Abstract
In a steady state, hematopoietic stem cells (HSC) exhibit very low levels of reactive oxygen species (ROS). Upon stress, HSC get activated and enter into proliferation and differentiation process to ensure blood cell regeneration. Once activated, their levels of ROS increase, as messengers to mediate their proliferation and differentiation programs. However, at the end of the stress episode, ROS levels need to return to normal to avoid HSC exhaustion. It was shown that antioxidants can prevent loss of HSC self-renewal potential in several contexts such as aging or after exposure to low doses of irradiation suggesting that antioxidants can be used to maintain HSC functional properties upon culture-induced stress. Indeed, in humans, HSC are increasingly used for cell and gene therapy approaches, requiring them to be cultured for several days. As expected, we show that a short culture period leads to drastic defects in HSC functional properties. Moreover, a switch of HSC transcriptional program from stemness to differentiation was evidenced in cultured HSC. Interestingly, cultured-HSC treated with 4-hydroxy-2,2,6,6-tetramethylpiperidin-1-oxyl (4-hydroxy-TEMPO or Tempol) exhibited a higher clonogenic potential in secondary colony forming unit cell (CFU-C) assay and higher reconstitution potential in xenograft model, compared to untreated cultured-HSC. By transcriptomic analyses combined with serial CFU-C assays, we show that Tempol, which mimics superoxide dismutase, protects HSC from culture-induced stress partly through VEGFα signaling. Thus, we demonstrate that adding Tempol leads to the protection of HSC functional properties during ex vivo culture.
Keywords: hematopoietic stem cells, antioxidants, reactive oxygen species, self-renewal
Graphical Abstract
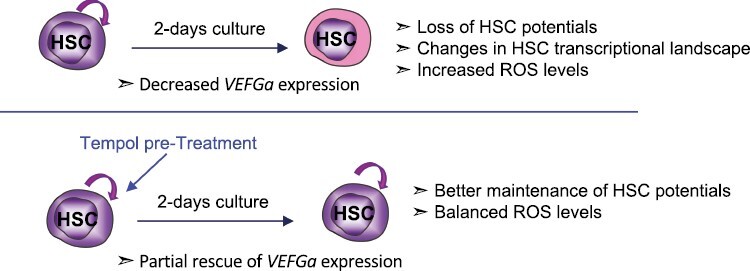
Significance Statement.
Reactive oxygen species (ROS) levels are very low in steady-state hematopoietic stem cells (HSC) and increase upon HSC activation or stress. At the end of the stress episode, ROS levels need to return to normal to avoid HSC exhaustion. Here, we show that a short culture period leads to drastic defects in HSC functional properties through increased ROS levels. Moreover, we show that 4-hydroxy-2,2,6,6-tetramethylpiperidin-1-oxyl (4-hydroxy-TEMPO or Tempol), which mimics super oxide dismutase, protects HSC from culture-induced stress partly through VEGFα signaling. These results open the way for optimized culture conditions of HSC and more efficient therapeutic engineering.
Introduction
Human hematopoietic stem cells (HSC) are a very rare cell population able to give rise to all hematopoietic cells. In humans, they are enriched CD34+CD38−/lowCD45RA−CD90+ cell population.1 HSC are largely quiescent and possess a self-renewal potential along with multipotent differentiation capacities.2,3 These characteristics make them powerful therapeutic tools for regenerative medicine, cell, and gene therapies.4,5 In transplantation settings, CD34+ cells are used as heterogenous cell products containing hematopoietic progenitors and precursors and HSC (responsible for short-term and long-term production of blood cells respectively).6 The efficacy of HSC graft capacity is tightly correlated with the number of cells transplanted.7 Cord blood HSC are increasingly used in transplantation, due to their easy recovery and their lower immunogenicity compared to other sources of allogeneic HSC. As for gene therapy, CD34+ cells are generally cultured for at least 2 days in a specific medium supplemented with cytokines and growth factors such as stem cell factor (SCF), thrombopoietin (TPO), and FLT3 ligand (FLT3L) to allow HSC survival and genetic modification by viral vectors.8 However, if growth factor signaling supports efficient CD34+ genetic correction, it can also drive partial differentiation and loss of HSC properties. Adult HSC are known to reside in a specific microenvironment that harbors low oxygen levels (called hypoxia), involved in the maintenance of HSC functional properties9 (for review10-12). Furthermore, the ex vivo culture of human CD34+ cells in hypoxia can participate in the HSC maintenance.13 Indeed, hypoxia allows HSC to display very low levels of reactive oxygen species (ROS).14 These very reactive molecules induce oxidative stress and damage to the cells when their levels are too high, but they also act as signaling molecules driving HSC differentiation and proliferation. Several cellular mechanisms are used to detoxify ROS generated both by physiological cues and by various external stresses. In particular, several enzymes act in concert to limit the accumulation of highly reactive species: O2− is transformed in H2O2 by the superoxide dismutase (SOD) family, then different families (thioredoxins, peroxiredoxins, catalase, or glutathione peroxidase 3) can metabolize H2O2 to avoid its transformation into HO●.15-17 These systems are fundamental since a ROS increase in HSC leads to differentiation and loss of functions, resulting in the decrease in their in vivo hematopoietic reconstitution capacities.18-20 When CD34+ cells (and among them HSC) are cultured for gene therapy procedures, they can partially lose their functions mostly because ROS level increase by non-physiologic O2 levels and growth factor signaling.21 Several methods may maintain HSC functions in these conditions. Recent studies proposed to perform HSC cultures in hypoxia,22,23 in hydrophobic hydrogels,24 or in the presence of small molecules such as UM171.25-27 Although very attractive, hypoxia and hydrogels are not easily adjustable in clinics and mechanisms of action are not clear. In a previous work, we showed that pretreatment with antioxidants allows the protection of HSC from ROS detrimental effects related to low doses of irradiation.28 Based on these results, we hypothesized that ex vivo antioxidant treatment of HSC prior to gene therapy procedures may help maintaining HSC functions. To this purpose, we used the 4-hydroxy-2,2,6,6-tetramethylpiperidin-1-oxyl (4-hydroxy-TEMPO or Tempol), a cell-permeable nitroxide compound that metabolizes O2− into H2O2 mimicking the action of the superoxide dismutase (SOD) enzymes. It is known to reduce oxidative stress in different organs and to protect cells from radiation effects.29 Tempol has been also shown to act as a reductive agent on other ROS species such as H2O2 via a catalase-like action or HO● by preventing its generation from H2O2 (for review30,31). In the present work, we show that Tempol is able to protect HSC from culture-mediated oxidative stress.
Materials and Methods
Cord Blood Samples and In Vivo Experiments
Cord blood (CB) samples were collected from healthy infants with the informed written consent of the mothers based on the declaration of Helsinki with the collaboration of Clinique des Noriets, Vitry, France and Cell Therapy Department of Hôpital Saint-Louis, Paris, France. Samplings and experiments were acknowledged by the Institutional Review Board of INSERM (Opinion number 13-105-1, IRB00003888). CD34+ cells were purified after standard procedures. Briefly, a Ficoll gradient was applied to the blood to isolate mononucleated cells. CD34+ cells were purified by immuno-magnetic selection using a CD34 MicroBeads kit according to manufacturer instructions (Miltenyi Biotech, Paris, France). Cells were then either directly used or frozen (liquid nitrogen) in serum with 10% DMSO for later experiments on thawed cells.
Cell Culture
Complete medium, used for cell transduction as previously described in32 is composed of BIT medium (STEMCELL Technologies) supplemented with 100 ng/mL SCF, 100 ng/mL FLT3L, 60 ng/mL interleukin-3 (IL3), and 10 nM TPO (all from Miltenyi Biotec). For long-term liquid culture experiments, we used StemSpan SFEM (STEMCELL Technologies) instead of BIT.
Drugs and Treatment
The following drugs were used as indicated: Tempol (Sigma, 300 µM), H202 (100 µM), VEGFa (peptide 165, Milteniy Biotec, 50 ng/mL), ZM32388 (Selleckchem, 10 nM), Brivanib (Selleckchem, 50 nM), and anti-VEGFa (Research Grade Bevacizumab Biosimilar, MAB9947, Biotechne, 10 ng/mL). Before long-term cultures, cells were treated with 300 µM Tempol (Sigma, diluted in water) for 2 h and washed, except when stated otherwise. In stated experiments, VEGFα (50 ng/mL, BioTechne) was added during the whole culture.
Flow Cytometry
Surface staining was performed for at least 15 min in the dark at room temperature before washing and resuspending cells in PBS. Antibodies are listed in Supplementary Material. Hoescht and ZombiAqua (Biolegend) were used as viability markers. Intracellular p38MAPK staining was done according to manufacturer instructions for phospho-flow staining (BD Biosciences). Briefly, cells were fixed with Fix Buffer I (BD Biosciences) for 10 min at 37 °C then permeabilized using Perm Buffer II (BD Biosciences) for 1 h on ice. Intracellular staining was then done in PBS with BSA and EDTA. Cells were generally analyzed either on a BD Canto II or a BD LSRII SORP (5 lasers). Cell sorting was done on a BD Influx or a BD Aria III SORP (5 lasers; all from BD Biosciences). Experiments were analyzed using FlowJo software.
Colony Forming Unit Cell (CFU-C) assay
CFU-C cultures were started with the progeny of 500 sorted HSC cultured for 2 days in triplicates as previously described.28 Briefly, they were seeded in methylcellulose medium H4435 (STEMCELL Technologies). After 12-14 days, colonies were identified and numerated. Plates with similar numbers and types of colonies were resuspended in warm PBS and 1% of the total recovered cells was seeded in secondary cultures.
Long-Term Culture Initiating Cell (LTC-IC) Assay
After 2 days of culture, HSC were seeded in limiting dilution in 96-well plates coated with MS5 stromal cells in Myelocult medium (STEMCELL Technologies, H5100) to perform LTC-IC assay as previously described.28 Briefly, they were maintained for 5 weeks with weekly half-medium change. Cells were then recovered and seeded in 500 µL of methylcellulose medium (STEMCELL Technologies, H4435). The presence of colonies was assessed 10-12 days later. For extended LTC, HSPC were cultured in 6-well plates for 5 weeks with weekly half-medium change. CD34+ cells were sorted at the end of the 5 initial weeks and then placed in culture in 96-well plates at different concentrations in limiting dilution.
Transplantation Experiments
NOD.Cg-Prkdc(scid) Il2rg(tm1Wjll)/SzJ (NSG; Jackson Laboratory) mice were housed in the pathogen-free animal facility of IRCM, CEA, Fontenay-aux-Roses, France. Adult 8-12-weeks-old NSG mice received a 2.5 Gy sublethal irradiation using a GSRD1-irradiator (137Cs source, GSM) and were anesthetized with isoflurane before intravenous retro-orbital injection (i.v.) of human cells. All experimental procedures were done in compliance with French Ministry of Agriculture regulations (animal facility registration number: D9203202, APAFIS #20464-201904301501699) for animal experimentation and in accordance with a local ethical committee (#44). CD34+ cells were cultured in bulk for 2 days in a transduction medium after pre-treatment with or without Tempol (300 µM). Total cells were counted and CD34+ cells were injected i.v. to irradiated mice (1-2.5 × 104/mouse). Mice were sacrificed after 16 weeks. The 4 long bones were recovered for BM and blood was sampled for analysis.
Microarray Transcriptomic Analysis
Affymetrix Human Clariom D chips were used for transcriptomic analysis, according to manufacturer instructions. Three samples of sorted HSC from different pools of CB were used for each condition. Results were analyzed on Affymetrix TAC Software, GSEA, and Molecular Signature. Differential expression of genes was considered true when fold change was ≥2 and P-value ≤ 0.05. Heatmap were done using http://shinyheatmap.com/. Data has been on Array express (E-MTAB-12121).
ROS Level and Mitochondrial Activation Measurements
ROS levels were measured at several days post-treatment, D0, D1, and D2. After staining with cell surface antibodies, CellRox DeepRED staining (Life Technologies) was performed for 30 min at 37 °C. Cells were washed and then fixed in BD cell fix buffer before being analyzed on a BD Canto II cytometer. For positive control of high ROS levels, some cells were first incubated with TBHP for 30 min before being incubated with CellRox DeepRED probe.
For some experiments, after staining with cell surface antibodies, CellRox Orange staining (Life Technologies) was performed for 30 min at 37 °C. The cells were then washed and incubated on ice and immediately analyzed on a BD Canto II FACS analyzer. Pre-treatments with Tempol were performed for 1 h prior to induction of ROS, which was either made before CellRox Orange staining with TBHP for 30 min or in the last 15 min of CellRox staining using H2O2 (100µM).
After staining with cell surface antibodies, cells were incubated either with TMRE (50 µM) and MTG (50 nM, Life Technologies) in PBS for 30 min at 37 °C and immediately analyzed on a BD Canto II or LSR-SORPFACS analyzer.
Expression of Antioxidant Genes or Antioxidogram
Antioxidogram is defined by the relative expression of 21 transcripts of key antioxidant genes by RT-qPCR. Analyses were performed by real-time PCR on the LightCycler 480 microwell plate-based cycler platform (Roche Applied Science) using Universal ProbeLibrary assays designed with the ProbeFinder software (Roche Applied Science), as previously described.33 The relative antioxidant gene expression was analyzed using the 2−ΔΔCt method34 and provided the antioxidogram profile.
Statistics
Statistical analyses were performed on Prism Software using predefined tests as indicated in figure legend. Limiting dilution analyses in vivo and in vitro were analyzed using L-Calc software.
Results
Culture in Cytokines-Enriched Medium Induces Major Changes in HSC Properties and HSC Gene Expression
To understand the effects of culture steps on HSC functional properties, we performed LTC-IC assay in limiting dilution. To this end, human HSC were purified by cell sorting based on the CD34+CD38−CD45RA−CD90+ phenotype, seeded directly in LTC-IC conditions (D0-HSC) or cultured 2 days (Fig. 1A) prior to being seeded in LTC-IC conditions (D2-HSC). We then compared the LTC-IC frequency within D2-HSC along with uncultured HSC (D0-HSC). The frequency of cells able to generate colonies after 5 weeks in LTC-IC conditions was 2.25 lower in D2-HSC condition (1/9, 95% CI [1/11-1/8]) compared to the D0-HSC condition (1/4 LTC-IC, 95% CI [1/5-1/4]) demonstrating that D2-HSC have lost stemness properties during the culture period (Fig. 1B). To understand further the molecular mechanisms accounting for these functional alterations, we performed transcriptomic analysis using CLARIOM D microarray technology and compared the transcriptional profile between D2-HSC and D0-HSC. D2 and D0-HSC were clustered separately in principal component analysis (Fig. 1C), revealing different transcriptomic landscapes. A total of 5476 genes were differentially regulated between both conditions, with 1868 and 3608 genes being respectively down-regulated and up-regulated in D2-HSC compared to D0-HSC (Fig. 1D and Supplementary Table S2). First, geneset enrichment analysis (GSEA) was performed, using some C2-CGP HSC and progenitor cell-specific genesets from https://www.gsea-msigdb.org/gsea/msigdb/index.jsp (Fig. 1E–1F and Supplementary Fig. S1B–S1C); HSC signatures were enriched in D0-HSC. Using genesets from Eppert et al,35 we found that HSC and progenitor gene signatures were respectively downregulated and upregulated in D2-HSC in comparison to D0-HSC, confirming that a 2-days-culture period induces HSC differentiation and leads to the loss of HSC identity (Fig. 1E–1F). Moreover, we uncovered that some genes involved in epigenetic modifications, such as TET2, DNMT3B, EZH1, and EZH2, were differentially expressed when comparing D2-HSC and D0-HSC conditions, exhibited respectively increased expression of EZH2, associated with activation and differentiation of HSC, and decreased expression of EZH1 that is associated with stemness36,37 in D2-HSC compared to D0-HSC. Similarly, TET2 expression was downregulated in D2-HSC compared to D0-HSC (Fig. 1G). Finally, this analysis showed that 2 days in culture-induced cell cycle/differentiation programs and oxidative stress in HSC. Indeed, when performing a gene ontology analysis on GSEA software, hallmark pathways enriched after 2 days of culture were related to cell cycle and oxidative phosphorylation. On the contrary, signatures related to hypoxia were enriched in D0-HSC (Fig. 1H; Supplementary Fig. S1A). Indeed, it was reported that HSC-displayed molecular signatures are associated with hypoxia regardless of O2 concentration.38,39 Thus ex vivo culture drove functional HSC alterations along with metabolic switch.
Figure 1.
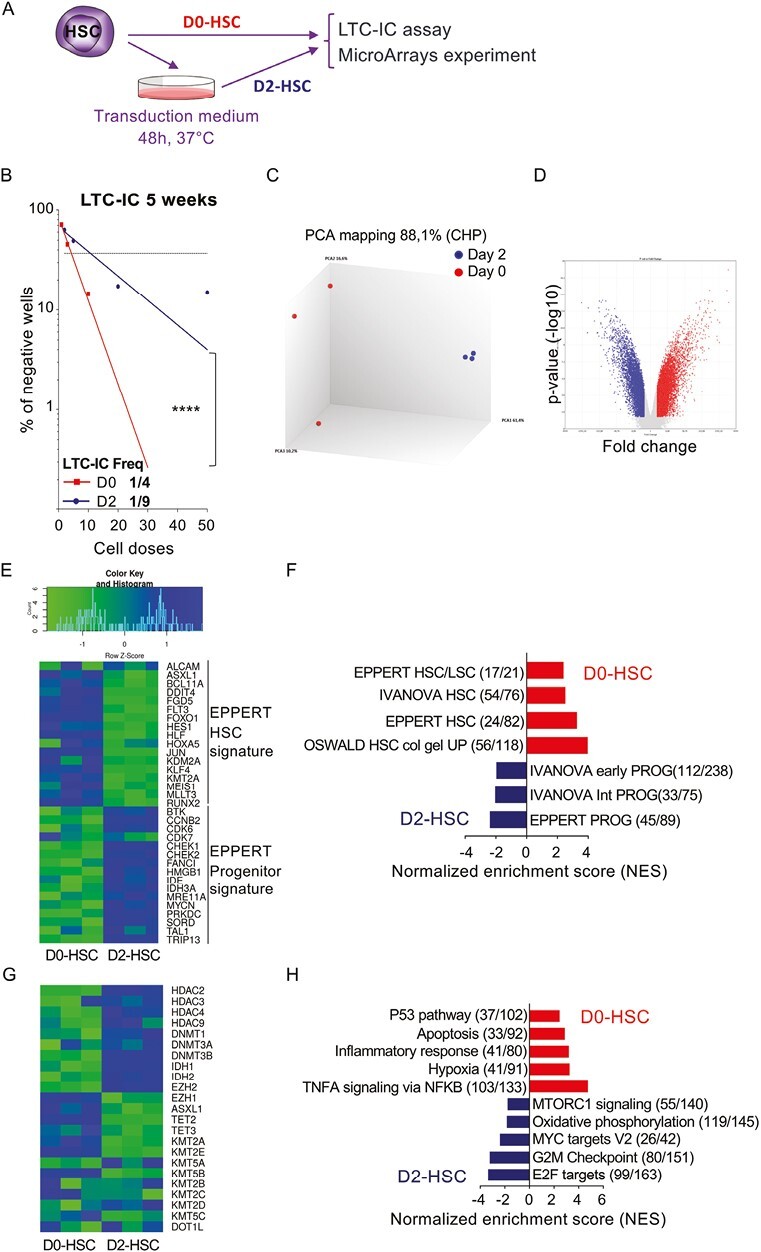
Two-days of culture induces major differences in gene expression and in HSC functionalities in vitro. (A) Experimental protocol: HSC (CD34+ CD38low CD45RA− CD90+) were cell sorted and either used directly (D0-HSC) to perform LTC-IC assay and microarray experiment or were cultured for 2 days (D2-HSC) before being used for experiments (B) LTC-IC assays confirming loss of HSC potential when cells are cultivated 2-days (D2-HSC, dark blue, r2 = 0.911) compared to uncultured HSC (D0-HSC, red, r2 = 0.996). Three independent experiments, ****P < .0001 (C) Principal component analysis of data: uncultured cells (D0-HSC, red) and 2-days cultured cells (D2-HSC, dark blue) cluster separately. (D) Volcano plot of data: more than 5000 of genes are differentially expressed between the 2 conditions (fold change > 2, P value < .05) (E) Heatmap showing differential expression of genes that characterized HSC versus progenitor signature defined by Eppert et al. Green reveals low expression and blue high expression level (fold change > 2, P value < .05). (F) GSEA analysis of the data using gene-sets specific to HSC and progenitor signatures from C2-CGP as indicated: FDR < 10-3. Red, gene-sets enriched in D0-HSC (uncultured HSC), blue, gene-sets enriched in D2-HSC (HSC that have been cultivated for 2 days). (G) Heatmap showing differential expression of genes involved in epigenetics in D0-HSC and D2-HSC (fold change > 2, P value < .05). (H) GSEA analysis of the data using hallmark gene-sets: several pathways are significantly different: FDR < 10−3. Red, gene-sets enriched in D0-HSC (uncultured HSC), blue, gene-sets enriched in D2-HSC (HSC that have been cultivated for 2 days).
Tempol Decreases Oxidative Stress
We previously showed that antioxidants such as N-acetyl cysteine (NAC) or catalase can protect HSC fundamental properties when exposed to stress such as low doses of irradiation.28 Strictly, 4-hydroxy-2,2,6,6-tetramethylpiperidin-1-oxyl or Tempol, which mimics the superoxide dismutase (SOD), not only protected HSC from low dose of irradiation but also increased non-irradiated and 20 mGy-irradiated HSC clonogenic potentials upon serial plating (secondary CFU-C, Supplementary Fig. S2A). Therefore, we postulated that Tempol may also preserve HSC potential during the 2-days stress-culture period. To tackle this hypothesis, we first checked in HSC the antioxidant effect of Tempol. HSC were pretreated with Tempol before inducing oxidative stress with Tert-butyl-hydroperoxyde (TBHP) and measuring ROS levels with CellRox Orange probe. Tempol inhibited TBHP-mediated ROS increase in human HSC (Supplementary Fig. S2B), therefore acting as an antioxidant. Therefore we measured ROS levels in HSC treated or not with Tempol at D0 (uncultured) and at D2 of culture. First, as expected, in non-treated conditions we observed an increase in ROS levels from D0 to D2 (Supplementary Fig. S2C). In addition, ROS levels tended to be lower when cells were treated with Tempol (Fig. 2A). To strengthen these results, we assessed the antioxidant effect of Tempol on HSC after 2 days of culture, using an “antioxidogram” assay. The “antioxidogram” test provides a synthetic view of the activation of many antioxidant pathways in response to an increase in intracellular ROS level, by following expression levels of transcripts of key antioxidant genes.33 Many antioxidant genes were significantly overexpressed in D2-HSC compared to uncultured cells (D0-HSC) confirming that oxidative stress was indeed induced in D2-HSC compared to D0-HSC (Fig. 2B, D2-HSC, black bold line vs. D0-HSC, dashed line). On the contrary, the curves of antioxidant gene expression in Tempol D2-HSC (Fig. 2B, Tempol D2-HSC, blue line) shifted back toward D0-HSC, indicating that many of the analyzed genes were down-regulated in the presence of Tempol. To further demonstrate the effect of Tempol in inhibiting ROS production in cultures, we investigated the phosphorylation status of p38MAPK, a secondary messenger of oxidative stress. After 2 days of culture, a low but significant decrease of the phosphorylation of p38MAPK in Tempol pre-treated D2-HSC was evidenced compared to untreated D2-HSC (Fig. 2C), indicating that ROS levels had diminished upon Tempol treatment.19,28 These results indicate that Tempol decreases ROS levels in D2-HSC. Altogether, these results showed that pre-treatment with Tempol is able to preserve HSC from the burst of oxidative stress during a 2 days-culture period.
Figure 2.
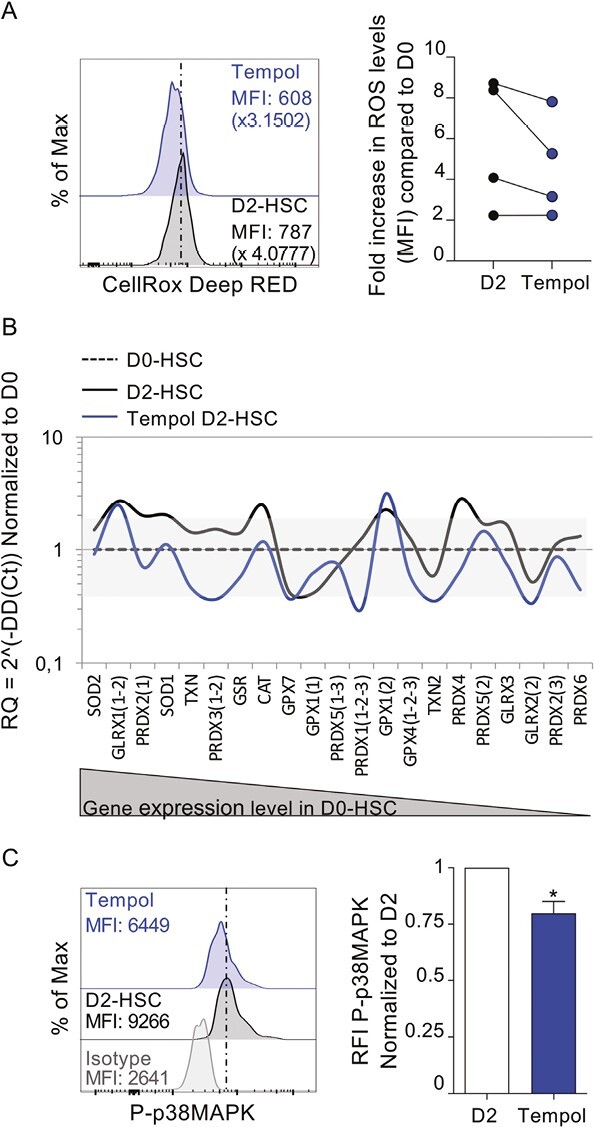
Tempol decreases ROS and oxidative stress response. (A) CD34+ cells were treated or not with Tempol, then ROS levels were measured using CellROX Deep red probe directedly after treatment (D0) or after 2 days of culture (D2). Left is represented ROS levels in HSC at D2 for one representative experiment, right the graft represents the fold increase in ROS levels compared to D0 HSC. Four independent experiments with 4 independent CB samples. (B) Antioxidogram is defined by the relative expression of 21 transcripts of key antioxidant genes by RT-qPCR in D2-HSC (black line) and Tempol treated-D2-HSC (blue line) compared to fresh cells (D0-HSC, dashed line). The grey zone corresponds to variations less than twice the normal expression. Transcripts are ranked in decreasing order of expression in D0-HSC. (C) Flow cytometry histograms (left) representing the level of phosphorylated p38MAPK (P-p38MAPK) in phenotypic D2-HSC. One representative example out of 4. Quantification (right) of MFI of P-p38MAPK relative to untreated condition. n = 4, Mann-Whitney test *P < .05.
Antioxidants Improve Functional Capacities In Vitro
We next investigated the impact of Tempol treatment on HSC functional capacities; we performed CFU-C experiments with D2-HSC and used their replating ability (serial CFU-C assay) to further dig into their immature functions. Briefly, sorted HSC, pre-treated or not with Tempol, were cultured for 2 days in a complete medium and then seeded in CFU-C medium. In primary cultures, the number of colonies generated was equivalent in both conditions (Fig. 3A), whereas in secondary CFU-C cultures, a significant increase in colony number was evidenced when D2-HSC were pre-treated with Tempol in comparison with the untreated ones (Fig. 3B). For 2 independent cord blood samples, the clonogenic potential of HSC was measured with (D2-HSC and tempol-treated D2-HSC) and without culture (D0-HSC). Secondary clonogenic potential of D2-HSC was decreased in comparison with the D0-HSC condition, and Tempol was allowed to prevent this loss (Supplementary Fig. S3A). For one experiment, we performed a tertiary CFU-C assay, there were more numerous colonies generated from Tempol-treated D2-HSC (10-20 times more; Supplementary Fig. S3B). This suggested either a better preservation of immature progenitor cells or an increased self-renewal potential of HSC, giving rise to a larger number of colonies in secondary (and tertiary) replating in the presence of Tempol. Moreover, no bias in the type of CFU-C was observed, demonstrating that Tempol did not impact HSC differentiation (Supplementary Fig. S3C–S3D). To confirm the effect of Tempol on HSC and to evaluate its implication on D2-HSC self-renewal potential,40,41 the frequency of LTC-IC (long-term culture-initiating cells, ie, the most immature cells) was quantified by limiting dilution assays. The results show that Tempol treatment of D2-HSC promoted the maintenance of LTC-IC comparatively to untreated D2-HSC, rising up LTC-IC frequency close to the one observed in D0-HSC (1/5, 95% CI [1/6-1/5], Figs. 3C,1F). To evaluate the capacity of antioxidants to maintain more immature cells, a similar experiment was conducted that included an extended (10 weeks) culture period. After the 5 first weeks of culture in bulk, LTC-persisting CD34+ cells were cell sorted (Supplementary Fig. S3E) and seeded in limiting dilution for 5 additional weeks before the evaluation of their clonogenic potential. After 10 weeks of culture, the LTC-IC frequency was 50% higher in Tempol-treated D2-HSC (1/66, 95% CI [1/55-1/79]) compared to untreated D2-HSC (1/98, 95% CI [1/81-1/118]; Fig. 3D), showing that Tempol protects HSC immature potentials.
Figure 3.
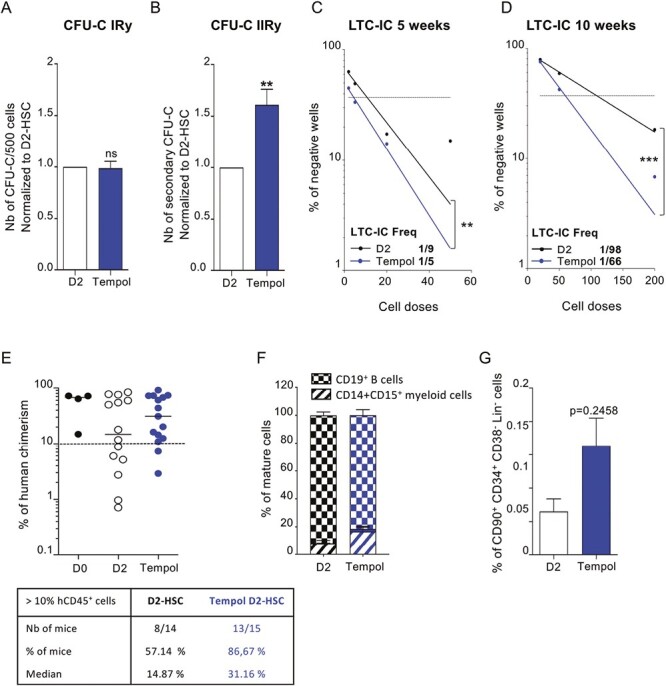
Tempol preserves long-term in vitro and in vivo HSC functionalities. (A) sorted-HSC were first pre-treated with Tempol (blue) or remained untreated (white) and then cultured for 2 days before being seeded in CFU-C medium at a density of 500 cells per plate in triplicate. Colonies (primary CFU-C) were quantified 10-12 days later. Five independent experiments. (B) Cells recovered from primary CFU-C plates were seeded in new CFU-C medium to perform serial CFU-C assay. Total cells (1%) of equivalent primary plates were seeded in triplicate. Secondary CFU-C were quantified 10-12 days later. Five independent experiments. *P < .05, **P < .01, Mann-Whitney test. (C) Representation of negative wells % from normal LTC-IC in limiting dilution (5 weeks) and quantification of LTC-IC frequency using L-CALC program (STEMCELL Technologies) between untreated D2-HSC (black, r2 = 0.911) and Tempol-treated D2-HSC (blue, r2 = 0.991). Three independent experiments (D) Representation of negative wells % from extended LTC-IC (10 weeks) in limiting dilution and quantification of LTC-IC frequency using L-CALC program (STEMCELL Technologies) between untreated D2-HSC (black, r2 = 0.997) and Tempol-treated D2-HSC (blue, r2 = 0.993). Three independent experiments. *P < .05, **P < .01, ***P < .001. (E-G) CD34+ cells were first pre-treated with Tempol (blue) or remained untreated (white) and then cultured for 2 days and grafted into NSG recipient mice. (10 000 to 25 000 CD34+ cells per mouse) 4 independent experiments. (E) % of human bone marrow chimerism obtained after 16 weeks (up) and table representing the % of mice with the % hCD45+ >10%. Each circle represents one independent mouse. (F) % of mature cells in the BM of recipient mice gated in hCD45+ fraction (Damier pattern B cells, hatched myeloid cells) (G) % of phenotypic HSC (CD34+CD38lowCD45RA−CD90+) in human cells recovered from the bone marrow.
Cultured-HSC Treated With Tempol Bear a Better Human Hematopoietic Reconstitution In Vivo
To confirm these results, CD34+ cells were pre-treated or not with Tempol, cultured for 2 days in complete medium (D2-CD34+ cells), and injected in vivo in immune-deficient NSG. Transplanted mice were sacrificed 16 weeks later and human chimerism was measured a using specific anti-human CD45 antibody to assess their long-term reconstitution potential (Supplementary Fig. S3F). D2-CD34+ cells generated a human hematopoietic progeny in vivo, but not as efficiently as fresh CD34+ cells (D0-CD34+), as a large heterogeneity of the hCD45+ cell levels was detected in NSG mouse BM transplanted with D2-CD34+ cells (Fig. 3E). This heterogeneity was reduced in Tempol treated D2-CD34+ condition. Indeed 86% of the mice injected with Tempol-treated D2-CD34+ cells exhibited a human chimerism >10% compared to 57% in mice injected with untreated D2-CD34+ cells (Fig. 3E). Neither a myeloid nor a lymphoid cell bias was detectable (Fig. 3F). However, using classical HSC markers, a trend, albeit not significant, to higher phenotypic HSC levels was observed in the BM of mice receiving Tempol-treated D2-CD34+ cells (Fig. 3G). Moreover, we performed one serial transplantation experiment. No difference in the % of chimerism was detected between both conditions in primary recipient mice, however, Tempol-treated D2 CD34+ cells were more efficient in secondary recipients suggesting a better maintenance of D2 cultured HSC functions when they were treated with Tempol (Supplementary Fig. S3G–S3H). Altogether, these data show that antioxidants may preserve the hematopoietic reconstitution potential of HSC during the 2-days culture (Fig. 3G).
Tempol Preserves HSC Maintenance In Vitro By Limiting HSC Cell Division
To understand how Tempol can protect HSC ex vivo, several parameters were monitored, such as proliferation, expression of immaturity cell surface markers, and cell division rate at different time points of culture after pretreatment with Tempol. No major impact of Tempol on cell growth was detected (Fig. 4A). As expected, a progressive loss of the HSC phenotype (CD34+CD90+) was observed with time in culture, and no difference was detected between non-treated and Tempol-treated D2-CD34+ cells (Fig. 4B). However, a decrease in phenotypic HSC cell division rate (CFSE staining) was evidenced when cells were pretreated with Tempol compared to untreated D2-HSC (Fig. 4C; Supplementary Fig. S4). We, therefore, checked for cell cycle status after 2 days of culture. Tempol-treated D2-HSC were significantly more quiescent explaining the delay observed in CFSE-cell division assay (Fig. 4D–4E). Finally, we analyzed mitochondria activation in D2-HSC treated or not with Tempol by measuring mitochondrial membrane potential using TMRE and mitochondrial mass using MTG probes. We observed that although Tempol had very limited effects on the mitochondrial mass (Fig. 4F), a delay in mitochondria activation occurred after 2, 4, and 7 days of culture in HSC pretreated with Tempol (Fig. 4G). Altogether, we observed that Tempol protected HSC maintenance by limiting their cell division rate and their metabolic activation.
Figure 4.
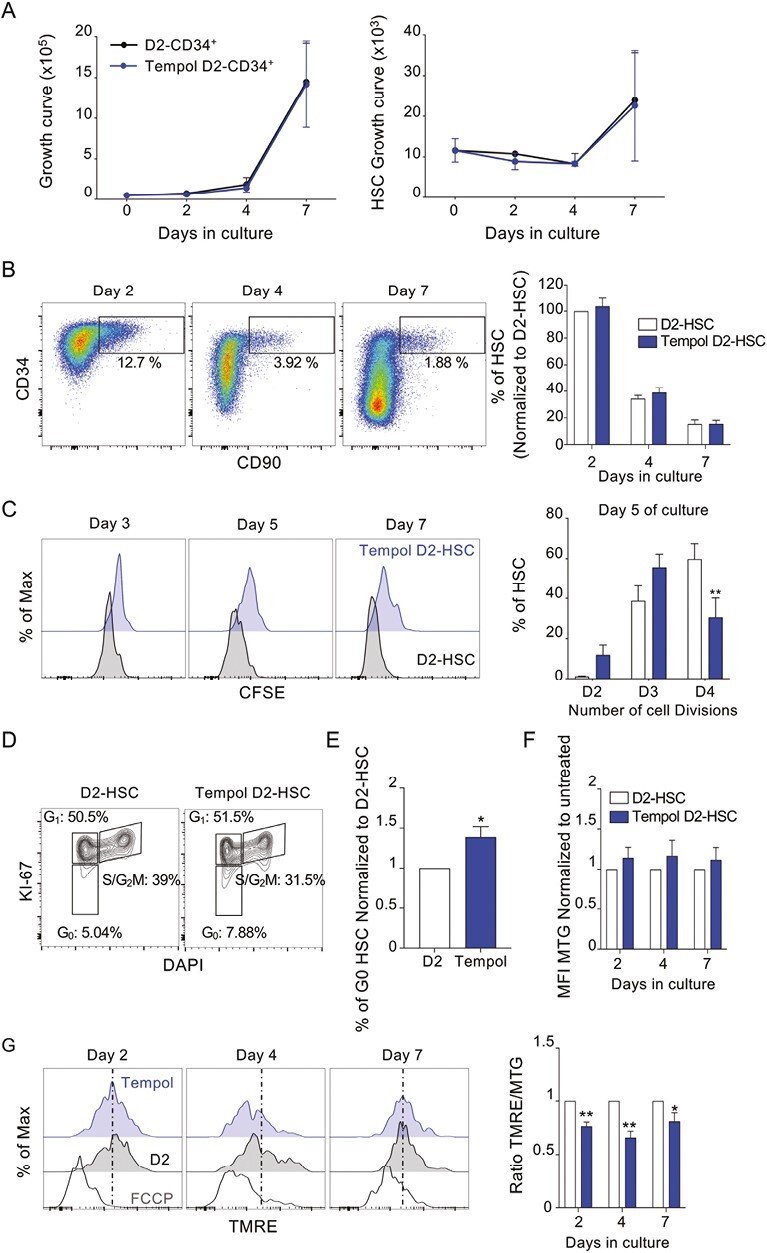
Tempol maintains HSC quiescence ex vivo and delays mitochondrial activity (A-B) CD34+ cells were first pre-treated with Tempol (blue) or remained untreated (white) and then cultured several days as indicated. (A) Curves representing the number of total cells (left) and phenotypic HSC (CD34+ CD90+, right) from CD34+ cells. (B) Gating strategy and progressive loss of CD34 and CD90 markers during culture (left), and histograms indicating % of phenotypic HSC normalized to % of untreated D2-HSC condition. (C) FACS histograms representing CFSE staining of sorted HSC pretreated with Tempol (blue) compared to untreated cells (white) after several days in culture. One representative example out of 3 independent experiments (left) and quantification of the % of HSC in the function of the number of cell divisions after 5 days of culture. Three independent experiments. *P < .05 and **P < .01, two-way ANOVA. (D) Flow cytometry cytograms representing the % of cells in each cell cycle state determined by Ki67 and Hoechst staining, one representative experiment (left) and sum up histograms (right) 4 independent experiments. (E) % of HSC in the G0 phase of the cell cycle for each condition after 2 days of culture. Four independent experiments. *P < .05 Mann-Whitney test. (F) Quantification of the mitochondrial mass showing MFI of MTG. Results are normalized to untreated conditions. Three independent experiments. Two-way ANOVA. (G) Flow cytometry histograms (left) representing mitochondria activation using the TMRE probe in MTG+ (MTG, mitochondrial mass) HSC pretreated with Tempol and after 2, 4, and 7 days of culture. One representative example out of 3. Quantification (right) of ratio TMRE/MTG at different days of culture. Three independent experiments, two-way ANOVA test. *P < .05 and **P < .01, two-way ANOVA. Results are normalized to untreated conditions.
Tempol Acts Through Regulation By VEGFα To Maintain HSC
To better understand antioxidant effects on HSC maintenance in vitro, we compared the transcriptional profile of untreated and Tempol-treated D2-HSC. Few genes (35) were differentially expressed between the 2 conditions (Fig. 5A), several of them (ACOT2, FAM134c, and EMRP1) were upregulated in the Tempol condition and were associated with oxidative stress modulation.42,43 Also, WIF and PADI4 genes, found downregulated in Tempol-treated D2-HSC, are known regulators of HSC exhaustion, proliferation, and differentiation.44,45 Furthermore, transcripts expression analysis showed that VEGFA was significantly more expressed after treatment with Tempol compared to untreated samples (Fig. 5A). This result is interesting considering the previously described role of VEGF signaling in HSC,46,47 and the fact that VEGFA is strongly downregulated in D2-HSC compared to D0-HSC (Fig. 5A; Supplementary Fig. S5A). To further validate the VEGFα role in the observed antioxidant-mediated effects, we first confirmed the enhanced VEGFA gene expression level in Tempol-treated D2-HSC (Supplementary Fig. S5B). Supplementation with exogenous VEGFα during the 2-days culture period did not change the number of colonies generated in primary CFU-C assay (Fig. 5B) but induced a significant increase in the number of colonies in secondary CFU-C cultures, similar to Tempol pre-treatment, suggesting that VEFGα mimics Tempol protection effects in D2-HSC (Fig. 5C). No significant additive effect was observed in secondary CFU-C assay when Tempol and VEGFα were combined. VEFGα treatment did not induce changes in the expression of the CD34 and CD90 immaturity markers nor in cell growth (Supplementary Fig. S5C). In line with these results, a comparison of ROS levels at D0 and D2 of culture in the presence of VEGFα indicated that VEFGα tends to maintain low levels of ROS in cultured-HSC as does Tempol (Supplementary Fig. S5D). To question whether Tempol acts on HSC maintenance via VEGFα signaling, HSC were pretreated with Tempol in combination with either an anti-VEGFα blocking antibody or 2 specific inhibitors of VEGFA signaling (ZM32388 and Brivanib). Anti-VEFGα antibody or VEGFα signaling inhibitors alone had no effect in the number of primary or secondary CFU-C (Fig. 5D–5E; Supplementary Fig. S5D). On the contrary, a combination of anti-VEFGα antibody or VEGFα inhibitors with Tempol inhibited Tempol effects on secondary CFU-C. Indeed, inhibiting VEGFα activity decreased the number of secondary CFU-C to the levels of untreated D2-cultured cells (Fig. 5E; Supplementary Fig. S5D), indicating that interfering with VEGFα signaling in Tempol-treated HSC reversed the beneficial effects of Tempol on HSC maintenance. Altogether, our data show that Tempol allows the maintenance of D2-cultured HSC functions partly through VEFGα signaling rescue (Fig. 5F).
Figure 5.
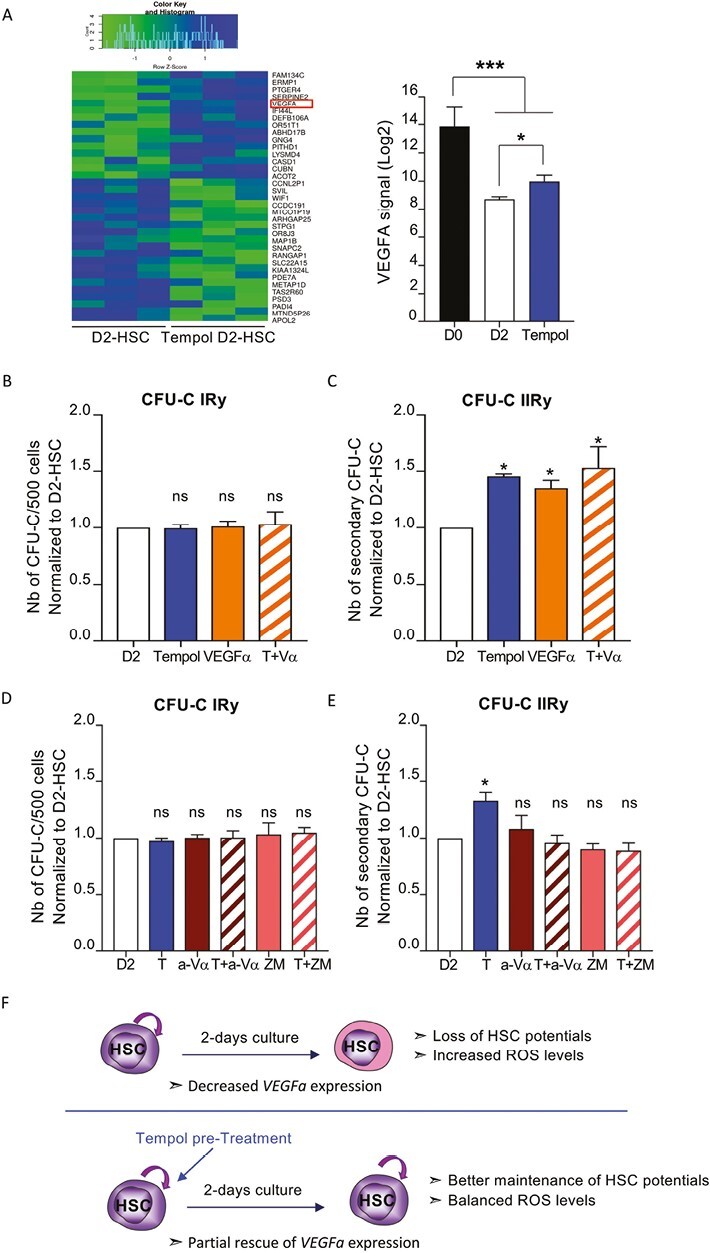
Tempol may work through VEGFa to preserve HSC functionalities (A) Heatmap showing gene differential expression between untreated D2-HSC and Tempol treated D2-HSC, n = 3 (green reveals low expression and blue high expression level, left, fold change < −2 and >2, P value < .05), and quantification of VEGFA expression obtained by transcriptomic data (right) n = 3, one-way ANOVA. (B-C) Sorted-HSC were first pre-treated with Tempol (blue), VEGFα (orange), with VEGFα and Tempol (hatched orange bar) or remained untreated (white) and then cultured for 2 days before being seeded in CFU-C medium at a density of 500 cells per plate in triplicate. For VEFGα conditions, VEGFα was also added to the culture medium. (B) Colonies (primary CFU-C) were quantified 10-12 days later. Three independent experiments. (C) Cells recovered from primary CFU-C plates were seeded in new CFU-C medium to perform serial CFU-C assay. Total cells (1%) of equivalent primary plates were seeded in triplicate. Secondary CFU-C was quantified 10-12 days later. Three independent experiments. (D-E) Sorted-HSC were first pre-treated with Tempol (blue), anti-VEGFα (brown), with anti-VEGFα, and Tempol (hatched brown bar), ZM32388 (pink), ZM32388 and Tempol (hatched pink bar) or remained untreated (white) and then cultured for 2 days before being seeded in CFU-C medium at a density of 500 cells per plate in triplicate. For VEFGα inhibition conditions, anti-VEGFα or ZM32388 were also added to the culture medium. (D) Colonies (primary CFU-C) was quantified 10-12 days later. Three independent experiments. (E) Cells recovered from primary CFU-C plates were seeded in new CFU-C medium to perform serial CFU-C assay. Total cells (1%) of equivalent primary plates were seeded in triplicate. Secondary CFU-C was quantified 10-12 days later. Three independent experiments. (B-E) *P < .05, Mann-Whitney test. (F) Schema representing the effect of 2 days of culture on HSC properties and the consequences of antioxidants treatment.
Discussion
Our results provide the proof of concept that antioxidant treatment is an interesting strategy to protect HSC loss of function during short-term (2 days) ex vivo culture. This can be useful in the case of gene therapy protocols for instance. Indeed, we show that Tempol harbors potent antioxidant activity and induces a decrease in oxidative stress endured by HSC after 2 days of culture (D2-HSC). This has beneficial consequences on HSC functional capacities. Indeed, Tempol pre-treatment enhanced in vitro generation of CFU-C, increased the frequency of LTC-IC, and rescued the loss of in vivo hematopoietic reconstitution abilities induced by culture, resulting in the maintenance of cultured HSC properties closer to the ones of D0-HSC. We also evaluated how culture conditions impact HSC gene expression and confirmed that cell cycle progression and oxidative stress are major drivers of the loss of HSC functionalities. The 2 days culture period induced a switch from the HSC state toward progenitor signature programs. Altogether, the transcriptomic data revealed that the culture period leads to HSC activation with a progressive loss of self-renewal potential and stemness properties. In addition, when considering the cell division assays (CFSE experiment), D2-HSC may have undergone up to 3 divisions whereas it is known that fresh CB HSC (D0 HSC) are mostly quiescent. Tempol-treated D2 HSC slowed down through the cell-division process. Tempol-treated D2-HSC were more quiescent and exhibited a delay in mitochondrial activation compared to untreated D2-HSC, suggesting that Tempol may partly limit HSC self-renewal potential loss during stress-induced culture. Finally, these data are in line with data obtained with murine HSC showing that upon activation, ROS levels increase in HSC to sustain HSC activation, leading to HSC cell division and differentiation. Then ROS levels have to be controlled to avoid HSC exhaustion.48,49 Recently, low mitochondrial activity has been associated with the high stemness potential of expanded HSC.50 We showed that Tempol effects are mediated at least in part by the inhibition of p38MAPK pathway and the increase of VEGFα signaling during culture. Indeed, a combination of Tempol with inhibitors of VEGFα signaling prevented Tempol-dependent D2-HSC maintenance ex vivo. Mechanisms by which Tempol may mediate VEGFα expression remain unknown but since VEGFα-dependent HSC maintenance is related to hypoxia,51 we can speculate that Tempol mechanism operates through the preservation of hypoxia-associated genetic program that in return favors HSC maintenance. Among other genes upregulated in Tempol-treated D2-HSC compared to untreated D2-HSC, several of them (ACOT2, FAM134c, and EMRP1) are associated with oxidative stress modulation.42,43 Moreover, WIF and PADI4 genes associated with HSC exhaustion, proliferation, and differentiation,44,45 were found downregulated in Tempol-treated HSC. Of note function of PADI4 on HSC maintenance is controversial.52 Therefore, gene expression modulated by Tempol may be associated with either oxidative stress modulation or with HSC exhaustion/proliferation balance. Our results are in favor of adding antioxidants such as Tempol to the culture medium to improve HSC maintenance in the context of cell/gene therapy protocols. This antioxidant model will allow the deciphering mechanisms involved in HSC maintenance.
Inhibition of ROS is a strategy that has also been evaluated by other groups. Hypoxia has long been considered a well-suited pathway to enhance to avoid ROS increase in HSC before transplantation. Indeed it is known that hypoxia preserves HSC during culture and also immediately at the time of HSC collection.21 However, keeping HSC in hypoxic conditions is difficult in clinical settings. A recent study using hydrophilic gels to culture HSC showed a drastic increase in their number without differentiation, related to a significant decrease in ROS levels in the gel-culture medium system.24 In fact, here we show that the culture step induces major stress-related changes in HSC transcriptomic landscape. We uncovered that some genes involved in epigenetic modifications in D2-HSC compared to D0-HSC indeed, compared to D0-HSC, D2-HSC exhibited increased expression of EZH2, associated with activation and differentiation of HSC, and decreased expression of EZH1 that is associated with stemness.36,37 Similarly, TET2 expression was downregulated in D2-HSC compared to D0-HSC. A recent study showed that modulating calcium efflux via calpain inhibition induced TET2 protein stabilization and HSC maintenance ex vivo.53 Therefore, it is tempting to speculate that the TET protein family and/or other proteins involved in epigenetic modifications, may be targets of antioxidants. Indeed, only a short contact with antioxidants triggers long-lasting functional effects. This relates to the fact that it is now more and more acknowledged that epigenetic modifications are involved in HSC fate and properties in vivo but also in HSC maintenance ex vivo.54 Furthermore, there is increasing evidence that ROS can trigger epigenetic modifications either on DNA or on histones.55,56 Several groups have shown that targeting epigenetic modifiers could maintain HSC properties ex vivo rather than promoting a great expansion. Therefore, focusing on putative epigenetic modifications triggered by antioxidants and exploring how combining Tempol with epigenetic modifiers could induce synergistic effects to expand HSC ex vivo is an exciting research avenue. In line with this idea, a link between epigenetics and mitochondrial activation/metabolism was described since valproic acid (VPA)-expanded HSC exhibited a lower mitochondrial activity while preserving their functional properties.50 Finally, while our study has mainly been completed on HSC from early post-natal CB samples, we believe it could be applicable to ontogenically different (older) HSC from bone marrow or peripheral blood. In fact, increased levels of ROS and p38MAPK activation are often linked to HSC aging and it is well known that adult HSC are less efficient to reconstitute the hematopoietic system compared to neonatal CB HSC. As bone marrow or mobilized-peripheral blood CD34+ are commonly used in the case of autologous graft and gene therapy protocols, protecting these cells from further oxidative stress may help to optimize gene therapy protocols.
Altogether, these results confirm that antioxidant treatment is helpful for HSC maintenance and validate Tempol as a novel antioxidant agent for HSC protection during ex vivo manipulations. Since Tempol is more and more studied for its protective effects in a wide range of pathologies, it is interesting now to validate its action in clinics and to approve it in pretreatment protocols to avoid oxidative stress-related disorders.
Supplementary Material
Acknowledgments
We acknowledge the midwives from Clinique des Noriets in Vitry-sur-Seine and the Cell Therapy department of Hôpital Saint Louis in Paris, France, for providing cord blood samples free of charge and the families who accepted to donate the samples for research purposes. This work was made possible thanks to multiple platforms (Flow cytometry, Microscopy, Irradiation, CIGEX, Microarray, and Animal Facilities) of IRCM, Fontenay-aux-Roses, France. We are thus grateful to D. Lewandowski and A. Chicheportiche for their great technical help during this project. A special thanks to V. Ménard and G. Piton, respectively from the irradiation platform and the CIGEX platform of the IRCM, for their devoted work. Finally, we would like to thank all F. Pflumio Lab members and more specifically Dr R. Haddad, Dr L. Renou and Dr J. Calvo. We are very grateful to Dr P.-H. Roméo, Dr C. Carles, T. Mercher, and O. Kosmider for their constant support and interest.
Contributor Information
Elia Henry, Université de Paris, INSERM, CEA, Stabilité Génétique Cellules Souches et Radiations, F-92260 Fontenay-aux-Roses, France; Université Paris-Saclay, INSERM, CEA, Stabilité Génétique Cellules Souches et Radiations, F-92260 Fontenay-aux-Roses, France; Laboratoire des cellules Souches Hématopoïétiques et des Leucémies, Equipe Niche et Cancer dans l’Hématopoïèse, équipe labellisée Ligue Nationale Contre le Cancer, UMR1274-E008, INSERM, CEA, F-92265 Fontenay-aux Roses, France.
Frédéric Picou, CNRS EMR 7001 LNOx Leukemic Niche and Redox Metabolism, Tours, France; EA 7501, Université de Tours, Tours, France; Department of Biological Hematology, Tours University Hospital, Tours, France.
Vilma Barroca, Université de Paris, INSERM, CEA, Stabilité Génétique Cellules Souches et Radiations, F-92260 Fontenay-aux-Roses, France; Université Paris-Saclay, INSERM, CEA, Stabilité Génétique Cellules Souches et Radiations, F-92260 Fontenay-aux-Roses, France; Laboratoire des cellules Souches Hématopoïétiques et des Leucémies, Equipe Niche et Cancer dans l’Hématopoïèse, équipe labellisée Ligue Nationale Contre le Cancer, UMR1274-E008, INSERM, CEA, F-92265 Fontenay-aux Roses, France.
Nathalie Dechamps, Université de Paris, INSERM, CEA, Stabilité Génétique Cellules Souches et Radiations, F-92260 Fontenay-aux-Roses, France; Université Paris-Saclay, INSERM, CEA, Stabilité Génétique Cellules Souches et Radiations, F-92260 Fontenay-aux-Roses, France.
Steicy Sobrino, Human Lymphohematopoiesis Laboratory, Université Paris Cité, Imagine Institute, INSERM UMR 1163, Paris, France.
Emmanuelle Six, Human Lymphohematopoiesis Laboratory, Université Paris Cité, Imagine Institute, INSERM UMR 1163, Paris, France.
Camille Gobeaux, Biochemistry Department/Diagnostic Biologique Automatisé (SDBA), DMU sBioPhyGen, Cochin Hospital-Université de Paris.
Patrick Auberger, OPALE Carnot Institute, The Organization for Partnerships in Leukemia, Saint-Louis Hospital, 75010 Paris, France; Université Côte d’Azur, C3M/INSERM U1065, Nice, France.
Olivier Hérault, CNRS EMR 7001 LNOx Leukemic Niche and Redox Metabolism, Tours, France; Department of Biological Hematology, Tours University Hospital, Tours, France.
Françoise Pflumio, Université de Paris, INSERM, CEA, Stabilité Génétique Cellules Souches et Radiations, F-92260 Fontenay-aux-Roses, France; Laboratoire des cellules Souches Hématopoïétiques et des Leucémies, Equipe Niche et Cancer dans l’Hématopoïèse, équipe labellisée Ligue Nationale Contre le Cancer, UMR1274-E008, INSERM, CEA, F-92265 Fontenay-aux Roses, France; OPALE Carnot Institute, The Organization for Partnerships in Leukemia, Saint-Louis Hospital, 75010 Paris, France.
Marie-Laure Arcangeli, Université de Paris, INSERM, CEA, Stabilité Génétique Cellules Souches et Radiations, F-92260 Fontenay-aux-Roses, France; Université Paris-Saclay, INSERM, CEA, Stabilité Génétique Cellules Souches et Radiations, F-92260 Fontenay-aux-Roses, France; Laboratoire des cellules Souches Hématopoïétiques et des Leucémies, Equipe Niche et Cancer dans l’Hématopoïèse, équipe labellisée Ligue Nationale Contre le Cancer, UMR1274-E008, INSERM, CEA, F-92265 Fontenay-aux Roses, France; OPALE Carnot Institute, The Organization for Partnerships in Leukemia, Saint-Louis Hospital, 75010 Paris, France; Université Côte d’Azur, C3M/INSERM U1065, Nice, France.
Funding
This study was supported by grants from INSERM, CEA (Radiobiology program), Ligue Nationale contre le Cancer, Ligue régionale contre le cancer (Comité 92), The foundation Cordons de Vie, European network RISK-IR and the EUR G.E.N.E (ref #ANR-17-EURE-0013), and is part of the Université de Paris IdEx #ANR—18-IDEX-0001 funded by the French Government through its “Investments for the Future” program. E. Henry was a PhD fellow from Ligue Nationale contre le Cancer and a doctoral Mentor of the EUR G.E.N.E.
Conflict of Interest
The authors declared no potential conflicts of interest.
Author Contributions
M.-L.A., O.H.: designed experiments. E.H., F.P., M.-L.A.: performed and analyzed experiments. V.B.: performed in vivo experiments. S.S.: provided help in transcriptomic analyses of microarray data. C.G.: helps with manuscript revisions. E.S., P.A., O.H., F.P.: provided scientific advice and critical look at the study. E.H., F.P., M.-L.A.: wrote the manuscript. M.-L.A.: supervised the whole study.
Data Availability
All data are incorporated into the article and its online Supplementary Material. Please contact the corresponding author for more details.
References
- 1. Doulatov S, Notta F, Laurenti E, Dick JE.. Hematopoiesis: a human perspective. Cell Stem Cell. 2012;10(2):120-136. 10.1016/j.stem.2012.01.006 [DOI] [PubMed] [Google Scholar]
- 2. Majeti R, Park CY, Weissman IL.. Identification of a hierarchy of multipotent hematopoietic progenitors in human cord blood. Cell Stem Cell. 2007;1(6):635-645. 10.1016/j.stem.2007.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pinho S, Frenette PS.. Haematopoietic stem cell activity and interactions with the niche. Nat Rev Mol Cell Biol. 2019;20(5):303-320. 10.1038/s41580-019-0103-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cavazzana M, Bushman FD, Miccio A, André-Schmutz I, Six E.. Gene therapy targeting haematopoietic stem cells for inherited diseases: progress and challenges. Nat Rev Drug Discov. 2019;18(6):447-462. 10.1038/s41573-019-0020-9 [DOI] [PubMed] [Google Scholar]
- 5. Hatzimichael E, Tuthill M.. Hematopoietic stem cell transplantation. Stem Cells Cloning Adv Appl. 2010;3:3105-3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Scala S, Aiuti A.. In vivo dynamics of human hematopoietic stem cells: novel concepts and future directions. Blood Adv. 2019;3(12):1916-1924. 10.1182/bloodadvances.2019000039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brunstein CG, Wagner JE.. Umbilical cord blood transplantation and banking. Annu Rev Med. 2006;57(1):403-417. 10.1146/annurev.med.57.051804.123642 [DOI] [PubMed] [Google Scholar]
- 8. Millington M, Arndt A, Boyd M, Applegate T, Shen S.. Towards a clinically relevant lentiviral transduction protocol for primary human CD34 hematopoietic stem/progenitor cells. PLoS One. 2009;4(7):e6461. 10.1371/journal.pone.0006461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Spencer JA, Ferraro F, Roussakis E, et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature. 2014;508(7495):269-273. 10.1038/nature13034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Testa U, Labbaye C, Castelli G, Pelosi E.. Oxidative stress and hypoxia in normal and leukemic stem cells. Exp Hematol. 2016;44(7):540-560. 10.1016/j.exphem.2016.04.012 [DOI] [PubMed] [Google Scholar]
- 11. Suda T, Takubo K, Semenza GL.. Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell. 2011;9(4):298-310. 10.1016/j.stem.2011.09.010 [DOI] [PubMed] [Google Scholar]
- 12. Boulais PE, Frenette PS.. Making sense of hematopoietic stem cell niches. Blood. 2015;125(17):2621-2629. 10.1182/blood-2014-09-570192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Danet GH, Pan Y, Luongo JL, Bonnet DA, Simon MC.. Expansion of human SCID-repopulating cells under hypoxic conditions. J Clin Invest. 2003;112(1):126-135. 10.1172/JCI17669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jang Y-Y, Sharkis SJ.. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood. 2007;110(8):3056-3063. 10.1182/blood-2007-05-087759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schieber M, Chandel NS.. ROSfunction in redox signaling and oxidative stress. Curr Biol. 2014;24(10):R453-R462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gupta R, Karpatkin S, Basch RS.. Hematopoiesis and stem cell renewal in long-term bone marrow cultures containing catalase. Blood. 2006;107(5):1837-1846. 10.1182/blood-2005-03-1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Herault O, Hope KJ, Deneault E, et al. A role for GPx3 in activity of normal and leukemia stem cells. J Exp Med. 2012;209(5):895-901. 10.1084/jem.20102386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tothova Z, Kollipara R, Huntly BJ, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128(2):325-339. 10.1016/j.cell.2007.01.003 [DOI] [PubMed] [Google Scholar]
- 19. Ito K, Hirao A, Arai F, et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12(4):446-451. 10.1038/nm1388 [DOI] [PubMed] [Google Scholar]
- 20. Ito K, Hirao A, Arai F, et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431(7011):997-1002. 10.1038/nature02989 [DOI] [PubMed] [Google Scholar]
- 21. Mantel CR, O’Leary HA, Chitteti BR, et al. Enhancing hematopoietic stem cell transplantation efficacy by mitigating oxygen shock. Cell. 2015;161(7):1553-1565. 10.1016/j.cell.2015.04.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cipolleschi MG, Dello Sbarba P, Olivotto M.. The role of hypoxia in the maintenance of hematopoietic stem cells. Blood. 1993;82(7):2031-2037. [PubMed] [Google Scholar]
- 23. Hermitte F, Brunet de la Grange P, Belloc F, Praloran V, Ivanovic Z.. Very low O2 concentration (0.1%) favors G0 return of dividing CD34+ cells. Stem Cells Dayton Ohio. 2006;24(1):65-73. 10.1634/stemcells.2004-0351 [DOI] [PubMed] [Google Scholar]
- 24. Bai T, Li J, Sinclair A, et al. Expansion of primitive human hematopoietic stem cells by culture in a zwitterionic hydrogel. Nat Med. 2019;25(10):1566-1575. 10.1038/s41591-019-0601-5 [DOI] [PubMed] [Google Scholar]
- 25. Fares I, Chagraoui J, Gareau Y, et al. Cord blood expansion. Pyrimidoindole derivatives are agonists of human hematopoietic stem cell self-renewal. Science. 2014;345(6203):1509-1512. 10.1126/science.1256337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cohen S, Roy J, Lachance S, et al. Hematopoietic stem cell transplantation using single UM171-expanded cord blood: a single-arm, phase 1-2 safety and feasibility study. Lancet Haematol. 2020;7(2):e134-e145. 10.1016/S2352-3026(19)30202-9 [DOI] [PubMed] [Google Scholar]
- 27. Dumont-Lagacé M, Feghaly A, Meunier M-C, et al. UM171 expansion of cord blood improves donor availability and HLA matching for all patients, including minorities. Transplant Cell Ther. 2022;28(7):410.e1-410.e5. 10.1016/j.jtct.2022.03.016 [DOI] [PubMed] [Google Scholar]
- 28. Henry E, Souissi-Sahraoui I, Deynoux M, et al. Human hematopoietic stem/progenitor cells display reactive oxygen species-dependent long-term hematopoietic defects after exposure to low doses of ionizing radiations. Haematologica. 2020;105(8):2044-2055. 10.3324/haematol.2019.226936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ramachandran L, Nair CKK.. Prevention of γ-radiation induced cellular genotoxicity by tempol: protection of hematopoietic system. Environ Toxicol Pharmacol. 2012;34(2):253-262. 10.1016/j.etap.2012.04.008 [DOI] [PubMed] [Google Scholar]
- 30. Wilcox CS, Pearlman A.. Chemistry and antihypertensive effects of tempol and other nitroxides. Pharmacol Rev. 2008;60(4):418-469. 10.1124/pr.108.000240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wilcox CS. Effects of tempol and redox-cycling nitroxides in models of oxidative stress. Pharmacol Ther. 2010;126(2):119-145. 10.1016/j.pharmthera.2010.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. de la Grange PB, Armstrong F, Duval V, et al. Low SCL/TAL1 expression reveals its major role in adult hematopoietic myeloid progenitors and stem cells. Blood. 2006;108(9):2998-3004. 10.1182/blood-2006-05-022988 [DOI] [PubMed] [Google Scholar]
- 33. Picou F, Vignon C, Debeissat C, et al. Bone marrow oxidative stress and specific antioxidant signatures in myelodysplastic syndromes. Blood Adv. 2019;3(24):4271-4279. 10.1182/bloodadvances.2019000677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Livak KJ, Schmittgen TD.. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods San Diego Calif. 2001;25(4):402-408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 35. Eppert K, Takenaka K, Lechman ER, et al. Stem cell gene expression programs influence clinical outcome in human leukemia. Nat Med. 2011;17(9):1086-1093. 10.1038/nm.2415 [DOI] [PubMed] [Google Scholar]
- 36. Hidalgo I, Herrera-Merchan A, Ligos JM, et al. Ezh1 is required for hematopoietic stem cell maintenance and prevents senescence-like cell cycle arrest. Cell Stem Cell. 2012;11(5):649-662. 10.1016/j.stem.2012.08.001 [DOI] [PubMed] [Google Scholar]
- 37. Bernitz JM, Rapp K, Daniel MG, et al. Memory of divisional history directs the continuous process of primitive hematopoietic lineage commitment. Stem Cell Rep. 2020;14(4):561-574. 10.1016/j.stemcr.2020.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Parmar K, Mauch P, Vergilio J-A, Sackstein R, Down JD.. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci U S A. 2007;104(13):5431-5436. 10.1073/pnas.0701152104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nombela-Arrieta C, Pivarnik G, Winkel B, et al. Quantitative imaging of hematopoietic stem and progenitor cell localization and hypoxic status in the bone marrow microenvironment. Nat Cell Biol. 2013;15(5):533-543. 10.1038/ncb2730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Petzer AL, Hogge DE, Landsdorp PM, Reid DS, Eaves CJ.. Self-renewal of primitive human hematopoietic cells (long-term-culture-initiating cells) in vitro and their expansion in defined medium. Proc Natl Acad Sci U S A. 1996;93(4):1470-1474. 10.1073/pnas.93.4.1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rizo A, Dontje B, Vellenga E, de Haan G, Schuringa JJ.. Long-term maintenance of human hematopoietic stem/progenitor cells by expression of BMI1. Blood. 2008;111(5):2621-2630. 10.1182/blood-2007-08-106666 [DOI] [PubMed] [Google Scholar]
- 42. Kumar D, Lak B, Suntio T, et al. RTN4B interacting protein FAM134C promotes ER membrane curvature and has a functional role in autophagy. Mol Biol Cell. 2021;32(12):1158-1170. 10.1091/mbc.E20-06-0409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Grandi A, Santi A, Campagnoli S, et al. ERMP1, a novel potential oncogene involved in UPR and oxidative stress defense, is highly expressed in human cancer. Oncotarget. 2016;7(39):63596-63610. 10.18632/oncotarget.11550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schaniel C, Sirabella D, Qiu J, et al. Wnt-inhibitory factor 1 dysregulation of the bone marrow niche exhausts hematopoietic stem cells. Blood. 2011;118(9):2420-2429. 10.1182/blood-2010-09-305664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nakashima K, Arai S, Suzuki A, et al. PAD4 regulates proliferation of multipotent haematopoietic cells by controlling c-myc expression. Nat Commun. 2013;4(1):1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gerber H-P, Malik AK, Solar GP, et al. VEGF regulates haematopoietic stem cell survival by an internal autocrine loop mechanism. Nature. 2002;417(6892):954-958. 10.1038/nature00821 [DOI] [PubMed] [Google Scholar]
- 47. Kirito K, Fox N, Komatsu N, Kaushansky K.. Thrombopoietin enhances expression of vascular endothelial growth factor (VEGF) in primitive hematopoietic cells through induction of HIF-1alpha. Blood. 2005;105(11):4258-4263. 10.1182/blood-2004-07-2712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cabezas-Wallscheid N, Buettner F, Sommerkamp P, et al. Vitamin A-retinoic acid signaling regulates hematopoietic stem cell dormancy. Cell. 2017;169(5):807-823.e19. 10.1016/j.cell.2017.04.018 [DOI] [PubMed] [Google Scholar]
- 49. Barroca V, Henry E, Dechamps N, et al. REDD1 is a gatekeeper of murine hematopoietic stem cell functions during stress responses. Leukemia. 2022;36(8):2140-2143. 10.1038/s41375-022-01609-x [DOI] [PubMed] [Google Scholar]
- 50. Papa L, Djedaini M, Martin TC, et al. Limited mitochondrial activity coupled with strong expression of CD34, CD90 and EPCR determines the functional fitness of ex vivo expanded human hematopoietic stem cells. Front Cell Dev Biol. 2020;8:592348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rehn M, Olsson A, Reckzeh K, et al. Hypoxic induction of vascular endothelial growth factor regulates murine hematopoietic stem cell function in the low-oxygenic niche. Blood. 2011;118(6):1534-1543. 10.1182/blood-2011-01-332890 [DOI] [PubMed] [Google Scholar]
- 52. Young C, Russell JR, Van De Lagemaat LN, et al. Intrinsic function of the peptidylarginine deiminase PADI4 is dispensable for normal haematopoiesis. Biol Open. 2022;11(6):bio059143. 10.1242/bio.059143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Luchsinger LL, Strikoudis A, Danzl NM, et al. Harnessing hematopoietic stem cell low intracellular calcium improves their maintenance in vitro. Cell Stem Cell. 2019;25(2):225-240.e7. 10.1016/j.stem.2019.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Goyama S, Kitamura T.. Epigenetics in normal and malignant hematopoiesis: an overview and update 2017. Cancer Sci. 2017;108(4):553-562. 10.1111/cas.13168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Guillaumet-Adkins A, Yañez Y, Peris-Diaz MD, et al. Epigenetics and oxidative stress in aging. Oxid Med Cell Longev. 2017;2017:9175806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ziech D, Franco R, Pappa A, Panayiotidis MI.. Reactive oxygen species (ROS)--induced genetic and epigenetic alterations in human carcinogenesis. Mutat Res. 2011;711(1-2):167-173. 10.1016/j.mrfmmm.2011.02.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are incorporated into the article and its online Supplementary Material. Please contact the corresponding author for more details.


