Abstract
Chronic pain is one of the most common, costly, and potentially debilitating health issues facing older adults, with attributable costs exceeding $600 billion annually. The prevalence of pain in humans increases with advancing age. Yet, the contributions of sex differences, age-related chronic inflammation, and changes in neuroplasticity to the overall experience of pain are less clear, given that opposing processes in aging interact. This review article examines and summarizes pre-clinical research and clinical data on chronic pain among older adults to identify knowledge gaps and provide the base for future research and clinical practice. We provide evidence to suggest that neurodegenerative conditions engender a loss of neural plasticity involved in pain response, whereas low-grade inflammation in aging increases CNS sensitization but decreases PNS sensitivity. Insights from preclinical studies are needed to answer mechanistic questions. However, the selection of appropriate aging models presents a challenge that has resulted in conflicting data regarding pain processing and behavioral outcomes that are difficult to translate to humans.
Keywords: Pain, Aged Adults, Aging, Neuroinflammation, Neuroplasticity, Opioids, Neuroimmune, NSAIDs
Introduction
With a predicted 30% increase of individuals 65 years and older in the U.S. in the next 10 years, understanding the prevalence of chronic pain, comorbidities, mechanisms, and therapeutic strategies in age-related pain conditions is more important than ever.1,2 Older adults are particularly vulnerable because of the increased prevalence of degenerative musculoskeletal disorders, frailty, and multimorbidity placing them at increased risk for pain states.3–5 Moreover, behavioral complications during aging, such as cognitive impairments could lead to decreased self-care, self-awareness, and worsening of health conditions, such as prolonged pain and increased hospitalization.6–8
The International Association for the Study of Pain (IASP) recently updated its definition of pain as “an aversive sensory and emotional experience typically caused by, or resembling that caused by, actual or potential tissue injury”. 9 Chronic pain is defined as pain that persists past the normal healing time, usually lasting more than 12 weeks, making it distinct from acute pain.10,11 Chronic pain is a complex condition that involves sensory, affective, cognitive, and behavioral components. 10 It has a heterogeneous etiology such as tissue injury, inflammation, autoimmunity, chemotherapeutics, and stress referred to as functional pain conditions. In addition, chronic pain is often comorbid with medical conditions like depression, anxiety, sleep disturbances, and fatigue. 12 Recovery from chronic pain is often bleak, with the most recent estimates by Li et al. at 30%. 13 Duration of previous chronic pain, age, chronic diseases, personality traits, and differences in cortico-limbic structural and functional connectivity are some factors that have been associated with a lower probability of recovery.13,14 Chronic pain syndromes are common in older adults and are associated with social isolation, functional disability, significant suffering, and increased healthcare resource utilization and costs. Chronic pain is also a risk factor for accelerated cognitive decline and premature death, suggesting a mechanism between pain and cognition. 15 As such, chronic pain has been increasingly recognized as a condition/disease of its own, particularly in older adults.16–18
This review begins with a brief overview of the epidemiology of pain, pain assessment, and pain management in older adults, as these have been reviewed excellently elsewhere. The main focus is on a thorough review of both pre-clinical research and clinical data on chronic pain among older adults to present a unifying concept, identify knowledge gaps, and provide the basis for future research and clinical practice.
Epidemiology of pain and aging
Chronic pain is an epidemic. In the U.S., one in five adults (20.4%, i.e., 50 million) report suffering from pain almost daily for at least 6 months, according to data from the 2016 National Health Interview Survey.19–21 The prevalence increases with advancing age: 27.8% of 45–64 year-olds, 27.6% of 65–84 year-olds, and 33.6% of 85 years and older have reported chronic pain. 20 By severity, 8% of U.S. adults (19.6 million) reported high-impact chronic pain, that is, pain that restricted life or major work activities on most days or every day during the past 6 months. 20 By sub-type, the broad prevalence of chronic neuropathic pain is 10% of U.S. adults, with significant variation based on age and ethnicity.22,23
Population-based pain prevalence estimates are often broad. Pain prevalence estimates from the National Health and Aging Trends Study (NHATS), a survey of a nationally representative sample of Medicare beneficiaries aged 65 and older in the U.S., employ conservative definitions of pain and activity-limiting pain and range between 13% and 49%. Several patient-level factors including comprehension and memory, attitude/behavioral problems, and language factors contribute to the wide variability in the reporting of chronic pain in older adults. In a sample of 7601 adults enrolled in NHATS, over half of the participants (52.9%) reported bothersome pain in the previous month, whereby back pain was most commonly reported (30.3%), followed by knee pain (24.8%), and shoulder pain (19.9%). 24 Moreover, a large British longitudinal cohort and meta-analysis found that chronic pain is a risk factor (57% greater risk) for early death. 25
Important to consider is the contribution of pain in dementia and how it is reported in Alzheimer’s patients. 26 Indeed, chronic pain can be a contributing factor to premature death 27 and has also been linked to increased cognitive decline and dementia (Whitlock EI et al., 2017). Therefore, it is important to assess the relationship and role of chronic pain in Alzheimer’s disease and dementia. 28 These findings suggest that shared mechanisms such as environmental exposures, genetics, or molecular targets could play a role in both conditions.28,29 Moreover, degeneration in brainstem regions modulating descending pain inhibition such as periaqueductal gray matter has been found in Alzheimer’s disease. 30 Cortico-limbic regions that have been implicated in pain modulation are severely affected by Alzheimer’s pathology and pain is associated with cognitive deficits that are more pronounced with aging.31–39 Furthermore, an NHATS study focused on older adults with pain and dementia, reported that out of 804 participants, nearly two-thirds (63.5%) reported bothersome pain in the last month (vs 54.5% among the matched cohort without dementia), and 43.3% reported activity-limiting pain (vs 27.2% among the matched cohort without dementia). 40 These studies in patients with dementia suggest potentially shared mechanisms between pain (or its treatment) and cognitive impairment. It may also suggest that patients with dementia are able to recognize and report pain, which is not necessarily to be expected with cognitive impairment, and therefore becomes a clinical concern.
Regardless, the burden of chronic pain and other comorbid conditions related to aging is expected to continue to increase with the growing proportion of older adults (≥65 years of age) representing 16.0% in 2018 to almost 20.6% by 2030. 41 The economic negative impact of chronic pain on the health care system and the economy is staggering. In the United States alone, estimated costs attributable to chronic pain, including direct medical costs, disability, and loss of productive time, exceeds $600 billion annually. 19 Along with the chronic pain crisis, the current opioid epidemic has resulted in a rising number of drug overdose deaths associated with prescription and synthetic opioid abuse/misuse. 42
Chronic pain assessment in older adults
A thorough assessment of chronic pain is an integral step toward developing an appropriate management plan for patients. Older adults are likely to underreport their pain for numerous reasons, including, but not limited to, assumptions that pain is a normal part of aging, fear of being labeled weak or identified as a complainer, fear of opioid addiction, and previous dismissal of pain report by healthcare providers.43–45
A comprehensive chronic pain assessment for older adults integrates a complete medical history, physical examination, relevant diagnostic tests, as well as an evaluation of sensory deficits, affective changes, and cognitive impairment.45,46 Patients with chronic pain should be assessed at each visit. An array of assessment tools are available to assess pain intensity among patients. Simple and easy-to-understand tools are the most effective for use with older adults, who are more likely to face cognitive impairments. 47 The most widely used pain assessment tools for older adults are the Numeric Rating Scale (NRS), Patient-Reported Outcomes Measurement Information System (PROMIS), Oswestry Disability Index (ODI), Verbal Descriptor Scale (VDS), Iowa Pain Thermometer (IPS), and Faces Pain Scale-Revised (FPS-R), all of which have been demonstrated to be valid and reliable. 43 NRS asks the patients to rate their pain from 0 to 10 with 0 indicating no pain and 10 representing the worst possible pain. 48 PROMIS is an NIH-funded rigorously tested patient-reported outcome (PRO) measurement tool. 49 The survey utilizes a computer adaptive testing system to target quality of life comprehensively and includes a pain assessment component. ODI assesses the functional limitations of low back pain in everyday life through a ten-question survey with each question targeting a specific activity of daily living (ADL). 50 Questions are scored to a maximum of five points and summed, with higher scores indicating a more severe disability. VDS asks the patient to describe his/her pain from “no pain,” to “mild,” “moderate,” “severe,” or “pain as bad as it could be”. 51 IPS is a modified VDS with seven pain descriptors of increasing pain intensity. 52 FPS-R asks patients to select one of six facial expressions that correspond with their pain, for a score between 0 to 10. 53 Additional comprehensive standardized pain assessment tools that assess the intensity and functional impact of pain are also available: Brief Pain Inventory, Geriatric Pain Measure, and Pain Disability Index. 44
When evaluating older adults with cognitive decline, observational methods along with self-report are used.24,54,55 Caregivers are also enlisted to provide their assessments of the patient’s pain. Reviews of various measures to assess pain in older adults have been summarized elsewhere.10,46,48
Managing chronic pain in older adults
Evidence-based guidelines for managing chronic pain in older adults embrace multi-model treatments (non-pharmacological and pharmacological interventions) to address the biopsychosocial nature of pain. Table 1 highlights U.S. standard of care guidelines for chronic pain in the elderly.56–59
Table 1.
U.S. Guidelines for standardized care of chronic pain in the elderly.
| Source | Recommendations |
|---|---|
| American geriatrics society panel 2009 56 | • Outlines recommendations for the use of drugs for persistent pain in older persons: Non-opioids, opioids, adjuvant analgesic drugs, other drugs |
| U.S. Department of health and human services 2019 57 | • Discusses recommendations with respect to: medications, restorative therapies (i.e., physical therapy, occupational therapy), interventional approaches, behavioral approaches for psychological, cognitive, emotional, and social aspects of pain, and complementary and integrative health |
| University of Iowa’s www.geriatricpain.org 2009-201258,59 | • Discusses recommendations related to: treatment plans, medication management, incorporating older adult and family teaching throughout assessment and treatment, and addressing pain using an interdisciplinary approach. |
Non-pharmacologic pain management strategies
Non-pharmacological interventions include psychosocial interventions, complementary and integrative health therapies, rehabilitation therapies, and exercise. 10 A recent meta-analysis of the efficacy of psychological interventions on chronic pain analyzed 22 studies on the impact of cognitive behavioral therapy (CBT)-based interventions on chronic pain outcomes. 60 The interventions produced small yet statistically significant benefits for pain relief, catastrophizing beliefs, and self-efficacy. Moreover, interventions were strongest when delivered using group-based approaches. The authors concluded that there is a need for psychological interventions that generate greater treatment effects for older adults.
Caregiver support is important in successful pain management programs for older adults, especially among those with cognitive decline. 61 Health professionals should partner with caregivers on proper medication management for patients. 62
Pharmacological treatments for chronic pain
Age-associated physiological changes can affect the way the body responds to pain medication. 63 Additionally, older adults tend to have more comorbidities, which can complicate pharmacologic pain management. 64 Therefore, in the elderly, pain medications need to be monitored carefully by a clinician to avoid side effects, adverse drug-drug interactions, and over- or under-use.63–66 Treatment plans vary according to origin and pain intensity. Paracetamol (acetaminophen) is the preferred treatment for older adults with mild-to-moderate pain, with minimal side effects. 10 NSAIDs are commonly used to treat pain and can be prescribed in combination with acetaminophen. However, NSAIDs pose increased risks with age and should therefore be prescribed for the shortest duration possible at the lowest effective dose, due to potential adverse effects, including gastrointestinal toxicity, nephrotoxicity, and cardiovascular risk. 67
Opioids are usually considered when pain is moderate-to-severe and other treatments have been unsuccessful. Opioid use needs to be monitored carefully due to the increased risk of adverse effects among older adults, which include dependence, ileus, and respiratory depression. In 2016, the Centers for Disease Control and Prevention issued 12 guidelines for prescribing opioids for chronic pain, which specify: (1) non-opioid therapies are preferred over opioids, (2) when opioids are used, prescribing the lowest possible effective dosage to reduce risks of opioid misuse and overdose, (3) and, monitoring all patients closely when prescribing opioids. 68 Precautions when using opiates in the elderly population have been reviewed elsewhere. 69
Chronic pain mechanisms and aging
Pain processing is not static but plastic, with the pain becoming chronic through long-term changes in the neural structures.15,70,71 The comprehensive understanding of the complexity of persistent pain and underlying mechanisms remains a challenge because studies are often focused on individual compartments of the nervous systems, such as nociceptors in the PNS, spinal circuits in the CNS, and to a lesser extent, different brain regions. There is still a lack of a system-wide understanding that integrates pain-related changes from nociceptor populations to higher-order processing of pain signals accounting for affective and cognitive aspects of behavior. 72 Peripheral sensory neurons undergo alterations with aging, leading to a decreased response to evoked stimuli73,74 and subsequent loss of pain sensation. CNS pain pathways such as the spinothalamic, spino-parabrachio-amygdaloid, and other pathways relay signals from the spinal cord to the brain. 15 The brain regions implicated in pain perception, cognitive processing, and aversive experiences include the posterior thalamus, sensorimotor cortex, limbic regions (medial thalamus, amygdala, ventral striatum/nucleus accumbens, hippocampus, medial prefrontal cortex), paralimbic regions (insular cortex), and pain modulatory centers (periaqueductal gray, PAG, and rostral ventromedial medulla, RVM).32,39,75–77
Compared to physiological pain, neuroplastic changes in the peripheral and central nervous system such as “central sensitization” play a prominent role in chronic pain development and persistence.78–80 Failure of descending inhibition or a switch to descending facilitation are believed to be mechanisms that allow the persistence of pain.81–84 The descending pain modulatory system converges on the PAG-RVM system that connects brain regions to spinal nociceptive processing. 84 Neuroplasticity in the corticolimbic circuitry that interconnects brain regions such as the medial prefrontal cortex and sub-cortical limbic areas such as the amygdala, contributes to the complexity of pain, its affective and cognitive dimensions, pain modulation, and comorbidities, and may predict pain persistence and resilience.14,32,33,77,85–88 Peripheral and central/spinal sensitization contributes to increased nociceptive signals to the brain to engage sensory (thalamo-cortical) and affective (cortico-limbic) systems. 89 Pain conditions are associated with functional and structural changes in these brain regions31–34,71 and differences in connectivity between prefrontal cortical and limbic regions such as the hippocampus and amygdala play a critical role in the prediction and amplification of chronic pain. Cortico-limbic reorganization amplifies nociceptive signals to the brain and drives chronification through emotional learning. 14
A better understanding of the mechanisms of pain transitioning from acute to chronic is critically important for pain management. Brain scans of patients with chronic pain have shown structural changes in these brain regions, including the reduced amygdala, hippocampus, and medial prefrontal cortex volumes.14,33,85,86 Studies using positron emission tomography (PET)-magnetic resonance in combination with radioligand tracer have been used to show glial activation in clinical studies in low back pain 90 and fibromyalgia 91 patients. These findings point towards neural and non-neuronal mechanisms of chronic pain.71,85
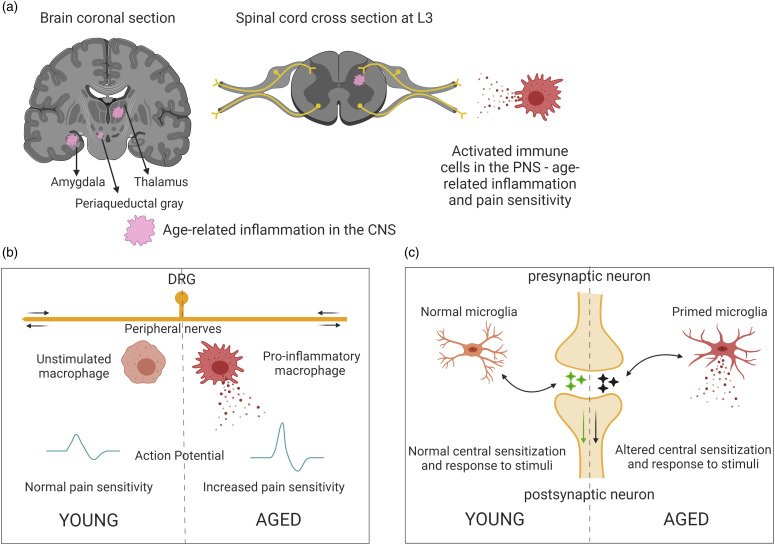
As we age, our immune system is primed, such that a low level of inflammation is prevalent throughout the body chronically in the absence of injury (inflammaging).92,93 Inflammatory mediators in the periphery, such as cytokines, chemokines, bradykinins, and other algogens can prolong the firing of nociceptors and reduce their threshold for activation.70,94 It has been well documented that systemic pro-inflammatory modulators are consistently upregulated in the elderly.92,93,95,96 Cytokines such as tumor necrosis factor alpha (TNFα), interleukins, and signaling molecules like inducible nitric oxide synthase (iNOS) are known activators of nociceptors for mediating pain responses.92,97 Aging also alters cell adhesion molecules via inflammation, which can affect wound healing. 98 Another major factor in age-related inflammation is the perturbance of anti-inflammatory and redox pathways. It has been shown that antioxidants are decreased with advanced age, leading to an upregulation of pro-inflammatory pathways.92,99 Currently, gerontologists and nutritionists recommend caloric restriction in the elderly, to help combat the activation of pro-inflammatory signaling and redox-responsive transcription factors like those of the peroxisome-proliferator-activated receptor (PPAR) family. 100 Downstream activation of relevant pathways such as prostaglandin E2 (PGE2), cyclic adenosine monophosphate (cAMP), and cyclooxygenase 2 (COX-2) can be prevented and pain hypersensitivity can be avoided through calorie restriction.70,101 Thus, it is likely that aging-associated chronic inflammation drives the development and persistence of chronic pain in geriatric individuals. Some unique aspects of inflammation and pain hypersensitivity in the PNS and CNS during aging are presented in Figure 1.
Figure 1.
Unique aspects of pain in aging. (a) Persistent low-grade inflammation (pink patches) in the aged is characterized by activated immune cells, namely microglia in the CNS and macrophages in the periphery. Secretion of pro-inflammatory factors affects nerves in the periphery as well as responses in the CNS. (b) Differences in PNS inflammatory state, neuronal activity, and pain response in aged peripheral tissues, compared to young. Chronic secretion of pro-inflammatory mediators damages nerves and alters pain sensitivity in the aged. (c) CNS immune sensitization or microglia become “primed”, leading to behavioral deficits in the aged.
Additionally, an increase in the frequency of injuries/disease/physiological degeneration is associated with increased persistent pain. In the elderly, chronic pain is mainly caused by musculoskeletal conditions; neuropathic pain, ischemic pain, and pain associated with cancer and its treatment are also major contributors. 64 Also, elderly women experience a high incidence of pain due to vertebral compression fractures. 64 The geriatric population is particularly vulnerable to these comorbidities and is consistently moving towards more independent/unassisted living. These contributing factors can lead to decreased care and increased painful outcomes. 102
The elderly have constant low-grade inflammation 103 and often aging-associated neurodegeneration, 104 which suggests that changes in brain structure play an important role in neuroplasticity associated with chronic pain. Age-associated comorbidities show decreased CNS plasticity and tissue degeneration.6,105,106 However, dynamic PNS plasticity with altered pain sensitization during aging and in response to inflammatory stimuli107–109 makes it especially challenging to develop a unifying hypothesis that captures these complex and seemingly opposing interactions (Figure 1). It is only logical that the research focus is now shifting toward investigations into aging-associated degeneration and inflammation and how these factors may facilitate chronic pain development.
Sex differences in pain
Epidemiological and clinical studies provide strong evidence for a greater prevalence of chronic pain syndromes such as fibromyalgia, osteoarthritis, and musculoskeletal pain, in females compared to males. 110 Also, it has been well-documented that males and females experience pain differently. 111 However, preclinical and clinical evidence for sex differences in pain sensitivity and tolerance is less clear.112–114 A meta-analysis of 122 articles published between 1998 and 2008 investigated sex differences in the perception of various experimental pain modalities in healthy subjects. 115 The study found that pressure pain thresholds were lower in females than in males, while cold and ischemic pain thresholds were similar in both sexes. Similarly, tolerance to thermal and pressure pain was lower in females than in males, but there was no sex difference in ischemic pain tolerance. Additionally, pain intensity did not show any sex differences in many of the pain modalities studied. Based on these findings, the authors proposed that sex differences in pain sensitivity show inconsistent patterns. However, a reanalysis of the same set of articles, which considered the direction of the sex difference reported by each article, showed that women were consistently more sensitive to pain than men, irrespective of pain modality or outcome measure. 116 Depression was not shown to mediate sex differences whereas the evidence for involvement of anxiety is further complicated in aged individuals as their hormonal levels are changed over time. Preclinical studies have shown that sex differences in pain processing and modulation exist at the molecular, cellular, and systems levels and involve genetic, hormonal, and neuroimmune factors. 113
The contribution of hormones to differences in pain sensitivity between sexes is unclear. Estrogen receptors are distributed throughout the peripheral and central nervous system in both males and females. Estrogen alpha and beta subtype receptors have been shown to be present in regions of the brain responsible for pain processing and modulation such as the amygdala, hypothalamus, periaqueductal gray, and regions of the dorsal spinal cord.117,118 In the peripheral nervous system, estrogen receptors punctate the cell surface of all nociceptors.
Preclinical studies in ovariectomized rodents suggest a role for estrogen in pain chronification that is yet to be well-defined. Some studies demonstrated increased mechanical and thermal hyperalgesia in ovariectomized rodents that were alleviated with estrogen administration.119,120 Other studies found estrogen-dependent hyperalgesic priming in female rodents but increased pain after knocking out or inhibiting estrogen receptors.121–123 This lack of clarity in the preclinical setting and a need to improve our understanding of hormonal influence in pain development offers exciting opportunities for further research. It should be noted that the role of other sex hormones, specifically androgens, remains to be clarified. Given this ambiguity, it is not clear if and how physiological or psychological factors could mediate sex differences in pain perception. However, cognitive and social factors appear to partially affect these differences, with individual history influencing pain responses in the female population. 124
Clinical studies found that sex differences in pain perception are subtle. A study conducted on both sexes of reproductive age determined that noxious laser-evoked potentials were lower in men than women, but there were no differences in subjective pain ratings. 125 Another study showed that behaviorally defined parameters had clear sex-based differences. 126 Measures of two such parameters, acceptance of pain and the fear of movement (kinesiophobia), showed that when men and women are subjected to the same magnitude of pain, women tend to be more active, accept the pain, and draw on social support while men have a lower activity level, kinesiophobia, and mood disturbances. 126
Thus, it becomes imperative to study sex differences with regard to different aspects of pain (sensorimotor, affective, cognitive, social) and at different ages in clinical pain conditions as well as in pre-clinical models to determine underlying mechanisms. Animal models of pain are well suited for mechanistic and preclinical studies, whereas experimental human pain models have limitations due to ethical concerns about invasive stimuli and because the prediction for clinical pain would be based on psychophysical readouts, 127 which would be particularly difficult in advanced age populations due to factors like cognitive impairment. Robust pain assessments need to be conducted to accurately classify chronic pain and detect sex- and age-related differences. This would include assessing the sensory and affective parameters of pain, the temporal aspect or location of pain, and the distribution of pain in a patient’s body. 128 Again, advanced-age individuals may have difficulties in accurately expressing such aspects, and therefore, more objective measures of pain mechanisms need to be included such as brain imaging, biomarkers, genetic factors, pharmacological phenotypes, and others to understand sex differences in pain and aging and to design effective strategies targeting the burden of pain. 128 Additional challenges include multiple comorbidities and ongoing medical treatments.
Preclinical pain research on aging
Animal models used for studying pain processing and testing therapeutic efficacy rely on non-report measures such as evoked responses (mechanical and thermal sensitivity tests or vocalizations) and non-evoked pain behaviors (grimace scales, conditioned place preference, and other operant assays) in models of inflammatory, neuropathic, or functional pain. A major challenge in the field is the need for pain-related parameters beyond nociceptive reflex measurements. 129
In pre-clinical studies, the findings related to age-differences in pain responses vary based on the pain test employed and are often equivocal (for a review of the literature before 2000 see). 130 More recent behavioral experiments demonstrate similar ambiguity in response to painful stimuli and are summarized in Table 2.74,131–145 For instance, studies using the hot plate test to examine age-related effects on nociception have reported an increase, decrease, as well as no difference in pain behaviors in older rodents compared to younger rodents.74,131,133,134,146–148 Similar inconsistencies in age-related changes in pain sensitivity have been reported in studies using inflammatory and neuropathic pain models.73,134–138,140–145,149,150 One of the possible reasons for the ambiguities in the results of preclinical studies on pain in aging (see Table 2) could be the use of animals of various ages from 10 months onwards classified as aged.73,109,130,141,151,152 Some investigations done on 10- to 11-month-old animals are misleadingly labeled as aging studies and confound the literature with conflicting findings. 151 These animals should be more accurately reported as middle-aged groups. Some groups have used 17- or 18-month-old rodents for aging studies.141,152,153 Other studies classified 22-month-old or older animals as aged for their investigations.154–156 While age is a relative parameter, the selection of an age range in animals that appropriately reflects aging in humans is paramount for determining aging-related changes in pain and related conditions.157–160 It is also important that appropriate models are selected that recapitulate specific clinical conditions and nociception upon manipulation by injury, application of chemical agents, or surgeries (see Table 2 for more details about preclinical studies). Therefore, the method of noxious stimulation and pain induction, and the end-point measurements from genes to systems levels are important considerations. 161
Table 2.
Preclinical studies assessing age-related differences in pain processing and behavior (2000 – present).
| Pain model | Rodent strain | Male (M) or female (F) | Age of rodent | Nociception in aging | Changes in aging rodents | Reference | |
|---|---|---|---|---|---|---|---|
| Young (Months/Specified) | Old (Months/Specified) | No Change, Increase, or Decrease | |||||
| Hot-plate | Mice C57BL/6J under genetically controlled condition | M | 2–11 | 17–23 | Increase | Reduced hot plate latency | 146 |
| Hot-plate | Mice C57BL/6J | M & F | 1.5 | 15 | Decrease | Longer thermal latency | 74 |
| 24 | |||||||
| Hot-plate | Mice C57BL/6J | M & F | 1.5–2 | 24 | Decrease | Decreased thermal sensitivity | 131 |
| Hot-plate | Mice C57BL/6J | M | 2–8 | 12 | No change | Similar thermal latency | 147 |
| Hot-plate | Mice 129Sv/Ev | M | 4–11 | 24 | Increase | Increased pain sensitivity (decreased pain threshold) | 148 |
| Hot-plate | Mice deletion of p66Shc in 129Sv/Ev | M | 4–11 | 24 | Decrease | Decreased pain sensitivity (increased pain threshold) | 148 |
| Hot-plate | Rat fischer 344/DuCrj | M | 7–13 | 29–34 | Decrease | Less paw licking behavior | 133 |
| Hot-plate | Rat fischer 344/DuCrj | M | 7–13 | 29–34 | Increase | Shorter thermal withdrawal latency | 133 |
| Hot-plate | Rat long -evans | F | 8 | 32 | No change | No change in paw-licking behavior to heat | 134 |
| Thermal escape (hot) | Rat long -evans | F | 8 | 32 | Increase | Increased thermal sensitivity (escape) | 134 |
| Thermal escape (cold) | Rat long -evans | F | 8 | 32 | Increase | Increased cold sensitivity (escape). Of note, is greater sensitivity to cold versus heat | 134 |
| Formalin test 20 µL of 5% formalin subcutaneous dorsal surface hindpaw | Mice C57BL/6 | M & F | 1.5–2 | 12 | Decrease | Reduced nociceptive response in aged mice | 144 |
| Formalin test (20 µL of 5% FormalinSubcutaneous left hindpaw) | Mice C57BL/6 | M | 2–6 | 13–20 | Increase | Temporal shift with an increase in the peak amplitudes | 145 |
| Formalin test (10 µL (young) and 50 µL (old) of 1.25%, 1.75%, and 2.25% formalin right hindpaw) | Rat wistar | M & F | 7–13–22 days | 82 days | Increase | At 82 days, both males and females showed biphasic patterns in response to 3 formalin concentrations | 149 |
| Formalin test (50 µL of 5% formalin subcutaneous dorsal hindpaw) | Rat long-evans | F | 8 | 16 | Increase | Increased sensitivity to thermal stimulation | 134 |
| 24 | |||||||
| Complete freund adjuvant (CFA) (30 µL of CFA subcutaneous left hindpaw) | Mice C57BL/6 | M | 2 | >18 | Increase | Young and old mice exhibit high pain behavior during acute (2 days) and chronic (8 weeks) inflammation. However, young mice exhibit higher sensitization to mechanical stimuli | 73 |
| Complete freund adjuvant (CFA) (30 µL of CFA subcutaneous left hindpaw) | Rat sprague-dawley | M | 3 | 18 | Increase | Increased thermal sensitivity at 48 h following CFA | 141 |
| Complete freund adjuvant (CFA) (50 µL of CFA subcutaneous left hindpaw) | Rat Fisher 344/DuCrj | M | 7–12 | 29–34 | No change | Aged rats showed enhanced peripheral inflammatory responses to CFA with only a slight change in dorsal horn neuronal activity | 140 |
| Chronic constriction model | Rat fischer 344/FBNF1 | M | 4–6 | 24–26 | Decrease | Decreased allodynic response on von-frey | 137 |
| Chronic constriction injury | Rat fischer F344 BNF1 hybrid | M | 4–6 | 14–16 | Decrease | Temporal shift with slower development of thermal hyperalgesia and tactile allodynia after injury | 142 |
| 24–26 | |||||||
| Chronic constriction injury | Rat fischer 344/FBNF1 hybrid | M | 4–6 | 14–16 | Decrease | Temporal shift with slower development of thermal hyperalgesia and tactile allodynia after injury | 143 |
| 24–26 | |||||||
| Chronic constriction model | Rat lou/cjall | M | 4–6 | 20–22 | Increase | Old and senescent rats are more sensitive to acute pain | 138 |
| 37–39 | |||||||
| IAN transection | Rats sprague -dawley rats | M | 9 | >29 | Increase | Increased background activity and activity following response to mechanical stimulus in the dorsal horn of aged rats | 136 |
| Plantar incision | Rat fischer 344/FBNF1 hybrid | M & F | 3–6 | >22 | Decrease | Aged males showed reduced mechanical hypersensitivity | 150 |
Aged rodents demonstrate altered neuronal activity in the spinal cord. 109 As compared to young adult dorsal horn neurons, aged dorsal horn neurons showed increased excitability but reduced excitatory synaptic input and increased GABAergic inhibitory synaptic input. 109 Studies on dorsal horn neural function in pain models have also shown increased background and evoked activity in aged (>29 months old) compared to young rodents.135,136 To that effect, temporal aspects of treatments or duration of testing on animal pain models become important for protocol relevance.
Numerous studies have shown increased neuroinflammation in aged rodents.106,155,162–164 It is known that chronic activation of immune cells, both in the periphery and the CNS, can lead to increased sensitization.105,106,164–166 However, opposing effects of inflammation and immunosenescence are reported for pain. Some preclinical studies have shown that inflammation and neuroinflammation are higher in the aged than young and decreased pain sensitivity was observed.130,154,156,167 Other preclinical studies have reported increased pain with inflammatory conditions.73,103 Peripheral infusion of lipopolysaccharide (LPS) into young and aged mice caused higher levels of neuroinflammation in the aged females compared to young females, as well as young and aged males, indicating sex and age differences. 168 In contrast, mechanical hypersensitivity responses to complete Freund’s adjuvant (CFA) are reduced in aged male and female mice, while pro-inflammatory cytokine levels in the spinal cord are higher, despite no changes in the dorsal root ganglia. 154 These data suggest that sex-specific differences may also be influenced by age in rodent models, which adds a layer of complexity and yet opens up opportunities for new research avenues. It has been shown that spinal microglia and astrocytes in aged rodents are in a relatively higher primed or reactive state and secrete cytokines that affect the PNS; however, their pain behavior does not necessarily reflect that change.152,156,168–170 This highlights an important gap in characterizing the responses of aging rodents to pain tests. Reduced pain sensitivity despite increased aged-induced inflammation with advanced age, may suggest that the response to pain-relieving therapies based on anti-inflammatory actions may be quite subtle in these animals. Thus, there is a need to re-evaluate methods used to assess pain behaviors and outcomes in aged rodent pain models.
Based on current literature, aged rodents display, in most studies, decreased pain behavior, but more activation of neurons and immune cells and higher levels of pro-inflammatory mediators.146,170–172 This suggests that there is a disconnect in age-induced inflammation, nociceptor signaling, and ascending facilitation to the brain, which may result in decreased pain prevalence in the aged. Interestingly, there are no studies assessing age differences in pain behaviors, neuronal function, and inflammation in the brain, and therefore studies addressing this knowledge gap are needed.
Clinical research on pain and aging models
In clinical studies, measuring experimentally-induced pain thresholds is the most commonly used approach to study age-related differences in pain perception or pain modulation (see Table 3). Healthy, informed adult volunteers comprise most of these studies due to ethical limitations. The two experimental methodologies prevalent in the literature are the response to direct painful stimulation and conditioned pain modulation (CPM). Noxious stimuli typically include thermal (hot or cold) or electrical stimulation. CPM measures inhibition of the effects of one painful test stimulus by another painful conditioned stimulus at an alternative site to study central mechanisms and integrity of pain inhibition [for example].173,174 Table 3 summarizes recent clinical literature studying age-related differences in pain perception and demonstrates considerable heterogeneity. The findings related to age-associated effects on pain perception vary depending on the type, intensity, duration, and site of the stimulus applied.175–187 The results from the CPM assessment suggest that older adults have decreased facilities in central inhibitory responses to pain, however, a study by Wrobel et al. showed that inhibitory controls were similar between old and young adults following placebo analgesia.175–179,182 Acute and isometric exercise has been shown to temporarily reduce pain sensitivity, and the magnitude of pain reduction has been shown to be associated with endogenous pain inhibition capacity.188,189 The magnitude of exercise-induced hypoalgesia was lower in older adults compared to younger adults. 190
Table 3.
Clinical studies that highlight age-related differences in pain perception and assessment (2000–Present).
| Methodology | Source | Human model type | Age category (mean) | Difference |
|---|---|---|---|---|
| Non-noxious (Mechanical)/Noxious (thermal/Electrical) stimulus | 186 | Healthy adults | Young (22) old (62) | Older adults exhibit higher ratings of the intensity and unpleasantness of thermal pain and enhanced temporal summation of thermal pain relative to younger adults |
| 187 | Healthy adults | Young (22) old (62.2) | Older adults have lower ischemic pain thresholds and tolerances assessed via the modified submaximal effort tourniquet procedure | |
| 183 | Healthy adults | Young (30) old (78.9) | The study finds an age-dependent temporal relationship with pain stimuli. There is a higher thermal and electrical stimulation threshold when the stimulus duration is short, but no differences when the stimulus duration is long | |
| 184 | Healthy adults | Young (27) old (71) | There are stimulus-specific age differences. Non-noxious stimuli thresholds increase with age whereas pressure pain thresholds decrease. Heat pain thresholds show no age-related changes. Older adults demonstrate greater temporal summation, but pain summation was not affected | |
| 181 | Healthy adults | Middle (45–56) older (57–79) | There are stimulus-specific age differences. Older adults are less sensitive to warm and painful heat stimuli than middle-aged adults. In addition, there is a greater decrease in sensitivity associated with aging in the lower extremities | |
| 103 | Healthy adults | Young (21.4) older (68.1) | Observed greater elevations in pro-inflammatory cytokines (TNF-α and IL-8) following cold pressor task and focal heat pain in older subjects. Only greater elevations of IL-6 after cold pressor task in older subjects Anti-inflammatory cytokines (IL-4, IL-5, and IL-10) peaked later in older subjects with increased elevations for focal heat pain only | |
| 182 | Healthy adults | Young (27) old (69) | Heat pain threshold increases with age, however, adult adults report more pain intensity. Inhibitory controls were identical between old and young adults following placebo analgesia | |
| 185 | Healthy adults | Young (34) old (67) | Older adults experienced greater temporal summation of spatial perception of cold stimuli compared with younger adults. Temporal summation of pain intensity for heat or cold stimuli showed no age differences | |
| Conditioned pain modulation | 178 | Healthy adults | Young (23) old (78) | Older adults needed a higher intensity of noxious stimulation to first report pain |
| 175 | Healthy adults | Young (21) old (63) | Older adults did not exhibit inhibitory controls whereas younger adults were able to on repetitive stimulus | |
| 176 | Healthy adults | Young (25) elderly (47) old (68) | Endogenous pain modulation was negatively correlated with advancing age | |
| 177 | Healthy adults | Young (25) old (65.2) | Older adults exhibit decreased inhibitory controls compared to young adults | |
| 179 | Healthy adults | Young (29) old (63) | Older adults have an age-related reduction in inhibitory processes | |
| 180 | Healthy adults | Young (24) old (64) | Older individuals experienced greater fluctuations in pain sensitivity following the varying intensity of the conditioned stimulus | |
| Exercise | 190 | Healthy adults | Young (22) old (64) | There are age-related differences in exercise-induced hypoalgesia. Younger adults experience greater hypoalgesia following exercise compared with older adults |
When devising a clinical study to address aging-related changes in general, there are important considerations. As study participants are enrolled voluntarily, there is an inherent participation bias towards healthier older patients who often have fewer comorbidities, less physical health decline, improved cognitive abilities, and better mental health than the average older adult. This is important in clinical practice and may affect the results of studies on pain perception and pain intensity in the elderly. Older patients have been found to report less pain from similar pathology regarding pain frequency and severity, further exacerbated by female gender and specific ethnicity differences. 181 As such, caution should be taken in generalizing the results of such studies.
Implications and future directions for clinical practice and pre-clinical and clinical research
To address chronic pain management among older adults in the clinical setting, the U.S. Pain Management Best Practices Inter-Agency Task Force has called for the development of pain management guidelines for older adults that address their unique risk factors; consideration of multidisciplinary approaches combining pharmacological and non-pharmacological approaches; and the establishment of appropriate pain management education for health care providers who treat older adults. 57
At present, there is a paucity of studies on pain during aging in the diagnostics and therapeutics field, with most published studies reporting outcomes of pain-relieving medications or therapy after various surgeries or disease modalities.40,67,191,192 While these are important areas of investigation required to address the need for novel treatments and their administration, the mechanistic underpinnings of pain in older adults remain unclear. Furthermore, the preponderance of published studies focused primarily on men, limiting our understanding of chronic pain syndromes in women.126,146,156 While using human experimental models for pain research has ethical considerations for inflicting or evoking pain to study pain responses, animal models offer a wider array of tools to examine different aspects of pain and underlying mechanisms in different pain conditions but rely on surrogate measures of pain.127,129,161 A unifying concept about altered pain sensitivity and neuroinflammation in aging has yet to emerge from preclinical studies. Moreover, studies assessing pain thresholds over the lifespan have been inconsistent.108,193 The understanding of the two inevitable conditions of the human, age and pain, has been complicated in a cloud of conflicting data from preclinical and translational studies, due to inconsistencies in pain modality testing (site, testing paradigm, and tools used), age of subjects, and lack of utilization of both sexes.175,193 A more robust characterization of aging pain models is necessary to document behavioral responses to pain, correlate them with neurobiological changes, and determine if these changes are age and/or sex-dependent. With the knowledge gap regarding age-related changes in pain processing in the brain, there are numerous advanced-age animal models for various neurodegenerative diseases 194 that could be repurposed to study various aspects of pain in aged animals and conditions of cognitive decline.
Acknowledgements
The authors would like to thank all present and previous lab. members of the Burton, Neugebauer, and Guindon labs.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by NIH grants R35GM147094 and R21DK130015 (M.D.B), Rita Foundation Award in Pain (M.D.B), and the University of Texas Rising STARS program research support grant (M.D.B). NIH grants R01 NS038261, R01 NS106902, R01NS118731, R01 NS120395, R01 NS129552 (V.N.) NIH grant R01 DA044999, Rita Allen Foundation, Division of Diabetes, Endocrinology, and Metabolic Diseases (DK130015), National Institute of General Medical Sciences (GM147094-01).
ORCID iDs
Josee Guindon https://orcid.org/0000-0002-2186-1745
Michael D Burton https://orcid.org/0000-0002-0628-824X
References
- 1.P IAftSo. Pain in older persons, 2020. https://www.iasp-pain.org/GlobalYear/PaininOlderPersons (accessed 11/19/2020 2020).
- 2.Bureau USC. In: census U. (ed). Projected age groups and sex composition of the population. Washington DC: US Census Bureau, Population Division, 2017. [Google Scholar]
- 3.Crews RT, Yalla SV, Fleischer AE, Wu SC. A growing troubling triad: diabetes, aging, and falls. Journal of aging research 2013; 2013: 342650. DOI: 10.1155/2013/342650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hughes S, Leary A, Zweizig S, Cain J. Surgery in elderly people: preoperative, operative and postoperative care to assist healing. Best Pract Res Clin Obstet Gynaecol 2013; 27: 753–765. DOI: 10.1016/j.bpobgyn.2013.02.006 [DOI] [PubMed] [Google Scholar]
- 5.Yang Y, Hu X, Zhang Q, Zou R. Diabetes mellitus and risk of falls in older adults: a systematic review and meta-analysis. Age Ageing 2016; 45: 761–767. Meta-Analysis; Review; Research Support Non-U.S. Gov. [DOI] [PubMed] [Google Scholar]
- 6.Simen AA, Bordner KA, Martin MP, Moy LA, Barry LC. Cognitive dysfunction with aging and the role of inflammation. Ther Adv Chronic Dis 2011; 2: 175–195. DOI: 10.1177/2040622311399145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bordier L, Doucet J, Boudet J, Bauduceau B. Update on cognitive decline and dementia in elderly patients with diabetes. Diabetes Metab 2014; 40: 331–337. DOI: 10.1016/j.diabet.2014.02.002 [DOI] [PubMed] [Google Scholar]
- 8.Sartori AC, Vance DE, Slater LZ, Crowe M. The impact of inflammation on cognitive function in older adults: implications for healthcare practice and research. J Neurosci Nurs 2012; 44: 206–217. DOI: 10.1097/JNN.0b013e3182527690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raja SN, Carr DB, Cohen M, Finnerup NB, Flor H, Gibson S, Keefe FJ, Mogil JS, Ringkamp M, Sluka KA, Song XJ, Stevens B, Sullivan MD, Tutelman PR, Ushida T, Vader K. The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain. 2020; 161: 1976–1982. doi: 10.1097/j.pain.0000000000001939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reid MC, Eccleston C, Pillemer K. Management of chronic pain in older adults. BMJ (Clinical research ed) 2015: 350: h532. DOI: 10.1136/bmj.h532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Treede R-D, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, Cohen M, Evers S, Finnerup NB, First MB, Giamberardino MA, Kaasa S, Kosek E, Lavandʼhomme P, Nicholas M, Perrot S, Scholz J, Schug S, Smith BH, Svensson P, Vlaeyen JWS, Wang S-J. A classification of chronic pain for ICD-11. Pain 2015; 156: 1003–1007, Research Support Non-U.S. Gov. DOI: 10.1097/j.pain.0000000000000160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dahan A, van Velzen M, Niesters M. Comorbidities and the complexities of chronic pain. Anesthes 2014; 121: 675–677. DOI: 10.1097/ALN.0000000000000402 [DOI] [PubMed] [Google Scholar]
- 13.Li R, Dworkin RH, Chapman BP, Becerra AZ, Yang L, Mooney CJ, Seplaki CL. Moderate to severe chronic pain in later life: risk and resilience factors for recovery. J Pain 2021; 22: 1657–1671. DOI: 10.1016/j.jpain.2021.05.007 [DOI] [PubMed] [Google Scholar]
- 14.Vachon-Presseau E, Centeno MV, Ren W, Berger SE, Tetreault P, Ghantous M, Baria A, Farmer M, Baliki MN, Schnitzer TJ, Apkarian AV. The emotional brain as a predictor and amplifier of chronic pain. J Dent Res 2016; 95: 605–612. Review; Research Support, N.I.H., Extramural; Research Support, Non-U.S. Gov't. DOI: 10.1177/0022034516638027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bushnell MC, Ceko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci 2013; 14: 502–511. DOI: 10.1038/nrn3516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, Cohen M, Evers S, Finnerup NB, First MB, Giamberardino MA, Kaasa S, Korwisi B, Kosek E, Lavand'homme P, Nicholas M, Perrot S, Scholz J, Schug S, Smith BH, Svensson P, Vlaeyen JWS, Wang SJ. Chronic pain as a symptom or a disease: the IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 2019; 160: 19–27. DOI: 10.1097/j.pain.0000000000001384 [DOI] [PubMed] [Google Scholar]
- 17.Stubbs B, Schofield P, Binnekade T, Patchay S, Sepehry A, Eggermont L. Pain is associated with recurrent falls in community-dwelling older adults: evidence from a systematic review and meta-analysis. Pain Med 2014; 15: 1115–1128. DOI: 10.1111/pme.12462 [DOI] [PubMed] [Google Scholar]
- 18.Stubbs B, West E, Patchay S, Schofield P. Is there a relationship between pain and psychological concerns related to falling in community dwelling older adults? A systematic review. Disabil Rehabil 2014; 36: 1931–1942. DOI: 10.3109/09638288.2014.882419 [DOI] [PubMed] [Google Scholar]
- 19.Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain 2012; 13: 715–724. DOI: 10.1016/j.jpain.2012.03.009 [DOI] [PubMed] [Google Scholar]
- 20.Dahlhamer J, Lucas J, Zelaya C, Nahin R, Mackey S, DeBar L, Kerns R, Von Korff M, Porter L, Helmick C. Prevalence of chronic pain and high-impact chronic pain among adults - United States, 2016. MMWR Morbid Mortal Week Rep 2018; 67: 1001–1006. DOI: 10.15585/mmwr.mm6736a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nahin RL, Feinberg T, Kapos FP, Terman GW. Estimated rates of incident and persistent chronic pain among US adults, 2019-2020. JAMA Netw Open 2023; 6: e2313563. DOI: 10.1001/jamanetworkopen.2023.13563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DiBonaventura MD, Sadosky A, Concialdi K, Hopps M, Kudel I, Parsons B, Cappelleri JC, Hlavacek P, Alexander AH, Stacey BR, Markman JD, Farrar JT. The prevalence of probable neuropathic pain in the US: results from a multimodal general-population health survey. J Pain Res 2017; 10: 2525–2538. DOI: 10.2147/jpr.s127014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yawn BP, Wollan PC, Weingarten TN, Watson JC, Hooten WM, Melton LJ, 3rd. The prevalence of neuropathic pain: clinical evaluation compared with screening tools in a community population. Pain Med 2009; 10: 586–593. DOI: 10.1111/j.1526-4637.2009.00588.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel KV, Guralnik JM, Dansie EJ, Turk DC. Prevalence and impact of pain among older adults in the United States: findings from the 2011 national health and aging trends study. Pain 2013; 154: 2649–2657. DOI: 10.1016/j.pain.2013.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gary JM, Maxwell SB, Gareth TJ. Persons with chronic widespread pain experience excess mortality: longitudinal results from UK Biobank and meta-analysis. Ann Rheum Dis 2017; 76: 1815. DOI: 10.1136/annrheumdis-2017-211476 [DOI] [PubMed] [Google Scholar]
- 26.Domenichiello AF, Ramsden CE. The silent epidemic of chronic pain in older adults. Prog Neuro Psychopharmacol Biol Psychiatr 2019; 93: 284–290, DOI: 10.1016/j.pnpbp.2019.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McLachlan AJ, Bath S, Naganathan V, Hilmer SN, Le Couteur DG, Gibson SJ, Blyth FM. Clinical pharmacology of analgesic medicines in older people: impact of frailty and cognitive impairment. Br J Clin Pharmacol 2011; 71: 351–364, DOI: 10.1111/j.1365-2125.2010.03847.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gagliese L, Gauthier LR, Narain N, Freedman T. Pain, aging and dementia: towards a biopsychosocial model. Prog Neuro Psychopharmacol Biol Psychiatr 2018; 87: 207–215, DOI: 10.1016/j.pnpbp.2017.09.022 [DOI] [PubMed] [Google Scholar]
- 29.Whitlock EL, Diaz-Ramirez LG, Glymour MM, Boscardin WJ, Covinsky KE, Smith AK. Association between persistent pain and memory decline and dementia in a longitudinal cohort of elders. JAMA Intern Med 2017; 177: 1146–1153. DOI: 10.1001/jamainternmed.2017.1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iseki E, Matsushita M, Kosaka K, Kondo H, Ishii T, Amano N. Distribution and morphology of brain stem plaques in Alzheimer's disease. Acta Neuropathol 1989; 78: 131–136. DOI: 10.1007/BF00688200 [DOI] [PubMed] [Google Scholar]
- 31.Neugebauer V, Presto P, Yakhnitsa V, Antenucci N, Mendoza B, Ji G. Pain-related cortico-limbic plasticity and opioid signaling. Neuropharmacology 2023; 231: 109510. DOI: 10.1016/j.neuropharm.2023.109510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neugebauer V. Amygdala physiology in pain. In: Urban JH, Rosenkranz JA. (eds) Handbook of behavioral neuroscience. DOI: 10.1016/b978-0-12-815134-1.00004-0: Elsevier, 2020, pp. 101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vachon-Presseau E, Tétreault P, Petre B, Huang L, Berger SE, Torbey S, Baria AT, Mansour AR, Hashmi JA, Griffith JW, Comasco E, Schnitzer TJ, Baliki MN, Apkarian AV. Corticolimbic anatomical characteristics predetermine risk for chronic pain. Brain 2016; 139: 1958–1970. DOI: 10.1093/brain/aww100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pfannmöller J, Lotze M. Review on biomarkers in the resting-state networks of chronic pain patients. Brain Cogn 2019; 131: 4–9. DOI: 10.1016/j.bandc.2018.06.005 [DOI] [PubMed] [Google Scholar]
- 35.Long JM, Holtzman DM. Alzheimer disease: an update on pathobiology and treatment strategies. Cell 2019; 179: 312–339. DOI: 10.1016/j.cell.2019.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moriarty O, Finn DP. Cognition and pain. Curr Opin Support Palliat Care 2014; 8: 130–136. DOI: 10.1097/spc.0000000000000054 [DOI] [PubMed] [Google Scholar]
- 37.Ji G, Sun H, Fu Y, Li Z, Pais-Vieira M, Galhardo V, Neugebauer V. Cognitive impairment in pain through amygdala-driven prefrontal cortical deactivation. J Neurosci 2010; 30: 5451–5464. DOI: 10.1523/jneurosci.0225-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moriarty O, Ruane N, O’Gorman D, Maharaj CH, Mitchell C, Sarma KM, Finn DP, McGuire BE. Cognitive impairment in patients with chronic neuropathic or radicular pain: an interaction of pain and age. Front Behav Neurosci 2017; 11: 100. DOI: 10.3389/fnbeh.2017.00100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neugebauer V, Galhardo V, Maione S, Mackey SC. Forebrain pain mechanisms. Brain Res Rev 2009; 60: 226–242. DOI: 10.1016/j.brainresrev.2008.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hunt LJ, Covinsky KE, Yaffe K, Stephens CE, Miao Y, Boscardin WJ, Smith AK. Pain in community-dwelling older adults with dementia: results from the national health and aging trends study. J Am Geriatr Soc 2015; 63: 1503–1511. DOI: 10.1111/jgs.13536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roberts AWOSU, Blakeslee L, Rabe MA. The population 65 years and older in the United States: 2016. 2018. https://www.census.gov/content/dam/Census/library/publications/2018/acs/ACS-38.pdf: U.S. Census Bureau, 2018. [Google Scholar]
- 42.Services USDoHH . National Pain Strategy outlines actions for improving pain care. https://www.census.gov/content/dam/Census/library/publications/2018/acs/ACS-38.pdf: HHS.gov, 2016. https://www.hhs.gov/ash/about-ash/news/2016/national-pain-strategy-outlines-actions-improving-pain-care/index.html. [Google Scholar]
- 43.Curtiss CP. Challenges in pain assessment in cognitively intact and cognitively impaired older adults with cancer. Oncol Nurs Forum 2010; 37(Suppl). DOI: 10.1188/10.ONF.S1.7-16 [DOI] [PubMed] [Google Scholar]
- 44.Hadjistavropoulos T, Martin RR, Sharpe D, Lints AC, McCreary DR, Asmundson GJ. A longitudinal investigation of fear of falling, fear of pain, and activity avoidance in community-dwelling older adults. J Aging Health 2007; 19: 965–984. DOI: 10.1177/0898264307308611 [DOI] [PubMed] [Google Scholar]
- 45.Malec M, Shega JW. Pain Management in the Elderly. Medi Clin Nor Ame 2015; 99: 337–350. DOI: 10.1016/j.mcna.2014.11.007 [DOI] [PubMed] [Google Scholar]
- 46.Booker SQ, Herr KA. Assessment and measurement of pain in adults in later life. Clin Geriatr Med 2016; 32: 677–692. DOI: 10.1016/j.cger.2016.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Horgas AL, Laframboise-Otto J, Aul K, Yoon SL. Pain management in the older adult. Evidence- Based Geriatric Nursing Protocols for Best Practice. New York, NY: Springer Publishing Company, 2020. doi: 10.1891/9780826188267.0022 [DOI] [Google Scholar]
- 48.Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: visual analog scale for pain (VAS Pain), numeric rating scale for pain (NRS Pain), mcgill pain questionnaire (MPQ), short-form mcgill pain questionnaire (SF-MPQ), chronic pain grade scale (CPGS), short form-36 bodily pain scale (SF-36 BPS), and measure of intermittent and constant osteoarthritis pain (ICOAP). Arthritis Care Res 2011; 63: S240–S252. DOI: 10.1002/acr.20543 [DOI] [PubMed] [Google Scholar]
- 49.Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, Amtmann D, Bode R, Buysse D, Choi S, Cook K, DeVellis R, DeWalt D, Fries JF, Gershon R, Hahn EA, Lai J-S, Pilkonis P, Revicki D, Rose M, Weinfurt K, Hays R. The patient-reported outcomes measurement information system (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol 2010; 63: 1179–1194. DOI: 10.1016/j.jclinepi.2010.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fairbank JCT, Pynsent PB. The Oswestry Disability Index. Spine 2000; 25: 2940–2952. [DOI] [PubMed] [Google Scholar]
- 51.Herr KA, Garand L. Assessment and measurement of pain in older adults. Clin Geriatr Med 2001; 17: 457–478, DOI: 10.1016/S0749-0690(05)70080-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Herr K, Spratt KF, Garand L, Li L. Evaluation of the iowa pain thermometer and other selected pain intensity scales in younger and older adult cohorts using controlled clinical pain: a preliminary study. Pain Med 2007; 8: 585–600. DOI: 10.1111/j.1526-4637.2007.00316.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hicks CL, von Baeyer CL, Spafford PA, van Korlaar I, Goodenough B. The faces pain scale-revised: toward a common metric in pediatric pain measurement. Pain 2001; 93: 173–183. DOI: 10.1016/s0304-3959(01)00314-1 [DOI] [PubMed] [Google Scholar]
- 54.Tsai IP, Jeong SY, Hunter S. Pain assessment and management for older patients with dementia in hospitals: an integrative literature review. Pain Manag Nurs 2018; 19: 54–71. DOI: 10.1016/j.pmn.2017.10.001 [DOI] [PubMed] [Google Scholar]
- 55.Achterberg W, Lautenbacher S, Husebo B, Erdal A, Herr K. Pain in Dementia. Pain Rep 2019; 5: e803. DOI: 10.1097/PR9.0000000000000803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hanlon JT, Backonja M, Weiner D, Argoff C. Evolving pharmacological management of persistent pain in older persons. Pain Med 2009; 10: 959–961. Editorial. DOI: 10.1111/j.1526-4637.2009.00698.x [DOI] [PubMed] [Google Scholar]
- 57.Services USDoHH. Pain Management Best Practices Inter-Agency Task Force Report: Updates, Gaps, Inconsistencies, and Recommendations, 2019, https://www.hhs.gov/ash/advisory- committees/pain/reports/index.html (accessed 12/14/2020 2020).
- 58.Ersek M, Polomano RA. Core principles of pain treatment. 2011, (accessed 12/14/2020 2020). [Google Scholar]
- 59.GeriatricPain.org . Clinical practice guidelines, 2009-2012, (accessed 12/14/2020 2020). [Google Scholar]
- 60.Niknejad B, Bolier R, Henderson CR, Delgado D, Kozlov E, Löckenhoff CE, Reid MC. Association between psychological interventions and chronic pain outcomes in older adults: a systematic review and meta-analysis. JAMA Intern Med 2018: 178: 830–839. DOI: 10.1001/jamainternmed.2018.0756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cravello L, Di Santo S, Varrassi G, Benincasa D, Marchettini P, de Tommaso M, Shofany J, Assogna F, Perotta D, Palmer K, Paladini A, di Iulio F, Caltagirone C. Chronic pain in the elderly with cognitive decline: a narrative review. Pain Ther 2019; 8: 53–65. DOI: 10.1007/s40122-019-0111-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Musich S, Wang SS, Slindee L, Kraemer S, Yeh CS. The association of pain locus of control with pain outcomes among older adults. Geriatric nursing 2019; 41: 521–529. DOI: 10.1016/j.gerinurse.2019.04.005 [DOI] [PubMed] [Google Scholar]
- 63.Pharmacological management of persistent pain in older persons. J Am Geriatr Soc 2009; 57: 1331–1346. DOI: 10.1111/j.1532-5415.2009.02376.x [DOI] [PubMed] [Google Scholar]
- 64.Ali A, Arif AW, Bhan C, Kumar D, Malik MB, Sayyed Z, Akhtar KH, Ahmad MQ. Managing chronic pain in the elderly: an overview of the recent therapeutic advancements. Cureus 2018; 10: e3293. DOI: 10.7759/cureus.3293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schneider J, Algharably E, Budnick A, Wenzel A, Dräger D, Kreutz R. Deficits in pain medication in older adults with chronic pain receiving home care: a cross-sectional study in Germany. PLoS One 2020; 15: e0229229. DOI: 10.1371/journal.pone.0229229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Potru S, Tang Y-l. Chronic pain, opioid use disorder, and clinical management among older adults. FOCUS 2021; 19: 294–302. DOI: 10.1176/appi.focus.20210002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging and disease 2018; 9: 143–150. DOI: 10.14336/AD.2017.0306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain - United States, 2016. MMWR Recommendations and reports 2016; 65: 1–49. Practice Guideline. DOI: 10.15585/mmwr.rr6501e1 [DOI] [PubMed] [Google Scholar]
- 69.Chau DL, Walker V, Pai L, Cho LM. Opiates and elderly: use and side effects. Clin Interv Aging 2008; 3: 273–278. DOI: 10.2147/cia.s1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fornasari D. Pain mechanisms in patients with chronic pain. Clin Drug Invest 2012; 32: 45–52. DOI: 10.2165/11630070-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 71.Apkarian AV, Hashmi JA, Baliki MN. Pain and the Brain: specificity and plasticity of the brain in clinical chronic pain. Pain 2011; 152. DOI: 10.1016/j.pain.2010.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Coghill RC. The Distributed Nociceptive System: A Framework for Understanding Pain. Trend neuroscie 2020; 43: 780–794. DOI: 10.1016/j.tins.2020.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weyer AD, Zappia KJ, Garrison SR, O’'Hara CL, Dodge AK, Stucky CL. Nociceptor sensitization depends on age and pain chronicity. Eneuro 2016; 3: 26. Article. DOI: 10.1523/eneuro.0115-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang S, Albers KM. Behavioral and cellular level changes in the aging somatosensory system. Ann N Y Acad Sci 2009; 1170: 745–749. DOI: 10.1111/j.1749-6632.2009.04011.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Apkarian AV, Bushnell MC, Treede R-D, Zubieta J-K. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 2005; 9: 463–484, DOI: 10.1016/j.ejpain.2004.11.001 [DOI] [PubMed] [Google Scholar]
- 76.Zhuo M. Cortical plasticity as a new endpoint measurement for chronic pain. Mol Pain 2011; 7: 1744–8069. DOI: 10.1186/1744-8069-7-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thompson JM, Neugebauer V, NIH, Extramural, Review. Cortico-limbic pain mechanisms. Neurosci Lett 2019; 702: 15–23. DOI: 10.1016/j.neulet.2018.11.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lopes DM, Malek N, Edye M, Jager SB, McMurray S, McMahon SB, Denk F. Sex differences in peripheral not central immune responses to pain-inducing injury. Sci Rep 2017; 7: 16460. DOI: 10.1038/s41598-017-16664-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Phillips K, Clauw DJ. Central pain mechanisms in chronic pain states--maybe it is all in their head. Best Pract Res Clin Rheumatol 2011; 25: 141–154. DOI: 10.1016/j.berh.2011.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Desmeules JA, Cedraschi C, Rapiti E, Baumgartner E, Finckh A, Cohen P, Dayer P, Vischer TL. Neurophysiologic evidence for a central sensitization in patients with fibromyalgia. Arthritis Rheum 2003; 48: 1420–1429. Article. DOI: 10.1002/art.10893 [DOI] [PubMed] [Google Scholar]
- 81.Chen Q, Heinricher MM. Descending control mechanisms and chronic pain. Curr Rheumatol Rep 2019; 21: 13. DOI: 10.1007/s11926-019-0813-1 [DOI] [PubMed] [Google Scholar]
- 82.Fields HL. Neurophysiology of Pain and Pain Modulation. Am J Med 1984; 77: 2–8. DOI: 10.1016/S0002-9343(84)80097-2 [DOI] [PubMed] [Google Scholar]
- 83.Bannister K, Dickenson AH. The plasticity of descending controls in pain: translational probing. J Physiol 2017; 595: 4159–4166, DOI: 10.1113/JP274165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ossipov MH, Morimura K, Porreca F. Descending pain modulation and chronification of pain. Curr Opin Support Palliat Care 2014; 8: 143–151. DOI: 10.1097/spc.0000000000000055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Baliki MN, Apkarian AV. Nociception, pain, negative moods, and behavior selection. Neuron 2015; 87: 474–491. DOI: 10.1016/j.neuron.2015.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vachon-Presseau E, Berger SE, Abdullah TB, Griffith JW, Schnitzer TJ, Apkarian AV. Identification of traits and functional connectivity-based neurotraits of chronic pain. PLoS Biol 2019; 17: e3000349. DOI: 10.1371/journal.pbio.3000349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ji G, Yakhnitsa V, Kiritoshi T, Presto P, Neugebauer V. Fear extinction learning ability predicts neuropathic pain behaviors and amygdala activity in male rats. Mol Pain 2018; 14: 1744806918804441. DOI: 10.1177/1744806918804441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Presto P, Ji G, Junell R, Griffin Z, Neugebauer V. Fear extinction-based inter-individual and sex differences in pain-related vocalizations and anxiety-like behaviors but not nocifensive reflexes. Brain Sci 2021; 11(10): 1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Colloca L, Ludman T, Bouhassira D, Baron R, Dickenson AH, Yarnitsky D, Freeman R, Truini A, Attal N, Finnerup NB, Eccleston C, Kalso E, Bennett DL, Dworkin RH, Raja SN. Neuropathic pain. Nat Rev Dis Primers 2017; 3: 17002. DOI: 10.1038/nrdp.2017.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Loggia ML, Chonde DB, Akeju O, Arabasz G, Catana C, Edwards RR, Hill E, Hsu S, Izquierdo-Garcia D, Ji R-R, Riley M, Wasan AD, Zürcher NR, Albrecht DS, Vangel MG, Rosen BR, Napadow V, Hooker JM. Evidence for brain glial activation in chronic pain patients. Brain 2015; 138: 604–615. DOI: 10.1093/brain/awu377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Albrecht DS, Forsberg A, Sandström A, Bergan C, Kadetoff D, Protsenko E, Lampa J, Lee YC, Höglund CO, Catana C, Cervenka S, Akeju O, Lekander M, Cohen G, Halldin C, Taylor N, Kim M, Hooker JM, Edwards RR, Napadow V, Kosek E, Loggia ML. Brain glial activation in fibromyalgia – A multi-site positron emission tomography investigation. Brain Behav Immun 2019; 75: 72–83, DOI: 10.1016/j.bbi.2018.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, Carter C, Yu BP, Leeuwenburgh C. Molecular inflammation: Underpinnings of aging and age-related diseases. Ageing Res Rev 2009; 8: 18–30. Review. DOI: 10.1016/j.arr.2008.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chung HY, Kim DH, Lee EK, Chung KW, Chung S, Lee B, Seo AY, Chung JH, Jung YS, Im E, Lee J, Kim ND, Choi YJ, Im DS, Yu BP. Redefining chronic inflammation in aging and age-related diseases: proposal of the senoinflammation concept. Aging Dis 2019; 10: 367–382. DOI: 10.14336/ad.2018.0324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell 2009; 139: 267–284. DOI: 10.1016/j.cell.2009.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rea IM, Gibson DS, McGilligan V, McNerlan SE, Alexander HD, Ross OA. Age and age-related diseases: role of inflammation triggers and cytokines. Front Immunol 2018; 9: 586. DOI: 10.3389/fimmu.2018.00586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bruunsgaard H, Pedersen M, Pedersen BK. Aging and proinflammatory cytokines. Curr Opin Hematol 2001; 8: 131–136. Article. DOI: 10.1097/00062752-200105000-00001 [DOI] [PubMed] [Google Scholar]
- 97.Haidar O, O’Neill N, Staunton CA, Bavan S, O’Brien F, Zouggari S, Sharif U, Mobasheri A, Kumagai K, Barrett-Jolley R. Pro-inflammatory cytokines drive deregulation of potassium channel expression in primary synovial fibroblasts. Front Physiol 2020; 11: 226. DOI: 10.3389/fphys.2020.00226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ashcroft GS, Horan MA, Ferguson MWJ. Aging alters the inflammatory and endothelial cell adhesion molecule profiles during human cutaneous wound healing. Lab Invest 1998; 78: 47–58. Article. [PubMed] [Google Scholar]
- 99.Zhao P, Sui BD, Liu N, Lv YJ, Zheng CX, Lu YB, Huang WT, Zhou CH, Chen J, Pang DL, Fei DD, Xuan K, Hu CH, Jin Y. Anti-aging pharmacology in cutaneous wound healing: effects of metformin, resveratrol, and rapamycin by local application. Aging Cell 2017; 16: 1083–1093. DOI: 10.1111/acel.12635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yu BP. Aging and oxidative stress: modulation by dietary restriction. Free Radic Biol Med 1996; 21: 651–668. Review. DOI: 10.1016/0891-5849(96)00162-1 [DOI] [PubMed] [Google Scholar]
- 101.Chung HY, Sung B, Jung KJ, Zou Y, Yu BP. The molecular inflammatory process in aging. Antioxidants Redox Signal 2006; 8: 572–581. Review. DOI: 10.1089/ars.2006.8.572 [DOI] [PubMed] [Google Scholar]
- 102.Platts-Mills TF, Dayaa JA. Musculoskeletal injures in older adults: preventing the transition to chronic pain and disability. N C Med J 2017; 78: 318–321. DOI: 10.18043/ncm.78.5.318 [DOI] [PubMed] [Google Scholar]
- 103.Cruz-Almeida Y, Aguirre M, Sorenson HL, Tighe P, Wallet SM, Riley JL, 3rd. Age differences in cytokine expression under conditions of health using experimental pain models. Exp Gerontol 2015; 72: 150–156. DOI: 10.1016/j.exger.2015.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Herrero MT, Morelli M. Multiple mechanisms of neurodegeneration and progression. Prog Neurobiol 2017; 155: 1. DOI: 10.1016/j.pneurobio.2017.06.001 [DOI] [PubMed] [Google Scholar]
- 105.Di Benedetto S, Muller L, Wenger E, Duzel S, Pawelec G. Contribution of neuroinflammation and immunity to brain aging and the mitigating effects of physical and cognitive interventions. Neurosci Biobehav Rev 2017; 75: 114–128. DOI: 10.1016/j.neubiorev.2017.01.044 [DOI] [PubMed] [Google Scholar]
- 106.Burton M, Rytych J, RA, Johnson R. Dietary luteolin reduces proinflammatory microglia in the brain of senescent mice. Rejuvenation Res 2016; 19: 286–292. DOI: 10.1089/rej.2015.1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Munoz-Espin D, Serrano M. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol 2014; 15: 482–496. DOI: 10.1038/nrm3823 [DOI] [PubMed] [Google Scholar]
- 108.El Tumi H, Johnson MI, Dantas PBF, Maynard MJ, Tashani OA. Age-related changes in pain sensitivity in healthy humans: a systematic review with meta-analysis. Eur J Pain 2017; 21: 955–964. DOI: 10.1002/ejp.1011 [DOI] [PubMed] [Google Scholar]
- 109.Mayhew JA, Callister RJ, Walker FR, Smith DW, Graham BA. Aging alters signaling properties in the mouse spinal dorsal horn. Mol Pain 2019; 15: 1744806919839860. DOI: 10.1177/1744806919839860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Casale R, Atzeni F, Bazzichi L, Beretta G, Costantini E, Sacerdote P, Tassorelli C. Pain in women: a perspective review on a relevant clinical issue that deserves prioritization. Pain and Therapy 2021; 10: 287–314. DOI: 10.1007/s40122-021-00244-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mogil JS. Qualitative sex differences in pain processing: emerging evidence of a biased literature. Nat Rev Neurosci 2020; 21: 353–365. DOI: 10.1038/s41583-020-0310-6 [DOI] [PubMed] [Google Scholar]
- 112.Bartley EJ, Fillingim RB. Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth 2013; 111: 52–58. DOI: 10.1093/bja/aet127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Presto P, Mazzitelli M, Junell R, Griffin Z, Neugebauer V. Sex differences in pain along the neuraxis. Neuropharmacology 2022; 210: 109030, DOI: 10.1016/j.neuropharm.2022.109030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sorge RE, Totsch SK. Sex differences in pain. J Neurosci Res 2017; 95: 1271–1281, DOI: 10.1002/jnr.23841 [DOI] [PubMed] [Google Scholar]
- 115.Racine M, Tousignant-Laflamme Y, Kloda LA, Dion D, Dupuis G, Choinière M. A systematic literature review of 10 years of research on sex/gender and experimental pain perception - part 1: are there really differences between women and men? Pain 2012; 153: 602–618. DOI: 10.1016/j.pain.2011.11.025 [DOI] [PubMed] [Google Scholar]
- 116.Mogil JS. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat Rev Neurosci 2012; 13: 859–866. DOI: 10.1038/nrn3360 [DOI] [PubMed] [Google Scholar]
- 117.Shughrue PJ, Merchenthaler I. Distribution of estrogen receptor beta immunoreactivity in the rat central nervous system. J Comp Neurol 2001; 436: 64–81. [PubMed] [Google Scholar]
- 118.Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology 1997; 138: 863–870. DOI: 10.1210/endo.138.3.4979 [DOI] [PubMed] [Google Scholar]
- 119.Mannino CA, South SM, Quinones-Jenab V, Inturrisi CE. Estradiol replacement in ovariectomized rats is antihyperalgesic in the formalin test. J Pain 2007; 8: 334–342. DOI: 10.1016/j.jpain.2006.10.002 [DOI] [PubMed] [Google Scholar]
- 120.Sarajari S, Oblinger MM. Estrogen effects on pain sensitivity and neuropeptide expression in rat sensory neurons. Exp Neurol 2010; 224: 163–169. DOI: 10.1016/j.expneurol.2010.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ferrari LF, Araldi D, Levine JD. Regulation of expression of hyperalgesic priming by estrogen receptor alpha in the rat. J Pain 2017; 18: 574–582. DOI: 10.1016/j.jpain.2016.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li L, Fan X, Warner M, Xu XJ, Gustafsson JA, Wiesenfeld-Hallin Z. Ablation of estrogen receptor alpha or beta eliminates sex differences in mechanical pain threshold in normal and inflamed mice. Pain 2009; 143: 37–40. DOI: 10.1016/j.pain.2009.01.005 [DOI] [PubMed] [Google Scholar]
- 123.Hall JM, Couse JF, Korach KS. The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem 2001; 276: 36869–36872. DOI: 10.1074/jbc.R100029200 [DOI] [PubMed] [Google Scholar]
- 124.Racine M, Tousignant-Laflamme Y, Kloda LA, Dion D, Dupuis G, Choinière M. A systematic literature review of 10 years of research on sex/gender and pain perception - part 2: do biopsychosocial factors alter pain sensitivity differently in women and men? Pain 2012; 153: 619–635. DOI: 10.1016/j.pain.2011.11.026 [DOI] [PubMed] [Google Scholar]
- 125.Staikou C, Kokotis P, Kyrozis A, Rallis D, Makrydakis G, Manoli D, Karandreas N, Stamboulis E, Moschovos C, Fassoulaki A. Differences in pain perception between men and women of reproductive age: a laser-evoked potentials study. Pain Med 2017; 18: 316–321. DOI: 10.1093/pm/pnw167 [DOI] [PubMed] [Google Scholar]
- 126.Rovner GS, Sunnerhagen KS, Björkdahl A, Gerdle B, Börsbo B, Johansson F, Gillanders D. Chronic pain and sex-differences; women accept and move, while men feel blue. PLoS One 2017; 12: e0175737. DOI: 10.1371/journal.pone.0175737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Oertel BG, Lotsch J. Clinical pharmacology of analgesics assessed with human experimental pain models: bridging basic and clinical research. Br J Pharmacol 2013; 168: 534–553. DOI: 10.1111/bph.12023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fillingim RB, Loeser JD, Baron R, Edwards RR. Assessment of chronic pain: domains, methods, and mechanisms. J Pain 2016; 17: T10–T20. DOI: 10.1016/j.jpain.2015.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Barrot M. Tests and models of nociception and pain in rodents. Neuroscience 2012; 211: 39–50. DOI: 10.1016/j.neuroscience.2011.12.041 [DOI] [PubMed] [Google Scholar]
- 130.Gagliese L, Melzack R. Age differences in nociception and pain behaviours in the rat. Neurosci Biobehav Rev 2000; 24: 843–854. DOI: 10.1016/s0149-7634(00)00041-5 [DOI] [PubMed] [Google Scholar]
- 131.Wang S, Davis BM, Zwick M, Waxman SG, Albers KM. Reduced thermal sensitivity and Nav1.8 and TRPV1 channel expression in sensory neurons of aged mice. Neurobiol Aging 2006; 27: 895–903. DOI: 10.1016/j.neurobiolaging.2005.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jourdan D, Boghossian S, Alloui A, Veyrat-Durebex C, Coudore MA, Eschalier A, Alliot J. Age-related changes in nociception and effect of morphine in the Lou rat. Eur J Pain 2000; 4: 291–300. DOI: 10.1053/eujp.2000.0188 [DOI] [PubMed] [Google Scholar]
- 133.Iwata K, Fukuoka T, Kondo E, Tsuboi Y, Tashiro A, Noguchi K, Masuda Y, Morimoto T, Kanda K. Plastic changes in nociceptive transmission of the rat spinal cord with advancing age. J Neurophysiol 2002; 87: 1086–1093. DOI: 10.1152/jn.00243.2001 [DOI] [PubMed] [Google Scholar]
- 134.Yezierski RP, King CD, Morgan D, Carter CS, Vierck CJ. Effects of age on thermal sensitivity in the rat. J Gerontol A Biol Sci Med Sci 2010; 65: 353–362. DOI: 10.1093/gerona/glq024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kitagawa J, Tsuboi Y, Ogawa A, Ren K, Hitomi S, Saitoh K, Takahashi O, Masuda Y, Harada T, Hanzawa N, Kanda K, Iwata K. Involvement of dorsal column nucleus neurons in nociceptive transmission in aged rats. J Neurophysiol 2005; 94: 4178–4187. DOI: 10.1152/jn.00243.2005 [DOI] [PubMed] [Google Scholar]
- 136.Iwata K, Tsuboi Y, Shima A, Harada T, Ren K, Kanda K, Kitagawa J. Central neuronal changes after nerve injury: neuroplastic influences of injury and aging. J Orofac Pain 2004; 18: 293–298. [PubMed] [Google Scholar]
- 137.Crisp T, Giles JR, Cruce WL, McBurney DL, Stuesse SL. The effects of aging on thermal hyperalgesia and tactile-evoked allodynia using two models of peripheral mononeuropathy in the rat. Neurosci Lett 2003; 339: 103–106. [DOI] [PubMed] [Google Scholar]
- 138.Pickering G, Jourdan D, Millecamps M, Chapuy E, Alliot J, Eschalier A. Age-related impact of neuropathic pain on animal behaviour. Eur J Pain 2006; 10: 749–755. DOI: 10.1016/j.ejpain.2005.12.002 [DOI] [PubMed] [Google Scholar]
- 139.Stuesse SL, Cruce WL, Lovell JA, McBurney DL, Crisp T. Microglial proliferation in the spinal cord of aged rats with a sciatic nerve injury. Neurosci Lett 2000; 287: 121–124. [DOI] [PubMed] [Google Scholar]
- 140.Kitagawa J, Kanda K, Sugiura M, Tsuboi Y, Ogawa A, Shimizu K, Koyama N, Kamo H, Watanabe T, Ren K, Iwata K. Effect of chronic inflammation on dorsal horn nociceptive neurons in aged rats. J Neurophysiol 2005; 93: 3594–3604. DOI: 10.1152/jn.01075.2004. [DOI] [PubMed] [Google Scholar]
- 141.Zhang RX, Lao L, Qiao JT, Ruda MA. Effects of aging on hyperalgesia and spinal dynorphin expression in rats with peripheral inflammation. Brain Res 2004; 999: 135–141. DOI: 10.1016/j.brainres.2003.11.042 [DOI] [PubMed] [Google Scholar]
- 142.Cruce WL, Lovell JA, Crisp T, Stuesse SL. Effect of aging on the substance P receptor, NK-1, in the spinal cord of rats with peripheral nerve injury. Somatosens Mot Res 2001; 18: 66–75. [DOI] [PubMed] [Google Scholar]
- 143.Stuesse SL, Crisp T, McBurney DL, Schechter JB, Lovell JA, Cruce WL. Neuropathic pain in aged rats: behavioral responses and astrocytic activation. Exp Brain Res 2001; 137: 219–227. [DOI] [PubMed] [Google Scholar]
- 144.King-Himmelreich TS, Moser CV, Wolters MC, Olbrich K, Geisslinger G, Niederberger E. Age-dependent changes in the inflammatory nociceptive behavior of mice. Int J Mol Sci 2015; 16: 27508–27519. DOI: 10.3390/ijms161126041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Scuteri D, Berliocchi L, Rombola L, Morrone LA, Tonin P, Bagetta G, Corasaniti MT. Effects of aging on formalin-induced pain behavior and analgesic activity of gabapentin in C57BL/6 Mice. Front Pharmacol 2020; 11: 663. DOI: 10.3389/fphar.2020.00663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shoji H, Miyakawa T. Age-related behavioral changes from young to old age in male mice of a C57BL/6J strain maintained under a genetic stability program. Neuropsychopharmacol Rep 2019; 39: 100–118. DOI: 10.1002/npr2.12052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Shoji H, Takao K, Hattori S, Miyakawa T. Age-related changes in behavior in C57BL/6J mice from young adulthood to middle age. Mol Brain 2016; 9: 11. DOI: 10.1186/s13041-016-0191-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Berry A, Capone F, Giorgio M, Pelicci PG, de Kloet ER, Alleva E, Minghetti L, Cirulli F. Deletion of the life span determinant p66Shc prevents age-dependent increases in emotionality and pain sensitivity in mice. Exp Gerontol 2007; 42: 37–45, DOI: 10.1016/j.exger.2006.05.018 [DOI] [PubMed] [Google Scholar]
- 149.Zouikr I, Tadros MA, Clifton VL, Beagley KW, Hodgson DM. Low formalin concentrations induce fine-tuned responses that are sex and age-dependent: a developmental study. PLoS One 2013; 8: e53384. DOI: 10.1371/journal.pone.0053384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dos Santos NL, Lenert ME, Castillo ZW, Mody PH, Thompson LT, Burton MD. Age and sex drive differential behavioral and neuroimmune phenotypes during postoperative pain. Neurobiol Aging 2023; 123: 129–144. DOI: 10.1016/j.neurobiolaging.2022.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Muralidharan A, Sotocinal SG, Austin J-S, Mogil JS. The influence of aging and duration of nerve injury on the antiallodynic efficacy of analgesics in laboratory mice. Pain reports 2020; 5: e824. DOI: 10.1097/pr9.0000000000000824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lee S, Wu Y, Shi XQ, Zhang J. Characteristics of spinal microglia in aged and obese mice: potential contributions to impaired sensory behavior. Immun Ageing 2015; 12: 10. DOI: 10.1186/s12979-015-0049-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gagliese L, Melzack R. Age differences in the response to the formalin test in rats. Neurobiol Aging 1999; 20: 699–707. Article. DOI: 10.1016/s0197-4580(99)00061-5 [DOI] [PubMed] [Google Scholar]
- 154.Mody PH, Dos Santos NL, Barron LR, Price TJ, Burton MD. eIF4E phosphorylation modulates pain and neuroinflammation in the aged. GeroScience 2020; 42: 1663–1674. DOI: 10.1007/s11357-020-00220-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sparkman NL, Buchanan JB, Dos Santos NL, Johnson RW, Burton MD. Aging sensitizes male mice to cognitive dysfunction induced by central HIV-1 gp120. Exp Gerontol 2019; 126: 110694. DOI: 10.1016/j.exger.2019.110694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Garner KM, Amin R, Johnson RW, Scarlett EJ, Burton MD. Microglia priming by interleukin-6 signaling is enhanced in aged mice. J Neuroimmunol 2018; 324: 90–99. DOI: 10.1016/j.jneuroim.2018.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sengupta P. The laboratory rat: relating its age with human’s. Int J Prev Med 2013; 4: 624–630. [PMC free article] [PubMed] [Google Scholar]
- 158.Jonas AM. The mouse in biomedical research. Physiol 1984; 27: 330–346. Historical Article. [PubMed] [Google Scholar]
- 159.Mitchell SJ, Scheibye-Knudsen M, Longo DL, de Cabo R, Intramural NIH. Animal models of aging research: implications for human aging and age-related diseases. Annual Revie Anim Bioscien 2015; 3: 283–303. DOI: 10.1146/annurev-animal-022114-110829 [DOI] [PubMed] [Google Scholar]
- 160.Millecamps M, Shi XQ, Piltonen M, Echeverry S, Diatchenko L, Zhang J, Stone LS. The geriatric pain experience in mice: intact cutaneous thresholds but altered responses to tonic and chronic pain. Neurobiol Aging 2020; 89: 1–11. DOI: 10.1016/j.neurobiolaging.2019.12.018 [DOI] [PubMed] [Google Scholar]
- 161.Gregory NS, Harris AL, Robinson CR, Dougherty PM, Fuchs PN, Sluka KA. An overview of animal models of pain: disease models and outcome measures. J Pain 2013; 14: 1255–1269. DOI: 10.1016/j.jpain.2013.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Rosczyk H, Sparkman NL, Johnson R. Neuroinflammation and cognitive function in aged mice following minor surgery. Exp Gerontol 2008; 43: 840–846. DOI: 10.1016/j.exger.2008.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mangold CA, Wronowski B, Du M, Masser DR, Hadad N, Bixler GV, Brucklacher RM, Ford MM, Sonntag WE, Freeman WM. Sexually divergent induction of microglial-associated neuroinflammation with hippocampal aging. J Neuroinflammation 2017; 14: 141. DOI: 10.1186/s12974-017-0920-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Matsuda M, Huh Y, Ji RR. Roles of inflammation, neurogenic inflammation, and neuroinflammation in pain. J Anesth 2019; 33: 131–139. DOI: 10.1007/s00540-018-2579-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ellis A, Bennett DL. Neuroinflammation and the generation of neuropathic pain. Br J Anaesth 2013; 111: 26–37. DOI: 10.1093/bja/aet128 [DOI] [PubMed] [Google Scholar]
- 166.Stevens AM, Liu L, Bertovich D, Janjic JM, Pollock JA. Differential expression of neuroinflammatory mRNAs in the rat sciatic nerve following chronic constriction injury and pain-relieving nanoemulsion NSAID delivery to infiltrating macrophages. Int J Mol Sci 2019; 20: 28. DOI: 10.3390/ijms20215269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Moy JK, Kuhn JL, Szabo-Pardi TA, Pradhan G, Price TJ. eIF4E phosphorylation regulates ongoing pain, independently of inflammation, and hyperalgesic priming in the mouse CFA model. Neurobiol Pain 2018; 4: 45–50. DOI: 10.1016/j.ynpai.2018.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Murtaj V, Belloli S, Di Grigoli G, Pannese M, Ballarini E, Rodriguez-Menendez V, Marmiroli P, Cappelli A, Masiello V, Monterisi C, Bellelli G, Panina-Bordignon P, Moresco RM. Age and sex influence the neuro-inflammatory response to a peripheral acute lps challenge. Front Aging Neurosci 2019; 11: 299. DOI: 10.3389/fnagi.2019.00299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Perkins AE, Piazza MK, Deak T. Stereological analysis of microglia in aged male and female Fischer 344 rats in socially-relevant brain regions. Neuroscience 2018; 377: 40–52. DOI: 10.1016/j.neuroscience.2018.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Dilger RN, Johnson RW. Aging, microglial cell priming, and the discordant central inflammatory response to signals from the peripheral immune system. J Leukoc Biol 2008; 84: 932–939. DOI: 10.1189/jlb.0208108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ye SM, Johnson RW. Increased interleukin-6 expression by microglia from brain of aged mice. J Neuroimmunol 1999; 93: 139–148. DOI: 10.1016/s0165-5728(98)00217-3 [DOI] [PubMed] [Google Scholar]
- 172.Norden DM, Godbout JP. Microglia of the aged brain: primed to be activated and resistant to regulation. Neuropathol Appl Neurobiol 2013; 39: 19–34. DOI: 10.1111/j.1365-2990.2012.01306.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kennedy DL, Kemp HI, Ridout D, Yarnitsky D, Rice ASC. Reliability of conditioned pain modulation: a systematic review. Pain 2016; 157: 2410–2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hoehn JL, Dahlquist LM, Zeroth JA. Conditioned pain modulation in children: the effects of painful and nonpainful conditioning stimuli. J Pain 2022; 23: 1208–1219. DOI: 10.1016/j.jpain.2022.02.004 [DOI] [PubMed] [Google Scholar]
- 175.Edwards RR, Fillingim RB, Ness TJ. Age-related differences in endogenous pain modulation: a comparison of diffuse noxious inhibitory controls in healthy older and younger adults. Pain 2003; 101: 155–165. DOI: 10.1016/s0304-3959(02)00324-x [DOI] [PubMed] [Google Scholar]
- 176.Lariviere M, Goffaux P, Marchand S, Julien N. Changes in pain perception and descending inhibitory controls start at middle age in healthy adults. Clin J Pain 2007; 23: 506–510. DOI: 10.1097/AJP.0b013e31806a23e8 [DOI] [PubMed] [Google Scholar]
- 177.Riley JL, 3rd, King CD, Wong F, Fillingim RB, Mauderli AP. Lack of endogenous modulation and reduced decay of prolonged heat pain in older adults. Pain 2010; 150: 153–160. DOI: 10.1016/j.pain.2010.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Washington LL, Gibson SJ, Helme RD. Age-related differences in the endogenous analgesic response to repeated cold water immersion in human volunteers. Pain 2000; 89: 89–96. DOI: 10.1016/S0304-3959(00)00352-3 [DOI] [PubMed] [Google Scholar]
- 179.Marouf R, Caron S, Lussier M, Bherer L, Piche M, Rainville P. Reduced pain inhibition is associated with reduced cognitive inhibition in healthy aging. Pain 2014; 155: 494–502. DOI: 10.1016/j.pain.2013.11.011 [DOI] [PubMed] [Google Scholar]
- 180.Naugle KM, Cruz-Almeida Y, Vierck CJ, Mauderli AP, Riley JL, 3rd. Age-related differences in conditioned pain modulation of sensitizing and desensitizing trends during response dependent stimulation. Behav Brain Res 2015; 289: 61–68. DOI: 10.1016/j.bbr.2015.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Riley JL, 3rd, Cruz-Almeida Y, Glover TL, King CD, Goodin BR, Sibille KT, Bartley EJ, Herbert MS, Sotolongo A, Fessler BJ, Redden DT, Staud R, Bradley LA, Fillingim RB. Age and race effects on pain sensitivity and modulation among middle-aged and older adults. J Pain 2014; 15: 272–282. DOI: 10.1016/j.jpain.2013.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wrobel N, Fadai T, Brassen S, Bingel U. Preserved capacity for placebo analgesia in the elderly. J Pain 2016; 17: 1318–1324. DOI: 10.1016/j.jpain.2016.08.012 [DOI] [PubMed] [Google Scholar]
- 183.Helme RD, Meliala A, Gibson SJ. Methodologic factors which contribute to variations in experimental pain threshold reported for older people. Neurosci Lett 2004; 361: 144–146. DOI: 10.1016/j.neulet.2003.12.014 [DOI] [PubMed] [Google Scholar]
- 184.Lautenbacher S, Kunz M, Strate P, Nielsen J, Arendt-Nielsen L. Age effects on pain thresholds, temporal summation and spatial summation of heat and pressure pain. Pain 2005; 115: 410–418. DOI: 10.1016/j.pain.2005.03.025 [DOI] [PubMed] [Google Scholar]
- 185.Naugle KM, Cruz-Almeida Y, Fillingim RB, Staud R, Riley JL, 3rd. Increased spatial dimensions of repetitive heat and cold stimuli in older women. Pain 2017; 158: 973–979. DOI: 10.1097/j.pain.0000000000000709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Edwards RR, Fillingim RB. Effects of age on temporal summation and habituation of thermal pain: clinical relevance in healthy older and younger adults. J Pain 2001; 2: 307–317. DOI: 10.1054/jpai.2001.25525 [DOI] [PubMed] [Google Scholar]
- 187.Edwards RR, Fillingim RB. Age-associated differences in responses to noxious stimuli. J Gerontol A Biol Sci Med Sci 2001; 56: M180–M185. DOI: 10.1093/gerona/56.3.m180 [DOI] [PubMed] [Google Scholar]
- 188.Naugle KM, Fillingim RB, Riley JL, 3rd. A meta-analytic review of the hypoalgesic effects of exercise. J Pain 2012; 13: 1139–1150. DOI: 10.1016/j.jpain.2012.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lemley KJ, Hunter SK, Bement MK. Conditioned pain modulation predicts exercise-induced hypoalgesia in healthy adults. Med Sci Sports Exerc 2015; 47: 176–184. DOI: 10.1249/mss.0000000000000381 [DOI] [PubMed] [Google Scholar]
- 190.Naugle KM, Naugle KE, Riley JL, 3rd. Reduced modulation of pain in older adults after isometric and aerobic exercise. J Pain 2016; 17: 719–728. DOI: 10.1016/j.jpain.2016.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Falzone E, Hoffmann C, Keita H. Postoperative analgesia in elderly patients. Drugs Aging 2013; 30: 81–90. DOI: 10.1007/s40266-012-0047-7 [DOI] [PubMed] [Google Scholar]
- 192.Reece AS. Relative and age-dependent stimulation of soluble and cellular immunity in opiate dependence. J Addict Med 2012; 6: 10–17. DOI: 10.1097/ADM.0b013e31822c3bf4 [DOI] [PubMed] [Google Scholar]
- 193.Lautenbacher S, Peters JH, Heesen M, Scheel J, Kunz M. Age changes in pain perception: a systematic-review and meta-analysis of age effects on pain and tolerance thresholds. Neurosci Biobehav Rev 2017; 75: 104–113, DOI: 10.1016/j.neubiorev.2017.01.039 [DOI] [PubMed] [Google Scholar]
- 194.Dawson TM, Golde TE, Lagier-Tourenne C. Animal models of neurodegenerative diseases. Nat Neurosci 2018; 21: 1370–1379. DOI: 10.1038/s41593-018-0236-8 [DOI] [PMC free article] [PubMed] [Google Scholar]