Abstract
In the past few decades, advancements in protein engineering, biotechnology, and structural biochemistry have resulted in the discovery of various techniques that enhanced the production yield of proteins, targetability, circulating half-life, product purity, and functionality of proteins and peptides. As a result, the utilization of proteins and peptides has increased in the treatment of many conditions, including ocular diseases. Ocular delivery of large molecules poses several challenges due to their high molecular weight, hydrophilicity, unstable nature, and poor permeation through cellular and enzymatic barriers. The use of novel strategies for delivering protein and peptides such as glycoengineering, PEGylation, Fc-fusion, chitosan nanoparticles, and liposomes have improved the efficacy, safety, and stability, which consequently expanded the therapeutic potential of proteins. This review article highlights various proteins and peptides that are useful in ocular disorders, challenges in their delivery to the eye, and strategies to enhance ocular bioavailability using novel delivery approaches. In addition, a few futuristic approaches that will assist in the ocular delivery of proteins and peptides were also discussed.
1. Introduction
The global therapeutic proteins market has witnessed significant growth in the past few years. It grew from 90.53 billion US dollar (USD) in 2020 to 98.1 billion USD in 2021 at a compound annual growth rate (CAGR) of 8.4%. The growth was observed because most pharmaceutical companies rearranged their operations while recovering from the COVID-19 impact. During the 2020–2021 years, COVID-19 restrictive containment measures like remote working, closure of commercial activities, and social distancing increased the operational challenges tremendously. However, it is projected that the global therapeutic market will hit the 155.06 billion USD mark by 2025 with a CAGR of 12.1% (Figure 1). The market of therapeutic proteins is dominated by major players such as Eli Lilly and Company, Baxter international, Amgen Inc., Abbott Laboratories, and F. Hoffmann-La Roche Ltd. Commercialization of therapeutic proteins has greatly benefited ophthalmology.1 In the eye, therapeutic proteins are employed for the neutralization of biomolecules, like cytokines and growth factors, prevention of angiogenesis, and protection of photoreceptors. Some of the commonly occurring ocular diseases include diabetic retinopathy (DR), retinal vein occlusion with cystoid macular edema (CMV), glaucoma, age-related macular degeneration (AMD), posterior uveitis, retinitis pigmentosa, and cytomegalovirus (CMV) retinitis. If these diseases are not treated in time, they can lead to blindness.2 In 2015, worldwide there were 253 million people suffering from visual impairment, out of which 217 million had moderate to severe impairment of vision and 36 million were blind. The number escalated to 596 million people with visual impairment in 2020, out of which 553 million had moderate to severe impairment of vision and 43 million people were blind. A projection indicates that visual impairment would affect 895 million people by 2050, which includes 61 million people completely blind (Table 1).3 Out of the total number of blind people worldwide, 26% of the people are blind due to disorders like AMD, glaucoma, and DR.4
Figure 1.
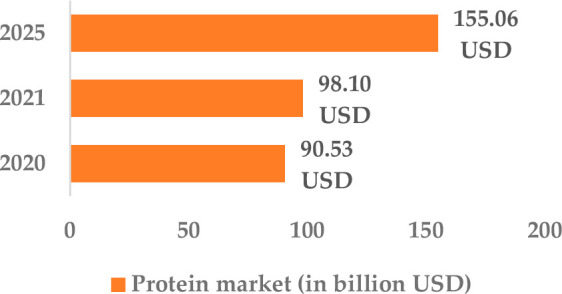
Projected growth of the therapeutic protein market.
Table 1. Projected Change in Vision Impairment 2015 to 2050.
| year | total people with vision impairment (in millions) | moderate to severe impairment (in millions) | blind (in millions) |
|---|---|---|---|
| 2015 | 253 | 217 | 36 |
| 2020 | 596 | 553 | 43 |
| 2050 (projected figures) | 956 | 895 | 61 |
The genetics and pathogenesis of ocular diseases are now better understood because of the research during the last few decades. For instance, the discovery of various complementary pathways and genetic associations has resulted in the development of effective therapies for retinal diseases.5 Cataracts, DR, and AMD are some of the diseases that affect the aging population in developed countries.6 Therapeutic proteins and peptides have emerged as one of the most novel therapeutics that have the potential to improve the treatment of numerous ocular diseases. They have various advantages over small molecules. These merits include lower toxicity, low off-target binding, high chemical, and biological diversity, minimal drug–drug interaction, high activity, and high potency. Apart from these benefits, biopharmaceutical companies earn hefty amounts from patented products containing proteins and peptides. For instance, Lucentis developed by Genentech is a patented product that was a blockbuster in the U.S. market. Although these molecules have tremendous benefits, there are numerous hurdles for developing biopharmaceutical products due to reasons such as short half- lives, instability due to chemical and physical degradation, lower permeability through the cell membrane due to large molecular weight and hydrophilicity, risk of immunogenicity, large molecular weight, complex structure, and clearance by reticuloendothelial system’s mononuclear phagocytes (MPS). Therefore, it is necessary to develop effective delivery ways to overcome these hurdles and successfully utilize these molecules in the treatment of eye disorders in order to improve overall patient well-being.6−9 This article presents a comprehensive overview of administering proteins and peptides through the ocular route, with a focus on their significance in treating various ocular disorders. The utilization of specific proteins that target ocular tissues for therapeutic purposes and associated delivery challenges were discussed. Additionally, the latest developments in formulation techniques and the use nanoparticles delivery systems to overcome these delivery challenges were discussed. The final segment of this review covers some futuristic approaches that will assist in the ocular delivery of proteins and peptides.
2. Proteins and Peptides in Eye Disorders
Proteins and peptides are mostly utilized in ocular diseases such as DR, glaucoma, and AMD. Proteins and peptides are classified into five categories according to their functions: 1. anti-VEGF (vascular endothelial growth factor) agents, 2. anti-TNF-α (tumor necrosis factor- alpha) agents, 3. GLP-1 (glucagon-like peptide 1) agonists, 4. tissue plasminogen activators, 5. neurotrophic growth factors (NGF)
2.1. Anti-Vascular Endothelial Growth Factor (VEGF) agents
Worldwide, many pharmaceutical companies are attempting to develop novel therapies for treating ocular disorders.10 In AMD, the disease progression leads to choroidal neovascularization (CNV), while in DR, the disease progression results in retinal neovascularization. Generally, the standard treatment of retinal neovascularization involves laser-assisted thermal photocoagulation or ablation of CNV so that the retina becomes anoxic. Currently, the standard treatment has been replaced by intravitreal injections of US Food and Drug Administration (FDA) approved anti-VEGF agents2 such as ranibizumab (Lucentis), pegaptanib (Macugen), conbercept (Lumitin), brolucizumab (Beovu),2,11 and aflibercept (Eylea) that act as “VEGF trap”.
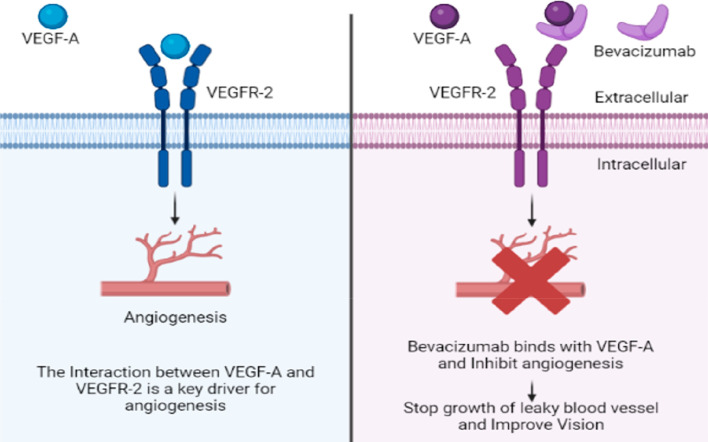
Bevacizumab (Avastin) is a humanized monoclonal IgG1 antibody (molecular weight 149 kDa) used off-label in the treatment of chorioretinal vascular disease. These agents act in two ways: (a) prevent the VEGF signaling peptide from binding to its receptor, and (b) neutralize the down streaming effect of VEGF-growth factors.10 The mechanism of action of bevacizumab is shown in Figure 2.
Figure 2.
Mechanism of action of bevacizumab.
The binding affinity of anti-VEGF proteins to different isoforms of VEGF receptors is different. Some bind to all isoforms, while others bind with specific receptors only. Bevacizumab and ranibizumab have the ability to bind with all isoforms of VEGF-A. A recombinant antibody fragment ranibizumab (Lucentis) when given in repeated doses, showed an excellent result in patients with visual problems. Ranibizumab was able to improve vision in 40% of patients while preventing vision loss in approximately 95% of patients.2 Ranibizumab (∼48 kDa) is one-third in size compared to bevacizumab because it contains only the Fab-portion of bevacizumab, but the smaller size enhances its clearance by 100-fold. Ranibizumab is better than bevacizumab only due to its better retina penetration and higher VEGF-binding ability.12,13 Pegaptanib (Macugen), developed by Eyetech Pharmaceuticals Inc. and Pfizer Inc., is a pegylated anti-VEGF aptamer that binds to major VEGF-A isoforms.6 It is utilized in wet AMD treatment for preventing neovascularization and its side effects include pallor, endotracheal tube reflux, need for dose interruption, and endotracheal tube obstruction.14 In 2011, Regeneron and Sanofi/Aventis developed an anti-VEGF antibody called aflibercept (Eylea) that contains a human immunoglobulin Fc portion. Aflibercept was approved by the FDA after the success of phase III (VISTA/VIVID) studies.15 The half-life of aflibercept is much longer than other agents. Conbercept (Lumitin) is one of the newly developed agents that has the ability to inhibit all isoforms of VEGF-A, VEGF-B, and VEGF-C. It has three parts: (1) Fc portion of IgG1, (2) extracellular domain 2 of VEGF receptor 1 (VEGFR-1), and (3) extracellular domains 3 and 4 of VEGFR-2.15
More recently, brolucizumab (RTH258, a single-chain small humanized antibody fragment) has received approval from the FDA for use in neovascular AMD. Brolucizumab has a small molecular size (26 kDa) with the ability to block all isoforms of VEGF-A.16 Clinical trials showed prolonged activity of brolucizumab as compared with anti-VEGF ranibizumab.16,17 Brolucizumab is currently being studied for indications such as DME and retinal vein occlusion.18 Faricimab is a newer type of FDA-approved anti-VEGF for the treatment of wet/neovascular AMD and DME, a bispecific antibody that can bind and neutralize VEGF-A as well as angiopoietin-2 (Ang-2).19 Ang-1 and Ang-2 are two angiopoietins that bind to the tyrosine-protein kinase receptor (Tie-2) complex. Ang-1 acts as an agonist and phosphorylates the receptor, which leads to vascular permeability inhibition and preservation of vascular stability.20,21 On the other hand, Ang-2 acts as a partial agonist or antagonist and blocks the phosphorylation of the receptor, causing the deactivation of the effects of the Ang-1-mediated pathway.20,22 Currently, available treatments for retinal vascular disease, such as nAMD, only target VEGF, leaving patients with poor visual acuity. However, faricimab has the ability to target both Ang-2 and VEGF resulting in better visual acuity.23,24 To date, in three phases, two studies were designed using faricimab for nAMD (AVENUE and STAIRWAY) and DME (BOULEVARD). In these trials, the efficacy and safety of faricimab were compared with ranibizumab (the current standard of care). In nAMD patients, it was reported that faricimab administered every 12 to 16 weeks had outcomes similar to those of monthly ranibizumab.25,26 Moreover, in DME patients, faricimab improved DR severity score (DRSS), central subfield thickness (CST), and best-corrected visual acuity (BCVA).27 A total of four phase 3 clinical trials has been conducted for faricimab, two in naïve nAMD patients (LUCERNE and TENEHAYA) and two in DME patients (RHINE and YOSEMITE). In naïve nAMD treatment, the efficacy of 6 mg faricimab administered every 16 weeks was compared with 2 mg aflibercept administered every 8 weeks by comparing the mean BCVA. The results showed that faricimab was able to sustain the effect with an ocular adverse effects incidence similar to aflibercept as shown by a letter difference of 0.7 letters (5.8 letters for faricimab and 5.1 letters for aflibercept) in TENEHAYA and 0 letters in LUCERNE (6.6 letters for faricimab and 6.6 letters for aflibercept).28 In DME patients, the same drug, dose, and administration were utilized with the end point being Early Treatment Diabetic Retinopathy Study [ETDRS] letters. The results reported a difference of 1.5 ETDRS letters in RHINE (11.8 letters for faricimab and 10.3 letters for aflibercept) and −0.2 ETDRS letters in YOSEMITE (10.7 letters for faricimab and 10.9 letters for aflibercept).29
Lampalizumab (INN) contains antigen-binding fragments of a humanized monoclonal antibody, which was proposed to reduce the degeneration of the macula as a part of late-stage AMD via blocking a complement D factor (CFD).2 However, in two phase 3 randomized clinical trials (975 Spectri participants and 906 Chroma participants), lampalizumab failed to reach the primary end point.30 Abicipar pegol, also known as AGN-150998 or MP0112, is a combination of 14 kDa recombinant protein with 20 kDa polyethylene glycol that can bind to various VEGF A isoforms such as VEGF A110, VEGF A121, VEGF A165, and VEGF A189.31−33 It belongs to the family of designed ankyrin repeat proteins (DARPin), which generally have four to six repeated motifs of naturally occurring proteins that have a high affinity for specific targets, typically in the picomolar range.34 Additionally, DARPin family molecules have high melting points that are above 80 °C and sometimes above 100 °C, which impart high stability.35 Moreover, these molecules have a structure that is one-third the mass of a Fab fragment or one-tenth the weight of an antibody.35 Due to these properties, DARPin family molecules required low concentrations for their biological effects as with abicipar.36 In various preclinical animal models, abicipar reduced neovascularization, vasodilation, vasculature tortuosity, and suppressed vascular growth.35 Further, in the initial phase I/II clinical trials of abicipar efficacy in improving fluorescein angiography leakage, retinal thickness and visual acuity were reported, along with the establishment of 1 mg as the maximum tolerated dose.37 Similar results were also obtained in two more phase I/II trials (NCT03335852 and NCT02859766).38 There were mainly two phase 3 clinical trials, SEQUOIA and CEDAR, where AMD patients with secondary active CNV were enrolled, and both had similar protocols.39 In both these trials, patients were divided into three groups. One group received 2 mg of abicipar every 4 weeks; the second group received 2 mg of abicipar every 8 weeks; and the third group received 0.5 mg of ranibizumab every 4 weeks. After 52 weeks, 93.2%, 91.3%, and 95.8% of patients in groups 1, 2, and 3 had stable vision. In the CEDAR trial, the proportion of patients with visual acuity of more than 15 letters was greater in the ranibizumab group; however, in the SEQUOIA trial, it was similar for both groups. Nevertheless, the main issue reported was the development of intraocular inflammation (IOI), which was 16.8% for group 1, 20.4% for group 2, and 4% for group 3. It was reported that the issue was due to the impurities of E. coli fragments; therefore, the manufacturing process was modified, and again a phase II clinical trial (MAPLE) was conducted in which the IOI rate was reduced to 8.9%.38−40 Abicipar pegol was not approved by the FDA due to the observed incidence of IOI.41
KSI-301 is another type of anti-VEGF that has a humanized IgG1 antibody covalently conjugated with phosphorylcholine polymer through a single-site specific linkage. The polymer is a high-molecular-weight, optically clear biopolymer that is conjugated to the immune effector antibody. This antibody-biopolymer conjugate (ABC) platform design has aided in enhancing intraocular durability through the optimization of molar dose and size.23,42,43 KSI-301 inhibits all isoforms of VEGF-A and it has a greater affinity toward VEGF-A compared to VEGFR1 and VEGFR2, which was established by the Kinetic Exclusion Assay (KinExA) and Surface Plasmon Resonance (SPR).44,45 In the phase Ia study in DME patients, the safety and efficacy of KSI-301 were evaluated. The study reported no drug-related adverse effects and an improvement of median optical coherence tomography (OCT) in central subfield thickness (CST), and a nine-letter improvement in BCVA was reported.43 However, the phase 2 trial (DAZZLE), comparing the safety and efficacy of KSI-301 with aflibercept in nAMD patients, was terminated as it was unable to improve mean BCVA.46 Nevertheless, various phase 3 trials in patients with DME, nonproliferative DR, wet nAMD, and macular edema (NCT04603937, NCT04611152, NCT05066230, and NCT04964089) are going on.47−50 Ziv-aflibercept is analogous to aflibercept with only difference is in the osmolarity. Ziv-aflibercept is hyperosmolar, whereas aflibercept is iso-osmolar. Despite being hyperosmolar, it does not alter intraocular and serum osmolarity, and its intravitreal administration does not cause inflammation, toxicity, or a higher cataract induction rate.51−54 Ziv-aflibercept is used off-label in ocular diseases such as AMD, RVO, and DME.55 Some of the anti-VEGF agents in clinical and preclinical stages are shown in Table 2. Despite the partial success of anti-VEGFs, the need for highly effective compounds that can reduce the burden of managing wet AMD still exists. The approved anti-VEGF therapies require patients to make regular visits to the clinics resulting in severe economic/psychological burdens on patients and the health care system. Biodegradable nanocarrier systems are being considered for the delivery of anti-VEGF agents in order to maintain long-term therapeutic effects through the continuous release of the medicine.
Table 2. Anti-VGEF Agents Which Are Currently in Clinical and Pre-clinical Stagesa.
| drug/protein name | molecular weight (kDa) | half-life | description | target | phase | company | current indication | clinical trails.gov identifier |
|---|---|---|---|---|---|---|---|---|
| Ranibizumab [Lucentis] | 48.35 | 9 days | recombinant humanized IgG1 kappa isotype monoclonal antibody | VEGF-CC1 | FDA approved | Genentech | DME, DR, AM | |
| Pegaptanib Sodium [Macugen] | 50 | 10 ± 4 days | polynucleotide aptamer | VEGF-165 | FDA approved | Gilead sciences | wet AMD | |
| Ocriplamin [Jetrea] | 27.2 | N.A. | recombinant human plasmin | fibronectin, Alpha-2 macroglobulin | FDA Approved | Thrombogenic NV | VMA, VRI | |
| Alpha-2 Antiplasmin | ||||||||
| Aflibercept [Eylea] | 115 | 7.13 days | recombinant fusion protein glycosylated | VEGF-A, B, and placenta growth factor | FDA Approved | Regeneron | DR, DME, AMD, CRC | |
| Bayer | ||||||||
| Brolucizumab [Beovu] | 26 | 4.4 ± 2 days | proteins-based monoclonal antibody | VEGF-A | FDA Approved | Novartis | nAMD | |
| Vgx-300 [opt 302] | N.A. | N.A. | recombinant fusion protein | VEGF - C, D | Phase- 3 [Recruiting] | Opthea limited | nAMD | NCT04757610 |
| Rn6g | N.A. | N.A. | antiamyloid beta antibody | amyloid beta fibrils in drusen | Phase-2 [Terminated] | Pfizer | AMD, GA | NCT01577381 |
| Conbercept [Lumitin] | 143 | 4.2 days [In rabbit] | recombinant fusion protein | VEGF-A/B placenta growth factor | Phase-3 [Rejected] | Chengdu kanghong Biotech | AMD | NCT03577899 |
| Lampalizumab | 47 | 6 days | fragment of humanized monoclonal antibody | CFD | Phase-3 [Rejected] | Roche | GA, AMD | NCT02247479 |
| Bevacizumab [Avastin] | 149 | estimated 20 days | humanized monoclonal IgG Antibody | VEGF-A | off label | Roche, Genentech | Wet AMD, Cancer |
VMA, Vitreomacular adhesion; GA, geographic atrophy; AMD, age-related macular degeneration; DR, diabetic retinopathy; VRI, vitreoretinal interface; nAMD, neovascular age-related macular degeneration; DME, diabetic macular edema; and CRVO, macular edema with central retinal vein occlusion.
2.2. Anti-TNF-α (Tumor Necrosis Factor-Alpha) Agents
TNF-α plays an important role in the pathogenesis of edematous, inflammatory, neurodegenerative diseases, and neovascularization.56 The mechanism of action of anti-TNF-α (tumor necrosis factor-alpha) agents is described in Figure 3.
Figure 3.
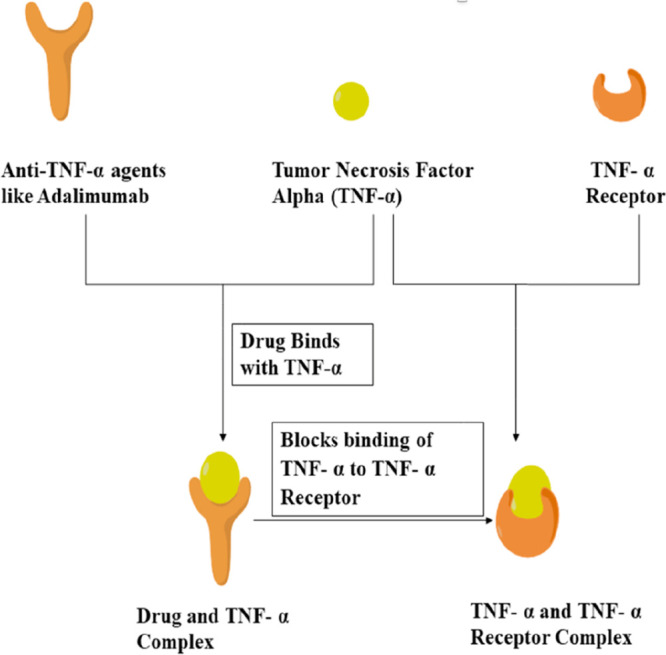
Mechanism of action of anti-TNF-α agents.
TNF-α is also involved in the pathogenesis of various ocular disorders such as proliferative vitreoretinopathy, macular edema, and experimentally induced retinal neovascularization.57−59 Anti-TNF-α agents such as infliximab (Remicade), adalimumab (Humira), golimumab (Simponi), and certolizumab pegol (Cimzia) are available for treating diseases like psoriasis arthritis, rheumatoid arthritis, and ankylosing spondylitis. In 2016, adalimumab (Humira) was approved by the FDA for the treatment of noninfectious intermediate, posterior, and panuveitis. Infliximab, a chimeric monoclonal antibody classified as TNF-α inhibitors. Anti-TNF-α agents, specifically adalimumab and infliximab, inhibit the binding of TNF-α with TNF-α receptors (TNFR) and thus block inflammatory responses. The use of anti-TNF-α agents to treat ocular inflammation is gradually increasing.19 Especially, these agents are considered widely for treating most forms of uveitis associated with Behçet’s disease and juvenile idiopathic arthritis. However, due to its high cost and enhanced risk of infections, it is less preferred when compared to other treatments. The initial infliximab dose ranged from 2.9 to 6.9 mg/kg with a median of 5.1 mg/kg. Doses were given at weeks 0, 2, and 4 and kept up at intervals of 4 weeks until the ocular inflammation decreased or disappeared.60 Further research in this field in terms of improving delivery strategies is required for treating retinal and choroidal infections, and treatment of vision problems.6
2.3. Glucagon-Like Peptide 1 (GLP-1) Agonists
Some of FDA-approved GLP-1 agonists use in Diabetes mellitus type 2 examples are albiglutide (Tanzeum), liraglutide (Victoza/Saxenda), dulaglutide (Trulicity), and exenatide (Beta/Bydureon). They exert their effect by binding to a receptor known as glucagon-like peptide 1 receptor (GLP1R). This leads to the activation of the adenylyl cyclase pathway and results in the enhanced synthesis and release of insulin. Pancreatic beta cells and the brain are the two regions where there is a high expression of GLP1R. It is believed that the retina also expresses GLP1R because it is an ontogenetic brain-derived tissue.61 Recently, Hernandez and coauthors reported that GLP1R is also found in nonketotic diabetic mice. By systemic administration of liraglutide, the treatment of retinal degeneration is possible. However, enhanced prosurvival signaling pathways and a decrease in extracellular glutamate levels are two major drawbacks of the therapy. Liraglutide was found to inhibit the upregulation of inflammatory cytokines in the retina in fatty rodents without provoking neovascularization of the eye and lowered retinal thickening of the inner nuclear layer of the retina when injected subcutaneously.62 GLP-1R agonists, such as dulaglutide, liraglutide, lixisenatide, exenatide, and semaglutide have shown a reduced risk of developing open-angle glaucoma.63 No increased risk of DR was observed in the AngioSafe 1 research NCT02671864, which aimed to clarify the relationship between exposure to GLP-1R agonists and DR through clinical and preclinical study methods. (NCT03811561).64 Another phase III interventional study by Novo Nordisk, the FOCUS trial, also investigated the long-term effects of injectable semaglutide in diabetic eye diseases.65 When native GLP-1 agonists were given topically to the patients, similar neuroprotective effects were achieved without having any effect on the blood glucose levels. This successful trial opened new ideas and approaches for the treatment of early stage diabetic retinopathy by arresting neurodegeneration of the retina with the clinical use of GLP-1 agonists.66
2.4. Tissue Plasminogen Activator
The naturally occurring serine protease known as tissue plasminogen activator (TPA) is produced by a range of tissues in mammals, particularly endothelial cells. Conjunctiva, cornea, trabecular meshwork, lens, vitreous, and retina contain TPA.67 The amount of TPA in the aqueous humor of healthy adult human eyes is about 30 times higher than that in plasma. Plasminogen is transformed into plasmin, an active serine protease that hydrolyses fibrin, by the main enzymatic action of TPA. Furthermore, TPA protects plasmin against antiplasmin inhibitors until complete clot dissolution is achieved.68 The delivery of ocular therapeutic proteins via implants has not yet received approval, although preclinical research with human recombinant tissue plasminogen activator demonstrated that TPA given intracamerally or intravitreally released the drug at a rate of 0.5 μg/day in the vitreous for 14 days. TPA is given as prophylactic use before surgery related to glaucoma.69 It may be sensible to use TPA prophylactically due to the reactivity of ocular tissues and fibrinous exudation, especially in children, and the fact that postsurgical intracameral injection of TPA in a child’s eye requires general anesthesia or brief sedation. Numerous researchers have supported the topical administration of TPA to dissolve fibrin clots in the anterior chamber, however, trials in human eyes and experimental animal models have yielded conflicting results. TPA injections with 25 μg or more have frequently been utilized intracamerally or intravitreally. The usefulness of 10 μg TPA for quick fibrinolysis in the anterior chamber is supported by numerous publications in the literature, and some researchers even advise a dose as low as 3 μg.70,71 The large molecular size of TPA (68 kDa) impedes its ability to traverse through an undamaged cornea.72,73
2.5. Neurotrophic Growth Factors (NGF)
The most advanced method of treating ocular surface illness at present is using NGF. NGF is hypothesized to control tear formation, immunological modulation, limbal stem cell proliferation, epithelial health, and ocular surface homeostasis. NGF is utilized as a treatment option in several ocular surface diseases because of its alleged impact on the ocular surface. It was found that rabbits with iatrogenic corneal epithelial defects had higher levels of corneal NGF expression, and topical NGF therapy quickly accelerated up the process of epithelial defect closure.74 Pilot clinical investigations were carried out in individuals with neurotrophic keratopathy (NK) in the late 1990s and early 2000s on the basis of the overwhelming amount of encouraging preclinical research. A phase I trial conducted in 2013 revealed that rhNGF was tolerable at increasing levels up to 180 μg/mL.75 In patients with moderate to severe NK disease, a phase I and subsequent double-masked phase II clinical trial (NGF0212/REPARO phase I/II) showed dramatically reduced epithelial defect healing time and lowered recurrence rate compared to control. Moreover, NGF has been examined in diabetic animal models. It is shown that retinal NGF and NGF receptor expression is initially elevated in streptozotocin-induced diabetic rats, which is expected to have a protective effect.76,77 A double-masked clinical randomized control trial was conducted after open-label pilot research to examine the effectiveness of topical NGF for treating visual loss caused by optic pathway gliomas. The superior colliculus (SC) of the central nervous system was shown to transport neurotrophins retrogradely (CNS).78,79 Research on rats showed that if IOP increases, it prevents brain-derived neurotrophic factor (BDNF) from traveling retrogradely from the SC to the soma of the RGCs. This RGCs’ loss of BDNF leads to degradation of the visual signal. RGC maintenance and survival have also been linked to other neurotrophic factors, including ciliary neurotrophic factor (CNTF) and glial cell-line derived neurotrophic factor (GDNF). It was also shown that RGCs can survive in cultures without exogenous BDNF, indicating that the loss of extrinsic BDNF due to cessation of retrograde transport is not the only cause of RGC death in glaucoma. Recently, a tropomyosin kinase receptor B (TrkB) agonist antibody (29D7) has been shown to increase RGCs survival in a dose-dependent manner and enhanced the cAMP elevation. The ability of antibody 29D7 to improve RGCs survival and regeneration in vivo following intravitreal injection was also established. Single antibody 29D7 injection boosted the density and survival of RGCs but to a smaller extent than in the BDNF-treated retinas. A phase 1B topical recombinant human nerve growth factor (rhNGF) randomized controlled study for neuroenhancement in glaucoma concluded the use of rhNGF in a topical 180-g/mL formulation is secure and acceptable. Despite the fact that no statistically significant short-term neuroenhancement was found in this trial, examination of efficacy in a neuroprotection trial is necessary given the potent effects of NGF in preclinical models and the patterns found in this study.80−82
3. Challenges for Ocular Delivery of Protein and Peptide
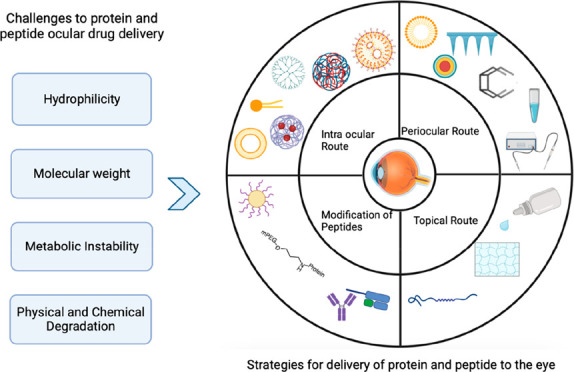
The success of most peptide and protein drugs depends on the ability to deliver the biologically active form at the action site of action. Ocular delivery of protein and peptides’ is challenging due to their poor permeation, large molecular weight, and susceptible to degradation. Proteins and peptides have complex structures and this complexity produces many challenges in their formulation and delivery. Another greatest challenge posed for biopharmaceutical companies is the low stability and short half-life, which leads to loss of activity at physiological pH and temperature.6 The challenges for the ocular delivery of protein and peptides could be either related to the physicochemical properties of proteins or the static, dynamic, and metabolic barriers of the eye.
3.1. Unfavorable Physicochemical Properties of Proteins
The physicochemical parameters of proteins and peptides such as hydrophilicity, molecular weight, and metabolic instability act as a barrier and ultimately lead to a decrease in the activity of formulation.
3.1.1. Hydrophilicity
Proteins and peptides are mostly hydrophilic in nature, hence their permeability through the biological membrane is very low. This directly affects the bioavailability of proteins and peptides. Macromolecules like proteins and peptides cannot be absorbed through simple or passive diffusion. Proteins are absorbed by active mechanisms such as active transport, pinocytosis, or endocytosis. These are the mechanisms by which hydrophilic substances can easily cross the membrane.83,84 The tight junctions of corneal epithelium hinder the permeation of hydrophilic molecules.85,86 Lipophilic molecules can easily pass through the corneal epithelium and collagen fibers present in the hydrophilic stroma. In some circumstances, small peptides are taken into the cells via active transport from the extracellular space, and that mechanism is known as receptor-mediated endocytosis.87 The major disadvantage of the endocytic pathway is the entrapment of proteins and peptides in the lysosomes and endosomes while entering the cell, and this can reduce the cytoplasmic concentration of proteins and peptides. To date, a lot of clinical trials have been conducted in which endocytic pathways were bypassed successfully and proteins and peptides were delivered directly to the cell cytoplasm. These methods involve microinjection and electroporation. By using these methods, it is possible to bypass endocytic pathways and drugs can be directly administered in the cell cytoplasm. However, specialized equipment is required to physically puncture the membrane, which is a cumbersome task to deliver the drug, especially via the oral route due to the acidic environment. Hence, they are administrated through other routes like parenteral (IV, IM, or SC), subconjunctival, and intravitreous. Sometimes the distribution of drugs occurs in normal tissues as a result the amount of the dose required increases leading to an increase in toxicity.6
3.1.2. High Molecular Weight
The molecular weight of protein is another major challenge because it has a direct impact on its permeability. High molecular weight proteins have poor permeability. To overcome this issue, a new approach has been adopted in which highly invasive intravitreal injections are employed as the primary mode of administration of proteins and peptides. Proteins have numerous donors/acceptors for hydrogen bonding with molecular weight generally above 1000 kDa.88 Hydrophilic large molecules cannot diffuse from corneal, retinal, and scleral tight junctions.89,90 Even though the tight junctional space in the conjunctival epithelium is much wider than the cornea, penetration of these large molecules is insufficient.91,92 Their ability to diffuse through the retina is limited only to those molecules whose molecular weight is more than 76 kDa due to their plexiform layers on the inner and outer sides. Macromolecules with a molecular weight greater than 150 kDa cannot reach the inner retina.89 Sometimes the molecules that can traverse through choroid are washed out through choriocapillaris thus reducing their therapeutic concentrations.
3.1.3. Metabolic Instability
The major reasons for the instability of proteins and peptides are complexity in structure, denaturation, adsorption, aggregation, and precipitation. These are the main pathways by which the physical degradation of proteins and peptides occurs which makes them physically unstable. Proteins are converted into inactive forms by pH, subunit proteins dissociation, high salt concentration, temperature, noncovalent complexation with ions, complexation of enzymes and cofactors, and proteolytic degradation through proteases and esterases. Various compounds chemically modify the proteins and degrade them. For example, oxidation of Fe (II) atoms in heme and SH-groups present in sulfhydryl-containing enzymes. Also, the exchange of thiol–disulfide and the breaking of susceptible side chains of methionine and tryptophan are also an example of chemical modification. All these changes result in the inactivation of proteins and peptides.9 In the body, physical and chemical degradation of proteins and peptides occurs through various pathways, which include reduction, oxidation, disulfide exchange, β-elimination, proteolysis, and deamination.93 If “active” confirmation of proteins and peptides is modified, then the activity is lost and aggregation of proteins occurs, which is irreversible. In the parenteral administration of proteins and peptides, half-lives are shortened due to degradation.94 Due to the short half-lives of proteins, maintenance of therapeutic levels of drugs requires the administration of frequent doses. Frequent intravitreal administrations may sometimes lead to complications including cataracts, retinal hemorrhage, and detachment.95Figure 4 describes the degradation pathways for proteins and peptides degradation.
Figure 4.
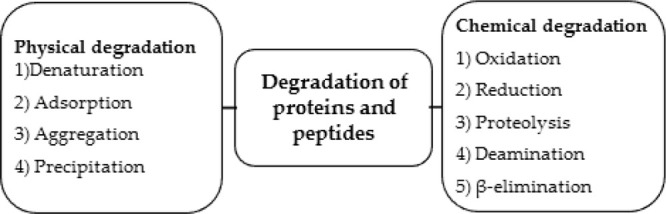
Ways through which proteins and peptides degrade.
3.2. Static and Dynamic Barriers Posed by the Eye
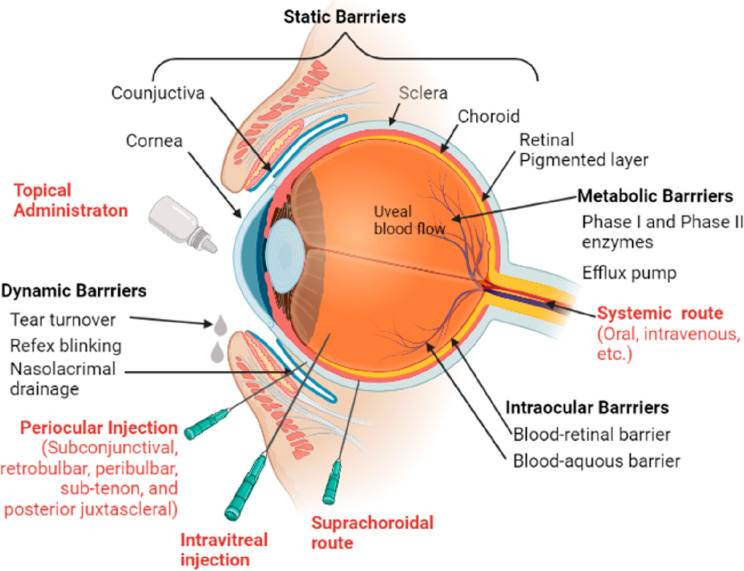
The human eye is one of the complex organs of the body. The tight junctions are present in between the epithelial or endothelial cells of various layers of the eye like the cornea, ciliary muscles, retina, iris, and conjunctiva. These junctions do not allow the drug diffusion. Topical administration results in a loss of the administered dose due to the rapid blinking of the eye (6–15 times/min) and tear turnover (0.5–2.2 μL/min) within 2–5 min. Less than 5% of the administered dose reaches the intraocular tissues because the rate of drug loss from the eye can be 500 to 700 times greater than the rate of drug absorption into the anterior chamber. Most of the administered drugs are washed out from the eye; decreasing the anterior segment bioavailability; by various mechanisms such as nasolacrimal drainage, tear dilution and tear turnover. The physicochemical properties of drug molecules and the delivery mode also affects the permeation and bioavailability of drugs.96,97 A conventional delivery system such as eye drops only achieves 1–3% bioavailability, so designing a formulation approach for targeting the posterior segment of the eye is very challenging. Hence, for the treatment of posterior segment diseases, intravitreal injections are preferred.98,99 Other barriers that prevent the entry of drug molecules into the eye include: the cornea and conjunctiva, blood-aqueous barrier and blood-retinal barrier.
3.2.1. Barrier Properties of Cornea, Conjunctiva, and Sclera
The cornea is composed of (from base to the surface) a layer of endothelial cells, Descemet’s membrane (posterior limiting membrane), stroma, Bowman’s layer (limiting lamina in the anterior part), basement membrane, and an epithelial layer. The cornea is avascular clear tissue.100 The stroma is composed of proteoglycans, keratocytes, and hydrated collagen, and it serves as a barrier for lipophilic drugs. On the other hand, the corneal epithelium acts as a barrier and prevents the entry of macromolecules and hydrophilic drugs into the aqueous humor.101−105 Generally, lipophilic drug molecules permeate through the transcellular pathway (through the cells), while hydrophilic molecules and small ions prefer paracellular route (through pores between the cells). The pore size is approximately 1 nm and permits the movement of drug molecules with molecular weight (MW) less than 700 Da. Large protein molecules find harder to get through the cornea when compared to small molecules. Conjunctiva plays an important role in the entry of macromolecules, nanomedicines, oligonucleotides, and peptides into deeper layers of the eye. However, it has a lesser role in drug absorption when compared to the cornea.106 Conjunctiva is the thin transparent membrane that covers the anterior part of the sclera and is present in the inner eyelid lining.107 It consists of three layers: first and outermost is the epithelium layer, then comes the substantia propria which has nerves, lymphatic, and blood vessels. The last and innermost layer is the submucosal layer, which is attached to the sclera.108 The bioelectrical resistance of conjunctival epithelium’s tight junctions is 1500 Ω·cm2, and it is responsible for controlling the permeation of hydrophilic drugs.109 Through conjunctival vasculature a large quantity of topically administered drugs enters the systemic circulation, hence drugs that are meant for targeting deeper tissue cannot be given via this route.110 For enhancing the penetration of protein, peptides, and other macromolecules, scientists/researchers have targeted the transporters of peptides, amino acids, and nucleosides, which are present in the conjunctival epithelium. Sclera is relatively more permeable when compared to the cornea and conjunctiva as it is mainly composed of a network of collagen fibers, proteoglycans, and glycoproteins in aqueous medium. Permeability of drug molecules through the sclera depends on several factors such as molecular radius, charge, molecular weight, and lipophilicity.2,12 Protein molecules with size greater than 150 kDa find it difficult to permeate through the sclera.111,112
3.2.2. Blood-Aqueous Barrier
The endothelia of nonpigmented ciliary epithelium and the iris-ciliary blood vessel have tight junctions which serve as a barrier known as the blood-aqueous barrier (BAB).113−117 BAB assists in the maintenance of transparency and chemical composition of intraocular fluids. Via the BAB, lipophilic drugs that are small in size enter the blood circulation of the uvea and are subsequently eliminated by the aqueous humor turnover.96 BAB restricts the entry of plasma proteins into aqueous humor making aqueous humor optically clear and essentially protein-free. For topically applied drugs, permeation from the anterior to posterior segment is limited due to the turnover phenomenon caused by the aqueous humor whose rate ranges from 2 to 3 mL/min.118−120 Generally, drug molecules with lower lipophilicity can permeate better through BAB when compared to drug molecules with higher lipophilicity.
3.2.3. Blood-Retinal Barrier
The retina is a light-sensitive thin film tissue made up of glial and neural cells. The eye’s innermost surface is covered by the retina. Intravitreal or intravenous injection can be given for drug delivery to the retina. However, for intravenous injection, a high dose should be given and only a small amount gets to the posterior segment of the eye because of the blood-retinal barrier (BRB).121 BRB consists of two types of cells: retinal pigmented epithelium (RPE) cells and retinal capillary endothelial (RCE).122 The retinal outermost layer is made up of RPE, which contains a single layer of cuboidal cells, and its main function is to manage the drug transport between the retina and choroid.123,124 Selective transport through the tight junctions present in the RCE protects the retina. Proteins, peptides, and small hydrophilic drugs have low permeability through the RCE.125 Ongoing research efforts are dedicated to the development of novel approaches with increased bioavailability, safety, and efficacy of ophthalmic drugs.126
3.3. Enzymatic Barriers
Generally, ocular tissue contains several enzymes like protease and aminopeptidase that are responsible for the degradation of protein and peptide molecules. Absorption of peptide-like large molecules is reduced in the ocular region because of peptidase-like enzymes, which metabolize the drug and decrease the bioavailability of protein and peptide molecules. Some endopeptidases [plasmin, collagenase] are also present in ocular tissues and fluids.127 For instance, Erb et al. demonstrated that there was a complete degradation of methionine enkephalin and 90% degradation of leucine enkephalin through hydrolyzation due to aminopeptidase within 5 min of instillation in the rabbit’s corneal epithelium.127
3.4. Formulation Issues
The key challenges in developing protein and peptide-based formulations as biotherapeutic agents are their structural properties and environmental factors. Agents like polysaccharides (dextrans) and sugars (trehalose) are incorporated with proteins and peptides to enhance their bioavailability.128,129 Proteins and peptides tend to form agglomerates which can be averted using low concentrations of Pluronic and nonionic surfactants such as polysorbates.130 Protein and peptide formulations tend to have a high viscosity to a variable degree. In topical ophthalmic formulations, contact time with the cornea can be increased by increasing viscosity up to 20 cPs (cP), but if the viscosity is increased further then it leads to activation of reflux blinking and tearing to reestablish the normal lachrymal viscosity, i.e., (1.05–5.97 cP). FDA does not allow the administration of large doses of protein formulations through the intravitreal route. Protein formulations when prepared in larger doses result in a concentrated formulation with higher viscosity. Hence, studies characterizing the delivery factors like the time required to complete the injection (syringeability) and forces needed to deliver the formulation with suitable needles (18 mm in length, 27–30G) are crucial. For such formulations, viscosity can be decreased by adding hydrophobic/inorganic salts or lysine and arginine.131 The pH of formulation should be the same as the pH of the lacrimal gland so that maximum activity is obtained. However, proteins and peptides are unstable at the physiological pH and get denatured by folding or aggregation. Considering the pH-dependent stability of proteins and peptides, buffers play a crucial role in preserving and maintaining their activity. However, the buffer capacity should be adequately maintained to stabilize the protein, while minimizing unwanted reactions. The high buffer capacity of the instilled fluid would tend to resist pH alteration by tear fluid to a greater degree and subsequently affect the drug absorption. Further, a hypertonic solution administered intravitreally can evoke anterior chamber transient desiccation, while a hypotonic solution can lead to edema and corneal clouding.6
4. Strategies to Enhance the Ocular Bioavailability of Proteins and Peptides
Generally, in the case of ocular administration of hydrophilic molecules such as proteins and peptides, bioavailability is a major challenge. The major hurdles during the formulation of proteins and peptides as biotherapeutic agents are related to their large molecular weight, metabolic instability, and hydrophilic nature. So, to overcome these difficulties and increase their bioavailability, the following methods are useful.
-
A
Selecting the optimal route of administration of protein and peptide
-
B
Protein and peptide modification
4.1. Selecting the Optimal Route of Administration of Protein and Peptide
Various routes for ocular protein and peptide administration are shown in Figure 5.
Figure 5.
Barriers to ocular drug delivery along with routes of ocular drug delivery. Reproduced with permission from Patel C et al.,132 CC BY 4.0.
4.1.1. Topical Route
Generally, drugs administered topically should follow corneal, conjunctival, or scleral pathways for absorption. Some of the limitations of this route include washout of drugs from the precorneal area, enzymatic degradation, limited dose administration (approximately 30 μL), and high clearance.133 The large size of protein and peptide drugs hinders their movement into ocular tissues when compared to small drug molecules. Typically, less than 1% of topically administered macromolecules enter the eye even with multiple doses per day.134 To overcome these problems bioadhesive polymers are used as they decrease the precorneal clearance and increase surface contact time with the cornea. To check the efficacy of bevacizumab drug through a topical application in patients with corneal neovascularization a study was conducted. As per the study, three patients were administered 10 mg/mL of bevacizumab twice a week. No ocular or systemic adverse effects and excellent efficacy were reported. In all three patients, bevacizumab prohibits further growth of corneal neovascularization and also regressed the disease. This study demonstrated that the topically long-term use of bevacizumab is safe as well as beneficial for patients with corneal neovascularization.135 Topical administration of drugs in the form of an eye fails to deliver drugs to the retina despite loading them in contact lenses that effectively increase drug residence time. For instance, a drug-eluting contact lens failed to deliver the required concentration of ranibizumab into the retina despite extended use for several days.136 Macromolecules administered systemically should overcome first-pass metabolism and the blood-aqueous/retinal barriers in order to reach the eye. Studies indicate that less than 0.1% of drugs administered systemically reach the eye.136 As a result, intraocular injections remain the most popular and effective administration route for delivering macromolecules.
4.1.2. Periocular Route
In this route, the drug reaches through the trans-scleral pathway to the choroid. One of the popular periocular routes is drug administration in the subconjunctival area, which is a space underneath the conjunctiva. In the periocular route, the drug has to pass through barriers like scleral thickness, choroidal blood circulation, and BRBs. The advantage of this route is that up to 500 μL of the drug, the solution can be delivered and this mode of administration may result in sustained effect.133 Multiple subconjunctival injections of conbercept which is useful as a therapy for pterygium surgery were found to be very safe as well as efficacious. The study was conducted on 96 patients by giving them 3 subconjunctival injections of conbercept (with 0.2 mL) and 5 subconjunctival injections of 5-fluorouracil (with 0.2 mL). All the study data were collected from the fifth day after pterygium which is taken as a baseline to 2 to 4 weeks of postoperation.137 Sub tenon injections are yet another popular mode of administration. In one of the studies, a 0.1 mL volume containing 2.5 mg of bevacizumab was administered using a subtenon injection in macular edema patients. The study reported an improvement in short-term vision in the eyes.138 Nanosize formulations may improve the diffusion of drugs. The combination of micro or nanoparticles with physical techniques such as ultrasound may help deliver a sufficient concentration of protein and peptide molecules.139
4.1.3. Intraocular Route
The intraocular route contains drug administration directly to the target site without passing through any tissue membrane and due to the bypass of membranes increase in bioavailability can be seen. Intraocular route is further classified into the following.
4.1.4. Intravitreal Route
Intravitreal administrated injections are given directly in the eye’s posterior segment (vitreous humor) and these are often in the form of suspension or solution. In the vitreous cavity accumulation of drugs is done up to a volume of 20–100 μL without affecting visuality. The elimination of drugs is decreasing due to the large molecular weight of proteins and peptides. Novel delivery methods and sustained-release formulations which contain proteins and peptides are employed for enhancing bioavailability.133 In the condition like neovascular age-related macular degeneration as per the study administration of faricimab (6 mg) through the intravitreal route successfully enhances the interval between two doses which shows its excellent sustain release property with efficacy which ultimately decreases the burden of treatment on the patient.140
4.1.5. Intrastromal Delivery
It involves the delivery of drugs directly into the corneal stroma so that the tear fluid drainage and corneal epithelial barrier can be bypassed easily. Alike other intraocular routes, the major advantage is barrier bypass.141 Ucgul et al. demonstrated in one of his studies that intrastromally administered anti-VEGFs for the treatment of corneal neovascularization are more beneficial compared to subconjunctival administration of anti-VEGFs. The study involved 24 New Zealand white rabbits that were divided into 4 groups, each containing 6 rabbits. Bevacizumab and aflibercept were used. In the first group of 6 rabbits, bevacizumab was given through the intrastromal route, followed by subconjunctival administration in the other group. Aflibercept was administered through the intrastromal route in the third group and by the subconjunctival route in group 4. The study results showed that there was an 82.5% and 88.1% reduction in corneal neovascularization for bevacizumab and aflibercept, respectively, after intrastromal administration. However, this number was only 69.9% and 64.5% for bevacizumab and aflibercept, respectively, after subconjunctival administration; hence, this concludes that intrastromal administration of anti-VEGF drugs has more effectively regressed corneal neovascularization compared to subconjunctival administration.142
4.1.6. Intracameral Delivery
Intracameral injections are made directly into the anterior part of the eye. When intracameral and intrastromal injections are given in combination, the fungal growth in the anterior region was reduced compared to conventional therapy. Additionally, it also reduced the invasion of fungus in the corneal and prevented the corneal perforation caused by the fungus.143 Intracameral delivery of bevacizumab (1.25) mg dose significantly improved trabeculectomy success rate compare to intraoperative mitomycin C. However, intracameral delivery increased the chance of early filtering bleb leakage.144
4.1.7. Suprachoroidal Delivery
The drug is injected into the suprachoroidal space, a space located between the choroid and sclera. Up to 1 mL of the drug solution or suspension can be delivered in the suprachoroidal space.124 A study was conducted to assess the distribution and safety of bevacizumab through the suprachoroidal route in rabbit eyes. Bevacizumab showed high efficacy and excellent bioavailability with the rapid distribution. Compared to intravitreal injection, suprachoroidal administration has 40 times higher Cmax value, i.e., 1043 ± 597 μg/g in choroid and retina. After 1 day of suprachoroidal administration of bevacizumab, it was detected in the posterior part of the eye with a two times lower concentration. After 1 week of bevacizumab administration, the concentration reduced from 1043 ± 597 μg/g (Cmax) to 2.36 ± 1.32 μg/g. Moreover, there were no adverse effects, such as a change in retinal function, inflammation, hemorrhages, retinal detachment, and suprachoroidal blebs, were reported up to 2 months after administration. The intraocular pressure was increased by more than 16 mm of Hg soon after suprachoroidal administration but returned to normal after 10 min. The major benefit of suprachoroidal delivery of bevacizumab was its rapid distribution in choroid layers and RPE, safety, and minimal invasiveness.145
4.2. Protein and Peptides Modification
Intracameral, suprachoroidal, intrastromal, and intravitreal injections are some of the intraocular techniques that are utilized for successful protein and peptide delivery through the ocular barriers. Of all these, the intravitreal is the most preferred method for the administration of protein and peptides to the posterior segment of the eye. No matter which injection is utilized for the delivery, the drug is cleared rapidly by the anterior aqueous humor and the posterior transretinal elimination pathways.146 As a result, repeated and frequent doses are required, which in turn increases the burden on patients and physicians. In addition, repeated injections are associated with adverse effects after each dose administration.6,147 These challenges can be overcome in two ways.
4.2.1. Chemical Modification
Clearance can be reduced and circulating half-life can be enhanced by chemically modifying the protein using hydrophilic polymers as this will improve their hydrodynamic diameter. An example of this technique is PEGylation. In the PEGylation approach, polyethylene glycol (PEG), a polymer approved by the FDA, is covalently attached to the sulfhydryl (−SH2) or primary amino (−NH2) groups of peptides and proteins. The immune response is reduced and biological activity is enhanced when PEG chains having a molecular weight from 5 to 40 kDa are used. Many PEGylated drugs, such as Pegaptanib, are approved by the FDA and are currently on the market.148 Large molecular weight PEG units are conjugated to a drug, increasing the hydrodynamic volume relative to the free drug and perhaps extending the residence period. For instance, Pegaptanib (Macugen), an anti-VEGF RNA aptamer conjugated with a high molecular weight (40 kDa) of PEG for the treatment of neovascular AMD, demonstrated extended tissue retention as a result of the molecular size increase. Another illustration is Pegcetacoplan, a cyclic peptide that is PEGylated to block complement C3 and contains a large molecular weight PEG moiety (40 kDa) for extended residence time.65 The intravitreal injections of 15 mg Pegcetacoplan monthly or every other month for a year produced a strong therapeutic benefit with AMD patients. Using a self-cleaving linker, Machinaga et al. attached small medicines to high molecular weight 4-arm PEG (40 kDa) while demonstrating a long cleavage half-life. Due to the prolonged retention period and gradual release of the free medicines, intravitreal injection of these PEGylated medications had a long-lasting effect on the rabbit vitreous.149 Apart from PEGylation, alternative approaches like negative charge on glycosaminoglycan HA, hydroxyl ethyl starch, and sialic acid also can enhance the half-life of protein and peptide and are currently under investigation.6
4.2.2. Genetic Engineering-Based Modifications
Fc fragment of the “IgG receptor and transporter” (FCGRT) gene in humans encodes for neonatal Fc receptor (FcRn) that is structurally similar to the major histocompatibility complex (MHC). A novel genetic fusion-based formulation development is using FcRn because of its potential to protect albumin and IgG from catabolism. Therapeutic protein and peptides’ half-life can be improved by using the FcRn approach because of its high expression in various ocular tissues like lens epithelium, conjunctiva lymphatic vessel, retinal blood vessel, optic nerve, blood vessel, nonpigmented ciliary epithelium, corneal epithelium and endothelium.150,151 Until now very few attempts to modify albumin- and IgG-FcRn interactions have been documented. The documented attempts have mutated the Fc-domain amino acid residues near the FcRn binding site and the albumin/antibody half-lives are increased by engineering the albumin- and IgG-FcRn interaction.152 Two amino acid residues of bevacizumab were mutated in a study done by Zalevsky and co-authors that showed a ∼11-fold increase in the binding affinity at pH 6 for the human FcRn gene.153 Bispecific antibodies (bsAb) are the antibody that can target both PDGF and VEGF pathways, this has improved AMD treatment. Despite the success, the bsAb is not clinically used because of its immunogenicity, processing, and manufacturing issues.6
5. Novel Approaches for the Delivery of Protein and Peptides
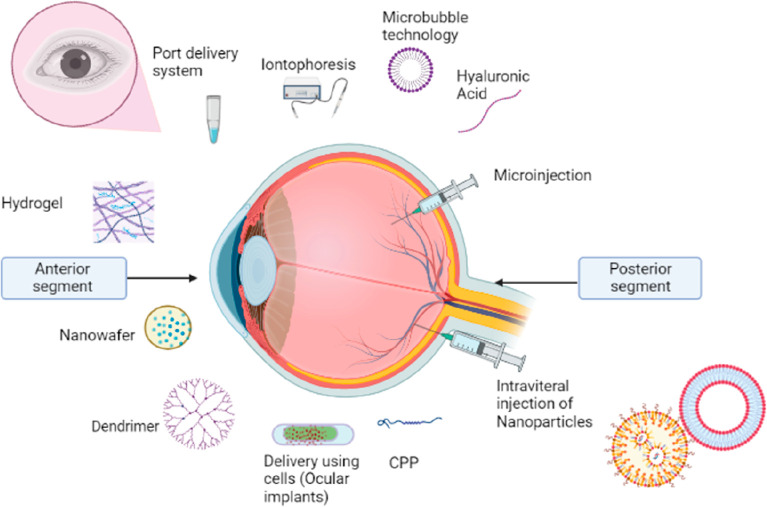
Over the last 20 years, the ophthalmology market has developed substantially because of the demand for newer therapeutic methods for the treatment of chronic ocular illnesses.154 The introduction of the antivascular endothelial growth factor (anti-VEGF), aptamer pegaptanib sodium, and monoclonal antibody ranibizumab in the early 21st century accelerated the development of proteins and peptides in the ophthalmic market. It is anticipated that the sales of biological medicines for ocular ailments would reach 35.7 billion US Dollar (USD) globally by the end of the year 2025.155 Protein therapies demonstrated tremendous potential for the treatment of ocular ailments, with benefits such as higher potency, low toxicity, decreased drug–drug interaction, and improved chemical and biological diversity. However, the distribution of these macromolecules is limited by degradation, limited permeability, short half-lives, and immunogenicity. So, it is crucial to develop novel ocular delivery systems for proteins and peptides in order to efficiently deliver these molecules to biological tissues.155−157 The application of nanotechnology for ocular therapy has produced positive results.158−162 Nanoparticulate systems can be used to entrap or encapsulate protein and peptide drugs. Further, these molecules can be adsorbed or covalently bonded to the nanosystem.160,163 Many types of nanoparticulate systems with distinct properties such as polymeric nanoparticles, dendritic structures micelles, solid lipid nanoparticles, nanostructured lipids, lipid nanocapsules, and liposomes, have been studied for the delivery of water-soluble macromolecules.161 Nanotechnology could enhance bioavailability by improving its drug solubility and permeability.164 In addition, the drug which is encapsulated in the nanosystems could make the drug less apparent to the immune system and provide sustain release.164−167 Nanosized carriers can defend encapsulated peptide drugs from enzymatic degradation and from tear turnover and thus provide sustained release of drugs. In addition, employing a mucoadhesive polymer in the preparation of nanocarriers enables the complex to adhere to the corneal epithelium for a long time.168 This strategy is primarily used for the monoclonal antibodies which are in clinical use for some time.169 Some of the most popular novel approaches for the ocular delivery of protein and peptides are shown in Figure 6.
Figure 6.
Novel approaches for ocular delivery of protein and peptides.
5.1. New Formulations and Nanosystems for the Delivery of Proteins and Peptides
5.1.1. Co-administration with Permeability Enhancer
These are various groups of agents that are co-administrated with protein and peptides because of their property of increasing either aqueous solubility or permeation of protein and peptides which leads to enhanced bioavailability. The following are groups of agents used:
5.1.1.1. Cyclodextrins
Cyclodextrins (CD) are truncated cone-shaped oligosaccharides that are water-soluble in nature. α-CD, β-CD, and γ-CD are naturally occurring cyclodextrins. The major difference between these naturally occurring cyclodextrins is in the number of “α-(1–4)-linked glucopyranose” subunits (six, seven, and eight, respectively).170 Semisynthetic derivatives like sulfobutylether β-CD, hydroxypropyl-β-CD, hydroxypropyl-γ-CD, and randomly methylated β-CD have been developed over a decade and have significantly enhanced properties like aqueous solubility.171 Cyclodextrins consist of hydrophilic hydroxyl functional groups attached to the external surfaces of their molecule and lipophilic Cavities inside it.172 A lipophilic drug that has a poor aqueous solubility can be entrapped inside the hydrophobic cavity, and weak hydrophobic interactions are seen between them but not bound covalently. In the cavity, they are protected and the complex is called the guest–host inclusion complex.173 The drug in the complex is allowed to interact with the corneal epithelium-like lipophilic membrane by the cyclodextrins as they cannot permeate through this membrane because of their large size. Cyclodextrins are considered GRAS (generally regarded as safe) molecules because of this they have various applications in the pharmaceutical industry for improving the drug’s bioavailability, and stability, enhancing solubility, and masking drug irritation effects.171 Formation/dissociation of the drug-CD inclusion complex is a dynamic process and, in this process, spontaneous drug uptake and release occur in the aqueous environment.174,175 The drug release from the complex and its absorption to the epithelial membrane occurs in the aqueous environment of tear film when lipid extraction causes a temporary disruption of the member due to cyclodextrins-members interactions.170,171 Laura Lorenzo-Soler et al. developed microsuspension-based eye drops of 3% cediranib maleate and γ-cyclodextrin through the autoclaving method. While autoclaving the drug degradation was prevented by the addition of heat stabilizers. The prepared eye drops were then administered in rabbits and cediranib concentration was measured after 3 h. Approximately, 10 ± 6 nM and 737 ± 460 nM of cediranib were found in the vitreous humor and retina, respectively. Cediranib levels obtained in the retina were 100 times higher than the reported IC50 value of the type-II VEGF receptor. This study demonstrated the ability of cyclodextrin to act as a permeability enhancer for ocular protein and peptide delivery.176
5.1.1.2. Chelating Agents
Corneal epithelium acts as a highly resistant barrier to all hydrophilic molecules e.g., proteins and peptides. The resistance to the movement of hydrophilic molecules is mainly due to the intercellular tight junction existing between epithelial cells which hinders paracellular transport. The activity of the tight junctions is dependent on the undetermined calcium ions availability.171,177 Chelating agents, specifically those which binds to the calcium ions, are used as formulation stabilizer. The use of chelating agents leads to disruption of adherents and tight junctions due to interstitial calcium ions segregation, hence resulting in the loss of barrier properties of the epithelium.171 Examples of calcium chelating agents include ethylene glycol-bis(β-aminoethyl)-N,N,N′,N′ tetraacetic acid (EGTA), ethylenediamine-N,N,N′,N′-tetraacetic acid (EDTA), ethylenediamine-N,N′-disuccinic acid (EDDS), and 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA). The major drawbacks of these agents are their toxicity implications following long-term use. Studies have shown that EDTA gets accumulated in the ciliary body and iris and also affects uveal tract-related endothelial cells and capillaries.178 Despite its extensive use in ocular delivery, chelating agents are not widely used for the ocular delivery of protein and peptides. However, there are studies in which chelating agents are used to enhancing the ocular delivery of bioactive proteins. For instance, in one study IFNβ was coupled with dextran using diethylenetriaminepentaacetic acid (DTPA), a chelating agent, for the treatment of choroidal neovascularization (CNV) in rabbits. IFNβ-dextran-DTPA and free IFNβ were administered for 4 weeks. The results reported that the complex had successfully inhibited the progression of CNV whereas free IFNβ had no significant effect on CNV.179
5.1.1.3. Surfactants
Surfactants are compounds that act on the surface present between aqueous and nonaqueous mediums, where they decrease the interfacial surface tension. Surfactants contain both hydrophilic and lipophilic moieties.171 In the pharmaceutical industry, they are generally used as an excipient that either increases the solubility of formulation components or enhances the permeability of the drug by altering the permeability of the membrane. In the eye the membrane permeation is altered by various methods like disrupting mucin and tear film, abolishing their protective properties, loosening the continuous intercellular tight junctions, or modifying the cell membrane of epithelium which leads to the annihilation of membrane integrity. The specific properties of surfactants and their type are determined by the polar group present in them. Based on the polar group, they have been classified into 4 groups: anionic, cationic, nonionic, and zwitter ion. Anionic surfactants have a negative polar group while cationic have a positive polar group and zwitter ion has both positive and negative charges. The charge present on the zwitter ion is dependent on the environmental conditions. The nonionic surfactants as the name suggest do not have any charge on their polar group, they are the preferred compound for the ocular delivery of drug as they enhance the formulation stability, drug solubility, biocompatibility, and also has very low toxicity compared to all the other type of surfactants.180,181 Bija et al.182 conducted a study to demonstrate the suitability of rabbit cornea as a substitute for the human cornea in in vivo applications. This group investigated the permeability-enhancing effects of 20% dimethyl sulfoxide (DMSO) and 0.01% benzalkonium chloride. The findings indicated that these substances effectively increased the permeability of cyclosporin A through the rabbit corneas. Sakshi et al.183 conducted a study using Saponin, benzalkonium chloride, EDTA, and paraben for permeation of luteinizing hormone-releasing hormone and thyrotropin-releasing hormone through rabbit’s cornea and conjunctiva. In this, two enhancers were surfactants, these were saponin (a natural surfactant) and benzalkonium chloride (BAC) (a cationic surfactant). It was reported that saponin 0.5%, BAC 0.05%, and BAC 0.1% enhanced the permeability of both hormones. Overall, all the penetration enhancers increased the permeability through the cornea more compare to the conjunctiva. While surfactants have been popularly used as solubility enhancers and permeation enhancers in ophthalmic systems, concerns related to toxicity/irritation of surfactants remains as a concern.
5.1.1.4. Other Amphiphilic Compounds
Many fatty acid substances are also utilized for facilitating drug permeation. They act by changing cell-membrane properties and by acting on junctions between tissues by making them loose. Clear solution-forming semifluorinated alkanes (SFAs) are amphiphilic compounds that have been used for ocular protein and peptide delivery.171 Agarwal et al.184 conducted a study comparing the topical ocular delivery of cyclosporin A (CsA) through two commercially available emulsions, Ikervis and Restasis, and two SFAs, perfluorobutylpentane (F4H5) and perfluorohexyloctane (F6H8). Corneal permeability was assessed by calculating the area under the curve (AUC) for 4 h and plotting a graph of time versus corneal concentration of CsA per gram of cornea (ng/g). The results showed that a single dose of CsA (0.05%) in F4H5 and F6H8 had nearly 8-fold higher permeability compared to Restasis. Apart from that, the permeability of 0.1% CsA in F4H5 is also approximately five times higher compared to the permeability of CsA from Ikervis. This study demonstrated that fatty acids substance has their application ocular delivery of protein and peptides.
5.1.2. Nanoparticles Based on Polymer
Polymeric nanoparticles are versatile drug delivery platforms with the ability to protect their cargo from rapid degradation, sustain the drug release, increase the drug half-life (t1/2), penetrate physiological barriers, and deliver the drug to the target cells by either passive or active targeting mechanisms.185
5.1.2.1. Poly(lactic-co-glycolic Acid) (PLGA) Based Nanoparticles
Poly(lactic-co-glycolic acid) PLGA has been studied extensively in polymeric nanoparticles because of its ability to transport molecules effectively. The biodegradability and biocompatibility of PLGA is the reason why it is being employed in drug delivery systems. PLGA is made up of lactic acid and glycolic acid.186 Intravitreal injection of bevacizumab showed similar efficacy and significant cost reduction compared to ranibizumab. Consequently, most efforts have been devoted to the creation of nanoparticles as carriers for bevacizumab.187,188 The size of bevacizumab-loaded PLGA nanoparticles is between 200 and 300 nm189−194 or smaller193 when prepared by a double emulsion solvent evaporation technique. The activity of protein and peptide drugs entrapped inside nanoparticles may alter with the formulation method, excipients used, and aggregate formation. For instance, the concentration and activity of bevacizumab decreased dramatically when it is encapsulated by the double-emulsion solvent evaporation method. To protect bevacizumab performance, several additives were explored. Albumin showed promising and most effective protection of bevacizumab against emulsification stress during the preparation of nanoparticles with a particle size of 197 nm, narrow size distribution, negative zeta potential, and higher encapsulation which is 82.4%.190 Bevacizumab encapsulation into PLGA nanoparticles prolonged the residency of bevacizumab in the vitreous and aqueous humor. Further, PLGA encapsulation had no significant toxicity effect in vitro and in vivo. Bevacizumab-encapsulated in PLGA nanoparticles showed significant antiangiogenic efficiency for treating corneal and retinal neovascularization.193 The effectiveness of bevacizumab-loaded PLGA nanoparticles was investigated on corneal and retinal neovascularization in mice. The maximum concentration of bevacizumab in the vitreous was reached 7 days after injection of bevacizumab-PLGA nanoparticles. No evidence of ocular toxicity was observed following the ocular injection of PLGA nanoparticles. The distribution in the posterior segment was seen with a reduction in concentration after 7–21 days of administration. The maximum concentration of bevacizumab in the vitreous and aqueous segments was observed after 6 days following the administration of bevacizumab-loaded PLGA nanoparticles. This clearly indicates that PLGA nanoparticles could prolong the residency of bevacizumab and produce long-lasting drug concentration compared with bevacizumab solution.193 A similar outcome was reported with bevacizumab nanoparticle-based on mesoporous silica, in which the maximum concentration was achieved after 7 days of administration.195 In different studies, the structure of bevacizumab after encapsulation in PLGA nanoparticles and after the drug release from PLGA nanoparticles was investigated. The secondary structure of bevacizumab is dominated by β-sheets (typical IgG), which may result due to the lyophilization procedure used in the formulation of PLGA nanoparticles as removal of water in lyophilization results in the development of intermolecular sheets. Bevacizumab exhibited conformational changes when combined with PLGA nanoparticles. Moreover, refolding of its structure might occur after release from nanoparticles as evidenced by the circular dichroism spectra of released bevacizumab, which was identical to the spectrum of native bevacizumab. For the long-term stability of bevacizumab-PLGA nanoparticles prepared by lyophilization, a study was conducted employing trehalose and bevacizumab for coencapsulation. A 10% w/v trehalose coating was done on nanoparticles to preserve the physical and chemical properties of the nanoparticles as well as the secondary and tertiary structure of bevacizumab. The antiangiogenic efficacy was maintained for at least six months.196
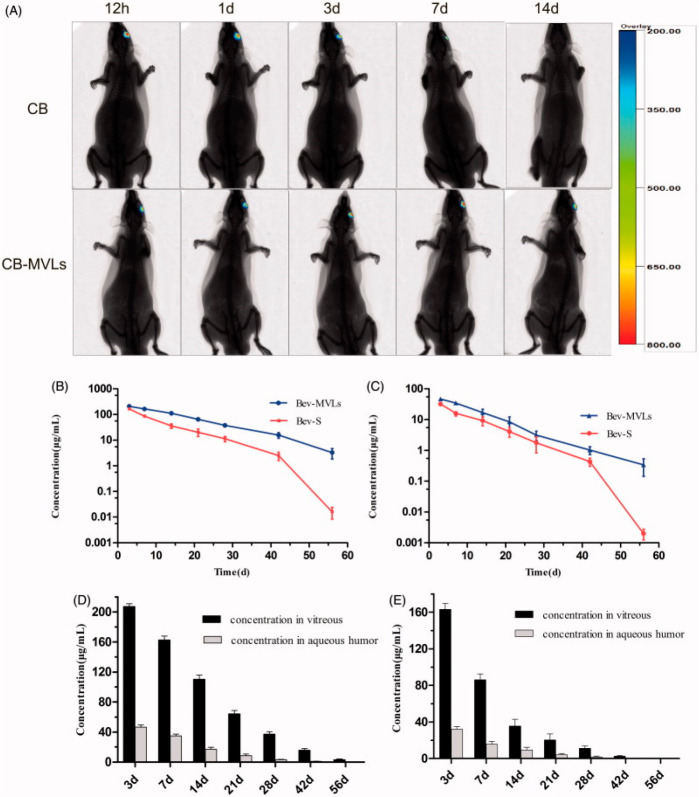
In an alternative approach, bevacizumab-coated polylactic acid (PLA) nanoparticles (265 nm) were encapsulated into the porous PLGA microparticles (11.61 μm). The VEGF-165 binding activity as well as the physical and chemical stability of bevacizumab was found to be preserved at 37 °C during the 4-month investigation. Additionally, no detectable aggregates of protein were found until the end of the study. Bevacizumab PLA nanoparticles inside the porous PLGA microparticles showed higher sustained distribution in the vitreous segment of rats after intravitreal injection, with a concentration of 21.1 μg/mL on day 1 and 13.96 μg/mL on day 45. After 2 months, the drug presence in the sclera, choroid-retinal pigment epithelial, vitreous, and lens tissue suggests sustained drug delivery.194 Coating of PLGA nanoparticles with mucoadhesive polymers such as chitosan has been investigated for topical and periocular routes of delivery. Chitosan is a hydrophilic polysaccharide with cationic properties and is commonly used in ophthalmic preparations as it can strongly bind with negatively charged cellular surfaces of the conjunctiva and corneal surface. Bevacizumab PLGA nanoparticle coated with chitosan exhibited very sluggish and steady release of the drug.192 Bevacizumab inhibited endothelial cell proliferation in vitro, whereas nanoparticle formulation inhibited VEGF-induced endothelial cell proliferation, migration, and tube formation more effectively than antibody solution.189 Recently, there was no significant in vitro or in vivo cytotoxicity or tissue harm in the case of bevacizumab encapsulated PLGA system. For treating corneal neovascularization and retinal neovascularization in an oxygen-induced model of retinal angiogenesis, it showed improved in vivo antiangiogenic efficacy of bevacizumab. Therefore, the scientist concluded that this formulation of BEV-PLGA-NPs could boost the bioavailability and decreases the toxicity of the drug during ocular angiogenesis. Similarly, the mesoporous silica-based nanoparticle demonstrated a higher antiangiogenic effect in in vitro assays of VEGF-induced endothelial cell proliferation. In a different study, mesoporous silica-based inorganic nanoparticles with layered double hydroxide (LDH) (SiO2@LDH-DOX) were formulated for the targeting of VEGF. SiO2@LDH-DOX NPs were modified with bevacizumab in order to minimize the toxicity of the DOX to the healthy surrounding structures and tissues. These modified nanoparticles will increase its targeting potential and will aid in the antiangiogenic properties provided by doxorubicin. The formulation showed an average diameter of 253 ± 10 nm. For the comparison between SiO2@LDH-BEV-DOX and SiO2@LDH-DOX confocal microscopy was utilized. Modified NPs were accumulated quickly into the nuclei and in higher quantity than that unmodified NPs, indicating their accurate targeting VEGF efficiency.197 Though PLGA NPs offer several advantages, they suffer from drawbacks such as the initial burst release of drugs, which may result in toxicity.198 Further, unanticipated inflammatory and immune responses were reported in the vitreous and retina following intravitreal injection due to the acidic degradation products from PLGA polymer.199 Several studies were reported in literature with an intent to sustain the release of bevacizumab using nanocarrier systems. PLGA nanoparticles of bevacizumab resulted in more than 40% of drug release in the first 2 h in a medium of phosphate buffer saline, followed by a continuous release over the next week and delayed release for up to 3 weeks.193 Bevacizumab PLGA nanoparticles coated with chitosan exhibits very sluggish and steady release that has not attained complete release for 3 days (maximum 25%).192 In an ex vivo study employing rabbit vitreous, bevacizumab PLGA nanoparticles showed burst release (10.3%) of encapsulated dose, followed by a gradual drug release.200 Bevacizumab PLA nanoparticles encapsulated within porous PLGA microparticles produce sustained release of bevacizumab in vitro, with a cumulative release of 67% to 81% after 4 months.194 PLGA nanoparticles have also been examined as a carrier system for aflibercept. About 75% of the drug was released after 7 days from the spherical nanoparticles, whereas the aflibercept solution released its whole payload in 24 h.201
The combination of PLGA and magnetite nanoparticles (Fe3O4) have been reported in literature as smart carriers of drugs. Efficient inhibition of the tube formation was shown by ranibizumab-conjugated iron oxide (Fe3O4)/polyethylene glycol-poly lactide-co-glycolide (PEG–PLGA) in the Matrigel-based assay method using human umbilical vein endothelial cells.202 Surface modification of the PLGA nanoparticles can enhance their ocular performance. As matter of fact, chitosan-coated BEV-PLGA-NPs resulted in an increase in the mucoadhesiveness of the system with pig mucin suspension. This resulted in enhanced scleral permeation, due to interaction between the negatively charged scleral surface and the positively charged amino group present in chitosan coating. This coated nanoparticulate systems resulted in nonirritant and well-tolerated by the chorioallantoic membrane, indicating safe ocular administration. Similarly, for VEGF targeting for both the diagnostic and therapeutic pathways, Goel et al. formulated sunitinib-loaded mesoporous silica nanoparticles. Sunitinib was selected because of its ability to inhibit receptor kinase. The surface of nanoparticles was modified with polyethylene glycol, anti-VEGFR ligand VEGF121 and radioisotope 64Cu. The determination of morphological changes after surface modification was done by the transmission electron microscope (TEM). More efficient targeting was achieved in the case of thiolated surface-modified VEGF sunitinib NPs as compared to nonsurface-modified NPs. Higher drug accumulation within the targeted site was achieved with little to no interaction with neighboring structures and cells.203
In another study, cetuximab (CTX) conjugated docetaxel (DTX) loaded nanoparticles were formulated by Patel et al. DTX is associated with toxicity and limited aqueous solubility. As compared to DTX nanoparticles and free drugs, CET-DTX-NPs showed highest reduction in proliferation. Higher intracellular uptake was achieved due to specific binding affinity of CET with EGFRs. These nanosystems showed site-specific sustained drug delivery when compared to DTX NPs. Enhancement of the safety margin was observed with the reduced dose-dependent toxicity.204 Though PLGA NPs offer several advantages, they suffer from drawbacks such as the initial burst release of drugs, which may result in toxicity.205 Further, unanticipated inflammatory and immune responses were reported in the vitreous and retina following intravitreal injection due to the acidic degradation products from PLGA polymer.206
5.1.2.2. Chitosan-Based Nanoparticles
Chitosan is a biocompatible, mucoadhesive, and biodegradable polymer widely used in ocular drug delivery. Due to its positively charged amino group, it can bind electrostatically with the negatively charged mucous layer.207 Cyclic peptide cyclosporine-loaded chitosan nanoparticles were investigated for the ocular mucosa. Upon topical instillation to rabbits, a therapeutic level of cyclosporine was achieved in the external ocular segment including cornea and conjunctiva for a minimum of 48 h with maintaining negligible to undetectable levels in the inner segment.208 Bevacizumab-loaded chitosan nanoparticle of 188 nm size showed a loading efficiency of 38% and the in vitro drug release lasted up to 21 days. Upon subtenon injection administration system penetrated into the sclera and achieved a high level of intravitreal concentration of peptide and did not show any significance allergic or inflammatory reaction.209 Similarly, bevacizumab chitosan nanoparticles formulated by emulsification evaporation method with a particle size of 90 nm were investigated for the production of VEGF and VEGF mRNA in the retinas of diabetic rats. Findings revealed that BEC–CS-NPs have a longer duration of action and lower VEGF expression than a solution.210 Chitosan-based nanoparticles ranging from 17 to 350 nm with ranibizumab as carrier drugs in the PLGA microparticles were investigated separately. In one study, nanoparticles incorporating chitosan N-acetyl l-cysteine, the system exhibited 69% of entrapment efficiency, and delayed drug release was released. The addition of tripolyphosphate (TPP) to this system accelerated protein release and reduced entrapment efficiency. Ranibizumab release form all systems protected its structural integrity and activity.211
5.1.2.3. Human Serum Albumin (HSA) Based Nanoparticles
Protein nanoparticles were also explored in ocular drug delivery as they offer several advantages such as biodegradability, ease of size control, surface modification, stability, and low immunogenicity.212 Human serum album (HSA)-based bevacizumab nanoparticles were reported in the literature as it is the most abundant protein found in the human body. Bevacizumab-NPs were formulated by desolvation and freeze-drying procedures without any physical or chemical modification. These nanoparticles were of size 310 nm and showed an initial burst effect followed by a sustained release at a rate of 6 μg/hour. The nanoparticles demonstrated a much higher ability to block VEGF than the free bevacizumab following once-daily topical treatment in a rat corneal neovascularization model. This nanosystem significantly reduced inflammation, edema, and fibroblast activity. PEGylation of these nanoparticles did not show any enhancement of the antiangiogenic properties of bevacizumab.213 The same formulation of HSA NPs with glutaraldehyde cross-linking was reported to be unsuitable for successfully loading bevacizumab because of the inactivation of the antibody.214 HSA NPs stabilized with Gantrez ES-425 exhibited a higher loading of the antibody with a burst release of the drug followed by sustained release.215 Polymeric nanoparticulate systems can be considered promising in ocular therapy based on the outcomes reported by different studies in the literature in terms of enhancement in efficacy, permeability, and controlled and targeted drug delivery of protein and peptide drugs.216
5.1.2.4. Toxicity of Polymeric Nanoparticles
Polymeric nanoparticles are widely in ophthalmic drug delivery. However, their long-term toxicity remains unclear. For example, chitosan nanoparticles are rapidly taken up by conjunctival and corneal epithelium within a few hours with causing inflammation of the ocular surface. Nevertheless, the long-term exposure these nanoparticles should be observed. The toxicity of PCL, PLGA, and PEGylated PLGA was studied in human retinal vascular endothelial progenitor cells and ARPE-19 cell lines. No toxicity was observed with PEGylated PLGA, while both PLGA and PCL exhibited toxic effects. Similarly, hyaluronic acid-PLGA NPs were found to be nontoxicity to RPE cells. These studies suggest that surface modification of PLGA nanoparticles might reduce their toxicity.
5.1.3. Nanoparticles Based on Lipids
Compared with the polymeric nano system, lipid-based nanoparticles are used to a lesser degree in the delivery of protein and peptides. This is due to the fact that lipids in the formulation tend to limit the loading of hydrophilic protein entities such as anti-VEGF agents. However, lipid-based systems offer low antigenicity and toxicity due to which some progress has been made.
5.1.3.1. Liposomes
Liposomes are spherical entities with an amphiphilic phospholipid bilayer and an aqueous core inside. Liposomes’ core–shell nanostructure allows them to load both hydrophobic and hydrophilic compounds. Hydrophobic medications are often enclosed in the shell’s lipophilic bilayers, while hydrophilic drugs are entrapped in the core’s aqueous phase.217,218 Stealth liposomes and conventional liposomes with DPPC:DPPE:DPPG:cholesterol with a composition of 60:10:0:30, 65:5:5:30, and 60:5:5:30 were developed for the bevacizumab drug delivery. All formulations showed extended drug release and prevented protein degradation under accelerated stability studies. Additionally, the presence of adjuvants such as trehalose and β-carotene maintained the stability of the antibody present in the formulation. Adjuvants also seemed to maintain cell viability after incubation of bevacizumab-encapsulated liposomes, whereas in the case of bevacizumab solution cell viability decreased. Upon single intravitreal administration, bevacizumab encapsulated within the liposomes minimum therapeutic concentration was maintained for up to weeks, whereas the solution was eliminated prior to 6 weeks. This indicates that liposomal formulation provides a slow release of bevacizumab and retains its activity.219 Similarly, another liposomal formulation of bevacizumab prepared, upon intravitreal injection showed high drug concentration in the posterior chamber (48 μg/mL) after 28 days compared to bevacizumab solution injection (28 μg/mL) after the same time and slower clearance was observed in case of the liposomal formulation.220
Multivesicular liposomes (MVLs) are known to provide high encapsulation efficiency for water-soluble drugs due to the high-volume ratio of 95:5 (aqueous: lipid). Further, MVLs form a drug-depot at the site of drug administration for prolonged release and minimize the initial burst release of drugs.221 A multivesicular liposome formulation was developed to improve bevacizumab bioavailability by the double emulsification method. The addition of 10% human serum albumin helped in maintaining the antibody activity. Bevacizumab multivesicular liposomes demonstrated a sustained release of the drug in various media and attained 80% encapsulation efficiency of the antibody. The structural integrity of the antibody was also maintained postrelease. Slower clearance of bevacizumab-loaded liposomes was observed compared to the solution. In vivo imaging tests in rats after intravitreal injection confirmed the sustained release capabilities of the formulation (Figure 7). Bevacizumab concentration and half-life were higher in the vitreous following bevacizumab-laden multivesicular liposomal injection compared to antibody solution. The area under the curve was twice higher as the antibody solution. Moreover, bevacizumab-loaded multivesicular liposomes could successfully decrease the thickness of choroidal neovascularization lesions in the animal model.221
Figure 7.
(A) In vivo imaging of SD rats after intravitreal injection of Cy7-labled bevacizumab (CB) and Cy7-labled bevacizumab multivesicular liposomes (CB-MVLs) at 0.5, 1, 3, 7, and 14 days, respectively. (B) The concentrations of bevacizumab multivesicular liposomes (Bev-MVLs) and bevacizumab solution (Bev-S) at 3, 7, 14, 21, 28, 42, and 56 days in the vitreous humor, and (C) in aqueous humor. (D) The comparison of bevacizumab concentrations between the vitreous and aqueous humor after intravitreal injection of Bev-MVLs, and (E) of Bev-S (mean ± SD, n = 3). Reproduced with permission from Mu et al.221 CC BY 4.0.
Short-term studies in rabbits following subconjunctival and vitreal injections indicate liposomes are safe and effective for ocular drug delivery. However, the long-term toxicity of liposomes should be assessed in order to realize its clinical applicability.
5.1.3.2. Solid Lipid Nanoparticles
Other lipid-based nanoparticles such as solid lipid nanoparticles (SLNs) are composed of crystallized lipids with drugs-incorporated in a highly ordered crystalline structure and stabilized with the help of emulsifiers. SLNs were first created utilizing lipids with melting points higher than room temperature and body temperature. Examples of such liquids include triglycerides, fatty acids, and waxes. SLNs offer various advantages, including enhanced stability, superior drug protection, controlled drug release and tunable features by lipid components.222−224 Various types of lipid nanoparticles are shown in Figure 8.
Figure 8.
Types of lipid nanoparticles: (a) liposomes, (b) solid lipid nanoparticles, and (c) nanostructured lipid carrier. Reproduced with permission from Xu et al.225 CC BY 4.0.
SLNs were investigated in the delivery of bevacizumab. SLNs of bevacizumab have been prepared by fatty acid coacervation technique using hydrophobic ion pair between antibody and sodium dioctyl sulfosuccinate to enhance lipophilicity of antibody and facilitate its entrapment inside SLNs. This homogeneous formulation showed an entrapment efficiency of nearly 30%. SLNs of bevacizumab dramatically reduced endothelial cell migration at a concentration 100–50 times lower than those of free bevacizumab. The nanocarrier formulation was 100–200 times more effective than the free drug at inhibiting the development of endothelial tubes. Although SLNs of bevacizumab were created as a therapeutic alternative for the treatment of glioblastoma, in vitro data indicated its usefulness in the treatment of ocular angiogenesis.226
5.1.3.3. Nanostructure Lipid Carriers
Nanostructure lipid carriers (NLCs) were developed as the second-generation SLNs system by replacing the fractional solid lipids components of SLNs with liquid lipids, resulting in a larger drug incorporation space and stability overtime.227 NLCs is a potential drug delivery method with better drug retention and higher loading capability.225,228 It has been suggested that NLC could increase drug loading and stability more than SLN formulations, probably due to the creation of a less ordered lipid matrix, by mixing solid lipid and oil. The mixed lipid matrix could prevent or reduce drug expulsion during storage.229 Andrade et al. formulated NLC of voriconazole by using solid (glyceryl behenate) and liquid lipids (capric caprylic triglycerides). Entrapment efficiency (EE) was found to be superior with 77.7% as compared to SLNs prepared by Kumar et al. (EE was reported in between 40% to 60%).230,231 Genistein loaded SLN and NLC were synthesized by Andrade at al. Based on the stability, drug release, skin permeation, and electron paramagnetic resonance studies, NLC of Genistein was found to be more flexible than SLN. Genistein was released more slowly from NLC than from SLN. This study demonstrated that NLC has a more fluid matrix than SLN, which would indicate that obtaining more fluid systems is desirable since more drug is retained inside the lipid matrixes.232 Lactoferrin-loaded NLCs as a new therapeutic alternative for the keratoconus treatment was synthesized by Fernandez et al., using double emulsion/solvent evaporation method. NLCs were spherical and uniform in shape with an average particle size of 119.45 ± 11.44 nm (PDI, 0.151 ± 0.045), and a zeta potential of 17.50 ± 2.53 mV. A controlled release of lactoferrin was observed suggesting their use as a possible delivery vehicle for hydrophilic drugs. This study also confirmed mucoadhesive properties of NLCs through electrostatic forces for at least 240 min with no evidence of tissue cytotoxicity. The versatility of these lipid nanosystems make them attractive vehicles in topical ophthalmic delivery.233
5.1.3.4. Lipid Nanocapsules
In order to improve ocular therapy, a novel hybrid formulation based on lipid nano capsules with bevacizumab on the surface and triamcinolone acetonide in the inner core was recently reported. This system was designed to show complementary and synergistic activity in the endotoxin-induced uveitis rabbit model.234 A phase inversion-insertion one-step approach for drug loading and surface-modification of nano capsules was utilized. This process involves post insertion of a bifunctional polymer, followed by antibody coupling via “click” chemistry. The nano capsules were negatively charged with a size of 102 nm and a drug loading of 56% in the lipid core. Additionally, the antibody retained its bioactivity following attachment to nano capsules as demonstrated through inhibition of endothelial cell migration and inhibition of in vitro VEGF-induced capillary formation. As a result, it offers a viable alternative for improving the treatment of eye disorders that are brought on by inflammation and/or neovascularisation.235 Lipid nanoparticles constitute an effective method for drug delivery due to their simple formation and the potential for postformulation improvements. Also, the lipids employed in the preparation are well-known for their safety and biocompatibility. Despite the significant challenge of loading hydrophilic molecules in lipid-based nanoparticles, progress has been seen in this area of study with promising outcomes regarding the impact of bevacizumab loaded both in vitro and in vivo.
5.1.4. Hydrogels
Hydrogel contains a multidimensional polymer network type of structure with physical or chemical bonding and is biocompatible for intraocular delivery.236,237 The physical bonding is explained through van der Waals forces, hydrogen bonds, and ionic bonds, while the presence of covalent bonds among the polymer chains is an example of chemical bonding.238,239 Maintenance of the drug concentration in the vitreous part of the ocular regions can be achieved via certain mechanisms like the sustained release of the drug from hydrogel which is ultimately possible because of these types of chemicals as well as physical bonding or interactions. Certain molecules like chitosan and hyaluronic acids are examples of polymers which are natural polysaccharides used in the preparation of hydrogels.240 In situ hydrogel system for drug delivery in the posterior region of the eye is adopted in recent times due to its various benefits like sustained drug release, prolonged half-lives in intravitreal drug delivery, ease of administration, and accurate dosing. Initially, it is given through the intravitreal route and further, it is converted into gel due to changes in certain parameters like temperature or pH.241−243 The hydrophilic nature of hydrogels prevents the potential destruction of labile biomacromolecules, unlike implants that are often composed of hydrophobic polymers. The adjustable nature of the hydrogel network permits the release of drugs at a desirable rate. According to one study conducted by Rauck et al., bevacizumab containing poly(ethylene glycol)-poly(serinol hexamethylene urethane) hydrogel showed sustained release of the drug until 9 weeks in rabbits following intravitreal injection. The presence of gel in the vitreous humor showed no evidence of inflammation histologically and IOP levels remained normal throughout the study. The levels of bevacizumab obtained from the gel were found to be 4.7 times higher than its concentrations from bolus injections.244 Hyaluronic acid and dextran are widely used polymers in ophthalmology due to their excellent biocompatibility. The property of hyaluronidase (Hyal) to catalyze the degradation of HA has been exploited for decades to increase the penetration of biopharmaceutical drugs across ocular tissue barriers. According to a study, a combination of anti-VEGF drugs along with hyaluronic acids (hyaluronan binding peptides) also makes drug release prolonged in the vitreous region which directly enhances the efficacy of a drug for 3–4 times in monkeys and rabbits for the treatment of corneal neovascularization.245 One of the five homologous hyaluronidases encoded in the human genome is called human Hyal-1 (hHyal-1). It cleaves HA substrates of various sizes in a size-independent manner to tetrasaccharides and is abundantly expressed in the majority of tissues. The bee venom hyaluronidase (bvHyal), whose structure has been demonstrated in conjunction with a HA tetrasaccharide, shares 31% sequence identity with the amino acid residues in the N-terminal of hHyal-1. Additionally, it is believed that Hyal-1’s EGF domains facilitate protein–protein interactions that are frequently linked to the control of growth and development.246 Another pure type of recombinant human hyaluronidase, rHuPH20, has demonstrated potential for increasing dexamethasone levels in serum and ocular tissues (choroid and retina). It is currently undergoing Phase I clinical research for multiple myeloma (NCT02519452). Recombinant human hyaluronidase’s potential to facilitate drug administration offers promise in the development of protein and peptide-based ocular formulations, even though blocking a vital stromal component like HA may result in some immunogenic reactions in the body.247 Furthermore, a combination of anti-VEGF drugs along with hyaluronic acids (hyaluronan binding peptides) resulted in prolonged drug release in the vitreous region. This strategy directly enhanced the efficacy of drugs 3–4 times in monkeys and rabbits for the treatment of corneal neovascularization.248
Moreover, in a study by Yu et al., in situ gel made up of hyaluronic acid/dextran was loaded with bevacizumab and tested in rabbits following intravitreal injection. The in situ gel was able to maintain therapeutic concentrations of bevacizumab in rabbit eyes at the relevant concentration for at least 6 months with no adverse events such as hemorrhage, retinal detachment, inflammation, or other gross pathological changes.249 The research conducted so far clearly indicates that hydrogels provide an option for far fewer intravitreal injections (weeks or months). Many of the hydrogel polymers are not included in commercial formulations as studies should be carried out in humans to evaluate their long-term biocompatibility.
5.2. New Strategies for the Delivery of Proteins and Peptides
5.2.1. Dendrimers
Dendrimers are monodispersed, well-defined, homogeneous tree-like branches containing structure that has the ability to conjugate and/or entrap high molecular weight compounds. Polyamidoamines (PAMAM), poly aryl ethers, polyamides (polypeptides), polyesters, and polyamines are some of the most utilized dendrimers.250,251 Dendrimers have application in targeted drug delivery because of the multiple functional groups present on their surface which strengthen ligand–receptor binding and causes accelerated response against dendrimer stimuli. Furthermore, the surface charge and molecular weight are also essential properties of dendrimers that influence their accumulation and rate of drug release. Compared to anionic and uncharged dendrimers, cationic dendrimers have greater absorption because of their better interaction with lipid bilayers.252
To date, very few studies are conducted utilizing dendrimers as a carrier for protein delivery due to the high cost of preparation, multistep synthesis, and poor-quality control. In a study, VEGF oligonucleotide (ODN-1) and a lipophilic amino acid dendrimer were conjugated and evaluated on a laser-induced CNV rat model for inhibition of CNV. This study reported that the lifespan of ODN-1 was extended due to the prevention of degradation through nucleases and also the conjugated system was able to penetrate deeper into the RPE. The conjugated system prevented CNV development for 4–6 months whereas an injection was able to prevent it for only 2 months.253 Recently, PAMAM dendrimers were modified using penetratin (PEN) and cyclic arginine-glycine-aspartate (RGD) hexapeptide and pegylated. These modified dendrimers showed 1.5 times higher penetration and distribution in the retina and cornea and resulted in retinal retention time greater than 12 h. Lastly, it was reported that these modified dendrimers could be utilized for neovascular targeting due to their high affinity toward αvβ3.254
5.2.2. Cell-Penetrating Peptides (CPPs)
Cell-penetrating peptides (CPPs) contain about a sequence of 5–40 amino acids between their structures. They are also known as Trojan horse peptides. CPPs are divided into three categories cationic (contains positive charge derived from arginine and lysine-like polar amino acids), hydrophobic (contains nonpolar amino acids), and amphipathic (contains both polar amino acids like lysine, arginine, and nonpolar amino acids like leucine, valine) on the basis of their physicochemical properties. Examples of cationic CPPs are TAT-derived peptides, polyarginine, and penetratin. The amphipathic nature and polycationic properties of CPPs decide their penetration type, either active (energy-dependent) or passive (energy-independent) across the membrane.255 The uptake mechanism of CPPs is explained through steps like contact with the cell membrane, interaction, and followed by the release of the drug. CPPs show extremely good affinity toward molecules associated with them for transfer across the membrane. Translocation of nanocarriers with CPPs happens via endocytosis (energy-dependent pathway).256 Recently, for ocular drug delivery, peptides that are novel have been developed for overcoming the selectivity issues in the case of TAT molecules. These POD molecules generally reduce side effects and are also useful as bacteriostatic agents via decreasing bacterial growth in the eye as they are useful in optic nerve diseases.257,258 Cell-penetrating peptides and nanoparticle carriers are showing no toxic or irritating effect when they are given in combination this was established in Draize test (in vivo study) and hen’s egg test (in vitro study).257 The efficacy of anti-VEGF drugs such as ranibizumab and bevacizumab (alone and complex of drug and CPP) following topical application or intravitreal injection in a choroidal neovascularization rodent model. The level of toxicity was found to be quite low with this combination and in vivo, experimentation in mice resulted in clinically relevant concentrations of bevacizumab in the posterior chamber of the rat eye following a single application. Further, testing was carried out in a CNV mouse model. Mice treated with either a single intravitreal injection of anti-VEGF (or) twice daily CPP + anti-VEGF eye drops (or) daily dexamethasone gavage for 10 days showed a significant reduction in areas of CNV when compared with lasered eyes without treatment.259 Elastin-like polypeptide (ELP) is another drug carrier useful in the transfer of drugs across the membrane when combined with SynB1 which is a cell-penetrating peptide (contains 18 amino acids) that leads to enhancement of membrane penetration. George and co-workers have concluded that ELP-CPPs complexes can enhance the penetration of drugs in human ocular tissues, but the same complex is not able to enhance the penetration of drugs across the membrane in rabbit-like animals. So, enhancement in permeability is completely dependent on the selectivity of any drug molecules to specific tissues.260 While the results published in the literature are promising, a general drawback of using CPPs in ocular drug delivery is their lack of cell and tissue specificity. A recent review by Pescina et al. summarized cell-penetrating peptides used in ocular drug delivery in a detailed manner with a special focus on noninvasive or minimally invasive administration along with toxicity issues.261
5.2.3. Delivery Using Living Cells
Encapsulated cell technology (ECT) consists of genetically engineered living cells encapsulated inside a semipermeable polymer membrane. The encapsulated cells are capable of manufacturing a specific therapeutic substance to target a specific disease. The semipermeable polymer membrane permits the outward movement of the therapeutic substance and further protects the encapsulated cells from rejection by the patient’s immune system while permitting the movement of oxygen and nutrients. RPE (human retinal pigment epithelial) cells are genetically engineered and encapsulated in an implant, these cells produce ciliary neurotrophic factor (CNTF) which protects the retina from degeneration. The modified RPE cell encapsulated implants known as NT-501 were reported by Renexus and Neurotech Pharmaceuticals.262 Similarly, NT-503 has the ability to neutralize VEGF 20–30-fold more compare to NT-501.263,264 ECT provides an alternative to the traditional administration of drugs to the eye as it allows any therapeutic agent to be engineered into the cells for delivery. However, the safety and efficacy of ECT should be demonstrated in clinical trials before this technology could be used in the delivery of drugs for incurable eye diseases.
5.2.4. Microneedles
Microneedles consist of microsized projections made up of polymer/metal with dimensions close to 200 μm. The tiny size of microneedles makes them minimal invasive in nature. Microneedles have the ability to bypass the epithelial transport barrier and conjunctival clearance while reducing retinal damage.265 They are less painful compared to traditional hypodermic needles and have the ability to release the drug over a period of extended time. Ocular microneedles are generally delivered in two categories: passive and active microneedles.6 In passive microneedles, solid microneedles coated with the formulation quickly dissolve after insertion following which the device is removed from the eye. Patel et al. have demonstrated the delivery of nanoparticles into the suprachoroidal space in whole rabbits, pigs, and human eyes ex vivo. This was done by applying pressure of 250–300 kPa to the borosilicate microneedles having a length of 800–1000 μm. The maximum volume deliverable by this method was 35 μL.266 Though this study was able to demonstrate the delivery of nanoparticle and microparticle suspensions into the suprachoroidal space using microneedles, the major drawback is their limited drug-loading capacity. To overcome this issue, titanium-based microneedles were developed using the titanium deep reactive ion Etching method by Aimi and co-workers. Drug carrying capacity was increased because of the uniformly thick fenestrate of titanium-based microneedles. These fenestrate are reservoirs that enhance titanium-based microneedles drug carrying capacity compared to solid microneedles.267 5-Fold enhancement compared to solid microneedles was seen in the drug-carrying capacity by using proper fenestration filing techniques. Fenestrated microneedles have the potential to be used as sustained biologics release delivery which can immensely affect the dosing frequency.264 Microneedles were also used in the anterior segment delivery for treating corneal neovascularization. Kim et al. compared microneedle-based intrastromal delivery of bevacizumab with conventional topical and subconjunctival delivery in New Zealand white rabbits. Bevacizumab-loaded stainless-steel hollow microneedles of 400 μm size were used in the study. Subconjunctival injection of bevacizumab showed similar results to microneedle-treated eyes, while the eyes treated with drops showed significantly lower suppression of corneal neovascularization than the single microneedle treatment. Neovascularization was reduced by 44% in microneedle-treated rabbits compared to untreated rabbits.268 To summarize, microneedles are successful in providing localized drug delivery to the eye in animals. However, further research is needed into the method of injection/retraction and force of injection to avoid discomfort in patients.269
5.2.5. Iontophoresis
Iontophoresis is a method in which a small amount of electric current is used for increasing the drug permeation through the ocular tissues such as the cornea and sclera. The electrical current is generally applied to the outer part of the eye, hence there is no need for surgical invasion.264 Ocular iontophoresis was first utilized for corneal ulcer treatment using salts of zinc by Wirtz in the year 1908. Currently, three iontophoretic devices have been developed for ocular delivery applications: Eyegate II (EyeGate Pharmaceuticals Inc., MA, U.S.A.), Ocuphor (IOMED Inc., UT, U.S.A.), and Visulex (Aciont Inc., UT, U.S.A.). Of these, the only patented device for transscleral delivery is EyeGate II. In this system, the electrode present inside the annular ocular applicator produces ions when current is applied. These ions cause the movement of ionized drug molecules into the anterior or posterior part of the eye by directly bypassing the conjunctiva and the sclera.270 Molokhia et al.271 used iontophoresis for transscleral administration of bevacizumab, immunoglobulin G, and gadolinium-labeled albumin (Galbumin) with Visulex-I system on a CNV animal model. Iontophoresis in 20 min successfully delivered nearly 0.6 mg of bevacizumab in rabbits’ eyes in vivo. Additionally, iontophoretic delivery of bevacizumab delayed retinal neovascularization by 4 weeks. Magnetic resonance imaging clearly showed the presence of Galbumin in the posterior tissues following iontophoresis. More recently, an iontophoresis device based on a hydrogel ionic circuit was reported by Zhao et al. for effectively delivering macromolecule and nanoparticles intraocularly. The hydrogel ionic circuit-based device was found to be capable of minimizing Joule heating, absorbing electrode overpotential-induced heating, and effectively buffering electrochemical reaction-generated pH changes. This study concluded that high-intensity iontophoresis increased (up to 300 times) macromolecule delivery to the anterior and posterior segments and bevacizumab reached target tissue compartments in 10–20 min of iontophoresis application. However, further evaluation of this technology is needed before its clinical translation.272
5.2.6. Port Delivery Systems
A port delivery system (PDS) is a system in which there is a controlled and sustained drug release through a porous metal element. These refillable, nonbiodegradable implants, are placed surgically either in pars plana or scleral plana, and the drug reaches the vitreous cavity through concentration gradient-mediated passive diffusion.273 Currently, 10 clinical trials on clinicaltrials.gov have the application of PDS in the ocular delivery of proteins/peptides. Out of these, 7 clinical trials are currently going on with NCT identifiers as follows: NCT04657289, NCT04108156, NCT04503551, NCT03683251, NCT05562947, NCT04853251, and NCT05476926. Two clinical trials have been completed, one of these was a phase III clinical trial (NCT03677934) that emphasized treatment satisfaction and patient preference between PDS delivery of ranibizumab and intravitreal injection of the same. The results illustrated that treatment satisfaction was achieved in both interventions but nearly all patients preferred PDS over an intravitreal injection.274 Another completed study was a phase II clinical trial (NCT02510794) of PDS-mediated delivery ranibizumab for the treatment of neovascular age-related macular degeneration. This study concluded that 100 mg/mL ranibizumab containing PDS implant was able to maintain a serum concentration of ranibizumab within the range of 130–2220 pg/mL for 12 months after the implantation. This was similar to ranibizumab concentration with monthly intravitreal injections.275 Lastly, a phase III trial (NCT05126966) with aflibercept or ranibizumab-loaded PDS was suspended because the PDS did not meet the required criteria.
5.2.7. Nanowafer
Small, transparent discs known as nano wafers are made of a variety of polymers, such as poly(vinyl alcohol), polyvinylpyrrolidone (PVP), (hydroxypropyl) methyl cellulose (HPMC), and carboxymethyl cellulose (CMC). In contrast to topical eye drops, these are applied with a fingertip to the ocular surface and can endure continuous blinking without moving. For the first time, Yuan and the team showed that a doxycycline-loaded PVA nanowafer released the drug steadily over the course of 24 h in mice while also increasing corneal permeability. The effectiveness of such PVA-fabricated nanowafers-loaded with axitinib for treating CNV in a mouse ocular burn model was reported by the same group.276 According to this study, a system of drug nanoreservoirs called nanowafers was developed and characterized. These nanowafers contained PnPP-19, a synthetic peptide made from a toxin found in the venom of the spider Phoneutria nigriventer that showed hypotensive effects for rat eyes when used topically. The result showed the evidence of PnPP-19 nanowafer system’s safety and possible therapeutic impact with safety in the treatment of retinal illnesses including glaucoma.277
5.2.8. Microbubbles Technology
As a minimally invasive technique, ultrasound and microbubbles (USMB) can be used to increase the effectiveness and targeting of ocular medicine administration.278,279 The delivery of therapeutic proteins to the anterior and posterior segments of the eye was found to be effective using a new ocular delivery technology using the microbubble, a stimuli-responsive intelligent polymeric carrier system that readily transforms to nanoscale microbubble vesicles in the presence of stimuli like pH, temperature, and magnetic field. They can temporarily disrupt the blood-retina barrier in order to enhance the delivery of systemically administered drugs into the eye for target delivery and ocular malignancies. In a size comparison study, custom-made nanobubbles of size greater than 200 nm were shown to exhibit higher gene transfection efficiency in the presence of 1 MHz-frequency ultrasound when compared to lower size nanobubbles.280 The potential of ultrasound-responsive nanobubbles in delivering macromolecules through various layers of the retina was studied in three retinal cell lines (human RPE cells, human-derived Müller glia, and mouse-derived photoreceptors). In comparison to ultrasound alone, ultrasound combined with nanobubbles (USNB) considerably improved the uptake of macromolecules in retinal cells. However, only the two cell lines of human origination showed a significant increase in intracellular uptake, demonstrating that induced effects are cell line-dependent.281 In another interesting work a GFP (growth factor plasmid) was injected intravitreally either alone or after loading into bubble liposomes. Plasmid transfer efficiency into the rabbit retina was studied using a miniature US transducer (SonoPore 4000). Rabbit retinas that received plasmid, plasmid-loaded in bubble liposomes, and ultrasound showed a significant increase in the GFP-positive cells. In addition, combining GFP plasmid with bubble liposomes greatly enhanced the gene delivery. Examining the eye physiology 1 and 3 days after treatment revealed no evident tissue damage as supported by histology. Endostatin (ES) is a fragment antibody with a molecular weight of 20 kDa. ES has been shown to suppress angiogenesis, endothelial proliferation, and tumor growth. To cure DR by preventing angiogenesis, Xu et al. created cationic microbubbles and investigated the transfer of endostatin-green fluorescent protein (ES-GFP) plasmid to human retinal vascular endothelial cells. This study concluded that ultrasound-mediated cationic microbubbles enhanced the transfection efficiency and this technology could be a useful tool for gene therapy targeting retinal neovascularization.282 Microbubble and nanobubble technologies have shown promising results in the delivery of macromolecules in vitro. Future studies must focus on translating these results and reproducing them in ex vivo and in vivo models of the eye.
5.2.9. Combination Systems
A few studies have shown that the use of combination approaches like nanoparticles, hydrogels, and microparticles for protein delivery in ocular diseases is more beneficial than their separate use. When nanoparticles and microparticles are given separately in the vitreous humor, distribution/clearance of particles occurs very fast resulting in a low therapeutic effect of the drug. Further, rapid particle dispersion in the vitreous humor can result in turbidity and affect vision. Likewise, with hydrogels, fast diffusion, as well as burst release of drugs, is reported in some studies.283 A combination strategy could be used to solve these problems, wherein hydrogel acts as a secondary barrier for drug release from nanoparticles and microparticles. Such combination systems can be used for the localized release of drugs. Injectable thermoresponsive PNIPAAm-based hydrogel along with PLGA microspheres developed by Liu et al. exhibited controlled release of the anti-VEGF drugs like aflibercept and ranibizumab over the period of 6 months.284,285 According to another study by Kim et al., a combination system of hydrogel and PLGA microspheres containing aflibercept showed sustained release of the drug for up to six months in healthy rhesus macaques following intravitreal administration of the drug. No abnormalities were reported in the anterior segment of rhesus macaques and IOP remained within the acceptable range during the study period.286−288
6. Other Promising Approaches
There are some techniques that can assist in enhancing the effective delivery of the current approaches, these are 3D printing, ocular microbots, teleophthalmology, microelectromechanical systems (MEMS), and quantum dots (QDs).289 3D printing (3DP) was developed by Charles W. Hull in 1980 and was represented as a technique of layering materials on top of each other for making any object. For biomedical applications, the development of bioinks for 3DP using biological cells and the material was an evolutionary step that has empowered the development of novel therapeutic delivery approaches.290 Won et al.291 developed a rod using coaxial 3DP that has a polymeric shell and a hydrogel core to which bevacizumab was added. These 3D-printed rods were able to release drugs at varied kinetics near the retina showing their controlled drug release property. Additionally, in the laser-induced choroidal neovascularization model, the efficacy of the system was evaluated and there was a significant improvement in therapeutic efficacy. At a small scale, 3DP is highly cost-effective but at a large scale, it is expensive.290
Ocular microbots are controllable and precisely movable bots that are used for intravitreal implants. They have the potential application to target the eye’s posterior segment and deliver drugs at millimeter and submillimeters levels. Microbots made up of biocompatible cobalt–nickel alloy with the highest magnetization was tested inside the eyeball of an anesthetized rabbit for long-term release of Rhodamine B (a model drug). However, there are a few challenges with this approach such as issues with its rotational and translational motion due to interaction with viscoelastic components present in the vitreous fluid.292,293 Teleophthalmology is an innovative approach that combines smartphone technology with basic diagnostic equipment. It is used for the prognosis and treatment of vision loss because of various diseases such as glaucoma and diabetic retinopathy. This technology can have mainstream applications in remote areas.294 Another approach is a microelectromechanical system (MEMS) that can be delivered to the target site surgically through a small incision in the eye. It has a transscleral parylene cannula that is flexible. The device can have a bolus or continuous drug delivery in both eye segments due to its target specificity. In this, the drug is released as the valve opens due to the overpressure produced manually through depression in the drug basin.295 Quantum dots are semiconductor nanocrystals that can produce an electrical stimulus when exposed to infrared, visible, or ultraviolet light.296 Quantum dots have their application in biolabeling and bioimaging and have replaced organic dyes for the same. These dots upon excitation of their electron emit a unit wavelength that depends on their size. These nanoparticulate semiconductors systems range from 2 to 10 nm.297,298
7. Future Directions and Conclusions
All the novel delivery techniques have enhanced drug delivery of protein and peptides in various ways. Some have provided controlled release of the drug, while others have increased the bioavailability or the half-life of the drug. But until now, no formulation or delivery system has been developed to simultaneously address all the challenges. Some of the molecules such as Lampalizumab and Conbercept discovered were not able to advance to the market. Therefore, there is still a need for more intensive research, specifically for novel drug delivery systems. As intravitreal injections are invasive in nature, patient compliance is very low. In addition, there are severe adverse effects to this mode of administration. In situ, delivery systems have been considered, but regulatory issues are a big hindrance to their approval. More studies should be carried out with novel carriers such as dendrimers, port systems, hydrogel, microneedle, and iontophoresis, for understanding their kinetics and also to establish their efficacy. On the other hand, nanocarriers such as liposomes, polymeric micelles, and cell-penetrating peptides have already proved their safety and efficacy and they should be commercialized to aid in the delivery of protein and peptides. Detailed experiments to understand the toxicity of nanocarriers loaded with protein and peptides should be explored in suitable animal models. This will help modify delivery systems and provide efficient access to the eye. In addition, imaging studies conducted in humans will help in the translation of animal studies. The future development of new biologic treatments for ocular diseases, that can enter clinical trials, will require a concerted effort in the innovation through the use of combination strategies as drug delivery technology platforms.
Acknowledgments
The authors would like to acknowledge GSBTM (GSBTM/JD(R&D)/618/21-22/00003682), Dept. of Science & Technology, Govt. of Gujarat, India, and Ajman University (2022-IRG-PH-8) for supporting this work. The authors would like to acknowledge Mr. Bharaneeswar Renukuntla for editing the manuscript.
Author Contributions
K.R., S.H.S.B., and C.P. conceptualized and developed the idea; D.A., V.H., S.P., J.R., M.S., and H.J., prepared the manuscript; K.R., C.P., S.P., and D.A. provided critical inputs and designs for figures. S.H.S.B., K.R., J.R., M.S., and C.P. edited the manuscript. All authors have read and agreed to the published version of the manuscript.
The authors declare no competing financial interest.
References
- Therapeutic Proteins Global Market Report 2021: COVID-19 Impact and Recovery to 2030 n.d. https://www.researchandmarkets.com/reports/5319142/therapeutic-proteins-global-market-report-2021 (accessed April 30, 2022).
- Radhakrishnan K.; Sonali N.; Moreno M.; Nirmal J.; Fernandez A. A.; Venkatraman S.; et al. Protein delivery to the back of the eye: barriers, carriers and stability of anti-VEGF proteins. Drug Discov Today 2017, 22, 416–423. 10.1016/j.drudis.2016.10.015. [DOI] [PubMed] [Google Scholar]
- Burton M. J.; Ramke J.; Marques A. P.; Bourne R. R. A.; Congdon N.; Jones I.; et al. The Lancet Global Health Commission on Global Eye Health: vision beyond 2020. Lancet Glob Health 2021, 9, e489–551. 10.1016/S2214-109X(20)30488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartnett T. E.; O’Connor A. J.; Ladewig K. Cubosomes and other potential ocular drug delivery vehicles for macromolecular therapeutics. Expert Opin. Drug Deliv. 2015, 12, 1513–1526. 10.1517/17425247.2015.1021680. [DOI] [PubMed] [Google Scholar]
- Zhang K.; Zhang L.; Weinreb R. N. Ophthalmic drug discovery: novel targets and mechanisms for retinal diseases and glaucoma. Nat. Rev. Drug Discov 2012, 11, 541–559. 10.1038/nrd3745. [DOI] [PubMed] [Google Scholar]
- Mandal A.; Pal D.; Agrahari V.; Trinh H. M.; Joseph M.; Mitra A. K. Ocular delivery of proteins and peptides: Challenges and novel formulation approaches. Adv. Drug Deliv Rev. 2018, 126, 67–95. 10.1016/j.addr.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitragotri S.; Burke P. A.; Langer R. Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nat. Rev. Drug Discov. 2014, 13, 655–672. 10.1038/nrd4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong-Le V.; Lovalenti P. M.; Abdul-Fattah A. M. Stabilization challenges and formulation strategies associated with oral biologic drug delivery systems. Adv. Drug Deliv. Rev. 2015, 93, 95–108. 10.1016/j.addr.2015.08.001. [DOI] [PubMed] [Google Scholar]
- Torchilin V. Intracellular delivery of protein and peptide therapeutics. Drug Discov Today Technol. 2008, 5, e95–103. 10.1016/j.ddtec.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Ferrara N.; Adamis A. P. Ten years of anti-vascular endothelial growth factor therapy. Nat. Rev. Drug Discov 2016, 15, 385–403. 10.1038/nrd.2015.17. [DOI] [PubMed] [Google Scholar]
- Lanzetta P. Anti-VEGF therapies for age-related macular degeneration: a powerful tactical gear or a blunt weapon? The choice is ours. Graefes Arch. Clin. Exp. Ophthalmol. 2021, 259, 3561–3567. 10.1007/s00417-021-05451-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakri S. J.; Snyder M. R.; Reid J. M.; Pulido J. S.; Ezzat M. K.; Singh R. J. Pharmacokinetics of intravitreal ranibizumab (Lucentis). Ophthalmology 2007, 114, 2179–2182. 10.1016/j.ophtha.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Cornel S.; Adriana I. D.; Mihaela T. C.; Speranta S.; Algerino D. S.; Mehdi B.; et al. Anti-vascular endothelial growth factor indications in ocular disease. Rom J. Ophthalmol. 2015, 59, 235. [PMC free article] [PubMed] [Google Scholar]
- THPdb: A Database of FDA approved Therapeutic Peptides and Proteins n.d. https://webs.iiitd.edu.in/raghava/thpdb/display.php?details=1529 (accessed May 1, 2022).
- Lang G. E. Diabetic macular edema. Ophthalmologica 2012, 227, 21–29. 10.1159/000337156. [DOI] [PubMed] [Google Scholar]
- Lu X.; Sun X. Profile of conbercept in the treatment of neovascular age-related macular degeneration. Drug Des Devel Ther 2015, 9, 2311. 10.2147/DDDT.S67536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz F. G.; Dugel P. U.; Weissgerber G.; Hamilton R.; Silva R.; Bandello F.; et al. Single-Chain Antibody Fragment VEGF Inhibitor RTH258 for Neovascular Age-Related Macular Degeneration. Ophthalmology 2016, 123, 1080–1089. 10.1016/j.ophtha.2015.12.030. [DOI] [PubMed] [Google Scholar]
- Tadayoni R.; Sararols L.; Weissgerber G.; Verma R.; Clemens A.; Holz F. G. Brolucizumab: A Newly Developed Anti-VEGF Molecule for the Treatment of Neovascular Age-Related Macular Degeneration. Ophthalmologica 2021, 244, 93–101. 10.1159/000513048. [DOI] [PubMed] [Google Scholar]
- Hussain R. M.; Neiweem A. E.; Kansara V.; Harris A.; Ciulla T. A. Tie-2/Angiopoietin pathway modulation as a therapeutic strategy for retinal disease. Expert Opin. Investig. Drugs 2019, 28, 861–869. 10.1080/13543784.2019.1667333. [DOI] [PubMed] [Google Scholar]
- Thurston G.; Daly C. The complex role of angiopoietin-2 in the angiopoietin-tie signaling pathway. Cold Spring Harbor Perspect. Med. 2012, 2, a006650. 10.1101/cshperspect.a006650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joussen A. M.; Ricci F.; Paris L. P.; Korn C.; Quezada-Ruiz C.; Zarbin M. Angiopoietin/Tie2 signalling and its role in retinal and choroidal vascular diseases: a review of preclinical data. Eye (Lond) 2021, 35, 1305–1316. 10.1038/s41433-020-01377-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahn G. M.; Khanani A. M. New Therapies of Neovascular AMD beyond Anti-VEGF Injections. Vision (Basel) 2018, 2, 15. 10.3390/vision2010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Khersan H.; Hussain R. M.; Ciulla T. A.; Dugel P. U. Innovative therapies for neovascular age-related macular degeneration. Expert Opin. Pharmacother. 2019, 20, 1879–1891. 10.1080/14656566.2019.1636031. [DOI] [PubMed] [Google Scholar]
- Muñoz-Ramón P v.; Hernández Martínez P.; Muñoz-Negrete F. J. New therapeutic targets in the treatment of age-related macular degeneration. Arch Soc. Esp Oftalmol. 2020, 95, 75–83. 10.1016/j.oftal.2019.09.011. [DOI] [PubMed] [Google Scholar]
- Sahni J.; Dugel P. U.; Patel S. S.; Chittum M. E.; Berger B.; del Valle Rubido M.; Sadikhov S.; Szczesny P.; Schwab D.; Nogoceke E.; Weikert R.; Fauser S.; et al. Safety and Efficacy of Different Doses and Regimens of Faricimab vs Ranibizumab in Neovascular Age-Related Macular Degeneration: The AVENUE Phase 2 Randomized Clinical Trial. JAMA Ophthalmol 2020, 138, 955–963. 10.1001/jamaophthalmol.2020.2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanani A. M.; Patel S. S.; Ferrone P. J.; Osborne A.; Sahni J.; Grzeschik S.; et al. Efficacy of Every Four Monthly and Quarterly Dosing of Faricimab vs Ranibizumab in Neovascular Age-Related Macular Degeneration: The STAIRWAY Phase 2 Randomized Clinical Trial. JAMA Ophthalmol 2020, 138, 964–72. 10.1001/jamaophthalmol.2020.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahni J.; Patel S. S.; Dugel P. U.; Khanani A. M.; Jhaveri C. D.; Wykoff C. C.; et al. Simultaneous Inhibition of Angiopoietin-2 and Vascular Endothelial Growth Factor-A with Faricimab in Diabetic Macular Edema: BOULEVARD Phase 2 Randomized Trial. Ophthalmology 2019, 126, 1155–70. 10.1016/j.ophtha.2019.03.023. [DOI] [PubMed] [Google Scholar]
- Heier J. S.; Khanani A. M.; Quezada Ruiz C.; Basu K.; Ferrone P. J.; Brittain C.; et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): two randomised, double-masked, phase 3, non-inferiority trials. Lancet 2022, 399, 729–740. 10.1016/S0140-6736(22)00010-1. [DOI] [PubMed] [Google Scholar]
- Wykoff C. C.; Abreu F.; Adamis A. P.; Basu K.; Eichenbaum D. A.; Haskova Z.; et al. Efficacy, durability, and safety of intravitreal faricimab with extended dosing up to every 16 weeks in patients with diabetic macular oedema (YOSEMITE and RHINE): two randomised, double-masked, phase 3 trials. Lancet 2022, 399, 741–755. 10.1016/S0140-6736(22)00018-6. [DOI] [PubMed] [Google Scholar]
- Lampalizumab - Wikipedia n.d. https://en.wikipedia.org/wiki/Lampalizumab (accessed May 1, 2022).
- Ferro Desideri L.; Traverso C. E.; Nicolò M. Abicipar pegol: an investigational anti-VEGF agent for the treatment of wet age-related macular degeneration. Expert Opin. Investig. Drugs 2020, 29, 651–658. 10.1080/13543784.2020.1772754. [DOI] [PubMed] [Google Scholar]
- Smithwick E.; Stewart M. W. Designed Ankyrin Repeat Proteins: A Look at their Evolving Use in Medicine with a Focus on the Treatment of Chorioretinal Vascular Disorders. Antiinflamm Antiallergy Agents Med. Chem. 2017, 16, 33–45. 10.2174/1871523016666170502115816. [DOI] [PubMed] [Google Scholar]
- Caputi A. P.; Navarra P. Beyond antibodies: ankyrins and DARPins. From basic research to drug approval. Curr. Opin Pharmacol 2020, 51, 93–101. 10.1016/j.coph.2020.05.004. [DOI] [PubMed] [Google Scholar]
- Steiner D.; Forrer P.; Plückthun A. Efficient selection of DARPins with sub-nanomolar affinities using SRP phage display. J. Mol. Biol. 2008, 382, 1211–1227. 10.1016/j.jmb.2008.07.085. [DOI] [PubMed] [Google Scholar]
- Rodrigues G. A.; Mason M.; Christie L. A.; Hansen C.; Hernandez L. M.; Burke J.; et al. Functional Characterization of Abicipar-Pegol, an Anti-VEGF DARPin Therapeutic That Potently Inhibits Angiogenesis and Vascular Permeability. Invest Ophthalmol Vis Sci. 2018, 59, 5836–46. 10.1167/iovs.18-25307. [DOI] [PubMed] [Google Scholar]
- Bragina O.; Chernov V.; Schulga A.; Konovalova E.; Garbukov E.; Vorobyeva A.; et al. Phase I Trial of 99mTc-(HE)3-G3, a DARPin-Based Probe for Imaging of HER2 Expression in Breast Cancer. J. Nucl. Med. 2022, 63, 528–35. 10.2967/jnumed.121.262542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souied E. H.; Devin F.; Mauget-Faysse M.; Kolar P.; Wolf-Schnurrbusch U.; Framme C.; Gaucher D.; Querques G.; Stumpp M. T.; Wolf S.; et al. Treatment of exudative age-related macular degeneration with a designed ankyrin repeat protein that binds vascular endothelial growth factor: a phase I/II study. Am. J. Ophthalmol 2014, 158, 724–732. 10.1016/j.ajo.2014.05.037. [DOI] [PubMed] [Google Scholar]
- Ghahvechian H.; Sallam A. B.; Jabbehdari S. A narrative review on the role of abicipar in age-related macular degeneration. Ann. Eye Sci. 2021, 6, 35–35. 10.21037/aes-21-45. [DOI] [Google Scholar]
- Kunimoto D.; Yoon Y. H.; Wykoff C. C.; Chang A.; Khurana R. N.; Maturi R. K.; et al. Efficacy and Safety of Abicipar in Neovascular Age-Related Macular Degeneration: 52-Week Results of Phase 3 Randomized Controlled Study. Ophthalmology 2020, 127, 1331–44. 10.1016/j.ophtha.2020.03.035. [DOI] [PubMed] [Google Scholar]
- Sharma A.; Kumar N.; Kuppermann B. D.; Bandello F. Abicipar pegol: the non-monoclonal antibody anti-VEGF. Eye (Lond) 2020, 34, 797–801. 10.1038/s41433-019-0607-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain R. M.; Shaukat B. A.; Ciulla L. M.; Berrocal A. M.; Sridhar J. Vascular Endothelial Growth Factor Antagonists: Promising Players in the Treatment of Neovascular Age-Related Macular Degeneration. Drug Des Devel Ther 2021, 15, 2653–65. 10.2147/DDDT.S295223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanta A.; Aziz A. A.; Jhingan M.; Singh S. R.; Khanani A. M.; Chhablani J. Emerging Therapies in Neovascular Age-Related Macular Degeneration in 2020. Asia Pac J. Ophthalmol (Phila) 2020, 9, 250–9. 10.1097/APO.0000000000000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran P. R.; Madanagopalan V. G. KSI-301: antibody biopolymer conjugate in retinal disorders. Ther Adv. Ophthalmol. 2021, 13, 251584142110277. 10.1177/25158414211027708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong L; Huang X.; Ngo W.; Dang D.; Lu J.; Jacobson R. D.; et al. KSI-301: An anti-VEGF antibody biopolymer conjugate with extended half-life for treatment of neovascular retinal diseases. Invest. Ophthalmol. Vis. Sci. 2018, 59, 211–211. [Google Scholar]
- Patel S. S.; Naor J.; Qudrat A.; Do D v; Buetelspacher D.; Hong L.; et al. Phase 1 first-in-human study of KSI-301: a novel anti-VEGF antibody biopolymer conjugate with extended durability. Invest. Ophthalmol. Vis. Sci. 2019, 60, 3670–3670. [Google Scholar]
- A Study to Evaluate the Efficacy and Safety of KSI-301, an Anti-VEGF Antibody Biopolymer Conjugate, Versus Aflibercept in Patients With Neovascular (Wet) Age-Related Macular Degeneration. - Full Text View - ClinicalTrials.gov n.d. https://clinicaltrials.gov/ct2/show/NCT04049266 (accessed March 1, 2023).
- A Study to Evaluate the Efficacy, Durability, and Safety of KSI-301 Compared to Aflibercept in Participants With Diabetic Macular Edema (DME) - Full Text View - ClinicalTrials.gov n.d. https://clinicaltrials.gov/ct2/show/NCT04603937?term=NCT04603937&draw=2&rank=1 (accessed March 1, 2023).
- A Study to Evaluate the Efficacy and Safety of Intravitreal KSI-301 Compared With Intravitreal Aflibercept in Participants With Neovascular (Wet) Age-related Macular Degeneration (wAMD) - Full Text View - ClinicalTrials.gov n.d. https://clinicaltrials.gov/ct2/show/NCT04964089?term=NCT04964089&draw=2&rank=1 (accessed March 1, 2023).
- A Study to Evaluate the Efficacy and Safety of Intravitreal KSI-301 in Participants With Moderately Severe to Severe Non-proliferative Diabetic Retinopathy (NPDR) - Full Text View - ClinicalTrials.gov n.d. https://clinicaltrials.gov/ct2/show/NCT05066230?term=NCT05066230&draw=2&rank=1 (accessed March 1, 2023).
- A Trial to Evaluate the Efficacy, Durability, and Safety of KSI-301 Compared to Aflibercept in Participants With Diabetic Macular Edema (DME) - Full Text View - ClinicalTrials.gov n.d. https://clinicaltrials.gov/ct2/show/NCT04611152?term=NCT04611152&draw=2&rank=1 (accessed March 1, 2023).
- de Oliveira Dias J. R.; Xavier C. O.; Maia A.; de Moraes N. S. B.; Meyer C.; Farah M. E.; et al. Intravitreal injection of ziv-aflibercept in patient with refractory age-related macular degeneration. Ophthalmic Surg Lasers Imaging Retina 2015, 46, 91–4. 10.3928/23258160-20150101-17. [DOI] [PubMed] [Google Scholar]
- Mansour A. M; Al-Ghadban S. I; Yunis M. H; El-Sabban M. E Ziv-aflibercept in macular disease. Br. J. Ophthalmol. 2015, 99, 1055–1059. 10.1136/bjophthalmol-2014-306319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhablani J. Intravitreal ziv-aflibercept for recurrent macular edema secondary to central retinal venous occlusion. Indian J. Ophthalmol 2015, 63, 469–70. 10.4103/0301-4738.159909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira Dias J. R.; Badaro E.; Novais E. A.; Colicchio D.; Chiarantin G. M. D.; Matioli M. M.; Verna C.; Penha F. M.; Barros N. M. T.; Meyer C. H.; Farah M. E.; Rodrigues E. B.; et al. Preclinical investigations of intravitreal ziv-aflibercept. Ophthalmic Surg. Lasers Imaging Retina 2014, 45, 577–584. 10.3928/23258160-20141118-15. [DOI] [PubMed] [Google Scholar]
- de Oliveira Dias J. R.; de Andrade G. C.; Novais E. A.; Farah M. E.; Rodrigues E. B.. Fusion proteins for treatment of retinal diseases: aflibercept, ziv-aflibercept, and conbercept. Int. J. Retina Vitreous 2016, 2 10.1186/s40942-016-0026-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues E. B.; Farah M. E.; Maia M.; Penha F. M.; Regatieri C.; Melo G. B.; et al. Therapeutic monoclonal antibodies in ophthalmology. Prog. Retin Eye Res. 2009, 28, 117–44. 10.1016/j.preteyeres.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Grant M. B.; Afzal A.; Spoerri P.; Pan H.; Shaw L. C.; Mames R. N. The role of growth factors in the pathogenesis of diabetic retinopathy. Expert Opin Investig Drugs 2004, 13, 1275–93. 10.1517/13543784.13.10.1275. [DOI] [PubMed] [Google Scholar]
- Theodossiadis P. G.; Markomichelakis N. N.; Sfikakis P. P. Tumor necrosis factor antagonists: preliminary evidence for an emerging approach in the treatment of ocular inflammation. Retina 2007, 27, 399–413. 10.1097/MAJ.0b013e3180318fbc. [DOI] [PubMed] [Google Scholar]
- Xiao R.; Lei C.; Zhang Y.; Zhang M. Interleukin-6 in retinal diseases: From pathogenesis to therapy. Exp. Eye Res. 2023, 233, 109556 10.1016/j.exer.2023.109556. [DOI] [PubMed] [Google Scholar]
- Bongartz T.; Sutton A. J.; Sweeting M. J.; Buchan I.; Matteson E. L.; Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA 2006, 295, 2275–85. 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- Puddu A.; Sanguineti R.; Montecucco F.; Viviani G. L. Retinal pigment epithelial cells express a functional receptor for glucagon-like peptide-1 (GLP-1). Mediators Inflamm. 2013, 2013, 1–10. 10.1155/2013/975032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K.; Yamada S.; Hoshino S.; Watanabe M.; Kimura K.; Kamijo-Ikemori A. Glucagon-like peptide-1 receptor agonist, liraglutide, attenuated retinal thickening in spontaneously diabetic Torii fatty rats. BMC Ophthalmol 2022, 22, 206. 10.1186/s12886-022-02413-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling J.; Hua P.; Dunaief J. L.; Cui Q. N.; VanderBeek B. L. Glucagon-like peptide 1 receptor agonist use is associated with reduced risk for glaucoma. British Journal of Ophthalmology 2023, 107, 215–20. 10.1136/bjophthalmol-2021-319232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaborit B.; Julla J.-B.; Besbes S.; Proust M.; Vincentelli C.; Alos B.; et al. Glucagon-like Peptide 1 Receptor Agonists, Diabetic Retinopathy and Angiogenesis: The AngioSafe Type 2 Diabetes Study. J. Clin Endocrinol Metab 2020, 105, e1549–60. 10.1210/clinem/dgz069. [DOI] [PubMed] [Google Scholar]
- Ji L.; Dong X.; Li Y.; Li Y.; Lim S.; Liu M.; Ning Z.; Rasmussen S.; Skjøth T. V.; Yuan G.; Eliaschewitz F. G.; et al. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as add-on to metformin in patients with type 2 diabetes in SUSTAIN China: A 30-week, double-blind, phase 3a, randomized trial. Diabetes Obes. Metab. 2021, 23, 404–414. 10.1111/dom.14232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández C.; Bogdanov P.; Corraliza L.; García-Ramírez M.; Solà-Adell C.; Arranz J. A.; et al. Topical administration of GLP-1 receptor agonists prevents retinal neurodegeneration in experimental diabetes. Diabetes 2016, 65, 172–87. 10.2337/db15-0443. [DOI] [PubMed] [Google Scholar]
- Mehta J. S. Recombinant tissue plasminogen activator following paediatric cataract surgery. Br. J. Ophthalmol. 2000, 84, 983–6. 10.1136/bjo.84.9.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. F. Use of Tissue Plasminogen Activator to Revive Blebs Following Intraocular Surgery. Arch. Ophthalmol. 2001, 119, 809. 10.1001/archopht.119.6.809. [DOI] [PubMed] [Google Scholar]
- Young J. B.; Buchberger A. R.; Bogaard J. D.; Luecke L. B.; Runquist M.; Skumatz C. M. B. Tissue Plasminogen Activator Effects on Fibrin Volume and the Ocular Proteome in a Juvenile Rabbit Model of Lensectomy. Transl. Vis. Sci. Technol. 2021, 10, 7. 10.1167/tvst.10.14.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedrich A.; Menapace R.; Ries E.; Polzer I. Intracameral tissue plasminogen activator to treat severe fibrinous effusion after cataract surgery. J. Cataract Refract. Surg. 1997, 23, 873–7. 10.1016/S0886-3350(97)80246-5. [DOI] [PubMed] [Google Scholar]
- Starck T.; Hopp L.; Held K. S.; Marouf L. M.; Yee R. W. Low-dose intraocular tissue plasminogen activator treatment for traumatic total hyphema, postcataract, and penetrating keratoplasty fibrinous membranes. J. Cataract Refract. Surg. 1995, 21, 219–24. 10.1016/S0886-3350(13)80514-7. [DOI] [PubMed] [Google Scholar]
- Bourges J. L.; Bloquel C.; Thomas A.; Froussart F.; Bochot A.; Azan F.; et al. Intraocular implants for extended drug delivery: Therapeutic applications. Adv. Drug Deliv Rev. 2006, 58, 1182–202. 10.1016/j.addr.2006.07.026. [DOI] [PubMed] [Google Scholar]
- Zamanlu M.; Eskandani M.; Barar J.; Jaymand M.; Pakchin P. S.; Farhoudi M. Enhanced thrombolysis using tissue plasminogen activator (tPA)-loaded PEGylated PLGA nanoparticles for ischemic stroke. J. Drug Deliv. Sci. Technol. 2019, 53, 101165 10.1016/j.jddst.2019.101165. [DOI] [Google Scholar]
- Liu Q.; McDermott A. M.; Miller W. L. Elevated Nerve Growth Factor in Dry Eye Associated With Established Contact Lens Wear. Eye & Contact Lens: Science & Clinical Practice 2009, 35, 232–7. 10.1097/ICL.0b013e3181b3e87f. [DOI] [PubMed] [Google Scholar]
- Lambiase A. Alterations of Tear Neuromediators in Dry Eye Disease. Archives of Ophthalmology 2011, 129, 981. 10.1001/archophthalmol.2011.200. [DOI] [PubMed] [Google Scholar]
- Lambiase A.; Rama P.; Bonini S.; Caprioglio G.; Aloe L. Topical Treatment with Nerve Growth Factor for Corneal Neurotrophic Ulcers. New Engl. J. Med. 1998, 338, 1174–80. 10.1056/NEJM199804233381702. [DOI] [PubMed] [Google Scholar]
- Bonini S.; Lambiase A.; Rama P.; Sinigaglia F.; Allegretti M.; Chao W.; et al. Phase II Randomized, Double-Masked, Vehicle-Controlled Trial of Recombinant Human Nerve Growth Factor for Neurotrophic Keratitis. Ophthalmology 2018, 125, 1332–43. 10.1016/j.ophtha.2018.02.022. [DOI] [PubMed] [Google Scholar]
- Mantelli F.; Lambiase A.; Colafrancesco V.; Rocco M. L.; Macchi I.; Aloe L. NGF and VEGF Effects on Retinal Ganglion Cell Fate: New Evidence from an Animal Model of Diabetes. Eur. J. Ophthalmol. 2014, 24, 247–53. 10.5301/ejo.5000359. [DOI] [PubMed] [Google Scholar]
- Claes M.; Geeraerts E.; Plaisance S.; Mentens S.; Van den Haute C.; De Groef L. Chronic Chemogenetic Activation of the Superior Colliculus in Glaucomatous Mice: Local and Retrograde Molecular Signature. Cells 2022, 11, 1784. 10.3390/cells11111784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada T.; Harada C.; Kohsaka S.; Wada E.; Yoshida K.; Ohno S.; et al. Microglia–Müller Glia Cell Interactions Control Neurotrophic Factor Production during Light-Induced Retinal Degeneration. J. Neurosci. 2002, 22, 9228–36. 10.1523/JNEUROSCI.22-21-09228.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitranshi N.; Dheer Y.; Abbasi M.; You Y.; Graham S. L.; Gupta V. Glaucoma Pathogenesis and Neurotrophins: Focus on the Molecular and Genetic Basis for Therapeutic Prospects. Curr. Neuropharmacol. 2018, 16, 1018–35. 10.2174/1570159X16666180419121247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanu L. N.; Ciolino J. B. Nerve Growth Factor as an Ocular Therapy: Applications, Challenges, and Future Directions. Semin. Ophthalmol. 2021, 36, 224–31. 10.1080/08820538.2021.1890793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muheem A.; Shakeel F.; Jahangir M. A.; Anwar M.; Mallick N.; Jain G. K.; et al. A review on the strategies for oral delivery of proteins and peptides and their clinical perspectives. Saudi Pharm. J. 2016, 24, 413–28. 10.1016/j.jsps.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renukuntla J.; Vadlapudi A. D.; Patel A.; Boddu S. H. S.; Mitra A. K. Approaches for enhancing oral bioavailability of peptides and proteins. Int. J. Pharm. 2013, 447, 75–93. 10.1016/j.ijpharm.2013.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi X.; Wang Y.; Fu-Shin X. Y. Corneal epithelial tight junctions and their response to lipopolysaccharide challenge. Invest. Ophthalmol. Vis. Sci. 2000, 41, 4093–4100. [PubMed] [Google Scholar]
- Mitic L. L.; van Itallie C. M.; Anderson J. M. Molecular physiology and pathophysiology of tight junctions I. Tight junction structure and function: lessons from mutant animals and proteins. Am. J. Physiol.,-Gastrointest. Liver Physiol. 2000, 279, G250–G254. 10.1152/ajpgi.2000.279.2.G250. [DOI] [PubMed] [Google Scholar]
- Zhao Z.; Ukidve A.; Kim J.; Mitragotri S. Targeting Strategies for Tissue-Specific Drug Delivery. Cell 2020, 181, 151–167. 10.1016/j.cell.2020.02.001. [DOI] [PubMed] [Google Scholar]
- Zelikin A. N.; Ehrhardt C.; Healy A. M. Materials and methods for delivery of biological drugs. Nat. Chem. 2016, 8, 997–1007. 10.1038/nchem.2629. [DOI] [PubMed] [Google Scholar]
- Tao Y.; Li X.; Jiang Y.; Bai X.; Wu B.; Dong J. Diffusion of macromolecule through retina after experimental branch retinal vein occlusion and estimate of intraretinal barrier. Curr. Drug Metab. 2007, 8, 151–6. 10.2174/138920007779815968. [DOI] [PubMed] [Google Scholar]
- Jackson T. L.; Antcliff R. J.; Hillenkamp J.; Marshall J. Human retinal molecular weight exclusion limit and estimate of species variation. Invest. Ophthalmol. Vis. Sci. 2003, 44, 2141–6. 10.1167/iovs.02-1027. [DOI] [PubMed] [Google Scholar]
- Delplace V.; Payne S.; Shoichet M. Delivery strategies for treatment of age-related ocular diseases: From a biological understanding to biomaterial solutions. J. Controlled Release 2015, 219, 652–68. 10.1016/j.jconrel.2015.09.065. [DOI] [PubMed] [Google Scholar]
- Leclercq B.; Mejlachowicz D.; Behar-Cohen F. Ocular Barriers and Their Influence on Gene Therapy Products Delivery. Pharmaceutics 2022, 14, 998. 10.3390/pharmaceutics14050998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilati U.; Allémann E.; Doelker E. Strategic approaches for overcoming peptide and protein instability within biodegradable nano-and microparticles. Eur. J. Pharm. Biopharm. 2005, 59, 375–88. 10.1016/j.ejpb.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Urtti A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1131–5. 10.1016/j.addr.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Vaishya R. D.; Khurana V.; Patel S.; Mitra A. K. Controlled ocular drug delivery with nanomicelles. Wiley Interdiscip Rev. Nanomed. Nanobiotechnol. 2014, 6, 422–37. 10.1002/wnan.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maulvi F. A.; Shetty Ditixa K. H.; Desai T.; Shah D. O.; Willcox M. D. P.. Recent advances in ophthalmic preparations: Ocular barriers, dosage forms and routes of administration. International Journal of Pharmaceutics Manuscript Draft-Powered by Editorial Manager and ProduXion Manager from Aries Systems Corporation. Int. J. Pharm. n.d. [Google Scholar]
- Maulvi F. A., Ranch K. M., Desai A. R., Desai D. T., Shukla M. R.. Ophthalmic Preparations; Elsevier: Remington; 2021; pp 565–75. [Google Scholar]
- Sirbat D.; Marchal-Heussler L.; Hoffman M.; Maincent P. Ways to improve ocular bioavailability for topical applications. J. Fr. Ophtalmol. 2000, 23, 505–509. [PubMed] [Google Scholar]
- Rodrigues G. A.; Lutz D.; Shen J.; Yuan X.; Shen H.; Cunningham J.; Rivers H. M.; et al. Topical drug delivery to the posterior segment of the eye: addressing the challenge of preclinical to clinical translation. Pharm. Res. 2018, 35, 1–5. 10.1007/s11095-018-2519-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DelMonte D. W.; Kim T. Anatomy and physiology of the cornea. J. Cataract. Refract. Surg. 2011, 37, 588–98. 10.1016/j.jcrs.2010.12.037. [DOI] [PubMed] [Google Scholar]
- Toropainen E.; Ranta V.-P.; Talvitie A.; Suhonen P.; Urtti A. Culture model of human corneal epithelium for prediction of ocular drug absorption. Invest. Ophthalmol. Vis. Sci. 2001, 42, 2942–2948. [PubMed] [Google Scholar]
- Cholkar K, Dasari S. R., Pal D, Mitra A. K.. Eye: Anatomy, Physiology and Barriers to Drug Delivery. In Ocular Transporters and Receptors; Elsevier; 2013; pp 1–36. [Google Scholar]
- Marfurt C. F.; Cox J.; Deek S.; Dvorscak L. Anatomy of the human corneal innervation. Exp. Eye Res. 2010, 90, 478–92. 10.1016/j.exer.2009.12.010. [DOI] [PubMed] [Google Scholar]
- Waring G. O. III; Bourne W. M.; Edelhauser H. F.; Kenyon K. R. The corneal endothelium: normal and pathologic structure and function. Ophthalmology 1982, 89, 531–90. 10.1016/S0161-6420(82)34746-6. [DOI] [PubMed] [Google Scholar]
- Mun E. A.; Morrison P. W. J.; Williams A. C.; Khutoryanskiy V v. On the barrier properties of the cornea: a microscopy study of the penetration of fluorescently labeled nanoparticles, polymers, and sodium fluorescein. Mol. Pharm. 2014, 11, 3556–3564. 10.1021/mp500332m. [DOI] [PubMed] [Google Scholar]
- Moss D. M.; Siccardi M. Optimizing nanomedicine pharmacokinetics using physiologically based pharmacokinetics modelling. Br. J. Pharmacol. 2014, 171, 3963–79. 10.1111/bph.12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida T.; Saika S. Cornea and sclera: anatomy and physiology. Cornea 2011, 1, 3–24. 10.1016/B978-0-323-06387-6.00008-8. [DOI] [Google Scholar]
- Kels B. D.; Grzybowski A.; Grant-Kels J. M. Human ocular anatomy. Clin Dermatol 2015, 33, 140–146. 10.1016/j.clindermatol.2014.10.006. [DOI] [PubMed] [Google Scholar]
- Saha P.; Kim K.-J.; Lee V. H. L. A primary culture model of rabbit conjunctival epithelial cells exhibiting tight barrier properties. Curr. Eye Res. 1996, 15, 1163–9. 10.3109/02713689608995151. [DOI] [PubMed] [Google Scholar]
- Ahmed I.The noncorneal route in ocular drug delivery. Ophthalmic Drug Delivery Systems; CRC Press: Boca Raton, FL, 2003; pp 356–385. [Google Scholar]
- Dey S.; Mitra A. K. Transporters and receptors in ocular drug delivery: opportunities and challenges. Expert Opin. Drug Deliv. 2005, 2, 201–204. 10.1517/17425247.2.2.201. [DOI] [PubMed] [Google Scholar]
- Geroski D. H.; Edelhauser H. F. Drug delivery for posterior segment eye disease. Invest. Ophthalmol. Vis. Sci. 2000, 41, 961–964. [PubMed] [Google Scholar]
- Lutty G. A.; Bhutto I; McLeod D. S.. Anatomy of the Ocular Vasculatures. In Ocular Blood Flow; Springer: New York, 2012; pp 3–21. [Google Scholar]
- Smith D. W.; Lee C.-J.; Gardiner B. S. No flow through the vitreous humor: How strong is the evidence?. Prog. Retin Eye Res. 2020, 78, 100845. 10.1016/j.preteyeres.2020.100845. [DOI] [PubMed] [Google Scholar]
- Ferroni M.; de Gaetano F.; Cereda M. G.; Boschetti F. A drug delivery analysis of large molecules in ocular vitreous chamber: Dependency on saccadic movements after intravitreal injection. Med. Eng. Phys. 2020, 82, 49–57. 10.1016/j.medengphy.2020.06.005. [DOI] [PubMed] [Google Scholar]
- Freddo T. F. A contemporary concept of the blood–aqueous barrier. Prog. Retin Eye Res. 2013, 32, 181–95. 10.1016/j.preteyeres.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrahari V.; Mandal A.; Agrahari V.; Trinh H. M.; Joseph M.; Ray A.; et al. A comprehensive insight on ocular pharmacokinetics. Drug Deliv Transl. Res. 2016, 6, 735–754. 10.1007/s13346-016-0339-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrimawithana T. R.; Young S.; Bunt C. R.; Green C.; Alany R. G. Drug delivery to the posterior segment of the eye. Drug Discov Today 2011, 16, 270–277. 10.1016/j.drudis.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Jooybar E.; Abdekhodaie M. J.; Farhadi F.; Cheng Y.-L. Computational modeling of drug distribution in the posterior segment of the eye: effects of device variables and positions. Math Biosci. 2014, 255, 11–20. 10.1016/j.mbs.2014.06.008. [DOI] [PubMed] [Google Scholar]
- Bron A. J.; Tiffany J. M.; Gouveia S. M.; Yokoi N.; Voon L. W. Functional aspects of the tear film lipid layer. Exp. Eye Res. 2004, 78, 347–360. 10.1016/j.exer.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Janoria K. G.; Gunda S.; Boddu S. H. S.; Mitra A. K. Novel approaches to retinal drug delivery. Expert Opin. Drug Deliv. 2007, 4, 371–388. 10.1517/17425247.4.4.371. [DOI] [PubMed] [Google Scholar]
- Fields M. A.; del Priore L v; Adelman R. A.; Rizzolo L. J. Interactions of the choroid, Bruch’s membrane, retinal pigment epithelium, and neurosensory retina collaborate to form the outer blood-retinal-barrier. Prog. Retin Eye Res. 2020, 76, 100803. 10.1016/j.preteyeres.2019.100803. [DOI] [PubMed] [Google Scholar]
- Runkle E. A.; Antonetti D. A. The blood-retinal barrier: structure and functional significance. Blood-Brain and Other Neural Barriers 2011, 686, 133–148. 10.1007/978-1-60761-938-3_5. [DOI] [PubMed] [Google Scholar]
- Kubo Y.; Akanuma S.; Hosoya K. Recent advances in drug and nutrient transport across the blood-retinal barrier. Expert Opin. Drug Metab. Toxicol. 2018, 14, 513–31. 10.1080/17425255.2018.1472764. [DOI] [PubMed] [Google Scholar]
- Hosoya K.; Tachikawa M. Inner blood-retinal barrier transporters: role of retinal drug delivery. Pharm. Res. 2009, 26, 2055–2065. 10.1007/s11095-009-9930-2. [DOI] [PubMed] [Google Scholar]
- Sahoo S. K.; Dilnawaz F.; Krishnakumar S. Nanotechnology in ocular drug delivery. Drug Discov. Today 2008, 13, 144–151. 10.1016/j.drudis.2007.10.021. [DOI] [PubMed] [Google Scholar]
- Vyas S. P., Paliwal R, Paliwal S. R.. Ocular delivery of peptides and proteins. Peptide and Protein Delivery; Elsevier Inc.: Amsterdam, 2011; pp 87–103. 10.1016/B978-0-12-384935-9.10005-7. [DOI] [Google Scholar]
- Daugherty A. L.; Mrsny R. J. Formulation and delivery issues for monoclonal antibody therapeutics. Adv. Drug Deliv. Rev. 2006, 58, 686–706. 10.1016/j.addr.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Sasahara K.; McPhie P.; Minton A. P. Effect of dextran on protein stability and conformation attributed to macromolecular crowding. J. Mol. Biol. 2003, 326, 1227–1237. 10.1016/S0022-2836(02)01443-2. [DOI] [PubMed] [Google Scholar]
- Kerwin B. A. Polysorbates 20 and 80 used in the formulation of protein biotherapeutics: structure and degradation pathways. J. Pharm. Sci. 2008, 97, 2924–2935. 10.1002/jps.21190. [DOI] [PubMed] [Google Scholar]
- Mitragotri S.; Burke P. A.; Langer R. Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nat. Rev. Drug Discov 2014, 13, 655–672. 10.1038/nrd4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel C.; Pande S.; Sagathia V.; Ranch K.; Beladiya J.; Boddu S. H. S.; et al. Nanocarriers for the Delivery of Neuroprotective Agents in the Treatment of Ocular Neurodegenerative Diseases. Pharmaceutics 2023, 15, 837. 10.3390/pharmaceutics15030837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomoto H.; Shiraga F.; Kuno N.; Kimura E.; Fujii S.; Shinomiya K.; et al. Pharmacokinetics of bevacizumab after topical, subconjunctival, and intravitreal administration in rabbits. Invest. Ophthalmol. Vis. Sci. 2009, 50, 4807–13. 10.1167/iovs.08-3148. [DOI] [PubMed] [Google Scholar]
- Zhao F.; Fan S.; Ghate D.; Romanova S.; Bronich T. K.; Zhao S. A Hydrogel Ionic Circuit Based High-Intensity Iontophoresis Device for Intraocular Macromolecule and Nanoparticle Delivery. Adv. Mater. 2022, 34, 2107315 10.1002/adma.202107315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo H. H.; Shen E. P. Long-term topical bevacizumab for prevention of corneal graft rejections. Eur. J. Ophthalmol. 2021, 31, NP48–52. 10.1177/1120672120939504. [DOI] [PubMed] [Google Scholar]
- Schultz C.; Breaux J.; Schentag J.; Morck D. Drug delivery to the posterior segment of the eye through hydrogel contact lenses. Clin Exp Optom 2011, 94, 212–218. 10.1111/j.1444-0938.2010.00553.x. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Tian Q.; Zheng T.; Chen D.; Wang Q.; Ke M. Effect of multiple subconjunctival conbercept injections as an adjuvant to the surgical treatment of pterygium: a prospective randomised comparative 6-month follow-up study. Eye (Lond) 2020, 34, 408–414. 10.1038/s41433-019-0596-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eljarrat-Binstock E.; Orucov F.; Aldouby Y.; Frucht-Pery J.; Domb A. J. Charged nanoparticles delivery to the eye using hydrogel iontophoresis. J. Controlled Release 2008, 126, 156–161. 10.1016/j.jconrel.2007.11.016. [DOI] [PubMed] [Google Scholar]
- Falavarjani K. G.; Khadamy J.; Karimi Moghaddam A.; Karimi N.; Modarres M. Posterior sub-tenon’s bevacizumab injection in diabetic macular edema; a pilot study. Saudi J. Ophthalmol. 2015, 29, 270–3. 10.1016/j.sjopt.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heier J. S.; Khanani A. M.; Quezada Ruiz C.; Basu K.; Ferrone P. J.; Brittain C.; et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): two randomised, double-masked, phase 3, non-inferiority trials. Lancet 2022, 399, 729–740. 10.1016/S0140-6736(22)00010-1. [DOI] [PubMed] [Google Scholar]
- Hashemian M. N.; Zare M. A.; Rahimi F.; Mohammadpour M. Deep intrastromal bevacizumab injection for management of corneal stromal vascularization after deep anterior lamellar keratoplasty, a novel technique. Cornea 2011, 30, 215–218. 10.1097/ICO.0b013e3181e291a6. [DOI] [PubMed] [Google Scholar]
- Ucgul R. K.; Celebi S.; Yilmaz N. S.; Bukan N.; Ucgul A. Y. Intrastromal versus subconjunctival anti-VEGF agents for treatment of corneal neovascularization: a rabbit study. Eye (Lond) 2021, 35, 3123–3130. 10.1038/s41433-020-01347-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J.; Zhang J.; Li Y.; Han X.; Zheng W.; Yang J.; Xu G.; et al. A combination of intrastromal and intracameral injections of amphotericin B in the treatment of severe fungal keratitis. J. Ophthalmol. 2016, 2016, 1–7. 10.1155/2016/3436415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahedian Z.; Mafi M.; Fakhraie G.; Zarei R.; Eslami Y.; Ghadimi H.; et al. Short-term Results of Trabeculectomy Using Adjunctive Intracameral Bevacizumab Versus Mitomycin C: A Randomized Controlled Trial. J. Glaucoma 2017, 26, 829–834. 10.1097/IJG.0000000000000741. [DOI] [PubMed] [Google Scholar]
- Sher I.; Goldberg Z.; Bubis E.; Barak Y.; Rotenstreich Y. Suprachoroidal delivery of bevacizumab in rabbit in vivo eyes: Rapid distribution throughout the posterior segment. Eur. J. Pharm. Biopharm. 2021, 169, 200–210. 10.1016/j.ejpb.2021.10.003. [DOI] [PubMed] [Google Scholar]
- Edelhauser H. F.; Rowe-Rendleman C. L.; Robinson M. R.; Dawson D. G.; Chader G. J.; Grossniklaus H. E.; et al. Ophthalmic drug delivery systems for the treatment of retinal diseases: basic research to clinical applications. Invest. Ophthalmol. Vis Sci. 2010, 51, 5403–5420. 10.1167/iovs.10-5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghate D.; Edelhauser H. F. Ocular drug delivery. Expert Opin Drug Deliv 2006, 3, 275–287. 10.1517/17425247.3.2.275. [DOI] [PubMed] [Google Scholar]
- Turecek P. L.; Bossard M. J.; Schoetens F.; Ivens I. A. PEGylation of biopharmaceuticals: a review of chemistry and nonclinical safety information of approved drugs. J. Pharm. Sci. 2016, 105, 460–475. 10.1016/j.xphs.2015.11.015. [DOI] [PubMed] [Google Scholar]
- Ng E. W. M.; Shima D. T.; Calias P.; Cunningham E. T.; Guyer D. R.; Adamis A. P. Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat. Rev. Drug Discov 2006, 5, 123–132. 10.1038/nrd1955. [DOI] [PubMed] [Google Scholar]
- Kuo T. T.; Baker K.; Yoshida M.; Qiao S.-W.; Aveson V. G.; Lencer W. I.; et al. Neonatal Fc receptor: from immunity to therapeutics. J. Clin. Immunol. 2010, 30, 777–789. 10.1007/s10875-010-9468-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oganesyan V.; Damschroder M. M.; Cook K. E.; Li Q.; Gao C.; Wu H.; et al. Structural insights into neonatal Fc receptor-based recycling mechanisms. J. Biol. Chem. 2014, 289, 7812–7824. 10.1074/jbc.M113.537563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sockolosky J. T.; Szoka F. C. The neonatal Fc receptor, FcRn, as a target for drug delivery and therapy. Adv. Drug Deliv Rev. 2015, 91, 109–124. 10.1016/j.addr.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalevsky J.; Chamberlain A. K.; Horton H. M.; Karki S.; Leung I. W. L.; Sproule T. J.; et al. Enhanced antibody half-life improves in vivo activity. Nat. Biotechnol. 2010, 28, 157–159. 10.1038/nbt.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal A.; Pal D.; Agrahari V.; My Trinh H.; Joseph M.; Mitra A. K.. Advanced Drug Delivery Reviews Ocular delivery of proteins and peptides: challenges and novel formulation approaches. 2018. [DOI] [PMC free article] [PubMed]
- Kim Y. C.; Chiang B.; Wu X.; Prausnitz M. R. Ocular delivery of macromolecules. J. Controlled Release 2014, 190, 172–181. 10.1016/j.jconrel.2014.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas S. P., Paliwal R, Paliwal S. R.. Ocular delivery of peptides and proteins. Peptide and Protein Delivery; Elsevier Inc.: Amsterdam, 2011; pp 87–103. 10.1016/B978-0-12-384935-9.10005-7. [DOI] [Google Scholar]
- Bajracharya R.; Song J. G.; Back S. Y.; Han H. K. Recent Advancements in Non-Invasive Formulations for Protein Drug Delivery. Comput. Struct. Biotechnol. J. 2019, 17, 1290–1308. 10.1016/j.csbj.2019.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal P, Huang D, Thakur S. S., Rupenthal I. D.. Nanotechnology for ocular drug delivery. Design of Nanostructures for Versatile Therapeutic Applications; Elsevier: Amsterdam; 2018; pp 137–188. 10.1016/B978-0-12-813667-6.00004-8. [DOI] [Google Scholar]
- Subrizi A.; del Amo E. M.; Korzhikov-Vlakh V.; Tennikova T.; Ruponen M.; Urtti A. Design principles of ocular drug delivery systems: importance of drug payload, release rate, and material properties. Drug Discov. Today 2019, 24, 1446–1457. 10.1016/j.drudis.2019.02.001. [DOI] [PubMed] [Google Scholar]
- Torchilin V.Introduction. Nanocarriers for Drug Delivery: Needs and Requirements. n.d.
- Fornaguera C.; García-Celma M. J. Personalized nanomedicine: A revolution at the nanoscale. J. Pers. Med. 2017, 7, 12. 10.3390/jpm7040012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza A. Z.; Siddiqui F. A. Nanomedicine and drug delivery: a mini review. Int. Nano Lett. 2014, 4, 94. 10.1007/s40089-014-0094-7. [DOI] [Google Scholar]
- Maulvi F. A., Ranch K. M., Desai A. R., Desai D. T., Shukla M. R.. Ophthalmic preparations. Remington, Elsevier: Amsterdam; 2021; pp 565–575 10.1016/B978-0-12-820007-0.00028-3. [DOI] [Google Scholar]
- Pakzad Y.; Fathi M; Omidi Y.; Zamanian A.; Mozafari M.. 21 - Nanotechnology for ocular and optic drug delivery and targeting. In Nanoengineered Biomaterials for Advanced Drug Delivery; Mozafari M., Ed.; Elsevier: Amsterdam; 2020; pp 499–523 10.1016/B978-0-08-102985-5.00021-8. [DOI] [Google Scholar]
- Weng Y.; Liu J.; Jin S.; Guo W.; Liang X.; Hu Z. Nanotechnology-based strategies for treatment of ocular disease. Acta Pharm. Sin B 2017, 7, 281–91. 10.1016/j.apsb.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S.; Franco Y. L.; Zhou Y.; Chen J. Nanotechnology in retinal drug delivery. Int. J. Ophthalmol. 2018, 11, 1038–1044. 10.18240/ijo.2018.06.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T.-C.; Hung K.-H.; Peng C.-H.; Liu J.-H.; Woung L.-C.; Tsai C.-Y.; Chen S.-J.; Chen Y.-T.; Hsu C.-C. Nanotechnology-based drug delivery treatments and specific targeting therapy for age-related macular degeneration. J. Chin. Med. Assoc. 2015, 78, 635. 10.1016/j.jcma.2015.07.008. [DOI] [PubMed] [Google Scholar]
- Ludwig P. E.; Freeman S. C.; Janot A. C. Novel stem cell and gene therapy in diabetic retinopathy, age related macular degeneration, and retinitis pigmentosa. Int. J. Retina Vitreous 2019, 5, 7. 10.1186/s40942-019-0158-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formica M. L.; Awde Alfonso H. G.; Palma S. D.. Biological drug therapy for ocular angiogenesis: Anti-VEGF agents and novel strategies based on nanotechnology. Pharmacol. Res. Perspect 2021, 9 10.1002/prp2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison P. W. J.; Connon C. J.; Khutoryanskiy V v. Cyclodextrin-mediated enhancement of riboflavin solubility and corneal permeability. Mol. Pharm. 2013, 10, 756–762. 10.1021/mp3005963. [DOI] [PubMed] [Google Scholar]
- Moiseev R v.; Morrison P. W. J.; Steele F.; Khutoryanskiy V v. Penetration enhancers in ocular drug delivery. Pharmaceutics 2019, 11, 321. 10.3390/pharmaceutics11070321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cal K.; Centkowska K. Use of cyclodextrins in topical formulations: Practical aspects. Eur. J. Pharm. Biopharm. 2008, 68, 467–478. 10.1016/j.ejpb.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Loftsson T.; Stefánsson E. Cyclodextrins in eye drop formulations: enhanced topical delivery of corticosteroids to the eye. Acta Ophthalmol. Scand. 2002, 80, 144–150. 10.1034/j.1600-0420.2002.800205.x. [DOI] [PubMed] [Google Scholar]
- Loftsson T.; Brewster M. E.; Masson M. Role of cyclodextrins in improving oral drug delivery. Am. J. Drug Deliv. 2004, 2, 261–275. 10.2165/00137696-200402040-00006. [DOI] [Google Scholar]
- Ghitman J.; Voicu S. I. Controlled drug delivery mediated by cyclodextrin-based supramolecular self-assembled carriers: From design to clinical performances. Carbohydr. Polym. Technol. Appl. 2023, 5, 100266 10.1016/j.carpta.2022.100266. [DOI] [Google Scholar]
- Lorenzo-Soler L.; Praphanwittaya P.; Olafsdottir O. B.; Kristinsdottir I. M.; Asgrimsdottir G. M.; Loftsson T.; et al. Topical noninvasive retinal drug delivery of a tyrosine kinase inhibitor: 3% cediranib maleate cyclodextrin nanoparticle eye drops in the rabbit eye. Acta Ophthalmol. 2022, 100, 788–796. 10.1111/aos.15101. [DOI] [PubMed] [Google Scholar]
- Kaur I. P.; Smitha R. Penetration enhancers and ocular bioadhesives: two new avenues for ophthalmic drug delivery. Drug Dev. Ind. Pharm. 2002, 28, 353–369. 10.1081/DDC-120002997. [DOI] [PubMed] [Google Scholar]
- Mahaling B.; Katti D. S. Understanding the influence of surface properties of nanoparticles and penetration enhancers for improving bioavailability in eye tissues in vivo. Int. J. Pharm. 2016, 501, 1–9. 10.1016/j.ijpharm.2016.01.053. [DOI] [PubMed] [Google Scholar]
- Yasukawa T.; Ogura Y.; Tabata Y.; Kimura H.; Wiedemann P.; Honda Y. Drug delivery systems for vitreoretinal diseases. Prog. Retin. Eye Res. 2004, 23, 253–281. 10.1016/j.preteyeres.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Jiao J. Polyoxyethylated nonionic surfactants and their applications in topical ocular drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1663–1673. 10.1016/j.addr.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Kumar G. P.; Rajeshwarrao P. Nonionic surfactant vesicular systems for effective drug delivery—an overview. Acta Pharm. Sin B 2011, 1, 208–219. 10.1016/j.apsb.2011.09.002. [DOI] [Google Scholar]
- van der Bijl P.; Engelbrecht A. H.; van Eyk A. D.; Meyer D. Comparative permeability of human and rabbit corneas to cyclosporin and tritiated water. J. Ocul. Pharmacol. Ther. 2002, 18, 419–427. 10.1089/10807680260362704. [DOI] [PubMed] [Google Scholar]
- Sasaki H.; Yamamura K.; Mukai T.; Nishida K.; Nakamura J.; Nakashima M.; et al. Modification of ocular permeability of peptide drugs by absorption promoters. Biol. Pharm. Bull. 2000, 23, 1524–1547. 10.1248/bpb.23.1524. [DOI] [PubMed] [Google Scholar]
- Agarwal P.; Scherer D.; Günther B.; Rupenthal I. D. Semifluorinated alkane based systems for enhanced corneal penetration of poorly soluble drugs. Int. J. Pharm. 2018, 538, 119–129. 10.1016/j.ijpharm.2018.01.019. [DOI] [PubMed] [Google Scholar]
- Sahoo S. K.; Dilnawaz F.; Krishnakumar S. Nanotechnology in ocular drug delivery. Drug Discov Today 2008, 13, 144–151. 10.1016/j.drudis.2007.10.021. [DOI] [PubMed] [Google Scholar]
- Jain R. A. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials 2000, 21, 2475–2490. 10.1016/S0142-9612(00)00115-0. [DOI] [PubMed] [Google Scholar]
- Hykin P.; Prevost A. T.; Vasconcelos J. C.; Murphy C.; Kelly J.; Ramu J.; et al. Clinical Effectiveness of Intravitreal Therapy With Ranibizumab vs Aflibercept vs Bevacizumab for Macular Edema Secondary to Central Retinal Vein Occlusion: A Randomized Clinical Trial. JAMA Ophthalmol 2019, 137, 1256–1264. 10.1001/jamaophthalmol.2019.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Real J. P.; Luna J. D.; Urrets-Zavalia J. A.; de Santis M. O.; Palma S. D.; Granero G. E. Accessibility as a Conditioning Factor in Treatment for Exudative Age-related Macular Degeneration. Eur. J. Ophthalmol. 2013, 23, 857–864. 10.5301/ejo.5000299. [DOI] [PubMed] [Google Scholar]
- Sousa F.; Cruz A.; Fonte P.; Pinto I. M.; Neves-Petersen M. T.; Sarmento B. A new paradigm for antiangiogenic therapy through controlled release of bevacizumab from PLGA nanoparticles. Sci. Rep. 2017, 7, 3736. 10.1038/s41598-017-03959-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshochian R.; Jeddi-Tehrani M.; Mahmoudi A. R.; Khoshayand M. R.; Atyabi F.; Sabzevari A.; et al. The protective effect of albumin on bevacizumab activity and stability in PLGA nanoparticles intended for retinal and choroidal neovascularization treatments. Eur. J. Pharm. Sci. 2013, 50, 341–352. 10.1016/j.ejps.2013.07.014. [DOI] [PubMed] [Google Scholar]
- Liu J.; Zhang X.; Li G.; Xu F.; Li S.; Teng L.; et al. Anti-angiogenic activity of bevacizumab-bearing dexamethasone-loaded PLGA nanoparticles for potential intravitreal applications. Int. J. Nanomed. 2019, 14, 8819–8834. 10.2147/IJN.S217038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandit J.; Sultana Y.; Aqil M. Chitosan-coated PLGA nanoparticles of bevacizumab as novel drug delivery to target retina: optimization, characterization, and in vitro toxicity evaluation. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1397–1407. 10.1080/21691401.2016.1243545. [DOI] [PubMed] [Google Scholar]
- Zhang X.-P.; Sun J.-G.; Yao J.; Shan K.; Liu B.-H.; Yao M.-D.; et al. Effect of nanoencapsulation using poly (lactide-co-glycolide) (PLGA) on anti-angiogenic activity of bevacizumab for ocular angiogenesis therapy. Biomed. Pharmaceutics 2018, 107, 1056–1063. 10.1016/j.biopha.2018.08.092. [DOI] [PubMed] [Google Scholar]
- Yandrapu S. K.; Upadhyay A. K.; Petrash J. M.; Kompella U. B. Nanoparticles in Porous Microparticles Prepared by Supercritical Infusion and Pressure Quench Technology for Sustained Delivery of Bevacizumab. Mol. Pharmaceutics 2013, 10, 4676–4686. 10.1021/mp400487f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J. G.; Jiang Q.; Zhang X. P.; Shan K.; Liu B. H.; Zhao C.; et al. Mesoporous silica nanoparticles as a delivery system for improving antiangiogenic therapy. Int. J. Nanomed. 2019, 14, 1489–1501. 10.2147/IJN.S195504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa F.; Cruz A.; Pinto I. M.; Sarmento B. Nanoparticles provide long-term stability of bevacizumab preserving its antiangiogenic activity. Acta Biomater. 2018, 78, 285–295. 10.1016/j.actbio.2018.07.040. [DOI] [PubMed] [Google Scholar]
- Zhu R.; Wang Z.; Liang P.; He X.; Zhuang X.; Huang R.; et al. Efficient VEGF targeting delivery of DOX using Bevacizumab conjugated SiO2@LDH for anti-neuroblastoma therapy. Acta Biomater 2017, 63, 163–180. 10.1016/j.actbio.2017.09.009. [DOI] [PubMed] [Google Scholar]
- Makadia H. K.; Siegel S. J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers (Basel) 2011, 3, 1377–1397. 10.3390/polym3031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prow T. W.; Bhutto I.; Kim S. Y.; Grebe R.; Merges C.; McLeod D. S.; et al. Ocular nanoparticle toxicity and transfection of the retina and retinal pigment epithelium. Nanomedicine 2008, 4, 340–349. 10.1016/j.nano.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshochian R.; Jeddi-Tehrani M.; Mahmoudi A. R.; Khoshayand M. R.; Atyabi F.; Sabzevari A.; et al. The protective effect of albumin on bevacizumab activity and stability in PLGA nanoparticles intended for retinal and choroidal neovascularization treatments. Eur. J. Pharm. Sci. 2013, 50, 341–352. 10.1016/j.ejps.2013.07.014. [DOI] [PubMed] [Google Scholar]
- Kelly S. J.; Hirani A.; Shahidadpury V.; Solanki A.; Halasz K.; Varghese Gupta S. Aflibercept Nanoformulation Inhibits VEGF Expression in Ocular In Vitro Model: A Preliminary Report. Biomedicines 2018, 6, 92. 10.3390/biomedicines6030092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J.; Peng X.; Cai Y.; Cong W. Development of facile drug delivery platform of ranibizumab fabricated PLGA-PEGylated magnetic nanoparticles for age-related macular degeneration therapy. J. Photochem. Photobiol. B 2018, 183, 133–136. 10.1016/j.jphotobiol.2018.04.033. [DOI] [PubMed] [Google Scholar]
- Goel S.; Chen F.; Hong H.; Valdovinos H. F.; Hernandez R.; Shi S.; et al. VEGF121-Conjugated Mesoporous Silica Nanoparticle: A Tumor Targeted Drug Delivery System. ACS Appl. Mater. Interfaces 2014, 6, 21677–21685. 10.1021/am506849p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel J.; Amrutiya J.; Bhatt P.; Javia A.; Jain M.; Misra A. Targeted delivery of monoclonal antibody conjugated docetaxel loaded PLGA nanoparticles into EGFR overexpressed lung tumour cells. J. Microencapsul. 2018, 35, 204–217. 10.1080/02652048.2018.1453560. [DOI] [PubMed] [Google Scholar]
- Sahoo S.; Dilnawaz F.; Krishnakumar S. Nanotechnology in ocular drug delivery. Drug Discov. Today 2008, 13, 144–151. 10.1016/j.drudis.2007.10.021. [DOI] [PubMed] [Google Scholar]
- Zhang X.-P.; Sun J.-G.; Yao J.; Shan K.; Liu B.-H.; Yao M.-D.; et al. Effect of nanoencapsulation using poly (lactide-co-glycolide) (PLGA) on anti-angiogenic activity of bevacizumab for ocular angiogenesis therapy. Biomed. Pharmacother. 2018, 107, 1056–1063. 10.1016/j.biopha.2018.08.092. [DOI] [PubMed] [Google Scholar]
- Başaran E.; Yenilmez E.; Berkman M. S.; Büyükköroğlu G.; Yazan Y. Chitosan nanoparticles for ocular delivery of cyclosporine A. J. Microencapsul. 2014, 31, 49–57. 10.3109/02652048.2013.805839. [DOI] [PubMed] [Google Scholar]
- de Campos A. M.; Sánchez A.; Alonso M. J. Chitosan nanoparticles: a new vehicle for the improvement of the delivery of drugs to the ocular surface. Application to cyclosporin A. Int. J. Pharm. 2001, 224, 159. 10.1016/S0378-5173(01)00760-8. [DOI] [PubMed] [Google Scholar]
- Ugurlu N.; Aşık M. D.; Çakmak H. B.; Tuncer S.; Turk M.; Çagıl N.; et al. Transscleral delivery of bevacizumab-loaded chitosan nanoparticles. J. Biomed. Nanotechnol. 2019, 15, 830–838. 10.1166/jbn.2019.2716. [DOI] [PubMed] [Google Scholar]
- Lu Y.; Zhou N.; Huang X.; Cheng J. W.; Li F. Q.; Wei R. L. Effect of intravitreal injection of bevacizumab-chitosan nanoparticles on retina of diabetic rats. Int. J. Ophthalmol. 2013, 7, 1–7. 10.3980/j.issn.2222-3959.2014.01.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsaid N.; Jackson T. L.; Elsaid Z.; Alqathama A.; Somavarapu S. PLGA Microparticles Entrapping Chitosan-Based Nanoparticles for the Ocular Delivery of Ranibizumab. Mol. Pharmaceutics 2016, 13, 2923–2940. 10.1021/acs.molpharmaceut.6b00335. [DOI] [PubMed] [Google Scholar]
- Pandit J.; Sultana Y.; Mohd A. Chitosan coated nanoparticles for efficient delivery of bevacizumab in the posterior ocular tissues via subconjunctival administration. Carbohydr. Polym. 2021, 267, 118217 10.1016/j.carbpol.2021.118217. [DOI] [PubMed] [Google Scholar]
- Luis de Redín I.; Boiero C.; Recalde S.; Agüeros M.; Allemandi D.; Llabot J. M.; et al. In vivo effect of bevacizumab-loaded albumin nanoparticles in the treatment of corneal neovascularization. Exp. Eye Res. 2019, 185, 107697 10.1016/j.exer.2019.107697. [DOI] [PubMed] [Google Scholar]
- Luis de Redín I.; Boiero C.; Martínez-Ohárriz M. C.; Agüeros M.; Ramos R.; Peñuelas I.; et al. Human serum albumin nanoparticles for ocular delivery of bevacizumab. Int. J. Pharm. 2018, 541, 214–223. 10.1016/j.ijpharm.2018.02.003. [DOI] [PubMed] [Google Scholar]
- Llabot J. M.; Luis de Redin I.; Agüeros M.; Dávila Caballero M. J.; Boiero C.; Irache J. M.; et al. In vitro characterization of new stabilizing albumin nanoparticles as a potential topical drug delivery system in the treatment of corneal neovascularization (CNV). J. Drug Deliv. Sci. Technol. 2019, 52, 379–385. 10.1016/j.jddst.2019.04.042. [DOI] [Google Scholar]
- Formica M. L.; Awde Alfonso H. G.; Palma S. D.. Biological drug therapy for ocular angiogenesis: Anti-VEGF agents and novel strategies based on nanotechnology. Pharmacol. Res. Perspect. 2021;9 10.1002/prp2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran R.; Wang H.; Hou F.; Liu Y.; Hui Y.; Petrovsky N.; et al. A Microfluidic Tumor-on-a-Chip for Assessing Multifunctional Liposomes’ Tumor Targeting and Anticancer Efficacy. Adv. Healthc Mater. 2019, 8, 1900015 10.1002/adhm.201900015. [DOI] [PubMed] [Google Scholar]
- Has C.; Sunthar P. A comprehensive review on recent preparation techniques of liposomes. J. Liposome Res. 2020, 30, 336–365. 10.1080/08982104.2019.1668010. [DOI] [PubMed] [Google Scholar]
- Karumanchi D. K.; Skrypai Y.; Thomas A.; Gaillard E. R. Rational design of liposomes for sustained release drug delivery of bevacizumab to treat ocular angiogenesis. J. Drug Deliv Sci. Technol. 2018, 47, 275–282. 10.1016/j.jddst.2018.07.003. [DOI] [Google Scholar]
- Abrishami M.; Ganavati S. Z.; Soroush D.; Rouhbakhsh M.; Jaafari M. R.; Malaekeh-Nikouei B. Preparation, Characterization, and In Vivo Evaluation of Nanoliposomes-Encapsulated Bevacizumab (Avastin) for Intravitreal Administration. Retina 2009, 29, 699–703. 10.1097/IAE.0b013e3181a2f42a. [DOI] [PubMed] [Google Scholar]
- Mu H.; Wang Y.; Chu Y.; Jiang Y.; Hua H.; Chu L.; et al. Multivesicular liposomes for sustained release of bevacizumab in treating laser-induced choroidal neovascularization. Drug Deliv. 2018, 25, 1372–1383. 10.1080/10717544.2018.1474967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekmann B.; Westesen K. Preparation and Physicochemical Characterization of Aqueous Dispersions of Coenzyme Q10 Nanoparticles. Pharm. Res. 1995, 12, 201–208. 10.1023/A:1016270724413. [DOI] [PubMed] [Google Scholar]
- Naseri N.; Valizadeh H.; Zakeri-Milani P. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Structure, Preparation and Application. Adv. Pharm. Bull. 2015, 5, 305–313. 10.15171/apb.2015.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur J.; Singh G.; Saini S.; Rana A. C.. Innovative Growth in Developing New Methods for Formulating Solid Lipid Nanoparticles and Microparticles. J. Drug Delivery Ther. 2012; 2 10.22270/jddt.v2i5.295. [DOI] [Google Scholar]
- Xu L.; Wang X.; Liu Y.; Yang G.; Falconer R. J.; Zhao C.-X. Lipid Nanoparticles for Drug Delivery. Adv. Nanobiomed. Res. 2022, 2, 2100109 10.1002/anbr.202100109. [DOI] [Google Scholar]
- Battaglia L.; Gallarate M.; Peira E.; Chirio D.; Solazzi I.; Giordano S. M. A.; et al. Bevacizumab loaded solid lipid nanoparticles prepared by the coacervation technique: preliminary in vitro studies. Nanotechnology 2015, 26, 255102 10.1088/0957-4484/26/25/255102. [DOI] [PubMed] [Google Scholar]
- Luo Q.; Zhao J.; Zhang X.; Pan W. Nanostructured lipid carrier (NLC) coated with Chitosan Oligosaccharides and its potential use in ocular drug delivery system. Int. J. Pharm. 2011, 403, 185–191. 10.1016/j.ijpharm.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Salvi V. R.; Pawar P. Nanostructured lipid carriers (NLC) system: A novel drug targeting carrier. J. Drug Deliv. Sci. Technol. 2019, 51, 255–267. 10.1016/j.jddst.2019.02.017. [DOI] [Google Scholar]
- Pardeike J.; Hommoss A.; Müller R. H. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int. J. Pharm. 2009, 366, 170–184. 10.1016/j.ijpharm.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Kumar R.; Sinha V. R. Fabrication Of Voriconazole Solid Lipid Nanoparticles For Effective Ocular Delivery. Value in Health 2014, 17, A613. 10.1016/j.jval.2014.08.2153. [DOI] [PubMed] [Google Scholar]
- Andrade L. M.; Rocha K. A. D.; De Sá F. A. P.; Marreto R. N.; Lima E. M.; Gratieri T.; et al. Voriconazole-Loaded Nanostructured Lipid Carriers for Ocular Drug Delivery. Cornea 2016, 35, 866–871. 10.1097/ICO.0000000000000825. [DOI] [PubMed] [Google Scholar]
- Andrade L. M.; de Fatima Reis C.; Maione-Silva L.; Anjos J. L. V.; Alonso A.; Serpa R. C.; Marreto R. N.; Lima E. M.; Taveira S. F. Impact of lipid dynamic behavior on physical stability, in vitro release and skin permeation of genistein-loaded lipid nanoparticles. Eur. J. Pharm. Biopharm. 2014, 88, 40–47. 10.1016/j.ejpb.2014.04.015. [DOI] [PubMed] [Google Scholar]
- Varela-Fernández R.; García-Otero X.; Díaz-Tomé V.; Regueiro U.; López-López M.; González-Barcia M.; et al. Lactoferrin-loaded nanostructured lipid carriers (NLCs) as a new formulation for optimized ocular drug delivery. Eur. J. Pharm. Biopharm. 2022, 172, 144–156. 10.1016/j.ejpb.2022.02.010. [DOI] [PubMed] [Google Scholar]
- Formica M. L.; Ullio Gamboa G. V.; Tártara L. I.; Luna J. D.; Benoit J. P.; Palma S. D. Triamcinolone acetonide-loaded lipid nanocapsules for ophthalmic applications. Int. J. Pharm. 2020, 573, 118795 10.1016/j.ijpharm.2019.118795. [DOI] [PubMed] [Google Scholar]
- Formica M. L.; Legeay S.; Bejaud J.; Montich G. G.; Ullio Gamboa G. V.; Benoit J.-P.; et al. Novel hybrid lipid nanocapsules loaded with a therapeutic monoclonal antibody – Bevacizumab – and Triamcinolone acetonide for combined therapy in neovascular ocular pathologies. Mater. Sci. Eng. C 2021, 119, 111398 10.1016/j.msec.2020.111398. [DOI] [PubMed] [Google Scholar]
- Burdick J. A.; Prestwich G. D. Hyaluronic Acid Hydrogels for Biomedical Applications. Adv. Mater. 2011, 23, H41–56. 10.1002/adma.201003963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.; Li X.; Zhou Y.; Wang X.; Zhang Y.; Fan Y.;. et al. Chitosan-based thermosensitive hydrogel as a promising ocular drug delivery system: Preparation, characterization, and in vivo evaluation. 2011;27:391–402 10.1177/0885328211406563. [DOI] [PubMed]
- Kushwaha S. K.; Saxena P.; Rai A. Stimuli sensitive hydrogels for ophthalmic drug delivery: A review. Int. J. Pharm. Investig. 2012, 2, 54. 10.4103/2230-973X.100036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermonden T.; Censi R.; Hennink W. E. Hydrogels for protein delivery. Chem. Rev. 2012, 112, 2853–2888. 10.1021/cr200157d. [DOI] [PubMed] [Google Scholar]
- Fathi M.; Barar J.; Aghanejad A.; Omidi Y. Hydrogels for ocular drug delivery and tissue engineering. Bioimpacts 2015, 5, 159. 10.15171/bi.2015.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi P.; Barros M.; Stansbury J. W.; Kompella U. B. Light-activated, in situ forming gel for sustained suprachoroidal delivery of bevacizumab. Mol. Pharm. 2013, 10, 2858–2867. 10.1021/mp300716t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al Khateb K.; Ozhmukhametova E. K.; Mussin M. N.; Seilkhanov S. K.; Rakhypbekov T. K.; Lau W. M.; et al. In situ gelling systems based on Pluronic F127/Pluronic F68 formulations for ocular drug delivery. Int. J. Pharm. 2016, 502, 70–79. 10.1016/j.ijpharm.2016.02.027. [DOI] [PubMed] [Google Scholar]
- Bisht R.; Jaiswal J. K.; Chen Y. S.; Jin J.; Rupenthal I. D. Light-responsive in situ forming injectable implants for effective drug delivery to the posterior segment of the eye. Expert Opin. Drug Deliv. 2016, 13, 953–962. 10.1517/17425247.2016.1163334. [DOI] [PubMed] [Google Scholar]
- Rauck B. M.; Friberg T. R.; Medina Mendez C. A.; Park D.; Shah V.; Bilonick R. A.; et al. Biocompatible Reverse Thermal Gel Sustains the Release of Intravitreal Bevacizumab In Vivo. Invest Ophthalmol. Vis. Sci. 2014, 55, 469–476. 10.1167/iovs.13-13120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbie S. J.; Lundh von Leithner P.; Ju M.; Lange C. A.; King A. G.; Adamson P.; Lee D.; Sychterz C.; Coffey P.; Ng Y.-S.; Bainbridge J. W.; Shima D. T.; et al. Assessing a Novel Depot Delivery Strategy for Noninvasive Administration of VEGF/PDGF RTK Inhibitors for Ocular Neovascular Disease. Invest. Ophthalmol. Vis. Sci. 2013, 54, 1490–1500. 10.1167/iovs.12-10169. [DOI] [PubMed] [Google Scholar]
- Kozak I.; Kayikcioglu O. R.; Cheng L.; Falkenstein I.; Silva G. A.; Yu D. X.; et al. The Effect of Recombinant Human Hyaluronidase on Dexamethasone Penetration into the Posterior Segment of the Eye After Sub-Tenon’s Injection. J. Ocular Pharmacol. Ther. 2006, 22, 362–369. 10.1089/jop.2006.22.362. [DOI] [PubMed] [Google Scholar]
- Chao K. L.; Muthukumar L.; Herzberg O. Structure of Human Hyaluronidase-1, a Hyaluronan Hydrolyzing Enzyme Involved in Tumor Growth and Angiogenesis. Biochemistry 2007, 46, 6911–6920. 10.1021/bi700382g. [DOI] [PubMed] [Google Scholar]
- Lynch C. R.; Kondiah P. P. D.; Choonara Y. E.; du Toit L. C.; Ally N.; Pillay V. Hydrogel Biomaterials for Application in Ocular Drug Delivery. Front. Bioeng. Biotechnol. 2020, 8, 228. 10.3389/fbioe.2020.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y.; Lau L. C. M.; Lo A. C.-y.; Chau Y. Injectable Chemically Crosslinked Hydrogel for the Controlled Release of Bevacizumab in Vitreous: A 6-Month In Vivo Study. Transl. Vis. Sci. Technol. 2015, 4, 5. 10.1167/tvst.4.2.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancina M. G.; Yang H. Dendrimers for Ocular Drug Delivery. Can. J. Chem. 2017, 95, 897–902. 10.1139/cjc-2017-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddu S. H. S.; Bhagav P.; Karla P. K.; Jacob S.; Adatiya M. D.; Dhameliya T. M.; et al. Polyamide/Poly(Amino Acid) Polymers for Drug Delivery. J. Funct. Biomater. 2021, 12, 58. 10.3390/jfb12040058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavuz B.; Bozdaǧ Pehlivan S.; Ünlü N. Dendrimeric systems and their applications in ocular drug delivery. Sci. World J. 2013, 2013, 1. 10.1155/2013/732340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marano R. J.; Toth I.; Wimmer N.; Brankov M.; Rakoczy P. E. Dendrimer delivery of an anti-VEGF oligonucleotide into the eye: a long-term study into inhibition of laser-induced CNV, distribution, uptake and toxicity. Gene Ther. 2005, 12, 1544–1550. 10.1038/sj.gt.3302579. [DOI] [PubMed] [Google Scholar]
- Yang X.; Wang L.; Li L.; Han M.; Tang S.; Wang T.; et al. A novel dendrimer-based complex co-modified with cyclic RGD hexapeptide and penetratin for noninvasive targeting and penetration of the ocular posterior segment. Drug Deliv. 2019, 26, 989–1001. 10.1080/10717544.2019.1667455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pescina S.; Ostacolo C.; Gomez-Monterrey I. M.; Sala M.; Bertamino A.; Sonvico F.; et al. Cell penetrating peptides in ocular drug delivery: State of the art. J. Controlled Release 2018, 284, 84–102. 10.1016/j.jconrel.2018.06.023. [DOI] [PubMed] [Google Scholar]
- Kaplan I. M.; Wadia J. S.; Dowdy S. F. Cationic TAT peptide transduction domain enters cells by macropinocytosis. J. Controlled Release 2005, 102, 247–53. 10.1016/j.jconrel.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Johnson L. N.; Cashman S. M.; Kumar-Singh R. Cell-penetrating peptide for enhanced delivery of nucleic acids and drugs to ocular tissues including retina and cornea. Mol. Ther. 2008, 16, 107–114. 10.1038/sj.mt.6300324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. N.; Cashman S. M.; Read S. P.; Kumar-Singh R. Cell penetrating peptide POD mediates delivery of recombinant proteins to retina, cornea and skin. Vision Res. 2010, 50, 686–697. 10.1016/j.visres.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cogan F.; Hill L. J.; Lynch A.; Morgan-Warren P. J.; Lechner J.; Berwick M. R.; et al. Topical Delivery of Anti-VEGF Drugs to the Ocular Posterior Segment Using Cell-Penetrating Peptides. Invest. Opthalmol. Vis. Sci. 2017, 58, 2578. 10.1167/iovs.16-20072. [DOI] [PubMed] [Google Scholar]
- George E. M.; Mahdi F.; Logue O. C.; Robinson G. G.; Bidwell G. L. Corneal Penetrating Elastin-Like Polypeptide Carriers. J. Ocular Pharmacol. Ther. 2016, 32, 163–171. 10.1089/jop.2015.0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pescina S.; Ostacolo C.; Gomez-Monterrey I. M.; Sala M.; Bertamino A.; Sonvico F.; et al. Cell penetrating peptides in ocular drug delivery: State of the art. J. Controlled Release 2018, 284, 84–102. 10.1016/j.jconrel.2018.06.023. [DOI] [PubMed] [Google Scholar]
- Emerich D. F.; Thanos C. G. NT-501: an ophthalmic implant of polymer-encapsulated ciliary neurotrophic factor-producing cells. Curr. Opin Mol. Ther 2008, 10, 506–515. [PubMed] [Google Scholar]
- Kuno N.; Fujii S. Biodegradable intraocular therapies for retinal disorders. Drugs Aging 2010, 27, 117–134. 10.2165/11530970-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Barar J.; Aghanejad A.; Fathi M.; Omidi Y. Advanced drug delivery and targeting technologies for the ocular diseases. Bioimpacts 2016, 6, 49. 10.15171/bi.2016.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P.; Yadav K. S. Applications of microneedles in delivering drugs for various ocular diseases. Life Sci. 2019, 237, 116907 10.1016/j.lfs.2019.116907. [DOI] [PubMed] [Google Scholar]
- Patel S. R.; Lin A. S. P.; Edelhauser H. F.; Prausnitz M. R. Suprachoroidal Drug Delivery to the Back of the Eye Using Hollow Microneedles. Pharm. Res. 2011, 28, 166–176. 10.1007/s11095-010-0271-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimi M. F.; Rao M. P.; MacDonald N. C.; Zuruzi A. S.; Bothman D. P. High-aspect-ratio bulk micromachining of titanium. Nat. Mater. 2004, 3, 103–105. 10.1038/nmat1058. [DOI] [PubMed] [Google Scholar]
- Kim Y. C.; Edelhauser H. F.; Prausnitz M. R. Targeted delivery of antiglaucoma drugs to the supraciliary space using microneedles. Invest. Ophthalmol. Vis Sci. 2014, 55, 7387–7397. 10.1167/iovs.14-14651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur Singh R. R.; Tekko I.; McAvoy K.; McMillan H.; Jones D.; Donnelly R. F. Minimally invasive microneedles for ocular drug delivery. Expert Opin. Drug Deliv. 2017, 14, 525–537. 10.1080/17425247.2016.1218460. [DOI] [PubMed] [Google Scholar]
- Perez V. L.; Wirostko B.; Korenfeld M.; From S.; Raizman M. Ophthalmic drug delivery using iontophoresis: Recent clinical applications. J. Ocular Pharmacol. Ther. 2020, 36, 75–87. 10.1089/jop.2019.0034. [DOI] [PubMed] [Google Scholar]
- Molokhia S.; Papangkorn K.; Butler C.; Higuchi J. W.; Brar B.; Ambati B.; et al. Transscleral Iontophoresis for Noninvasive Ocular Drug Delivery of Macromolecules. J. Ocul. Pharmacol. Ther. 2020, 36, 247–256. 10.1089/jop.2019.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F.; Fan S.; Ghate D.; Romanova S.; Bronich T. K.; Zhao S. A Hydrogel Ionic Circuit Based High-Intensity Iontophoresis Device for Intraocular Macromolecule and Nanoparticle Delivery. Adv. Mater. 2022, 34, 2107315 10.1002/adma.202107315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campochiaro P. A.; Marcus D. M.; Awh C. C.; Regillo C.; Adamis A. P.; Bantseev V.; et al. The Port Delivery System with Ranibizumab for Neovascular Age-Related Macular Degeneration: Results from the Randomized Phase 2 Ladder Clinical Trial. Ophthalmology 2019, 126, 1141–1154. 10.1016/j.ophtha.2019.03.036. [DOI] [PubMed] [Google Scholar]
- Chang M. A.; Kapre A.; Kaufman D.; Kardatzke D. R.; Rabena M.; Patel S.; et al. Patient Preference and Treatment Satisfaction With a Port Delivery System for Ranibizumab vs Intravitreal Injections in Patients With Neovascular Age-Related Macular Degeneration: A Randomized Clinical Trial. JAMA Ophthalmol. 2022, 140, 771–778. 10.1001/jamaophthalmol.2022.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykoff C. C.; Campochiaro P. A.; Pieramici D. J.; Khanani A. M.; Gune S.; Maia M.; et al. Pharmacokinetics of the Port Delivery System with Ranibizumab in the Ladder Phase 2 Trial for Neovascular Age-Related Macular Degeneration. Ophthalmol. Ther. 2022, 11, 1705–1717. 10.1007/s40123-022-00532-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dourado L. F. N.; Silva CN da; Gonçalves R. S.; Inoue T. T.; de Lima M. E.; Cunha-Júnior A da S. Improvement of PnPP-19 peptide bioavailability for glaucoma therapy: Design and application of nanowafers based on PVA. J. Drug Deliv. Sci. Technol. 2022, 74, 103501 10.1016/j.jddst.2022.103501. [DOI] [Google Scholar]
- Yuan X.; Marcano D. C.; Shin C. S.; Hua X.; Isenhart L. C.; Pflugfelder S. C.; et al. Ocular Drug Delivery Nanowafer with Enhanced Therapeutic Efficacy. ACS Nano 2015, 9, 1749–1758. 10.1021/nn506599f. [DOI] [PubMed] [Google Scholar]
- Mandal A.; Bisht R.; Rupenthal I. D.; Mitra A. K. Polymeric micelles for ocular drug delivery: From structural frameworks to recent preclinical studies. J. Controlled Release 2017, 248, 96–116. 10.1016/j.jconrel.2017.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Jawadi S.; Thakur S. S. Ultrasound-responsive lipid microbubbles for drug delivery: A review of preparation techniques to optimize formulation size, stability and drug loading. Int. J. Pharm. 2020, 585, 119559 10.1016/j.ijpharm.2020.119559. [DOI] [PubMed] [Google Scholar]
- Kida H.; Feril L. B.; Irie Y.; Endo H.; Itaka K.; Tachibana K. Influence of nanobubble size distribution on ultrasound-mediated plasmid DNA and messenger RNA gene delivery. Front. Pharmacol. 2022, 13, 855495. 10.3389/fphar.2022.855495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousou C.; Schuurmans C. C. L.; Urtti A.; Mastrobattista E.; Storm G.; Moonen C.; et al. Ultrasound and Microbubbles for the Treatment of Ocular Diseases: From Preclinical Research towards Clinical Application. Pharmaceutics 2021, 13, 1782. 10.3390/pharmaceutics13111782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y.; Xie Z.; Zhou Y.; Zhou X.; Li P.; Wang Z. Experimental endostatin-GFP gene transfection into human retinal vascular endothelial cells using ultrasound-targeted cationic microbubble destruction. Mol. Vis 2015, 21, 930. [PMC free article] [PubMed] [Google Scholar]
- Osswald C. R.; Kang-Mieler J. J. Controlled and Extended Release of a Model Protein from a Microsphere-Hydrogel Drug Delivery System. Ann. Biomed. Eng. 2015, 43, 2609–2617. 10.1007/s10439-015-1314-7. [DOI] [PubMed] [Google Scholar]
- Liu W.; Borrell M. A.; Venerus D. C.; Mieler W. F.; Kang-Mieler J. J. Characterization of Biodegradable Microsphere-Hydrogel Ocular Drug Delivery System for Controlled and Extended Release of Ranibizumab. Transl Vis Sci. Technol. 2019, 8, 12. 10.1167/tvst.8.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W.; Lee B. S.; Mieler W. F.; Kang-Mieler J. J.. Biodegradable Microsphere-Hydrogel Ocular Drug Delivery System for Controlled and Extended Release of Bioactive Aflibercept In Vitro. 2018;44, pp 264–274. 10.1080/02713683.2018.1533983. [DOI] [PMC free article] [PubMed]
- Kim S.; Kang-Mieler J. J.; Liu W.; Wang Z.; Yiu G.; Teixeira L. B. C.;. et al. Safety and Biocompatibility of Aflibercept-Loaded Microsphere Thermo-Responsive Hydrogel Drug Delivery System in a Nonhuman Primate Model. Transl. Vis Sci. Technol. 2020, 9, p 30. 10.1167/tvst.9.3.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P.; Tyagi M.; Kumar Y.; Gupta K.; Sharma P. Ocular chemical injuries and their management. Oman J. Ophthalmol 2013, 6, 83. 10.4103/0974-620X.116624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott L.; Eskes C.; Hoffmann S.; Adriaens E.; Alepée N.; Bufo M.; et al. A proposed eye irritation testing strategy to reduce and replace in vivo studies using Bottom–Up and Top–Down approaches. Toxicol. In Vitro 2010, 24, 1–9. 10.1016/j.tiv.2009.05.019. [DOI] [PubMed] [Google Scholar]
- Bhatt M.; Shende P. Modulated approaches for strategic transportation of proteins and peptides via ocular route. J. Drug Deliv Sci. Technol. 2021, 66, 102835 10.1016/j.jddst.2021.102835. [DOI] [Google Scholar]
- Tan G.; Ioannou N.; Mathew E.; Tagalakis A. D.; Lamprou D. A.; Yu-Wai-Man C. 3D printing in Ophthalmology: From medical implants to personalised medicine. Int. J. Pharm. 2022, 625, 122094 10.1016/j.ijpharm.2022.122094. [DOI] [PubMed] [Google Scholar]
- Won J. Y.; Kim J.; Gao G.; Kim J.; Jang J.; Park Y. H.; et al. 3D printing of drug-loaded multi-shell rods for local delivery of bevacizumab and dexamethasone: A synergetic therapy for retinal vascular diseases. Acta Biomater 2020, 116, 174–185. 10.1016/j.actbio.2020.09.015. [DOI] [PubMed] [Google Scholar]
- Fusco S.; Ullrich F.; Pokki J.; Chatzipirpiridis G.; Özkale B.; Sivaraman K. M.; et al. Microrobots: a new era in ocular drug delivery. Expert Opin. Drug Deliv. 2014, 11, 1815–1826. 10.1517/17425247.2014.938633. [DOI] [PubMed] [Google Scholar]
- Smirnov R. Microrobots: 21st century eye surgeons. Biochem (Lond) 2020, 42, 76–80. 10.1042/BIO20200068. [DOI] [Google Scholar]
- Kapoor R.; Walters S. P.; Al-Aswad L. A. The current state of artificial intelligence in ophthalmology. Surv. Ophthalmol. 2019, 64, 233–240. 10.1016/j.survophthal.2018.09.002. [DOI] [PubMed] [Google Scholar]
- Li P.-Y.; Shih J.; Lo R.; Saati S.; Agrawal R.; Humayun M. S.; Tai Y.-C.; Meng E.; et al. An electrochemical intraocular drug delivery device. Sens. Actuators A 2008, 143, 41–48. 10.1016/j.sna.2007.06.034. [DOI] [Google Scholar]
- Jackson T. L.; Mandava N.; Quiroz-Mercado H.; Benage M.; Garcia-Aguirre G.; Morales-Canton V.; et al. Intravitreal quantum dots for retinitis pigmentosa: a first-in-human safety study. Nanomedicine 2021, 16, 617–626. 10.2217/nnm-2020-0471. [DOI] [PubMed] [Google Scholar]
- Matea C. T.; Mocan T.; Tabaran F.; Pop T.; Mosteanu O.; Puia C.; et al. Quantum dots in imaging, drug delivery and sensor applications. Int. J. Nanomedicine 2017, 12, 5421–5431. 10.2147/IJN.S138624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinaud F.; King D.; Moore H. P.; Weiss S. Bioactivation and cell targeting of semiconductor CdSe/ZnS nanocrystals with phytochelatin-related peptides. J. Am. Chem. Soc. 2004, 126, 6115–6123. 10.1021/ja031691c. [DOI] [PMC free article] [PubMed] [Google Scholar]