Abstract
Background:
Metastatic triple-negative breast cancer (mTNBC) is an aggressive subtype of breast cancer with poor survival. Currently, the literature lacks comprehensive real-world evidence on locally recurrent and mTNBC patients. To validate the optimal treatment for patients with mTNBC, real-world evidence in combination with data from clinical trials must be evaluated as complementary.
Objectives:
The objective of the study is to examine outcomes and treatment patterns of patients with advanced triple-negative breast cancer (TNBC) utilizing real-world data of patients from all oncology sites across Denmark.
Design:
This is a retrospective, non-interventional, multi-site, population-based observational study conducted across all oncology departments in Denmark.
Methods:
We included all women diagnosed with metastatic or locally recurrent TNBC from January 1, 2017, to December 31, 2019, using the national Danish Breast Cancer Group database. The primary endpoints were overall survival (OS) and progression-free survival (PFS) in the first to third treatment line.
Results:
The study included 243 women diagnosed with metastatic or recurrent TNBC. The median OS (mOS) was 11.6 months after the first line of treatment, 6.5 months after the second line, and 6.5 months after the third line. De novo mTNBC was associated with shorter OS (mOS: 8.3 vs 14.2 months), and those with a relapse within 18 months of primary diagnosis had shorter OS than those with a relapse after 18 months (mOS: 10.0 vs 18.2). In the first line, taxane was the preferred choice of treatment for patients with de novo mTNBC, whereas capecitabine was preferred for patients with recurrent TNBC.
Conclusions:
This real-world, nationwide study demonstrated poor OS among patients with metastatic or recurrent TNBC, with a mOS of 11.6 months (95% CI, 9.9-17.3). Patients who presented with de novo mTNBC or who had a relapse of their breast cancer within 18 months of primary diagnosis had shorter OS.
Registration
The study was registered and approved by the Danish Capital Regions research overview (P-2021-605).
Keywords: Triple-negative breast cancer, metastatic, survival, treatment
Introduction
Triple-negative breast cancer (TNBC) is a subtype of breast cancer characterized by the absence of estrogen receptor (ER), progesterone receptor, and human epidermal growth factor receptor 2 (HER2). It is an aggressive subtype with a high risk of early relapse, large tumor size, high malignancy grade, and early onset.1,2
Despite optimal treatment, some patients experience a metastatic relapse, with TNBC known for having an early peak of recurrence within the first 3 years after diagnosis. 3 Metastases are often aggressive and more likely to occur in viscera, particularly in the lungs, liver, and brain. Prior to 2020, targeted treatments for TNBC were limited, but new treatment options such as immunotherapy and sacituzumab govitecan have emerged.
The current guidelines for treating locally recurrent inoperable or metastatic TNBC (mTNBC) primarily involve chemotherapy and immunotherapy for PD-L1 positive patients. 4
In 2020, atezolizumab was approved as the first-line treatment in combination with nab-paclitaxel for patients with PD-L1-positive advanced TNBC breast cancer, either as de novo mTNBC or as recurrence at least 12 months after ending primary treatment. Atezolizumab in combination with nab-paclitaxel improved both progression-free survival (PFS) and overall survival (OS) in PD-L1-positive patients compared with placebo plus nab-paclitaxel. 5
To validate the optimal treatment for patients with metastatic breast cancer (mBC), real-world evidence in combination with data from clinical trials must be evaluated complementary. Furthermore, there is a significant gap between the patient populations included in phase III clinical trials and the real-life patient group. Patient populations in randomized clinical trials tend to be highly selected through strict inclusion and exclusion criteria, which rarely represent the patients in a real-world clinical setting. 5
Currently, the literature lacks comprehensive real-world evidence on locally recurrent or mTNBC patients, including patient characteristics, clinical profiles, treatment patterns, and outcomes. Our study aims to provide a comprehensive description of treatment patterns and outcomes for locally recurrent or mTNBC patients in Denmark, utilizing a unique nationwide database.
Methods
Study design
This is a retrospective, non-interventional, multi-site, population-based observational study conducted across all oncology departments in Denmark. The study uses data obtained from the Danish Breast Cancer Group (DBCG) national database.
Patient selection
The study includes all women aged 18 years or above who were diagnosed with either de novo mTNBC or recurrent TNBC in Denmark between January 1, 2017, and December 31, 2019.
Data source
The DBCG database is a nationwide clinical database that contains information on diagnosis, demographic, pathology, treatment, follow-up, location of metastasis, ER and HER2 status, date of progression, treatment modalities, start and end dates for treatment, as well as reason for discontinuation of treatment. All hospital departments of surgery, pathology, and oncology in Denmark report data to the database through electronic case report forms. Patients are identified from pathology reports, reports from electronic patient charts, and hospital pharmacies.
The DBCG database lacks specification regarding the type of surgery and surgery site, and it also includes cases where no treatment, including surgery or radiotherapy, was administered.
Follow-up
Patients are followed from index date (the date of diagnosis of either de novo mTNBC or recurrent TNBC) until their last clinical follow-up or death, whichever occurs first. The vital status of patients was tracked until July 31, 2021, whereas patients who did not experience any event in the form of death or disease progression were censored on June 30, 2020. To ensure complete follow-up on vital status, data were linked with the Danish Civil Registration System.
Measures
The study’s primary objectives were OS and PFS in first-, second-, and third-line treatment. The secondary objectives included describing treatment patterns and analyzing OS and PFS stratified by disease presentation (de novo metastatic vs recurrent breast cancer), time to relapse, and age. Time to relapse was defined as the duration between the primary date of diagnosis and the date of relapse. De novo mTNBC was defined as patients who had distant metastases at diagnosis or within 90 days of (neo)adjuvant therapy initiation. Visceral disease was defined as metastases to lever, lung, pleura, or ovaries. Non-visceral disease was defined as metastases to bone, distant lymph nodes, and skin. Brain metastases were defined as the spread of primary cancer to the central nervous system including leptomeningeal carcinomatosis. ER- and HER2-status was defined based on the pathological information obtained from a biopsy of the metastatic site. In cases where pathological information specific to the metastatic site was unavailable, the subtype was determined using the pathological information derived from the primary tumor. Progression was based on radiological, clinical, and biochemical examination from the treating departments. The switch of therapy due to toxicity was distinguished from the switch of therapy due to disease progression in the DBCG database. Patients would not count as switching treatment line when switching due to toxicity.
Statistical analysis
Progression-free survival and OS were assessed for first-, second-, and third-line treatment. Overall survival was estimated from the index date until the death of any cause or end of follow-up for vital status. Subsequently, for the second and third lines, OS was estimated from the date of start of the specific treatment line. Progression-free survival and OS were assessed for first-, second-, and third-line treatment. Overall survival was estimated from the index date until the death of any cause or end of follow-up for vital status. Subsequently, for the second and third lines, OS was estimated from the date of the start of the specific treatment line. PFS was estimated from the index date until progression, death of any cause, or end of follow-up, whichever occurred first. For second- and third-line PFS, the researchers estimated the date from the start of the specific line. Overall survival and PFS were estimated using the Kaplan-Meier method and reported as median. Regarding the choice of treatment, it was registered how many patients began that treatment as first-, second-, or third-line treatment. Time on treatment was estimated from initiation of the first treatment until termination of the last treatment within the first line. If no date of termination of treatment was recorded, patients were censored on June 30. Progression-free survival was estimated from the index date until progression, death of any cause, or end of follow-up, whichever occurred first. For second- and third-line PFS, the researchers estimated the date from the start of the specific line. Overall survival and PFS were estimated using the Kaplan-Meier method and reported as median. Regarding the choice of treatment, it was registered how many patients began that treatment as first-, second-, or third-line treatment. Time on treatment was estimated from initiation of the first treatment until termination of the last treatment within the first line. If no date of termination of treatment was recorded, patients were censored on June 30, 2020. The median time on treatment was estimated using the Kaplan-Meier method. Estimated potential follow-up time was calculated using the reverse Kaplan-Meier method applied to the censored times reversing the roles of event status and censored. Follow-up time was presented as medians with interquartile ranges (IQR). No formal power analysis was performed for this retrospective, observational study primarily being descriptive with no hypotheses specified. All patients registered for the specific time period were included.
Approvals
The study was registered and approved by the Capital Regions research overview (P-2021-605).
Results
Patient population
Between January 1, 2017, and December 31, 2019, 243 patients in Denmark were registered with mTNBC. Table 1 displays patient characteristics.
Table 1.
Characteristics of patients.
| Women | 243 (100%) |
|---|---|
| Age (years) | |
| Median age (range) | 64 (30-92) |
| <55 | 70 (29%) |
| ⩾55 and <65 | 53 (22%) |
| ⩾65 and <75 | 58 (24%) |
| ⩾75 | 62 (25%) |
| Site of cancer | |
| Visceral | 168 (69%) |
| Non-visceral | 73 (30%) |
| Unknown | 2 (1%) |
| Brain metastases | |
| Yes | 21 (9%) |
| No | 220 (91%) |
| Unknown | 2 (1%) |
| Number of sites | |
| 1-2 | 146 (60%) |
| ⩾3 | 95 (39%) |
| Unknown | 2 (1%) |
| Stage | |
| De novo | 48 (20%) |
| Recurrent | 195 (80%) |
| Prior treatment | |
| Neoadjuvant | 40 (21%) |
| Adjuvant | 112 (57%) |
| None | 17 (9%) |
| Unknown | 26 (13%) |
| (Neo)adjuvant therapy | |
| Anthracyclin | 128 (84%) |
| Taxane | 113 (74%) |
| Capecitabine | 7 (5%) |
| Time to relapse | |
| >3 and <6 months | 8 (4%) |
| 6-12 months | 24 (12%) |
| 12-18 months | 32 (16%) |
| >18 months | 131 (67%) |
At the time of mTNBC diagnosis, the median age was 64 years. De novo mTNBC was diagnosed in 48 (20%) patients, whereas recurrent TNBC was diagnosed in 195 (80%) patients. Among the patients with recurrent TNBC, 33 (17%) had received neoadjuvant therapy, 110 (56%) had received adjuvant therapy, and 17 (9%) had not received any (neo)adjuvant therapy. Notably, 64 patients (33%) experienced breast cancer relapse within 18 months of primary diagnosis, whereas 131 (67%) patients had a relapse beyond 18 months after ending (neo)adjuvant therapy.
At baseline, 69% of the patients presented with visceral metastases, whereas 21 (9%) patients had brain metastases. The location of metastases was unknown for 2 patients.
Treatment patterns
This study recorded 243 patients in the first line, out of which 143 (59%) proceeded to the second line, and 89 (62%) to the third line.
Out of the 243 patients in the first line, 224 (92%) received treatment for advanced disease, whereas 19 (8%) did not. Similarly, 12 (8%) patients in the second line and 7 (8%) patients in the third line did not receive treatment.
In the first line, taxane was the preferred choice of treatment for patients with de novo mTNBC, whereas capecitabine was preferred for patients with recurrent TNBC. In the second line, capecitabine remained the preferred treatment for patients with recurrent disease, whereas eribulin was the preferred treatment in the third line. A total of 27 (52%) of primary metastatic patients were treated with either a taxane or an anthracycline in the first line.
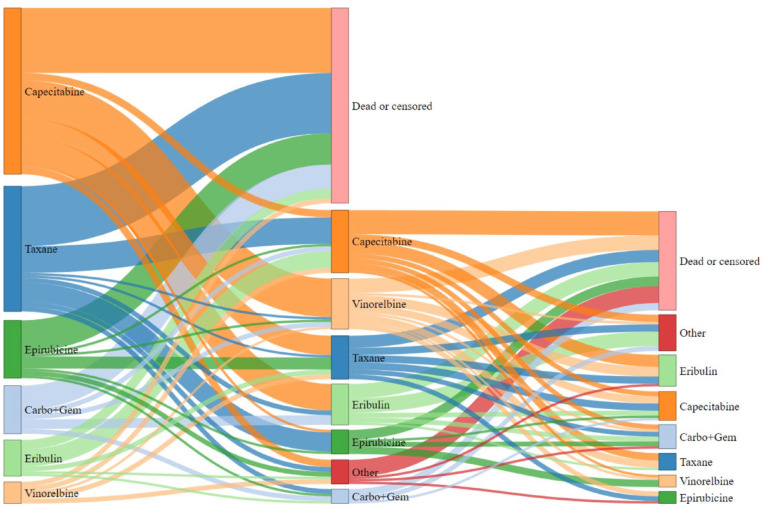
The median time on treatment for the first line was found to be 122 days (range 1-972). Treatment choices for the first 3 lines of treatment are presented in Table 2. The course of treatment is shown through a Sankey diagram in Figure 1.
Table 2.
Treatment received by patients within each of the first 3 lines.
| Treatment | Line | |||||
|---|---|---|---|---|---|---|
| First | Second | Third | ||||
| Stage: | De novo | Recurrent | De novo | Recurrent | De novo | Recurrent |
| Epirubicin | 9 (19%) | 15 (8%) | 8 (24%) | 2 (2%) | 3 (16%) | 3 (4%) |
| Taxane | 16 (33%) | 36 (18%) | 7 (11%) | 12 (11%) | 1 (5%) | 6 (9%) |
| Capecitabine | 12 (25%) | 57 (29%) | 5 (15%) | 24 (22%) | 2 (11%) | 11 (16%) |
| Carboplatin + gemcitabine | 1 (2%) | 19 (10%) | 1 (3%) | 6 (5%) | 3 (16%) | 7 (10%) |
| Vinorelbine | 4 (8%) | 5 (3%) | 3 (9%) | 19 (17%) | 3 (16%) | 2 (3%) |
| Eribulin | 0 (0%) | 15 (8%) | 1 (3%) | 19 (17%) | 3 (16%) | 13 (19%) |
| Surgery | 0 (0%) | 20 (10%) | 0 (0%) | 2 (2%) | 0 (0%) | 2 (3%) |
| No treatment | 5 (10%) | 14 (7%) | 4 (12%) | 8 (7%) | 3 (16%) | 4 (6%) |
| Other | 1 (2%) | 14 (7%) | 4 (12%) | 18 (16%) | 1 (5%) | 22 (31%) |
| Total | 48 | 195 | 33 | 110 | 19 | 70 |
| 243 | 143 | 89 | ||||
Other = CMF, Caelyx, PARP inhibitor, carboplatin, gemcitabine, radiotherapy, and experimental treatment.
Figure 1.
Sankey diagram illustration of the course of treatment followed by patients from the first to the third line. The size of the box in the diagram is relative to the number of patients.
Out of 195 patients with recurrent breast cancer, 57 (29%) received capecitabine as the first line of treatment. Among the patients that received capecitabine in the first line, only 1 (2%) had a relapse within 6 months after initiation of adjuvant therapy, and 8 (14%) had a relapse within 12 months.
Progression-free survival
During the study period, 152 patients in the first line experienced progression and 49 died, whereas in the second line, the numbers were 92 and 38, respectively, and in the third line, the numbers were 65 and 18.
The median PFS (mPFS) in the first, second, and third lines were 4.9 months (95% CI, 4.2-6.3), 2.5 months (95% CI, 2.3-2.8), and 2.1 months (95% CI, 1.9-3.4), respectively (Figure 2).
Figure 2.
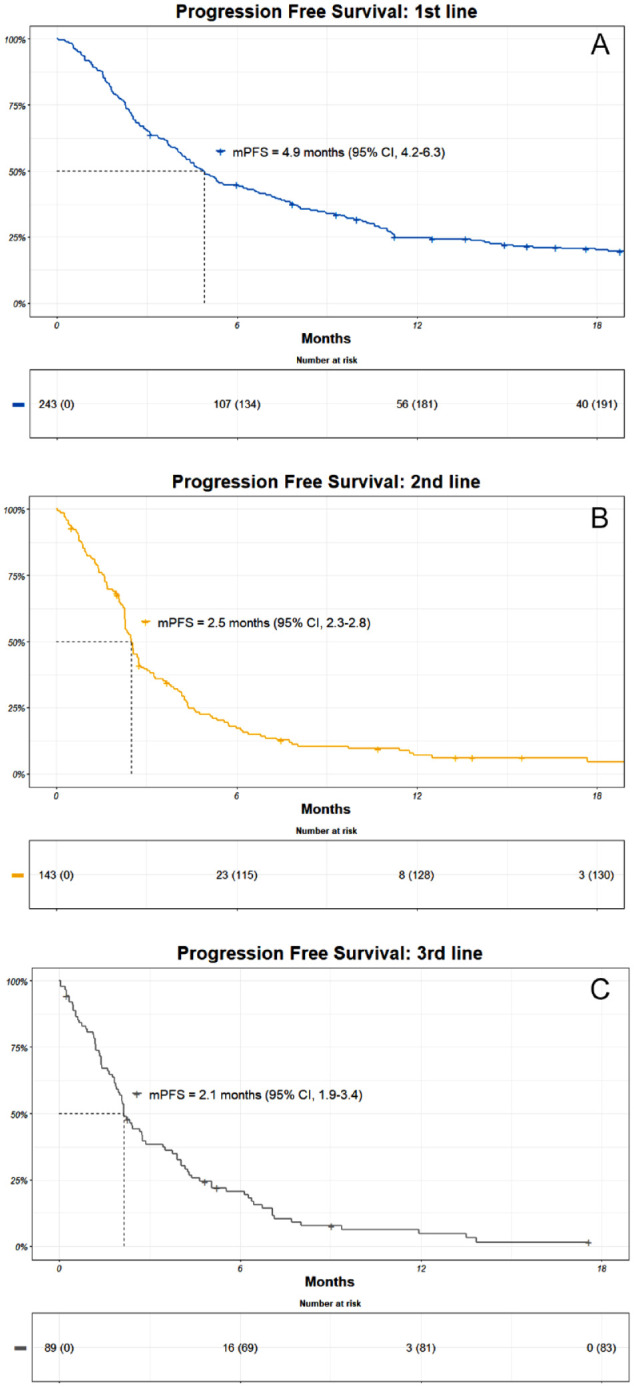
Kaplan-Meier (KM) estimates of progression-free survival (PFS) in the (A) first, (B) second, and (C) third line. Patients at risk are included together with an estimate of mPFS.
CI indicates confidence interval; mPFS, median progression-free survival.
Overall survival
A total of 185 (76%) patients died during the study period, with a median OS (mOS) of 11.6 months (95% CI, 9.9-17.3). In the second line, the mOS was 6.5 months (95% CI, 4.9-9.0), and in the third line, it was 6.5 months (95% CI, 4.0-10.0) (Figure 3). Median follow-up for OS was 38.1 months (IQR, 29.3-45.9).
Figure 3.
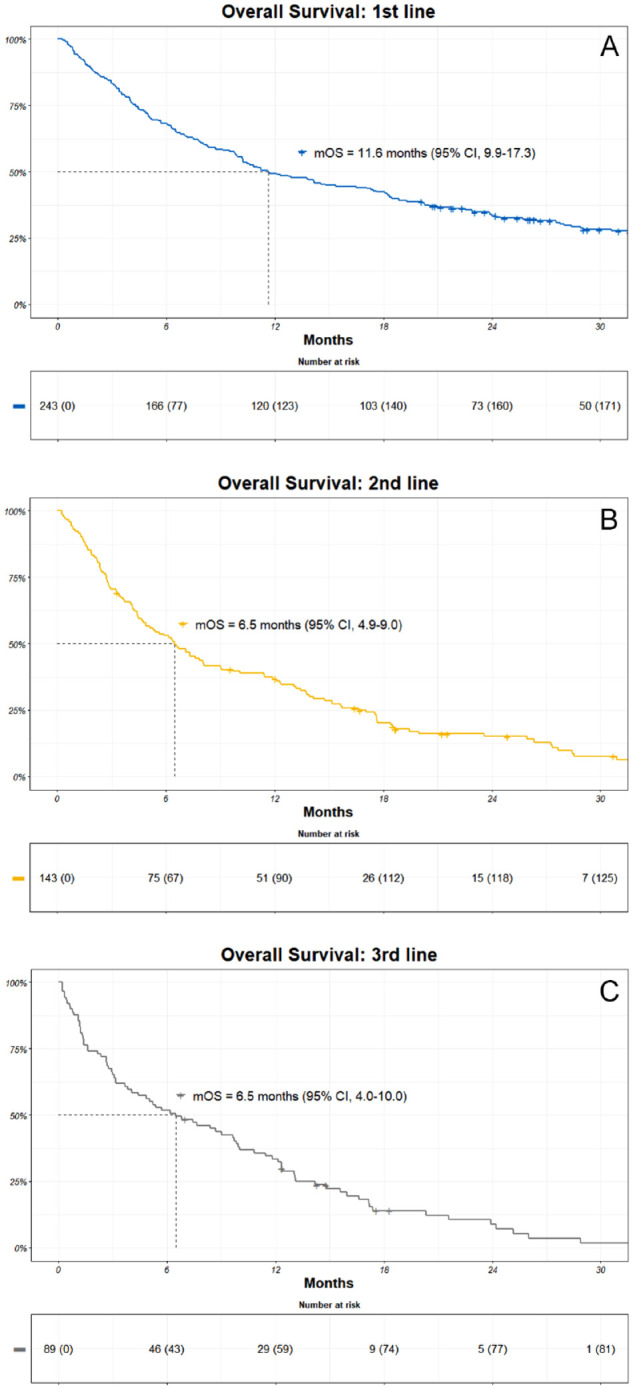
Kaplan-Meier (KM) estimates of overall survival (OS) among patients in the (A) first, (B) second, and (C) third line. Patients at risk are included together with an estimate of mOS.
CI indicates confidence interval; mOS, median overall survival.
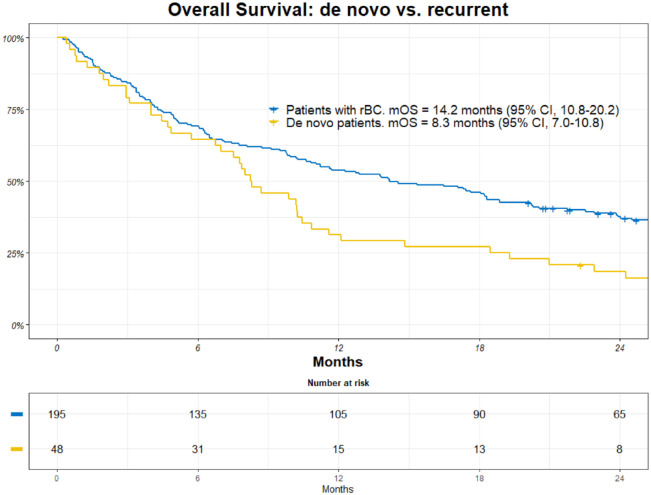
The de novo mTNBC group had a mOS of 8.3 months (95% CI, 7.0-10.8), and the recurrent patients had a mOS of 14.2 months (95% CI, 10.8-20.2) (Figure 4).
Figure 4.
Kaplan-Meier (KM) estimates of overall survival (OS) among patients with de novo metastatic breast cancer vs recurrent breast cancer. Patients at risk are included together with an estimate of mOS.
CI indicates confidence interval; mOS, median overall survival; rBC, recurrent breast cancer.
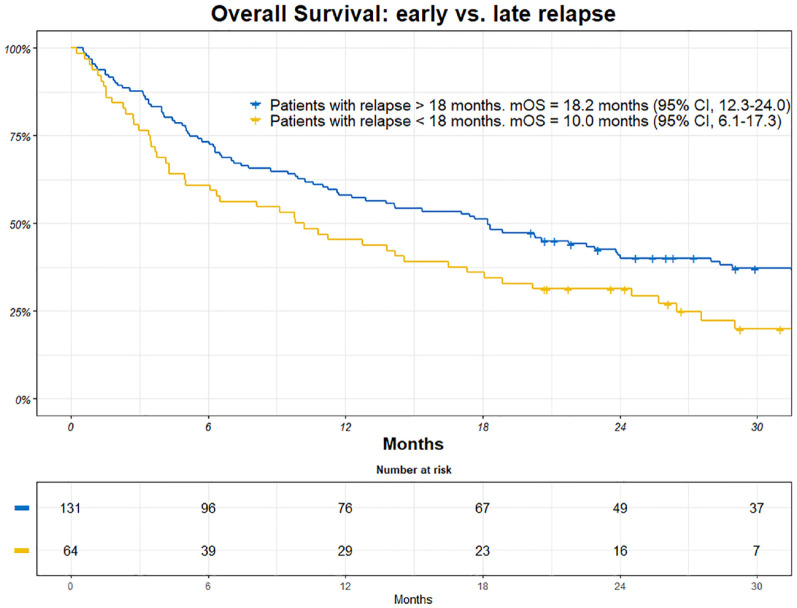
Patients who experienced early relapse (within 18 months) had a mOS of 10.0 months (95% CI, 6.1-17.3). Patients who had late relapses had a mOS of 18.2 months (95% CI, 12.3-24.0), (Figure 5).
Figure 5.
Kaplan-Meier (KM) estimates of overall survival (OS) among patients with early vs late reoccurrence of breast cancer. Patients at risk are included together with an estimate of mOS.
CI indicates confidence interval; mOS, median overall survival.
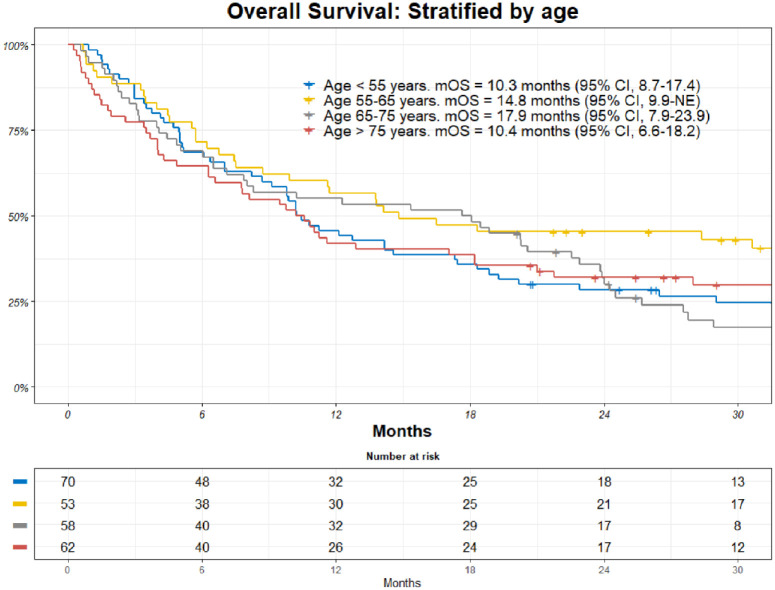
There was no significant difference in mOS based on age. mOS for patients under 55 years was 10.3 months (95% CI, 8.7-17.4) and 14.8 months for patients aged 55-65 (95% CI, 9.9 to not estimable). mOS for patients aged 65 to 75 years was 17.9 months (95% CI, 7.9-23.9), and 10.4 months (95% CI, 6.6-18.2) for patients over the age of 75 years (Figure 6).
Figure 6.
Kaplan-Meier (KM) estimates of overall survival (OS) among all patients stratified on age. Patients at risk are included together with an estimate of mOS.
CI indicates confidence interval; mOS, median overall survival.
Discussion
In this retrospective, multi-site, population-based study involving all departments of oncology in Denmark, we examined all women diagnosed with advanced TNBC between January 1, 2017, and December 31, 2019. Our study found a mOS of 11.6 months (95% CI, 9.9-17.3) and an mPFS of 4.9 months (95% CI, 4.2-6.3).
Real-world data on outcomes for patients with mTNBC is scarce. A 2020 retrospective observational study of community oncology centers in the United States from January 2010 to January 2016 included 608 patients and reported a comparable mOS of 11.8 months (95% CI, 10.2-13.1) and mPFS of 4.2 months (95% CI, 3.7-4.6). 6 It is important to note, however, that, although both studies are based on real-world data, differences exist between the study cohorts. The previous study differs from ours in age (mean age 57.5 vs median of 64 in ours) and patients going without therapy in the first line (17% vs 8%).
A meta-analysis of mTNBC subgroups from 3 phase III trials in first line mTNBC reported a mOS of 17.5 months and an mPFS of 5.4 months with single-agent chemotherapy. 7 Although the mOS from this study is considerably longer than the mOS found in our study (11.8 months, 95% CI, 10.2-13.1), the mPFS reported in the meta-analysis is comparable with our study (4.2 months, 95% CI, 3.7-4.6). The difference in OS could reflect the stricter inclusion criteria known to be present in phase III trials. Furthermore, the percentage of de novo mTNBC patients was higher in our real-world study, and these patients had a significantly worse OS. As patients with these characteristics were not excluded from our study, this could explain the shorter mOS observed in our study.
In our study, only 52% of de novo mTNBC patients received a taxane or anthracycline as the first line of treatment, whereas 35% received another type of chemotherapy. This heterogeneity in first-line chemotherapy choices likely reflects patient preferences, physician guidance, and experience. However, it is concerning that only approximately half of the patients received the recommended first-line treatment, and this should prompt reflection in clinical practice.
In contrast to the real-world study conducted in the United States, our study diverges considerably in the first-line treatment. 6 Notably, 8% of our patients underwent combination therapy as their first-line treatment, whereas the US study recorded a substantially higher percentage of 57% for this modality. Furthermore, our study showed a larger number of patients receiving single-agent capecitabine as first-line treatment (28%), as opposed to the 14% reported in the other study. The preferred first-line treatment in the US study was carboplatin in combination with gemcitabine (15.1%), whereas a taxane or anthracycline was the preferred first-line therapy in our study (31%).
It should be noted that this study included patients from January 2017 to December 2019, a period during which atezolizumab, pembrolizumab, and sacituzumab govitecan were not reimbursed in Denmark. Atezolizumab has been approved since January 2020, but it is unlikely to significantly increase the overall median survival for the group of TNBC patients, as it is introduced to a subgroup of patients.
Patients with de novo mTNBC, who are treatment naïve, were found to have a shorter mOS than patients with recurrent breast cancer (8.3 vs 14.2 months). This finding is somewhat surprising, given that patients with other breast cancer subtypes, such as HER2-positive or ER-positive patients with de novo mBC, have a better prognosis than patients with relapsed disease.8,9 This may be due to the more aggressive biology of mTNBC, but it may also reflect that less effective chemotherapy regimens are chosen, in consultation with the patient, knowing the short expected remaining lifetime when the disease has metastasized.
Patients with a relapse of primary disease within 18 months after ending adjuvant therapy had worse survival than patients with a relapse after 18 months (10.0 vs 18.2 months). This could reflect either a more indolent tumor type or, after all, some sensitivity to adjuvant chemotherapy.
mOS from initiation of the second and third line of treatment was 6.5 months (95% CI, 4.9-9.0) and 6.5 months (95% CI, 4.0-10.0), respectively. mPFS from the initiation of the second and third line was 2.5 months (95% CI, 2.3-2.8) and 2.1 months (95% CI, 1.9-3.4), respectively. The progression-free interval significantly diminishes as seen with a PFS of 4.9 months (95% CI, 4.2-6.3) and 2.5 months (95% CI, 2.3-2.8) in the first and second lines, respectively. These results should cause some reflections in clinical practice as to the number of lines initiated and data such as these should be shared with the patient before starting a new treatment.
This study contains certain strengths and limitations. The DBCG national database was used in data collection, which means that every known woman in Denmark diagnosed with mTNBC in the study period was included in this study. This further supports reducing geographical and socioeconomic bias from the study. Furthermore, analyses regarding the first to the third line of treatment were made. This is to the best of our knowledge the first real-world study to examine the outcome differences between the first 3 lines of treatment in mTNBC. No information regarding performance status, objective response rates (that for some patients could have a palliative impact), quality of life during treatment compared with no systemic treatment, and data concerning the safety of treatment was available.
Conclusions
This observational, nationwide, retrospective, population-based study included 243 patients who were diagnosed with mTNBC. The study population had a mOS and mPFS of 11.6 months (95% CI, 9.9-17.3) and 4.9 months (95% CI, 4.2-6.3), respectively. Furthermore, patients with de novo mTNBC as well as patients with early recurrence had a worse mOS than patients with recurrent breast cancer and patients with late recurrence, respectively.
Acknowledgments
None.
Declarations
Ethics approval and consent to participate: The study was approved by the Danish Breast Cancer Group’s oncological committee. The study was also registered and approved by the Capital Regions research overview (P-2021-605) which constitutes informed consent.
Consent for publication: Not applicable.
Author contributions: Alan Celik: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing—original draft, and Writing—review & editing.
Tobias Berg: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Supervision, Visualization, and Writing—review & editing.
Maj-Britt Jensen: Conceptualization, Data curation, Methodology, Supervision, and Writing—review & editing.
Erik Jakobsen: Investigation and Writing—review & editing.
Hanne Nielsen: Investigation and Writing—review & editing.
Iben Kümler: Investigation and Writing—review & editing.
Vesna Glavicic: Investigation and Writing—review & editing.
Jeanette Dupont Jensen: Investigation and Writing—review & editing.
Ann Knoop: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, and Writing—review & editing.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article: This study was funded by Merck Sharp and Dohme.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: TB: Institutional grants from Danish Cancer Society, Neye-fonden, Roche, Novartis, Samsung Bioepis, Pfizer, AstraZeneca, Merck Eisai, and Venture Oncology Personal; Invited Speaker: Pfizer; and Advisory Board: Merck. MJ: Institutional grants from Samsung Bioepis, Nanostring Technologies, and Venture Oncology. AK: Institutional grants from Roche and Venture Oncology Personal and Advisory Board: Novartis, AstraZeneca, MDS, Roche, Pfizer, and Eli Lilly Danmark A/S. Other authors had no conflict of interest to declare.
Availability of data and materials: All data are stored in the DBCG database. The dataset can be made available to qualified researchers through application to the Danish Breast Cancer Group. Please contact dbcg.rigshospitalet@regionh.dk.
References
- 1. Aysola K, Desai A, Welch C, et al. Triple negative breast cancer—an overview. Hered Genet. 2013;2013(Suppl 2):001. doi: 10.4172/2161-1041.S2-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang L, Huang Y, Feng Z, et al. Comparison of breast cancer risk factors among molecular subtypes: a case-only study. Cancer Med. 2019;8:1882-1892. doi: 10.1002/cam4.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Peart O. Metastatic breast cancer. Radiol Technol. 2017;88:519M-539M. [PubMed] [Google Scholar]
- 4. Cardoso F, Paluch-Shimon S, Senkus E, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol. 2020;31:1623-1649. doi: 10.1016/j.annonc.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim HS, Lee S, Kim JH. Real-world evidence versus randomized controlled trial: clinical research based on electronic medical records. J Korean Med Sci. 2018;33:1-7. doi: 10.3346/jkms.2018.33.e213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Skinner KE, Haiderali A, Huang M, Schwartzberg LS. Real-world effectiveness outcomes in patients diagnosed with metastatic triple-negative breast cancer. Futur Oncol. 2021;17:931-941. doi: 10.2217/fon-2020-1021. [DOI] [PubMed] [Google Scholar]
- 7. Li CH, Karantza V, Aktan G, Lala M. Current treatment landscape for patients with locally recurrent inoperable or metastatic triple-negative breast cancer: a systematic literature review. Breast Cancer Res. 2019;21:143. doi: 10.1186/s13058-019-1210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Güth U, Magaton I, Huang DJ, Fisher R, Schötzau A, Vetter M. Primary and secondary distant metastatic breast cancer: two sides of the same coin. Breast. 2014;23:26-32. doi: 10.1016/j.breast.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 9. Yamamura J, Kamigaki S, Fujita J, Osato H, Komoike Y. The difference in prognostic outcomes between de novo stage IV and recurrent metastatic patients with hormone receptor-positive, HER2-negative breast cancer. In Vivo. 2018;32:353-358. doi: 10.21873/invivo.11245. [DOI] [PMC free article] [PubMed] [Google Scholar]