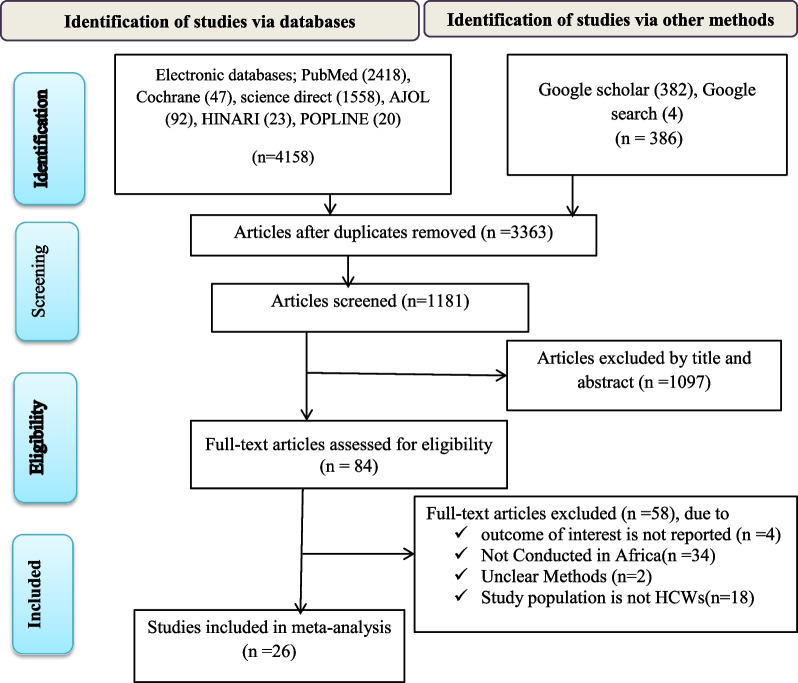
A total of 4544 articles were identified after a thorough literature search. Of these articles, 3363 duplicates were removed, and 1181 were screened only based on their titles and abstracts. Following the exclusion of 1097 articles, 84 full-text papers were verified for eligibility using the pre-determined criteria, with 58 articles excluded. Finally, 26 articles [20–24, 41–61] that satisfied the criteria were included in the meta-analysis (Fig. 1).
Fig. 1.
Flow chart of study selection for systematic review and meta-analysis of bacterial contamination of mobile phones among healthcare workers in Africa
Descriptions of the included studies
All included studies were cross-sectional by design and were published between July 2009 and October 2022. The current meta-analysis used 2887 mobile phones from healthcare professionals to estimate the pooled proportion of bacterial contamination. In terms of sub-regional distribution, nine studies were from Eastern Africa [21, 23, 24, 41–45, 57], four studies were from Western [20, 50, 51, 54], eight studies were from Northern [22, 46–49, 53, 56, 59], two studies from Southern [55, 60], and three studies from central African countries [52, 58, 61]. The overall bacterial contamination rate of mobile phones reported by all studies included in this review ranges from 10.3% to 99.9% in Africa (Table 1).
Table 1.
Descriptive summary of 26 studies included in the meta-analysis to estimate the pooled prevalence of bacterial contamination of mobile phones of HCWs in Africa
| Study ID | Authors (year) |
Country | Isolate type | Bacterial isolation method | Temperature for growth in Co | Incubation time in hours | Sample size | Overall mobile phones bacteria contamination rate with 95%CI |
|---|---|---|---|---|---|---|---|---|
| 1 | Asfaw et al. 2021 [21] | Ethiopia | Moistened swab | MacConkey agar | 35–37 | 24 | 65 | 99.9 (99.3,100.67) |
| 2 | Gashaw et al. 2014[44] | Ethiopia | not specified |
MacConkey agar, chocolate agar, and blood agar plates |
37 | 24–48 | 57 | 98.3 (94.9, 101.66) |
| 3 | Daka 2014 [41] | Ethiopia | Moistened swab | Blood agar | 37 | 18–24 | 100 | 62 (52.5, 71.5) |
| 4 | Ayalew et al. 2019 [42] | Ethiopia | Moistened swab | Blood agar | 37 | 18–24 | 422 | 59.4 (54.7, 64.1) |
| 5 | Misgana et al. 2014 [43] | Ethiopia | Moistened swab | Blood agar | 37 | 24–48 | 66 | 86.4 (78.1, 94.7) |
| 6 | Bodena et al. 2019 [45] | Ethiopia | Moistened swab | MacConkey Agar | 37 | 18–24 | 226 | 94.2 (91.2, 97.3) |
| 7 | Araya et al. 2021[23] | Ethiopia | not specified | MacConkey and Blood agar | 37 | 24–48 | 572 | 79.4 (76.1, 82.7) |
| 8 | Mohamedin et al. 2019 [46] | Egypt | not specified | MacConkey and Blood agar | 37 | 24–48 | 150 | 79.3 (72.8, 85.8) |
| 9 | Elgabeery 2021 [47] | Egypt | not specified | MacConkey’s agar, nutrient agar, blood agar | 37 | 24 | 160 | 84.4 (78.8, 90.0) |
| 10 | Selim et al. 2015 [48] | Egypt | Moistened swab | MacConkey’s and Blood agar plates | 37 | 24 | 40 | 99.9 (98.9, 100.9) |
| 11 | Shahaby et al. 2012 [49] | Egypt | Dry swab | MacConkey agar plates | 37 | 48 | 8 | 10.3 (− 10.8, 31.4) |
| 12 | Shahlol et al. 2015 [59] | Libya | Moistened | MacCkonkeyNutrient agar | 37 | 24 | 120 | 52.5 (43.6, 61.4) |
| 13 | Mohamadou et al. 2021 [52] | Cameroon | Moistened swab | Blood, Chocolate, and Mannitol Salt agar | 37 | 24–48 | 156 | 95.7 (92.5, 98.9) |
| 14 | Bissong et al. 2022 [61] | Cameroon | Moistened swab | Blood, Chocolate, and Mannitol Salt agar | 37 | 24 | 78 | 96.2 (92.0, 100.4) |
| 15 | Christelle et al. 2019 [58] | DR Congo | not specified | MacConkey Agar | NR | NR | 54 | 99.9 (99.1, 100.7) |
| 16 | Yar et al. 2021 [50] | Ghana | Moistened swab |
Blood and MacConkey Agar |
37 | 24 h | 35 | 99.9 (98.9, 100.9) |
| 17 | Fandoh 2018 [51] | Ghana | Dry | RODAC plate | NR | NR | 39 | 97.5 (97.5, 102.4) |
| 18 | Daoudi et al. 2017 [53] | Morocco | not specified | Blood agar | 37 | 72 | 17 | 99.9 (98.4, 101.4) |
| 19 | Nwankwo et al. 2014 [20] | Nigeria | Moistened swab | MacConkey and blood agar plates | 37 | 18–24 | 56 | 94.6 (88.7, 100.5) |
| 20 | Akinyemi et al. 2009 [54] | Nigeria | not specified | blood agar and eosin methylene blue agar plates | 37 | 24 | 38 | 15.3 (3.9, 26.8) |
| 21 | Bobat et al. 2016 [55] | South Africa | Moistened swab | Colistin, nalidixic acid agar, and MacConkey agar plates | 37 | 18–24 | 100 | 30.0 (21.0, 39.0) |
| 22 | Dibetso 2018 [60] | South Africa | Moistened swab | Blood agar | 4 | 48 | 66 | 99.9 (99.1, 100.7) |
| 23 | Haghamad 2021 [22] | Sudan | not specified | Blood agar and MacConkey agar | 37 | 18–24 | 100 | 87 (80.4, 93.6) |
| 24 | Osman et al. 2018 [56] | Sudan | Moistened swab | blood agar, MacConkey agar, and chocolate agar | 37 | 24 | 60 | 95 (89.3, 100.7) |
| 25 | Tusabe et al. 2021 [57] | Uganda | Moistened swab | MacConkey agar plates | 37 | 24 | 13 | 93 (79.1, 106.9) |
| 26 | Mushabati et al. 2021 [24] | Zambia | Moistened swab | MacConkey, chocolate, and blood agar | 35–37 | 18–24 | 92 | 79 (70.7, 87.3) |
NR not reported, RODAC Replicate Organism Detection and Count Plates
Prevalence and types of bacterial isolates
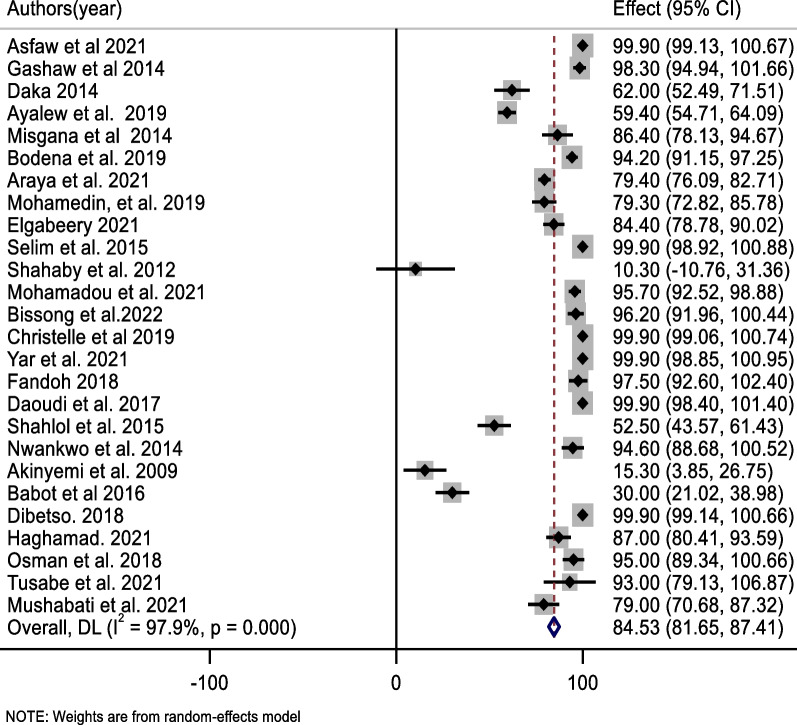
The pooled prevalence of bacterial contamination of mobile phones used by healthcare professionals in Africa was 84.5%; 95% CI (81.7, 87.4%) (Fig. 2). the high heterogeneity was showed among included studies (I2 = 97.9%, p < 0.001). As a result, a random effect model was used to estimate the pooled prevalence of bacterial contamination of healthcare workers’ mobile phones. A univariate meta-regression analysis was performed using variables such as year of publication, quality score, and sample size to identify credible sources of heterogeneity. Accordingly, the sample size and year of publication were a significant source of variability among the variables included in the studies (Table 2).
Fig. 2.
Forest plot of pooled bacterial contamination rate of mobile phones used by healthcare workers in Africa
Table 2.
Possible source of the heterogeneity of mobile phone bacterial contamination among HCWs based on univariate meta-regression
| Variable | Coefficient | p value | 95% CI |
|---|---|---|---|
| Year of publication | 3.47 | < 0.001 | 2.48, 4.46 |
| Sample size | − 0.035 | < 0.001 | − 0.054, -0.016 |
| Sub-region | 0.465 | 0.581 | − 2.73, 3.67 |
| Culture media | − 2.28 | 0.414 | − 7.74, 3.19 |
| Quality score | 1.98 | 0.622 | − 8.48, 12.28 |
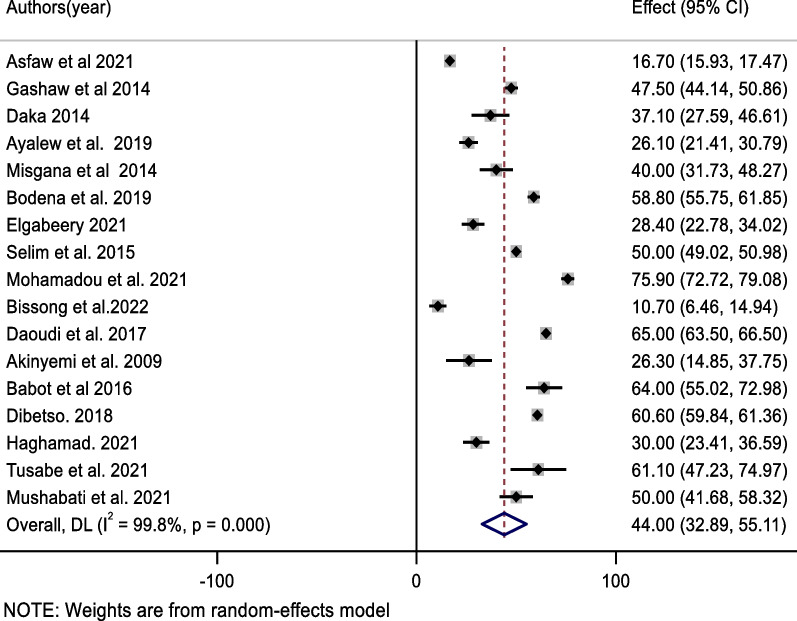
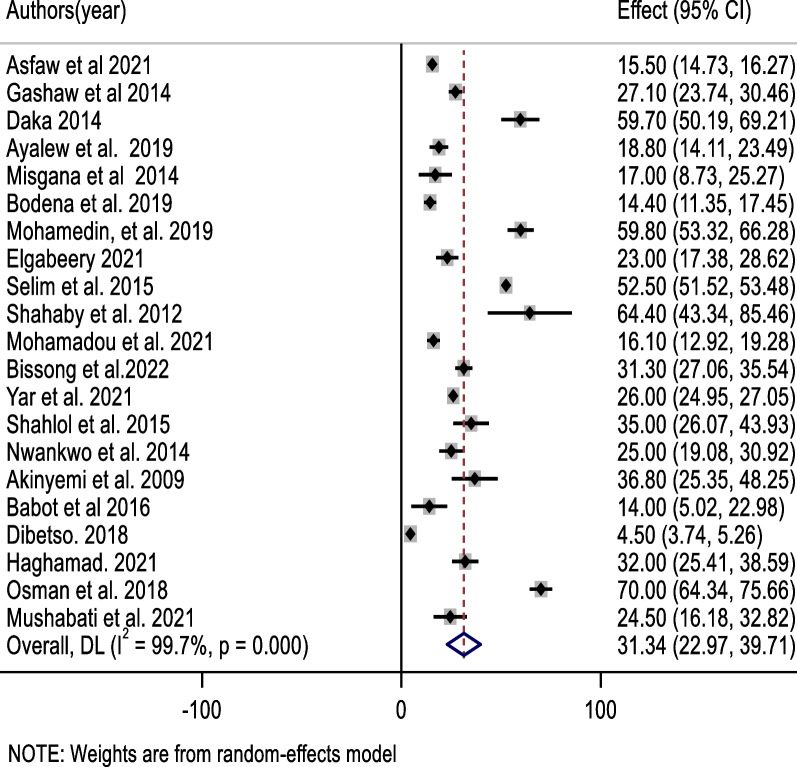
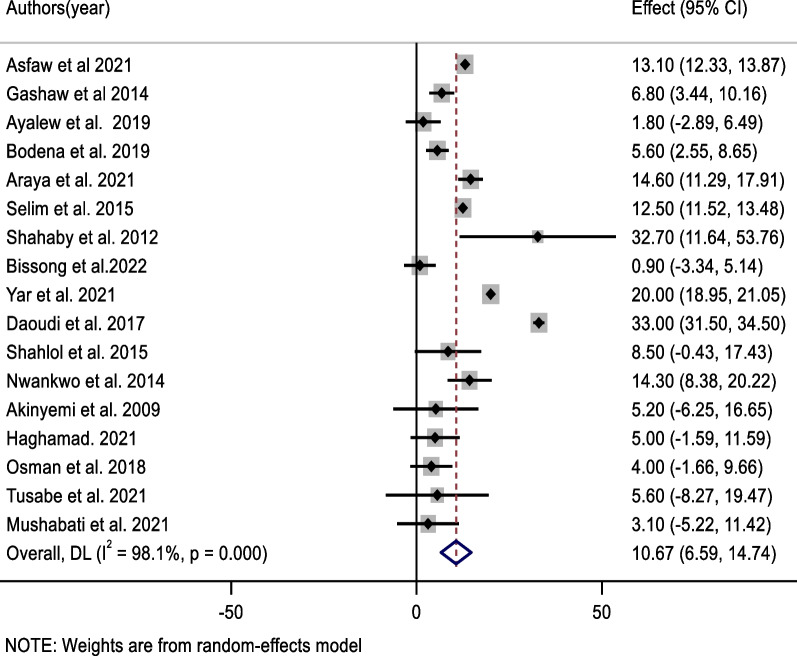
The most prevalent bacteria in this review were coagulase-negative staphylococci (CONS), which accounted for 44.0% of the pooled contamination rate (95% CI 32.9, 55.1%) of mobile phones used by healthcare workers, followed by Staphylococcus aureus, which accounted for 31.3% of the pooled contamination rate of mobile phones used by healthcare workers (23.0, 39.7%). On the other hand, the Gram-negative bacterium Escherichia coli was found in 10.7% of mobile phones used by healthcare workers [95% CI (6.6, 14.7%)] (Figs. 3, 4, 5).
Fig. 3.
Forest plot of pooled contamination rate of mobile phones of healthcare workers by coagulase-negative staphylococci in Africa
Fig. 4.
Forest plot of pooled contamination rate of mobile phones of healthcare workers by Staphylococcus aurous in Africa
Fig. 5.
Forest plot of pooled contamination rate of mobile phones of healthcare workers by Escherichia Coli in Africa
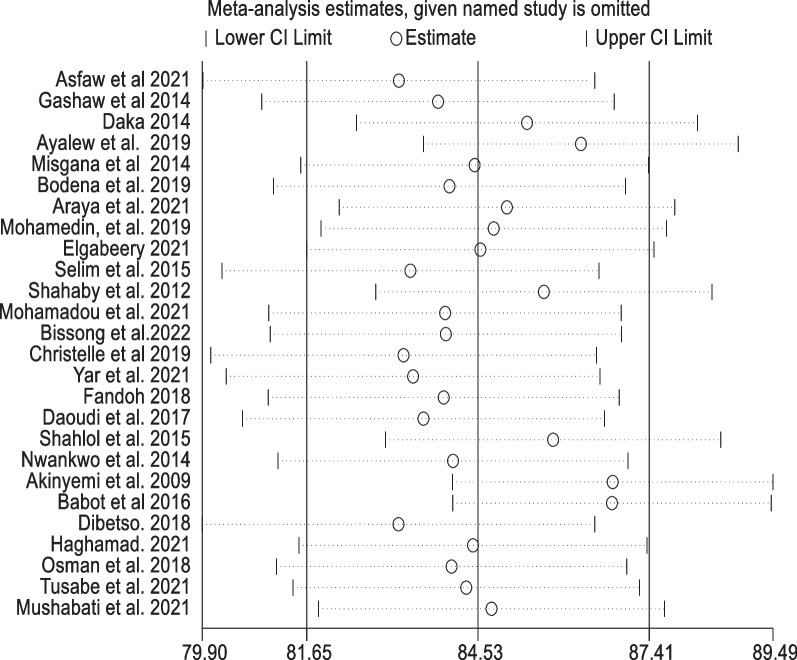
Sensitivity analysis
The findings were put to the test using a leave-one-out sensitivity analysis. The random-effects model was robust, and according to the sensitivity analyses, no single study affected the pooled rate of bacterial contamination of mobile phones used by healthcare workers. The pooled mobile phone bacterial contamination was nearly equal to the real effect magnitude when a single study was eliminated from a meta-analysis (Fig. 6).
Fig. 6.
Sensitivity analysis of mobile phones bacterial contamination removed at a time: contamination rate and 95% confidence interval among healthcare workers in Africa
Publication bias
The funnel plot was used to examine the publication bias. The funnel plot demonstrated that the item distribution was consistent. We employed Begg's and Egger's tests to objectively confirm the symmetry. In the prevalence of bacterial contamination of mobile phones used by healthcare workers, Egger's and Begg's test indicated no evidence of publication bias (p = 0.645) and (p = 0.052) (Fig. 7).
Fig. 7.
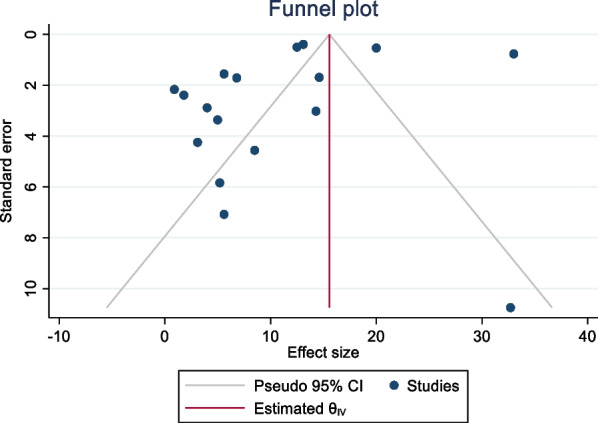
Funnel plot with 95% confidence limits of the pooled bacterial contamination rate of mobile phones used by healthcare workers in Africa
Subgroup analysis
This meta-analysis used subgroup analysis based on the country's sub-regions, study setting, and sample size. As a result, the northern African countries had the greatest pooled prevalence of bacterial contamination of mobile phones, at 87.3% (95% CI (81.6, 93.0%), followed by the eastern African countries, at 83.62% (95% CI 74.40, 92.84%). A subgroup analysis depending on the year of publication was also performed. The combined percentage of bacterial contamination in mobile phones among studies conducted from 2009 to 2014 and 2015 to 2022 was 62.5% and 88%, respectively. The prevalence of bacterial contamination on mobile phones was 95.2% in studies that used a selective bacterial isolation method. However, in studies that used a non-selective bacterial isolation method, it was found to be 70.4%, and in studies that used both (selective + non-selective) bacterial isolation methods, it was found to be 86.3%. A substantial variability was observed across the country's sub-regions, year of publication, types of healthcare facilities, and bacterial isolation methods of included studies in all subgroup analyses (Table 3).
Table 3.
Subgroup rate of mobile phone bacterial contamination among healthcare workers in Africa (2009–2022)
| Variables | Subgroup | No of included study | Sample size | mobile phone's bacterial contamination rate (95% CI) | Heterogeneity across the studies | Heterogeneity between group (p value) |
|
|---|---|---|---|---|---|---|---|
| I2 (%) | p value | ||||||
| Sub-region | Eastern | 9 | 1613 | 83.62(74.40, 92.84) | 98.4 | < 0.001 | < 0.001 |
| Western | 4 | 168 | 77.97(58.86, 97.07) | 98.6 | < 0.001 | ||
| Northern | 8 | 632 | 87.32(81.64, 92.99) | 96.0 | < 0.001 | ||
| Southern | 2 | 166 | 30.0(21.0, 38.94) | 0 | |||
| Middle | 3 | 288 | 98.1(94.02, 102.17) | 84.0 | 0.012 | ||
| Year of publication | 2009–2014 | 6 | 325 | 62.46(39.97, 84.96) | 98.2 | < 0.001 | 0.027 |
| 2015–2022 | 20 | 2298 | 83.03(84.78, 91.29) | 97.9 | < 0.001 | ||
| Types of Health facility | Hospital | 24 | 2822 | 84.54(81.16, 87.93) | 98.1 | < 0.001 | < 0.001 |
| Health center | 1 | 57 | 98.3(94.94, 101.66) | 0 | |||
| Clinic | 1 | 8 | 10.30(-10.76, 31.36) | 0 | |||
| Sample size | ≤ 114 | 19 | 1081 | 86.10(82.87, 89.32 | 97.4 | < 0.001 | 0.481 |
| > 114 | 7 | 1786 | 82.15(71.65, 92.64) | 97.6 | < 0.001 | ||
| Bacteria isolation method | Selective | 5 | 366 | 95.19(91.32, 99.06) | 95.0 | < 0.001 | 0.001 |
| non-selective | 6 | 827 | 70.39(53.14, 87.64) | 99.0 | < 0.001 | ||
| Selective and non-selective | 15 | 1794 | 86.33(81.13, 91.53) | 97.6 | < 0.001 | ||
Narrative review
Antimicrobial susceptibility and multidrug resistance patterns
We descriptively explained bacterial isolates' antimicrobial susceptibility and multidrug resistance using 14 studies [20, 21, 23, 24, 41–45, 52–55, 57]. According to an Ethiopian study, bacterial isolates had a greater rate of resistance to penicillin (84%), ampicillin (81%), and tetracycline (81%). Nevertheless, a study conducted in Nigeria revealed that over 75% of bacterial isolates were sensitive to Fluoroquinolone and Ceftriaxone (Table 4).
Table 4.
Summary of antimicrobial susceptibility and multidrug resistance pattern of bacterial isolates in Africa
| Authors (year) | Country | Antimicrobial susceptibility of the bacterial Isolates | MDR Pattern of Bacterial isolates |
|---|---|---|---|
| Asfaw et al. 2021 [21] | Ethiopia | Not reported |
The overall multidrug resistance prevalence was 42.9% -Bacterial isolates (CoNS, E. coli) showed higher resistance to Penicillin (84%), Ampicillin (81%), and Tetracycline (42%) |
| Gashaw et al. 2014 [44] | Ethiopia |
About 87.5% of S. aureus, 89.3% of CONS, and all S. pyogenes isolates were sensitive to Ciprofloxacin E. coli was 100% sensitive to Ciprofloxacin, Gentamycin, and Trimethoprim–sulfamethoxazole |
-More than half (52.2%) and 60.9% of Gram-positive bacteria were resistant to Amoxicillin and Trimethoprim–sulfamethoxazole -E. cloacae were 100% resistant to Ceftriaxone, Ciprofloxacin, Amoxicillin, and Chloramphenicol |
| Misgana et al. 2014 [43] | Ethiopia | The antimicrobial susceptibility of CoNS was 55.60% for methicillin, and S. aureus was 70.30% for Vancomycin | -About 39.40% of S. aureus isolates were MRSA, of which 38.50% were Vancomycin-resistant |
| Bodena et al. 2019 [45] | Ethiopia | Ceftriaxone (80.6%), Ciprofloxacin (77.3%), and Gentamicin (72.7%) showed higher activity against bacterial isolates (CONS, E. coli and S.aureus) |
The overall prevalence of multidrug resistance (MDR) bacterial isolates was 69.9% Amongst all the bacterial isolates, Pseudomonas sp. (87.5%), Klebsiella sp. (86.7%), and Citrobacter sp. (75%) showed MDR |
| Araya et al. 2021 [23] | Ethiopia | Citrobacter and E. coli are sensitive to Chloramphenicol and Cotrimoxazole |
About 79.2% of the ESBL-producing isolates showed multidrug resistance K. oxytoca, Salmonella spp., P. vulgaris, and P.mirabilis showed 100% multidrug resistance |
| Mohamedin et al. 2019 [46] | Egypt | About 100% of S. aureus was sensitive to Kanamycin and Trimethoprim–sulphamethoxazole | Around 98.2% of S.aureus was resistant to Methicillin, Oxacillin, and Ampicillin antibiotics |
| Mohamadou et al. 2021 [52] | Cameroon | Ceftazidim, Norfloxacin, Imipeneme, Netilmicin and Azthreonam) were efficient against the P. aeruginosas | The prevalence of MDR (≥ 3 antibiotic classes) of identified bacteria (S. aureus and Gram-negative bacteria) was 71.4% |
| Daoudi et al. 2017 [53] | Morocco | Coagulase-negative Staphylococcus sensible to Methicillin | Staphylococcus aureus strains were methicillin-resistant |
| Nwankwo et al. 2014 [20] | Nigeria | 42.8% and 71.4% of S.aureus was sensitive to Amoxicillin and Gentamicin, respectively | High level of bacterial isolates (S.aureus, S. epidermidis) resistance against Cotrimoxazole, Tetracycline and Ampicillin, Gentamicin, Ceftriaxone, and Ciprofloxacin |
| Akinyemi et al. 2009 [54] | Nigeria | Over 75% of the isolates (CONS, E. coli and S.aureus, were susceptible to the Fluoroquinolone and Ceftriaxone antibiotics | Not reported |
| Bobat et al. 2016 [55] | South Africa | All of the S. aureus isolated were Methicillin/Cloxacillin sensitive | Not reported |
| Osman et al. 2018 [56] | Sudan | 40% of Staphylococcus aureus isolates' sensitivity to Oxacillin | Staphylococcus aureus isolates were 98.6% resistant to Oxacillin |
| Tusabe et al. 2021 [57] | Uganda | All bacterial isolates (E. coli Micrococcus spp, CoNS, and Bacillus spp) are susceptible to gentamicin | About 60%, 80% and 90% of the CoNS isolates were resistant to Ciprofloxacin, penicillin, and cotrimoxazole, respectively |
| Mushabati et al. 2021 [24] | Zambia |
S. aureus was susceptible to Ciprofloxacin (88%), Clindamycin (88%), Gentamicin (84%), Cotrimoxazole (50%) and Erythromycin (50%) |
Resistance to cefoxitin was detected in 25% of S. aureus and 48% of CoNS |
CONS Coagulase-negative staphylococci, ESBL Extended-spectrum beta-lactamase, MDR Multidrug resistance
Discussion
Healthcare workers’ (HCWs') continuous handling of MPs promotes the spread of healthcare-associated illnesses. In addition, pathogenic organisms colonizing mobile phones may increase antibiotic resistance [3, 62–64]. This systematic review and meta-analysis aimed to estimate the pooled prevalence of bacterial contamination of mobile phones used by healthcare workers in Africa. As a result, 84.5% of mobile phones were contaminated with bacteria. Mobile phone bacterial contamination is responsible for different infectious illnesses and increases the burden of nosocomial infections unless standard guidelines for using and cleaning mobile phones in healthcare settings are established [1, 3, 26, 27].
On the other hand, bacterial contamination of MPs could be a significant concern influencing the execution of efficient infection prevention measures, thus jeopardizing efforts to limit cross-contamination [65]. This review's result was slightly higher than a meta-analysis in Egypt, which reported a pooled prevalence of bacterial contamination of mobile phones, 78% [25]. Similarly, this review finding was consistent with a systematic review published in Peru [66]. The variation in bacterial contamination of mobile phones could be due to the fluctuating of hand hygiene practiced by healthcare workers, the different types of mobile phones utilized, and the bacterial isolation methods [16, 59]. Furthermore, the type and load of bacterial contamination are known to be influenced by the design of touchscreen phones and the type of keypad surface. The previous evidences had shown the presence of small crevices or micro texture on touchscreen phone surfaces can provide a conducive environment for bacterial colonization. In addition, certain keypad surfaces, particularly those made of porous materials, have been associated with higher bacterial loads compared to non-porous surfaces [67–69].
We conducted a sub-group analysis based on the country sub-region, finding that research from northern African countries had the highest incidence of bacterial contamination of mobile phones. In contrast, studies from southern African countries had the lowest prevalence. Compared to research conducted in other sub-regional countries, most of the papers included in this review were from eastern and northern African countries. One of the possible explanations for the regional heterogeneity in bacterial contamination levels among healthcare workers mobile phones is variations in healthcare facilities, particularly differences in sterilization practices, availability of hand hygiene resources, or adherence to infection control protocols might have influenced the observed disparities. As a result of our findings, it may be necessary to encourage all African countries to achieve a zero prevalence of bacterial contamination in mobile phones.
A subgroup analysis was also performed using the year of publication and the method of bacterial isolation. As a result, studies conducted from 2015 to 2021 found a higher incidence of bacterial contamination in mobile phones than those conducted from 2009 to 2014, demonstrating a lower frequency of bacterial contamination. This disparity could be because smartphones or screen-touch mobile phones, which have a high contamination rate and have been used by healthcare workers in recent years, have a high contamination rate. In terms of bacterial isolation methods, studies using selective bacterial isolation methods, such as MacConkey, had the highest frequency of bacterial contamination on mobile phones when compared to non-selective and combined (selective and non-selective) methods. These differences could be related to competition among bacteria as selective media inhibit other contaminating organisms.
Coagulase-negative staphylococci (CONS) were the most common bacteria isolated in this review, followed by Gram-positive bacteria, such as Staphylococcus aureus. However, Staphylococcus aureus is the most common bacterial infection in most countries and is responsible for over 1 million worldwide deaths, with no focus on global public health expenditure [10]. Escherichia coli were one of the commonest isolated Gram-negative bacteria from mobile phones used by healthcare workers. The possible reason for the high isolation of Staphylococci species might be related to their residence on skin surface and mucosa, on the other hand, the isolation of E. coli, possibly due to cross-contamination with gastrointestinal samples. This finding was in line with findings from other studies [1, 2, 10, 15, 70].
The review's second objective was to describe antimicrobial susceptibility and resistance patterns among African bacterial isolates from healthcare workers' mobile phones. In a study conducted in Ethiopia, Ceftriaxone and Ciprofloxacin were effective against 71.7% and 89.1% of Gram-positive bacterial isolates, such as CONS and S. aureus, respectively, while E. coli was 100% sensitive to Ciprofloxacin, Gentamycin, and Trimethoprim–sulfamethoxazole [41]. However, a study conducted in Nigeria found substantial resistance levels to Cotrimoxazole, Tetracycline, Ampicillin, Gentamicin, Ceftriaxone, and Ciprofloxacin [20]. Most patients treated at home are resistant to one or more antimicrobials [67]. Different bacterial strains, hospital environment, empirical treatment practice, use of antibacterial as a prophylactic, easy availability of some drugs without a prescription, drug dose, and indiscriminate/prolonged use of common antibiotics could all contribute to discrepancies in antimicrobial susceptibility in the included studies [71].
Implication of the study
Mobile phones are constantly infected with microorganisms from the hands of users, hundreds of times per day, even while in toilets. Out of the common bacterial contaminants, Coagulation-negative staphylococci and Staphylococcus aurous have been linked to skin and soft tissue infections, whereas Escherichia coli has been linked to gastrointestinal and urinary tract infections. Sanitizing mobile phones as frequently as we wash our hands, through the use of new technology-driven solutions such as safety-certified enclosed ultraviolet-C emitting mobile phone sanitizers that clean phones in 10–20s is crucial. This fast and effective technology-driven phone sanitization is practical and could be performed in all healthcare settings as health care professionals practice hand hygiene. The installation of stations that can disinfect both hands and mobile phones in healthcare facilities would reduce cross-contamination hazards and should be included in the five critical times of hand washing. This study's findings also offer a strong message to the general public to prevent further microbial spread in Africa.
Limitations of the study
All the studies examined were cross-sectional designs; it could be difficult to establish a cause–effect relationship. The study's findings were only generalizable to the included country's sub-regions. Gram-negative bacterial isolates were not described according to their resistance phenotype.
Conclusion
The contamination of mobile phones used by HCWs with various bacterial isolates was shown to be considerable in this review. The most prevalent bacteria isolated were coagulase-negative staphylococci, Staphylococcus aurous, and Escherichia coli. The prevalence of bacterial contamination in mobile phones varies by country and sub-region. Healthcare workers should practice proper hand hygiene and disinfect their phones after using them in healthcare facilities. Thus, healthcare planners and policymakers should establish norms to manage healthcare workers' hand hygiene, disinfection, sterilization, and washing after using mobile phones in healthcare facilities.
Supplementary Information
Additional file 2. Search results of all databases.
Additional file 3. Risk of bias assessment of included studies.
Acknowledgements
Not applicable.
Author contributions
DZ, BS, and GB contributed to the conception, design, and data extraction; AM, TD, FD, FN, DA, BG, WN, MM, ZT, and VC evaluated the methodological quality of the included articles, participated in data analysis, interpretation and writing the first draft of the paper. All the authors read, commented on, edited, and approved the final submitted manuscript.
Funding
There was no funding for this work from any entity.
Availability of data and materials
The manuscript contains all pertinent information.
Declarations
Ethics approval and consent to participate
The ethical approval and consent to participate in this study are not applicable, because, as stated in the title, it is a systematic review and meta-analysis with no direct participation of study subjects as in a primary study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kotris I, Drenjanèeviæ D, Talapko J, Bukovski S. Identification of microorganisms on mobile phones of intensive care unit health care workers and medical students in the tertiary hospital. Med Glas. 2017;14(1):85–90. doi: 10.17392/878-16. [DOI] [PubMed] [Google Scholar]
- 2.Heyba M, Ismaiel M, Alotaibi A, Mahmoud M, Baqer H, Safar A, et al. Microbiological contamination of mobile phones of clinicians in intensive care units and neonatal care units in public hospitals in Kuwait. BMC Infect Dis. 2015;15(1):1–9. doi: 10.1186/s12879-015-1172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ulger F, Dilek A, Esen S, Sunbul M, Leblebicioglu H. Are healthcare workers' mobile phones a potential source of nosocomial infections? Review of the literature. J Infect Dev Ctries. 2015;9(10):1046–1053. doi: 10.3855/jidc.6104. [DOI] [PubMed] [Google Scholar]
- 4.Prgomet M, Georgiou A, Westbrook JI. The impact of mobile handheld technology on hospital physicians' work practices and patient care: a systematic review. J Am Med Inform Assoc. 2009;16(6):792–801. doi: 10.1197/jamia.M3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ventola CL. Mobile devices and apps for health care professionals: uses and benefits. P T. 2014;39(5):356. [PMC free article] [PubMed] [Google Scholar]
- 6.Manning ML, Davis J, Sparnon E, Ballard RM. iPads, droids, and bugs: Infection prevention for mobile handheld devices at the point of care. Am J Infect Control. 2013;41(11):1073–1076. doi: 10.1016/j.ajic.2013.03.304. [DOI] [PubMed] [Google Scholar]
- 7.Brady RR, Verran J, Damani N, Gibb A. Review of mobile communication devices as potential reservoirs of nosocomial pathogens. J Hosp Infect. 2009;71(4):295–300. doi: 10.1016/j.jhin.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Smibert O, Aung A, Woolnough E, Carter G, Schultz M, Howden B, et al. Mobile phones and computer keyboards: unlikely reservoirs of multidrug-resistant organisms in the tertiary intensive care unit. J Hosp Infect. 2018;99(3):295–298. doi: 10.1016/j.jhin.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 9.Christensen G, Bruggemann H. Bacterial skin commensals and their role as host guardians. Benef Microbes. 2014;5(2):201–215. doi: 10.3920/BM2012.0062. [DOI] [PubMed] [Google Scholar]
- 10.Shah PD, Shaikh NM, Dholaria KV. Microorganisms isolated from mobile phones and hands of health-care workers in a tertiary care hospital of Ahmedabad, Gujarat, India. Indian J Public Health. 2019;63(2):147. doi: 10.4103/ijph.IJPH_179_18. [DOI] [PubMed] [Google Scholar]
- 11.Ustun C, Cihangiroglu M. Health care workers’ mobile phones: a potential cause of microbial cross-contamination between hospitals and community. J Occup Environ Hyg. 2012;9(9):538–542. doi: 10.1080/15459624.2012.697419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saxena S, Singh T, Agarwal H, Mehta G, Dutta R. Bacterial colonization of rings and cell phones carried by health-care providers: are these mobile bacterial zoos in the hospital? Trop Doct. 2011;41(2):116–118. doi: 10.1258/td.2010.100186. [DOI] [PubMed] [Google Scholar]
- 13.Bhat SS, Hegde SK, Salian S. Potential of mobile phones to serve as a reservoir in spread of nosocomial pathogens. Online J Health Allied Sci. 2011;10(2).
- 14.Parthasarathy A, Wong NH, Weiss AN, Tian S, Ali SE, Cavanaugh NT, et al. Selfies and cellfies: Whole genome sequencing and annotation of five antibiotic resistant bacteria isolated from the surfaces of smartphones, an inquiry based laboratory exercise in a genomics undergraduate course at the rochester institute of technology. J Genomics. 2019;7:26. doi: 10.7150/jgen.31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pal S, Juyal D, Adekhandi S, Sharma M, Prakash R, Sharma N, et al. Mobile phones: Reservoirs for the transmission of nosocomial pathogens. Adv Biomed Res. 2015;4:144. doi: 10.4103/2277-9175.161553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galazzi A, Panigada M, Broggi E, Grancini A, Adamini I, Binda F, et al. Microbiological colonization of healthcare workers’ mobile phones in a tertiary-level Italian intensive care unit. Intensive Crit Care Nurs. 2019;52:17–21. doi: 10.1016/j.iccn.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Pillet S, Berthelot P, Gagneux-Brunon A, Mory O, Gay C, Viallon A, et al. Contamination of healthcare workers' mobile phones by epidemic viruses. Clin Microbiol Infect. 2016;22(5):456.e1–e6. doi: 10.1016/j.cmi.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tannhäuser R, Nickel O, Lindner M, Bethge A, Wolf J, Borte S, et al. Bacterial contamination of the smartphones of healthcare workers in a German tertiary-care hospital before and during the COVID-19 pandemic. Am J Infect Control. 2021;50:414–419. doi: 10.1016/j.ajic.2021.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tambe NN, Pai C. A study of microbial flora and MRSA harboured by mobile phones of health care personnel. Int J Rec Tre Sci Tech. 2012;4:14–18. [Google Scholar]
- 20.Nwankwo EO, Ekwunife N, Mofolorunsho KC. Nosocomial pathogens associated with the mobile phones of healthcare workers in a hospital in Anyigba, Kogi state, Nigeria. J Epidemiol Global Health. 2014;4(2):135–140. doi: 10.1016/j.jegh.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asfaw T, Genetu D. High rate of bacterial contamination on healthcare worker’s mobile phone and potential role in dissemination of healthcare-associated infection at Debre Berhan Referral Hospital, North Shoa Zone, Ethiopia. Risk Manag Healthc Policy. 2021;14:2601. doi: 10.2147/RMHP.S313387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haghamad A. Prevalence of bacterial contamination on mobile phones of medical staff in Shendi Hospitals-Sudan. SAR J Pathol Microbiol. 2021;2(3):28–31. [Google Scholar]
- 23.Araya S, Desta K, Woldeamanuel Y. Extended-spectrum beta-lactamase-producing gram-negative bacteria on healthcare workers' mobile phones: evidence from Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia. Risk Manag Healthc Policy. 2021;14:283–291. doi: 10.2147/RMHP.S291876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mushabati N, Samutela M, Yamba K, Ngulube J, Nakazwe R, Nkhoma P, et al. Bacterial contamination of mobile phones of healthcare workers at the University Teaching Hospital, Lusaka, Zambia. Infect Prev Pract. 2021;3(2):100126. doi: 10.1016/j.infpip.2021.100126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Omran A, Taha MS. Bacterial contamination of mobile phones among health care workers: a meta-analysis study. J Med Sci Res. 2020;3(2):87. doi: 10.4103/JMISR.JMISR_50_20. [DOI] [Google Scholar]
- 26.Pérez-Cano H, Santos MR, Moreno BC. Microbiota in mobile phones of medical ophthalmologists. Arch Soc Esp Oftalmol. 2019;94(2):55–59. doi: 10.1016/j.oftal.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 27.Curtis A, Moore Z, Patton D, O'Connor T, Nugent L. Does using a cellular mobile phone increase the risk of nosocomial infections in the Neonatal Intensive Care Unit: a systematic review. J Neonatal Nurs. 2018;24(5):247–252. doi: 10.1016/j.jnn.2018.05.008. [DOI] [Google Scholar]
- 28.Leong XYA, Chong SY, Koh SEA, Yeo BC, Tan KY, Ling ML. Healthcare workers' beliefs, attitudes and compliance with mobile phone hygiene in a main operating theatre complex. Infect Prev Pract. 2020;2(1):100031. doi: 10.1016/j.infpip.2019.100031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhardwaj N, Khatri M, Bhardwaj SK, Sonne C, Deep A, Kim KH. A review on mobile phones as bacterial reservoirs in healthcare environments and potential device decontamination approaches. Environ Res. 2020;186:109569. doi: 10.1016/j.envres.2020.109569. [DOI] [PubMed] [Google Scholar]
- 30.Nejad SB, Allegranzi B, Syed SB, Ellis B, Pittet D. Health-care-associated infection in Africa: a systematic review. Bull World Health Organ. 2011;89:757–765. doi: 10.2471/BLT.11.088179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pasquarella C, Pitzurra O, Savino A. The index of microbial air contamination. J Hosp Infect. 2000;46(4):241–256. doi: 10.1053/jhin.2000.0820. [DOI] [PubMed] [Google Scholar]
- 32.Website. Your Cell Phone Is 10 Times Dirtier Than a Toilet Seat [Internet], Time 2021. http://time.com/4908654/cell-phone-bacteria/. Accessed 16 Mar 2019.
- 33.Simmonds R, Lee D, Hayhurst E. Mobile phones as fomites for potential pathogens in hospitals: microbiome analysis reveals hidden contaminants. J Hosp Infect. 2020;104(2):207–213. doi: 10.1016/j.jhin.2019.09.010. [DOI] [PubMed] [Google Scholar]
- 34.Beer D, Vandermeer B, Brosnikoff C, Shokoples S, Rennie R, Forgie S. bacterial contamination of health care workers’pagers and the efficacy of various disinfecting agents. Pediatr Infect Dis J. 2006;25(11):1074–1075. doi: 10.1097/01.inf.0000242649.27400.94. [DOI] [PubMed] [Google Scholar]
- 35.Huffman S, Webb C, Spina SP. Investigation into the cleaning methods of smartphones and wearables from infectious contamination in a patient care environment (I-SWIPE) Am J Infect Control. 2020;48(5):545–549. doi: 10.1016/j.ajic.2019.09.009. [DOI] [PubMed] [Google Scholar]
- 36.Stern C, Lizarondo L, Carrier J, Godfrey C, Rieger K, Salmond S, et al. Methodological guidance for the conduct of mixed methods systematic reviews. JBI Evid Synth. 2020;18(10):2108–2118. doi: 10.11124/JBISRIR-D-19-00169. [DOI] [PubMed] [Google Scholar]
- 37.Getachew H, Derbie A, Mekonnen D. Surfaces and air bacteriology of selected wards at a referral hospital, Northwest Ethiopia: a cross-sectional study. Int J Infect Dis. 2020;101:44–45. doi: 10.1016/j.ijid.2020.09.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wayne P. Clinical and Laboratory Standards Institute: Performance Standards for Antimicrobial Susceptibility Testing: Informational Supplement, M100. Clinical and Laboratory Standards Institute (CLSI); 2018.
- 39.Moola S. Chapter 7: systematic reviews of etiology and risk. In: Aromataris E, Munn Z, eds. Joanna Briggs institute reviewer's manual. The Joanna Briggs Institute, 2017.
- 40.Higgins J, Altman D, Gøtzsche P, Jüni P, Moher D, Oxman A, et al. Cochrane bias methods group; cochrane statistical methods group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343(7829):d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Daka D. Antibiotic-resistant Staphylococcus aureus isolated from mobile phone and hands of Health care workers in the Hawassa referral Hospital, South Ethiopia. J Microbiol Antimicrob. 2014;6(4):72–78. doi: 10.5897/JMA2014.0303. [DOI] [Google Scholar]
- 42.Ayalew W, Mulu W, Biadglegne F. Bacterial contamination and antibiogram of isolates from health care workers’ fomites at Felege Hiwot Referral Hospital, northwest Ethiopia. Ethiop J Health Dev. 2019;33(2).
- 43.Misgana GM, Abdissa K, Abebe G. Bacterial contamination of mobile phones of health care workers at Jimma University Specialized Hospital, Jimma, South West Ethiopia. Int J Infect Control. 2015;11(1).
- 44.Gashaw M, Abtew D, Addis Z. Prevalence and antimicrobial susceptibility pattern of bacteria isolated from mobile phones of health care professionals working in Gondar town health centers. ISRN Public Health. 2014;2014:1–6. doi: 10.1155/2014/205074. [DOI] [Google Scholar]
- 45.Bodena D, Teklemariam Z, Balakrishnan S, Tesfa T. Bacterial contamination of mobile phones of health professionals in Eastern Ethiopia: antimicrobial susceptibility and associated factors. Trop Med Health. 2019;47:15. doi: 10.1186/s41182-019-0144-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mohamedin A, Elsayed A, Nashnoush HA. Bacterial contamination of mobile phones healthcare versus non-healthcare workers at Mansoura City, Egypt. J Plant Protect Pathol. 2019;10(2):101–109. doi: 10.21608/jppp.2019.40886. [DOI] [Google Scholar]
- 47.Elgabeery RE. Healthcare workers’ mobile phones as a possible vehicle of nosocomial pathogens and the role of different disinfectants in their decontamination. Egypt J Med Microbiol. 2021;30(3):29–36. doi: 10.51429/EJMM30304. [DOI] [Google Scholar]
- 48.Selim HS, Abaza AF. Microbial contamination of mobile phones in a health care setting in Alexandria, Egypt. GMS Hyg Infect Control. 2015;10:Doc03-Doc. [DOI] [PMC free article] [PubMed]
- 49.Shahaby A, Awad N, El-Tarras A, Bahobial A. Mobile phone as potential reservoirs of bacterial pathogens. Afr J Biotech. 2012;11(92):15896–15904. doi: 10.5897/AJB12.1836. [DOI] [Google Scholar]
- 50.Yar DD, Francis G, Roland K, Collins O-A, Balali GI, Kuffour RA, et al. Mobile phones of healthcare workers are possible source of nosocomial infections: evidence from asante-mampong municipal government hospital Ghana. Microbiol Res J Int. 2021;31(6):54–61. [Google Scholar]
- 51.Fandoh ME. Mobile Phone Use and Associated Bacterial Contamination in the Neonatal Intensive Care Unit of the Korle-Bu Teaching Hospital: University Of Ghana; 2018. http://ugspace.ug.gh.
- 52.Mohamadou M, Kountchou L, Mbah C, Bamia A, Abdouraman B, Ngoutane A. Social habits of health professionals and their mobile phones as source of MDR nosocomial bacteria in cameroon, Sub Saharan Africa. J Infect Dis Prev Med. 2021;9:214. [Google Scholar]
- 53.Daoudi A, Nadia El Idrissi S, Bennaoui F, Alaoui M, Soraa N, Fadl Mrabih Rabou M. Study of Bacterial Contamination of Mobile Phones and Stethoscopes in Neonatal Intensive Care Unit. Int J Pediatr. 2017;5(11):6139–42.
- 54.Akinyemi KO, Atapu AD, Adetona OO, Coker AO. The potential role of mobile phones in the spread of bacterial infections. J Infect Dev Ctries. 2009;3(08):628–632. doi: 10.3855/jidc.556. [DOI] [PubMed] [Google Scholar]
- 55.Bobat R, Archary M, Lawler M, Mawlana S, Naidoo KL, Maphumulo S, et al. The presence and spectrum of bacteria colonising mobile phones of staff and caregivers in high disease burden paediatric and neonatal wards in an urban teaching hospital in Durban, South Africa. South Afr J Infect Dis. 2017;32(1):9–11. [Google Scholar]
- 56.Osman M, Omer S, Almugadam B, Ahmed H. Frequency of MRSA isolates in mobile phones, ears and hands of healthcare workers. J Antimicrob Agents. 2018;4(161):2472–1212.1000161.
- 57.Fred T. Bacterial contamination of healthcare worker’s mobile phones; a case study at two referral hospitals in Uganda. Glob Secur. 2021 doi: 10.21203/rs.3.rs-955201/v1. [DOI] [Google Scholar]
- 58.Christelle KM, Hendrick ML, Henri MT. Microbial Ecology of Mobile Phones Staff Maternity Hospital Public Lubumbashi, DR Congo. Microbiol Infect Dis. 2019;3(3):1–4. doi: 10.33425/2639-9458.1063. [DOI] [Google Scholar]
- 59.Shahlol AM, Khalifallah HM, Shahlol EM, Dastidar R, Halder MT, Lamichhane G. Bacterial contamination of mobile phones and hands of health care workers in Sabha Medical Center Hospital, Fazzan area in southwestern of LIBYA. Int J Res Med Sci. 2015;1(4):1–8. [Google Scholar]
- 60.Dibetso T. Microbial contamination of mobile phones of theatre staff at Chris Hani Baragwanath academic hospital (Doctoral dissertation, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg).
- 61.Bissong ME, Moukou M. Mobile phones of hospital workers: a potential reservoir for the transmission of pathogenic bacteria. Afr J Clin Exp Microbiol. 2022;23(4):407–415. doi: 10.4314/ajcem.v23i4.9. [DOI] [Google Scholar]
- 62.Goldblatt JG, Krief I, Klonsky T, Haller D, Milloul V, Sixsmith DM, et al. Use of cellular telephones and transmission of pathogens by medical staff in New York and Israel. Infect Control Hosp Epidemiol. 2007;28(4):500–503. doi: 10.1086/513446. [DOI] [PubMed] [Google Scholar]
- 63.Kirkby S, Biggs C, Ikuta L, Zukowsky K. Cell phones in the neonatal intensive care unit. Adv Neonatal Care. 2016;16(6):404–409. doi: 10.1097/ANC.0000000000000328. [DOI] [PubMed] [Google Scholar]
- 64.Koroglu M, Gunal S, Yildiz F, Savas M, Ozer A, Altindis M. Comparison of keypads and touch-screen mobile phones/devices as potential risk for microbial contamination. J Infect Dev Ctries. 2015;9(12):1308–1314. doi: 10.3855/jidc.6171. [DOI] [PubMed] [Google Scholar]
- 65.Shakir IA, Patel NH, Chamberland RR, Kaar SG. Investigation of cell phones as a potential source of bacterial contamination in the operating room. JBJS. 2015;97(3):225–231. doi: 10.2106/JBJS.N.00523. [DOI] [PubMed] [Google Scholar]
- 66.Loyola S, Gutierrez LR, Horna G, Petersen K, Agapito J, Osada J, et al. Extended-spectrum β-lactamase-producing Enterobacteriaceae in cell phones of health care workers from Peruvian pediatric and neonatal intensive care units. Am J Infect Control. 2016;44(8):910–916. doi: 10.1016/j.ajic.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morvai J, Szabó R. The role of mobile communication devices in the spread of infections. Orv Hetil. 2015;156(20):802–807. doi: 10.1556/650.2015.30147. [DOI] [PubMed] [Google Scholar]
- 68.Brady RR, Chitnis S, Stewart RW, Graham C, Yalamarthi S, Morris K. NHS connecting for health: healthcare professionals, mobile technology, and infection control. Telemed J E Health. 2012;18(4):289–291. doi: 10.1089/tmj.2011.0147. [DOI] [PubMed] [Google Scholar]
- 69.Al Momani W, Khatatbeh M, Altaany Z. Antibiotic susceptibility of bacterial pathogens recovered from the hand and mobile phones of university students. Germs. 2019;9(1):9. doi: 10.18683/germs.2019.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.World Health Organization . Antimicrobial resistance: global report on surveillance. Geneva: World Health Organization; 2014. [Google Scholar]
- 71.Control CfD Prevention . Antibiotic resistance threats in the United States, 2013. Atlanta: CDC; 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 2. Search results of all databases.
Additional file 3. Risk of bias assessment of included studies.
Data Availability Statement
The manuscript contains all pertinent information.