Abstract
Stress is a state of disrupted homeostasis, triggered by intrinsic or extrinsic factors, the stressors, which are counteracted by various physiological and behavioural adaptive responses. Stress has been linked to cancer development and incidence for decades; however, epidemiological studies and clinical trials have yielded contradictory results. The present review discusses the effects of stress on cancer development and the various underlying mechanisms. Animal studies have revealed a clear link between stress and cancer progression, revealing molecular, cellular and endocrine processes that are implicated in these effects. Thus, stress hormones, their receptor systems and their intracellular molecular pathways mediate the effects of stress on cancer initiation, progression and the development of metastases. The mechanisms linking stress and cancer progression can either be indirect, mediated by changes in the cancer microenvironment or immune system dysregulation, or direct, through the binding of neuroendocrine stress-related signalling molecules to cancer cell receptors. Stress affects numerous anti- and pro-cancer immune system components, including host resistance to metastasis, tumour retention and/or immune suppression. Chronic psychological stress through the elevation of catecholamine levels may increase cancer cell death resistance. On the whole, stress is linked to cancer development and incidence, with psychological stressors playing a crucial role. Animal studies have revealed a better link than human ones, with stress-related hormones influencing tumour development, migration, invasion and cell proliferation. Randomized controlled trials are required to further evaluate the long-term cancer outcomes of stress and its management.
Keywords: cancer, stress, pathogenesis, hormones, pathophysiology
1. Introduction
Stress can be defined as a state of disrupted homeostasis triggered by intrinsic or extrinsic stressors, which is counteracted by a plethora of physiological and behavioural adaptive responses aiming to re-establish the altered equilibrium of the organism (1-3). The concept of a corporal 'steady state' that was defined with the Greek-derived term 'homeostasis' in the beginning of the 20th century, was initially described by ancient Greek natural philosophers, with the words 'harmonious balance', and 'isonomia', later termed 'eucrasia' by Hippocrates and 'eustatheia', by Epicurus, revealing an intellectual understanding of this fundamental concept (1). Lifestyle in contemporary civilizations has evolved and changed considerably from that of our forefathers, and in combination with the lengthening of human life expectancy, has allowed the currently high incidence of 'affluence-related' disorders (1,4). External stressors of modern life, mainly chronic psycho-socio-economic stress and protracted crises, such as the circumstances encountered with the COVID-19 pandemic, economic conditions and climate change, underscore the need to further comprehend stress and its effects on humanity (5-10). This is of particular importance, as uncontrolled chronic stress can have unfavourable, potentially hazardous outcomes, as evidenced by an ever-growing list of stress-related disorders, including several forms of cancer (2,5,11).
Stress has been linked to cancer development and incidence for a number of decades, if not millennia; however, epidemiological studies and clinical trials have produced contradictory results (3,11,12). Thus, psychological stressors have been linked to the development of cancer since the 2nd century CE, with the ancient Greek physician, Galen, noticing that tumours of the reproductive organs were more frequent in women with 'melancholic natures' (12). Other researchers have noted the importance of psychological variables in the occurrence of cancer in women, such as those with 'greater sensitivity and frustration'; however, these studies were based on limited observations and/or personal concerns (12,13).
It has been consistently demonstrated that the immune system plays a critical role in inhibiting cancer progression (12,14). Numerous preclinical and clinical psychoneuroimmunological and neurobiological investigations have been published over the past three decades, delving into the processes behind the linkages between stress and cancer, and have revealed molecular, cellular and endocrine processes that may be implicated in these effects (12,15-18). Animal studies have revealed a clearer link than clinical human studies, indicating that stress can exacerbate the hallmarks of cancer, promoting tumour growth and metastasis by directly altering the molecular properties of malignant tissue, its microenvironment, its anti-host immune reaction activity, and other indirect cancer progression modifiers, as will be further analysed in the present review (12). Of note, discrepancies in preclinical and clinical or epidemiological research observations may be explained as follows: Firstly, preclinical studies link stressful conditions or stress-relieving activities with phases of cancer development on natural or transplanted tumours that are particularly susceptible to the effects of stress; secondly, theoretical and methodological challenges in carrying out human studies that obscure the influence of stress on cancer development (12).
The aim of the present review was to discuss stress and its relation to cancer, examining various pathways that drive carcinogenesis. The review initially summarizes epidemiological data from human studies examining the risk of cancer development and progression related to stress. Focus is then paid to the mechanistic aspects of stress physiology and the discussion of the mechanisms through which stress affects the molecular features of malignant tissue, its microenvironment and its anti-host immune reaction. Furthermore, other, indirect cancer progression modifiers, that promote the growth and spread of numerous cancer forms are also reviewed. The synthesis of the present review may have practical clinical implications.
2. Epidemiological observations
There is accumulating evidence to indicate that stress increases the risk of developing cancer; nevertheless, not all human studies on this topic are consistent (19). A meta-analysis of 12 European cohort studies found no association between work-related stress and overall cancer risk or, more specifically, colorectal, lung, breast, or prostate cancer risk (20). However, another meta-analysis that investigated the association between work-related stress and cancer risk, and focused on colorectal, lung and oesophageal cancers, found a significant association between stress and the risk of cancer development in populations primarily from North America and Europe (21). In addition, another meta-analysis of 53 studies indicated that stress-related psychosocial variables were linked to an increased cancer incidence in healthy populations, a decreased survival time and an increased mortality rate in patients with cancer (22). Stress-prone personalities, unfavourable coping mechanisms, negative emotional responses and a poor quality of life have also been linked to an increased cancer incidence and mortality rate, as well as to a decreased survival time (22). Of note, the same meta-analysis found that there were publication biases and methodological heterogeneity and potential errors in the studies examined; the authors of that study suggested caution in interpreting the findings and emphasized the need for further investigations (11,23). Previous studies have linked specific stressors, such as cold climates, bereavement, war and depression, to a higher incidence of cancers (24-27). However, others studies have shown no association between stress and ovarian or breast cancer (28,29).
Examining stress and cancer progression (often by evaluating the survival rates of patients with cancer) can be relatively challenging. Stress, including life events, is often assessed without regarding the time of cancer detection, while its impact on cancer progression is not assessed (11). In addition, the majority of patients with cancer experience some levels of distress, which may influence cancer progression regardless of baseline stress levels; this may mask the association between stress levels and cancer progression, but could allow for the observation of the beneficial effects of stress-reducing interventions (11,30). Emotional distress in patients with cancer increases mental health issues, which, in turn, may affect cancer prognosis and increase mortality rates (31,32). Psychological stress and discomfort have also been linked to increased mortality rates (33). A recent meta-analysis demonstrated that stressor-specific and cancer-specific effects on survival were evident (11). Depression in patients with breast cancer increases the risk of cancer-specific mortality, while low social support in combination with depression may increase the risk of cancer-related mortality (11,34-36).
The inconsistent effects of stress on cancer development and incidence and heterogeneous approaches, preclude a solid aetiological relation. The subjective stress perception of patients with cancer is influenced by disease burden, resulting in biased retrospective assessments (11). Malignant transformation in humans is a prolonged process characterized by long-term 'dormancy' and a high prevalence of subclinical cancer (37). Cancer incidence may be increased by disease initiation, escape from dormancy, or a more rapid progression to clinical manifestation (11). Despite the controversies associated with human studies, data from animal models are more consistent, as is presented below, in the pathophysiological sections of the present review, following a brief description of stress physiology.
3. Physiological stress responses
Stress was initially described Walter Cannon, followed by Hans Selye (1,38-40). The latter scientist, defined stress as the body's response to stimuli to preserve homeostasis, obviously meaning the adaptive response of the organism to stress (11,40,41). As aforementioned, living organisms maintain homeostasis, a term introduced by Cannon, i.e., a complex dynamic equilibrium, which is constantly challenged by stressors inherent to life's demands, such as routine activities and life-changing or threatening events (1,3,42-44). Thus, stress, is a state where homeostasis is threatened or perceived to be threatened, and is re-established through the complex repertoire of behavioural and physiological adaptive responses, which, among others, includes the mobilisation of metabolic energy to sustain crucial physiological adaptive responses (1,3,42-45). Stressor magnitude and chronicity are crucial; the adaptive homeostatic systems of organisms activate compensatory responses when stressors exceed thresholds, ensuring adaptive responses to the stressor (1). A summary of the neuroendocrine systems involved in the physiological stress response is presented in Fig. 1.
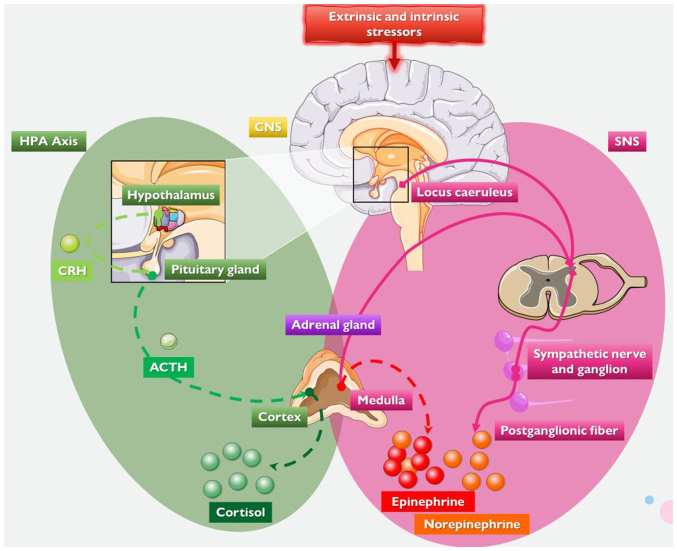
Figure 1.
The neuroendocrine systems involved in the physiological stress response. Stress may be induced by a variety of psychological, physiological, or environmental factors which are processed by the CNS. The two principal neuroendocrine response systems involved are the SNS and the HPA axis. The HPA axis components are illustrated in the green scheme. The hypothalamus secretes CRH, which stimulates the pituitary gland to synthesise and secrete ACTH, which triggers adrenal cortex glucocorticoid, (cortisol or corticosterone), secretion and release. The components of the SNS are shown in the purple scheme. The locus caeruleus and other brainstem nuclei release NE and activate the SNS in response to stress. Sympathetic postganglionic nerve terminals generate vesicles containing NE when triggered. The SNS innervates the adrenal medulla, causing it to generate and release NE and epinephrine. CNS, central nervous system; SNS, sympathetic nervous system; HPA axis, hypothalamus-pituitary-adrenal axis; CRH, corticotropin-releasing hormone; ACTH, adrenocorticotropic hormone; NE, norepinephrine. Parts of this image were derived from the free medical site http://smart.servier.com/ (accessed on July 15, 2023) by Servier, licenced under a Creative Commons Attribution 3.0 Unported Licence.
The 'stress syndrome' corresponds to the physiological adaptive response that coordinates homeostasis and protects organisms during acute stress (1,46). It involves the central nervous system (CNS) and peripheral organs and tissues, and it facilitates adaptive functions, such as arousal and cardiopulmonary function, and inhibits non-adaptive ones, such as eating, growth and reproduction (47). Stress-related changes increase oxygenation and nutrient supply to the brain, heart and skeletal muscles, crucial for central coordination and the 'fight or flight' reaction (1,48). The CNS retains basal homeostasis and processes and integrate responses to various stimuli, including physiological reactions to external or internal stressors (1,3,42-44). The CNS orchestrates the complex adaptation to stress by stimulating the sympathetic nervous system (SNS) and the hypothalamic-pituitary-adrenal (HPA) axis, inducing the secretion respectively of cortisol and adrenergic hormones, primarily noradrenaline (norepinephrine) and adrenaline (epinephrine), while it withdraws the activity of the parasympathetic nervous system (3,42-46).
The HPA axis is activated or dysregulated by mental health issues and/or behavioural changes, including depression and social isolation, influencing pro-inflammatory and anti-inflammatory stress-related immunomodulatory molecules and pathways [including the cellular glucocorticoid signalling system, interleukin (IL)-2 and interferon-γ] (1,11,43,49-52). The hypothalamus, part of the HPA axis, secretes corticotropin-releasing hormone, stimulating adrenocorticotropic hormone secretion by the anterior pituitary gland, which stimulates the production of glucocorticoids by the adrenal cortex, i.e., cortisol in primates and corticosterone in rodents (1,11,43,53).
During stress, the SNS increases the release of the catecholamines norepinephrine and epinephrine by the systemic sympathetic system and the adrenal medulla (1,11,43). The neurohormones norepinephrine and epinephrine cause arousal, increase the metabolic rate, stimulate the cardiopulmonary system, enhance gluconeogenesis, glycogenolysis, proteolysis and lipolysis, and increase catabolism (1,3,11,42-44). Stress-induced hormones also affect other key physiological and biochemical procedures, including various brain networks (e.g., reward), and the water and electrolyte equilibrium (1,3,11,42-44).
The dose-response curve for homeostatic processes, including the stress system, has an inverted U shape (1,46,48,54). Basal, healthy homeostasis occurs in the centre of the curve, the ideal region (1,46,48). Inadequate adaptation, known as low allostasis or 'cacostasis', or an excessive response, known as high allostasis, or 'cacostasis', may yield suboptimal consequences (1). In high allostasis, both the intensity and duration of stressors are key predictors of their effects. Thus, both hypofunction and hyperfunction of the homeostatic systems may have negative consequences, including a decreased survival and higher morbidity (1,46,48). When the stress exceeds the ability of the individual to manage it, it becomes deleterious, and the risk for illness increases by ~20% (11,55-57).
The interaction of homeostasis-disrupting stressors and stressor-activated adaptive responses can result in one of three outcomes: normal match, which yields the organism to its basal homeostasis or 'eustasis'; defective match, which results in 'cacostasis'; or improved match, which results in a new, more resilient equilibrium, 'hyperstasis' (1,46,48). Patients are at a greater risk when allostasis becomes demanding and the allostatic load exceeds overload thresholds (11,55-57). The duration and intensity of the response to stress vary significantly among individuals, and are influenced by physiological factors, psychosocial characteristics and previous stressful life events, such as childhood trauma (11,55-57). As a result, patients may respond differently to stressors such as cancer diagnosis, treatment and survival.
Overall, the predominant effects of deleterious stress can lead to the development of various chronic diseases and comorbidities, including dysmetabolic conditions and cardiometabolic diseases, which predispose to cancer development via various mechanisms, indirect or direct (vide infra) (2,58-65). The following sections focus on cancer pathophysiology and its hallmarks, and thereafter, on the direct impact of stress on these.
4. Cancer hallmarks and the determination of the phases of oncogenesis
Multiple pathways are involved in the development of cancer, including the upregulation of pro-oncogenes and the suppression of onco-suppressor genes, as reviewed extensively elsewhere (66-73). In oncogenesis, the shift from a cell's original state to a malignant state is a process that requires the cell to surmount its anti-oncogenic milestones (74,75). Cancer is caused by various genetic and epigenetic alterations in (stem) cells, primarily involving mutations, deletions, inversions, amplifications and chromosome translocations resulting, among others, in an altered oncogene activity (66,76-78). Based on these characteristics, the hallmarks of cancer have been compiled, as described Hanahan and Weinberg (79,80). These features, which are distinguishing characteristics with evolutionary benefits (80,81), include the capability of infinite cell proliferation, persistent angiogenesis, resilience to cell death, the potential of invasion and metastasis, the ability to evade growth inhibitors, and self-sufficiency in growth factors (74,79,82,83).
The dysregulation of metabolism, a mechanism that plays a critical role in cellular stress signalling pathways and procedures (including mitochondrial functions), and the avoidance of the immune system are two additional characteristics of cancer that have recently emerged (74,80,84). One of the most essential qualities of tumour cells is their capacity to withstand environmental stresses, such as hypoxia, nutritional deprivation and DNA-damaging agents (74,83). Cellular stress is an extrinsic element that influences cancer formation and development. It consists of oxidative stress generated by reactive oxygen species, metabolic stress owing to increased metabolic demands and genotoxic stress, which involves DNA damage (74,85). Cellular stress generally initiates the process of cell death (74,85). Nevertheless, cancer cells can tolerate cellular stress by modifying gene expression, metabolism and escaping growth inhibitory signals (74,80,81,83,86). Notably, pre-malignant or malignant foci can be eliminated, become dormant or grow slowly, or progress to clinical manifestation (37).
Some phases of this heterogeneous non-linear process may theoretically be more important than others (11). Examples include activating the angiogenic switch, allowing growth or escape from dormancy, interacting with immune cells, circulating tumour cells passing through capillaries, and the survival of tumour-associated lymphocytes (87-92). During such potentially critical times, the impact of stress may be amplified (11,74,85). Furthermore, whether stress exacerbates or alleviates malignant processes may be affected by the stage of malignant growth, unique tumour features and the range of stress responses (11). Additionally, immune-tumour interactions may either attenuate or accelerate tumour development, and stress hormones can influence both processes (15,51,93-96). Consequently, it is anticipated that interactions between stress and cancer would be non-linear, with the impact of stress potentially altering the anticipated responses, depending on the stage of cancer progression (11).
Synchronized acute or chronic stress events with a critical cancer phase may, in theory, exert a more prominent effect on cancer growth than non-synchronized events (11). Animal models can focus on critical times by using specific cancer types and stress paradigms, optimizing the understanding of the effects of stress on cancer cells (11). For instance, stressing animals before and after tumour cell injection has been shown to exacerbate the unfavourable effects on the ability of marginating pulmonary natural killer (NK) cells to prevent experimental lung metastasis (89,97,98). On the contrary, chronic stressors do not affect initial breast cancer tumour formation in animal models, but accelerate dissemination and metastasis. Social isolation prior to inoculation does not affect primary tumour development but, after already-palpable tumours were present, it accelerates their growth (99-101). Of note, some of the aforementioned key oncogenic pathophysiological phases may not be identified in a clinical setting, whereas others, particularly those associated with cancer therapeutic interventions, are known to influence cancer progression and may be exploited to reduce the effects of stress on cancer growth (11).
The sections that follow concentrate on various hallmarks and stages of oncogenesis and cancer development, which may be affected by stress. A summary of the effects of stress on cancer initiation, progression, metastasis and selected underlying mechanisms is illustrated in Fig. 2.
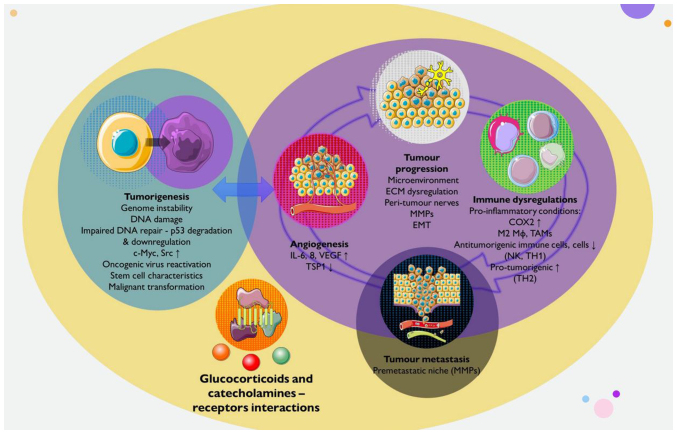
Figure 2.
Effects of stress on cancer initiation, progression, metastasis and underlying mechanisms. The schematic diagram presents the effects of stress on the biological behaviours of tumours. Glucocorticoids and catecholamines produced by the activated neuroendocrine stress system are involved in tumour regulation by binding to their respective receptors. Stress may promote tumorigenesis through genomic instability, DNA damage, the reactivation of latent oncogenic infections, the upregulation of oncogenes, the acquisition of stem cell-like characteristics and eventually, malignant transformation. Stress can facilitate tumour progression through various mechanisms, including alterations in the tumour microenvironment through the stimulation of MMPs, ECM remodelling, neo-angiogenesis and neurogenesis. Stress can promote tumour metastasis by establishing a premetastatic microenvironment and premetastatic niches, and by remodelling the lymphatic vasculature. Distress can induce pro-inflammatory conditions, as well as immune suppression, by reducing the infiltration and function of effector immune cells, such as TH and NK cells, and by promoting the infiltration and function of suppressive cells, including TH2 cells, M2 Mφ (macrophages), and TAMs. C-Myc, cellular myelocytomatosis oncogene; COX2, cyclooxygenase 2; Src, proto-oncogene tyrosine-protein kinase Src; IL, interleukin; VEGF, vascular endothelial growth factor; TSP1, thrombospondin 1 (anti-angiogenic factor); MMPs, matrix metalloproteinases; ECM, extracellular matrix; TH1, T-helper 1 cells; TH2, TH1, T-helper 2 cells; NK, natural killer; TAMs, tumour-associated macrophages. Parts of this image were derived from the free medical site http://smart.servier.com/ (accessed on July 15, 2023) by Servier, licenced under a Creative Commons Attribution 3.0 Unported Licence.
5. Pathophysiological mechanisms of the effects of stress on cancer
Cancer pathogenesis/tumorigenesis
Stress-cancer interactions have been studied via various strategies, including exposure to stressors in laboratory animals and retrospective clinical studies in humans (12). A considerable body of evidence suggests that stress can aggravate the majority of hallmarks of cancer, including genome instability, tumour-promoting inflammation, immune resistance, proliferative signalling, resistance to cell death, angiogenesis, and invasion and metastasis (11,12,80). Stress hormones, their receptor systems and intracellular molecular pathways of adrenergic receptors (ARs) and glucocorticoid receptors (GCRs) have been shown to mediate these effects (102-105). Stress may contribute to cancer initiation, progression and the development of metastasis (12). Herein, tumour initiation is defined and referred to as the transition of non-malignant to malignant tissue, as opposed to tumour progression, which arises after this transformation, despite the fact that the majority of cancer hallmarks may affect both the onset and progress of the disease (11). Recently, it was also shown that tumour-derived cytokines, immune mediators and neurotransmitters can potentially control neuroendocrine centres and modulating body homeostasis (106). These findings suggest a bidirectional communication between local autonomic and sensory nerves, and the tumour may affect the brain and HPA and other axes (106).
i) Stress ignites cancer initiation
The ancient Greek physician, Galen, observed a higher incidence of cancer in women who were melancholy, and early experiments revealed increased cancer incidence in stressed animals (12,107,108). However, the direct effect of stress on cancer initiation remains a matter of debate. It is also unclear whether stress is responsible for new cancer induction or only leads to the clinical manifestation of previously present dormant cancer through other processes, for instance, neo-angiogenesis (12). Retrospective and/or prospective clinical studies have demonstrated higher stress levels in relation to breast cancer, while others have shown no association (12,28,34,109,110). However, these studies use a flawed case-control design due to the presence or suspicion of cancer at the time of stress assessment (12,109). Prospective studies have found that women with high stress levels had a 2-fold higher risk of developing breast cancer than those with no stress, while, by contrast, women with low stress levels had a reduced risk of developing breast cancer (12,109,110). Of general note, the outcomes of clinical studies have been inconclusive, as they result from highly individual differences in perception of stressor intensity in humans, leading to significant differences in neuroendocrine stress responses between individuals (12).
Preclinical data published over the years have highlighted the potential role of mediators of the neuroendocrine stress response (particularly norepinephrine, epinephrine and cortisol) in processes related to carcinogenesis which act directly on cancer cells and promote tumour growth (5,12,102,111-113). Inflammation, angiogenesis, genomic instability, metastasis and the expression of stem cell-like genes are all facilitated by the binding of stress hormones to their receptors (5,102,111). This occurs through the epigenetic alteration or the activation of a variety of mechanisms. Moreover, chronic stress-induced tumour cells become resistant to apoptosis and cancer therapy (5,103).
ii) Stress, genomic instability, DNA damage and the onset of cancer
Cancer is primarily caused by random mutations in non-cancerous stem cells during DNA replication, with environmental factors and inherited predispositions accounting for only a third of variation among tissues (74,76,85,114-116). Notably, carcinogenic infections account for 13-15% of human cancers, and stress may increase the risk of developing cancer by promoting the spread or reactivation of latent oncogenic viruses (68,115,117-122). Stress hormones can reactivate oncogenic viruses, such as human papilloma virus, Epstein-Barr virus, Kaposi sarcoma-associated herpesvirus, and hepatitis B and C viruses, stimulate oncogenes in infected cells, reduce interferon production and impair antiviral immunity (123-126). Psychosocial stress is an environmental factor that contributes to cancer induction by increasing somatic DNA mutation frequency and sensitizing cells to environmental carcinogens (127). Research has demonstrated that exposure to swim stress, noise, or foot shock increases chromosomal aberrations and sister chromatid exchanges in bone marrow cells (128). In healthy female workers, poor stress-coping behaviours have been shown to result in increased levels of cancer-related oxidative DNA damage in leukocytes, and these biomarkers of DNA damage are associated with average working hours, self-blame coping strategies and recent family loss (128). Highly distressed individuals have a reduced DNA repair in lymphocytes exposed to γ-irradiation, while chronically stressed individuals are more sensitive to DNA damage induction by external factors, such as H2O2 and γ-radiation (129,130). These findings suggest that psychological stress may increase susceptibility to environmental mutagenic agents (130).
Studies using mechanistic approaches have been carried out in order to obtain an understanding of the processes and pathways linking stress and mutation, including AR and GCR signalling pathways and the ATR-p21 pathway (11,12). It has been demonstrated that some stressors cause DNA damage and hinder DNA repair, which may favour malignant transformation (11). Specifically, it has been shown that serum from stressed animals or adrenaline, noradrenaline and cortisol (each component alone and synergistically when combined) enhances DNA damage and/or inhibits DNA repair following UV irradiation (131). In non-cancerous murine and human cell lines, adrenaline receptor AR-mediated reactive oxygen species production and arrestin-MDM2-dependent p53 degradation aggravated DNA damage and hindered DNA repair (132).
Chronic stress induces these two AR-mediated processes, and glucocorticoid-mediated responses can also boost MDM2-dependent p53 downregulation and increase apoptosis resistance in response to ionising radiation (133,134). In mice expressing c-Myc, a proto-oncogene, the HPA axis was investigated in cancer induction (135,136). Mice developed intraepithelial prostate neoplasia (PIN) and this progressed to invasive adenocarcinoma (135). Chemical sympathectomy, administered at a young age, reduced the incidence of PIN by 83%. However, the effect of sympathectomy was not observed in adult individuals, as the PIN had already developed in adult mice (135). β2- and β3-ARs play a primary role in prostate adenocarcinoma development, and the deletion of genes for these receptors leads to a significant reduction in the incidence of adenocarcinoma in genetically modified mice (135).
Norepinephrine, epinephrine and cortisol increase the formation of oxygen radicals, causing DNA damage and reducing cell repair processes (12). It has been shown that the short-term exposure to physiological concentrations of these substances induces at least a 5-fold increase in DNA damage in treated murine 3T3 cells (113,131,132). Pre-treatment with an antagonist of ARs or GCRs protected these cells from DNA damage (131). Norepinephrine and cortisol reduced DNA repair in 3T3 cells exposed to UV radiation (131). The infusion of isoproterenol, an β-AR agonist, induces the degradation of the tumour suppressor protein, p53, in mouse thymus cells, while propranolol increases the gene expression of p53 in primary melanoma-derived and metastasis-derived tumours in mice (132,137). Chronic restraint stress attenuates p53 functions and promotes ionizing radiation-induced tumorigenesis in p53+/− mice, with glucocorticoids playing a central role in these processes (134). β-adrenergic signalling also participates in the activation of oncogenes, with the stimulation of ovarian cancer cells with norepinephrine significantly activates oncogene Src (138). A similar effect of adrenergic signalling on the gene expression of Her2 has also been described (139).
Rather than focusing on intermediate signs, such as DNA damage or the reactivation of carcinogenic viruses, several in vivo animal investigations on the influence of stress on carcinogenesis have been conducted (11). Restraint stress, social isolation and a cold ambient temperature, all lead to carcinogen-induced cancer development (134,140-142). Repetitive restraint stress enhances pancreatic carcinogenesis in transgenic models of spontaneous cancer via AR signalling (143), although sympathetic denervation results in a reduction in carcinogenesis in models of prostate cancer (135). Models based on accelerated cancer induction struggle to differentiate the effects of stress on tumour initiation and development due to the overlap between the time course of stress and the initiation and progression periods (134,143-145).
Stress promotes cancer progression
Hans Selye (40) also suggested that stress may increase cancer growth. Animals studies have shown that stress can lead to cancer progression (146). The mechanisms linking stress and cancer progression are either indirect, mediated by changes in the cancer microenvironment, immune system dysregulation/inhibition, or direct, through the binding of neuroendocrine stress-related signalling molecules to cancer cell receptors (12):
i) Tumour microenvironment (TME)
The neuroendocrine system modifies the TME in a manner that favours cancer progression (5,111). The TME is composed of immune cells and other stromal cells, such as fibroblasts, adipocytes and endothelial cells, in addition to extracellular components (extracellular matrix) (5,147). These components pertain to the circulatory system, lymphatic system and peripheral nerves, and they support cancer cells (148). Chronic stress-related hormones influence tumour development, including interactions between cancer cells, and invading immune and stromal cell populations in the TME; recent research indicates that tumours can attract nerves into the TME and form peri-tumour nerves, which influence carcinogenesis, angiogenesis, invasion and metastasis (5,149,150).
ii) Stress and the induction of (lymph)angiogenesis
The in vitro activation of AR on cancer cells increases the synthesis and release of angiogenic factors (12,151). Adrenaline and noradrenaline promote angiogenesis by increasing the production and synthesis of angiogenic factors, such as vascular endothelial growth factor (VEGF), IL-8, IL-6 and enzymes, such as matrix metalloproteinases (MMPs) (151-155). The potentiating effect of VEGF production by cancer cells is induced by neuropeptide Y, a co-transmitter of norepinephrine (156). It has been shown that ovarian orthotopic tumours exhibit an increased vascularization and VEGF expression in stressed mice and the effect is mediated through β2-AR (18). In the same study, 2-AR-cyclic AMP-protein kinase A signalling was shown to boost tumour VEGF production, vascularization and proliferation (18). It has been found that stress-induced AR signalling significantly decreases the expression of the anti-angiogenic factor, thrombospondin 1, in prostate cancer xenografts through epigenetic regulation, resulting in similar outcomes in pancreatic, colorectal and breast cancer models (99,157-160). Finally, chronic restraint stress in mice has been shown to remodel the lymphatic vessels around tumour tissue, mediated by the activation of β-AR on tumour-associated macrophages (161). This effect stimulates the production of VEGFC by tumour cells (161).
iii) Inflammation and immunomodulation are hampered by stress
Stress causes inflammation, anti-inflammation, as well as immune evasion (15). In animal models and patients with cancer, the effects of stress on the activity and distribution of ARs, prostaglandin receptors and GCRs have been extensively investigated (15,94,105,162,163). The pro-inflammatory effects of stress, as well as its implications on immune system dysregulation that allows cancer to avoid destruction are addressed in the following sections.
a) Stress and cancer-promoting inflammation
Pro-inflammatory molecules released by immune cells can lead to mutagenic processes, transforming normal cells into cancer cells (164). Stress can affect inflammation-related mutagenic processes by activating the sympathetic nervous system (165). A previous study on diethyl nitrosamine-induced hepatocarcinogenesis in rats demonstrated that sympathectomy with 6-hydroxydopamine reduced the development of hepatocellular carcinoma (165). However, prazosin, an α-AR antagonist, led to carcinoma development in up to 64% of rats. Sympathetic innervation is crucial for maintaining the liver inflammatory microenvironment, which initiates hepatocellular carcinoma development (165). Stress-induced-adrenergic signalling increases inflammation in malignant cells and tumour-associated macrophages (TAMs), leading to an increased expression of cyclooxygenase 2, prostaglandin secretion and increase levels of pro-inflammatory cytokines such as IL-6, which in turn increases macrophage recruitment and M2-pro-inflammatory-polarization (99,151,153,161,166-169). Social isolation and depression are linked to an increased M2 polarization in breast tumours, while higher levels of depression and an increased expression of genes encoding AR and prostaglandins predict a decreased survival rate of cancer patients (166,170).
b) Stress and avoidance of immune destruction
According to animal and human research, stress affects numerous anti- and pro-cancer immune system components (12). Chronic stress in humans has been shown to suppress host resistance to metastasis, causing lung tumour retention and metastasis (98). An acute stressor, such as swimming stress, suppresses NK cell activity and increases lung tumour retention (98). This effect can be attenuated or prevented by reducing the release of norepinephrine and epinephrine or using β1- and β2-blockers (98). However, the administration of epinephrine or other agonists of β-AR can also suppress NK cell activity and increase lung tumour retention (17). This suggests that acute stress suppresses NK cell activity and compromises resistance to NK-sensitive metastasis (17,98).
Stress-induced-adrenergic signalling or agonists can inhibit NK cell activity against tumour cells, leading to increased lung metastases (17,89,97,98,171,172). A lower NK cytotoxicity in patients with ovarian cancer is linked to less social support and depression (173). Stress induces a shift from T-helper 1 cell (TH1) to T-helper 2 cell (TH2) cytokine production, increasing tumour growth in colorectal and squamous cell carcinoma mouse models (140,174,175). A depressive and worried mood are related to a decreased TH1 cell/TH2 cell-type cytokine ratio in patients with ovarian cancer (176). The stress-induced-adrenergic response promotes tumour growth by upregulating suppressive immune cells, such as myeloid-derived suppressor cells (MDSCs) and regulatory T-cells (140,158,163,175,177). Higher levels of stress are associated with a decrease in the number of circulating MDSCs (178). Additionally, chronic sound stress has been shown to increase colon cancer progression, plasma norepinephrine and corticosterone levels, and to induce a shift in the TH1 to TH2 response (158,174). Similar effects of stress on immunological functions have been observed in clinical investigations, including a reduction in protective immunity, the exacerbation of chronic inflammation, and the enhancement of immunosuppressive processes (15).
iv) Direct effects of stress on cancer cells
Stress hormones, which can be generated systemically or even secreted locally in the TME by infiltrating sympathetic nerve endings, immune cells, or tumour cells per se can have direct effects on cancer cells and boosting their malignant properties (139,179-182). Both noradrenaline and adrenaline enhance cancer cell proliferation, as well as survival (through anti-apoptosis), cell migration and invasion, epithelial-mesenchymal transition (EMT), the production of prostaglandins and the activation of MMPs (100,152,166,183-190). In animal models, psychological or physiological stressors (such as social confrontation, restraint and surgery) have been shown to enhance tumour development and metastasis by activating tumour the AR, as demonstrated by pharmacological or molecular blockage, or genetic knockout (18,99,175,183,188,191-193).
A recent study demonstrated that tumour innervation led to the advancement of cancer (194). Tumours can generate neural growth agents that promote sympathetic tumour innervation. During stress-induced sympathetic activation, higher tumour noradrenaline levels establish a feedforward loop that promotes cancer progression (143). Multiple cancer forms express AR, and higher levels of tumour noradrenaline and/or plasma adrenaline are associated with larger tumour size, advanced stage, lymph node metastases and/or a shorter survival (143,152,166,167,183,184,187,189,190,195).
In vitro and in vivo, stress-related hormones may promote the growth of cancer cells (12). AR activation has been shown to stimulate the proliferation of lung adenocarcinoma, pancreatic cancer and fibrosarcoma cells (196-198). Glucocorticoids have also been found to exert a stimulatory effect on cancer cell proliferation. Studies have demonstrated that physiological concentrations of hydrocortisone promote the proliferation of cell lines from metastatic breast carcinomas (199,200). Cortisol and its metabolite cortisone have been shown to stimulate prostate cancer cell growth in the absence of androgens (18).
Chronic behavioural stress can increase tissue catecholamine levels and promote the growth and invasiveness of ovarian carcinoma cells, primarily through the activation of β2-AR (18). The effect of stress on cancer cell resistance to death supports previous findings, such as the inhibition of the apoptosis of prostate and breast cancer cells by epinephrine, chemical sympathectomy increasing the gene expression of apoptotic factors in mouse melanoma tumours, and selective antagonists of β1- and β2-AR on colorectal cancer cell growth (16,201,202). Psychologic stress can inhibit apoptosis, but this effect is prevented by administration of a selective β2-AR antagonist (16).
Stress and metastasis
Numerous of the aforementioned and other stress-induced processes contribute to metastasis, in addition to promoting initiation and progression. Stress significantly increases the development of metastases, as demonstrated by studies on colon carcinoma, nasopharyngeal carcinoma, and ovarian cancer cells (203-205). As previously demonstrated, norepinephrine treatment increases the locomotor activity of colon carcinoma cells; however, this effect is blocked by propranolol (203). MMP-2 and MMP-9 levels have also been shown to be increased in the cell culture supernatant, whereas this effect is blocked by propranolol (206). Other examples include studies in mice, demonstrating that stress-induced AR activation promotes circulating tumour cell migration to the bones via the increased expression of RANKL by bone marrow-derived stem cells or to the lungs via the CC-chemokine ligand 2-CC-chemokine receptor 2-mediated attraction of macrophages, thereby forming pre-metastatic niches and increasing organ-specific metastasis (191,207). Additionally, stress increases tumour and stromal cell MMP production, tumour cell anoikis resistance, and cancer cell EMT, thus boosting malignant cell detachment, invasiveness and circulation survival (99,100,157,183, 186,188-190,208).
Perceived stress, depressive symptoms, or social isolation have been shown to be associated with the increased expression of EMT-related genes in tumours in patients with breast and ovarian cancer, as well as higher levels of MMP-9 in tumour cells and/or TAMs (99,206). In numerous mouse models, AR inhibition significantly reduces experimental and spontaneous metastases (89,99,161,183,191,207,209). Similar to how tumour AR expression levels have been associated with lymph node metastasis in patients with gastric and lung cancer, incidental AR-blocker use has been linked to a lower risk of developing metastasis or recurrence in patients with breast and ovarian cancer, as well as to the improved survival of patients with melanoma and breast cancer, but not lung and ovarian cancer (161,183,210-216).
It has been demonstrated that chronic stress in cancer patients with elevated depressive symptoms and low social support leads to a 30-fold increase in metastasis to distant tissues (99). Stress-induced lymphatic vessel rearrangement may also contribute to cancer cell dissemination (161). β2-AR activation reduces deformability in metastatic human breast cancer cells, rendering them more invasive (217). Randomized controlled trials are required to evaluate the effects of AR-blockers on long-term cancer outcomes due to the discrepancies in the analysed indices (such as metastasis vs. survival), the diversity of cancer types and the uncontrolled settings of correlational research (10).
6. Conclusions and future perspectives
The present review indicates that stress has been linked to cancer development and incidence for a number of decades. Psychological stressors have been linked to cancer development, with the immune system playing a critical role in inhibiting cancer progression. Recent research has advanced the current understanding of the role of stress in cancer induction, growth and metastasis development, with the SNS and HPA axis playing crucial roles. Animal studies have revealed a clearer link than clinical human studies, suggesting that stress factors can exacerbate cancer hallmarks and promote growth and metastasis by directly affecting malignant tissue, its microenvironment, antitumor immune activity and other indirect cancer progression modifiers. Stress-related hormones can influence tumour development, migration, invasion, and cancer cell proliferation. The therapeutic potential of these pathophysiological mechanisms is shown by the discovery of numerous procedures that are triggered by stress in patients with cancer; however, these are beyond the scope of this review and can be further investigated in the future. Randomized controlled trials are required to evaluate the effects of stress on long-term cancer outcomes. Psychological therapies can potentially target stress and benefit individuals. To minimize cancer recurrence and associated mortality, chronic stress-management interventions need to be tested during critical periods, accompanied by pharmacological approaches, and include individualized modules.
Acknowledgments
Not applicable.
Funding Statement
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
DAS and VEG conceptualized the study. IGL, VEG, PP, GPC and DAS made a substantial contribution to the interpretation and analysis of data to be included in the review, and wrote and prepared the draft of the manuscript. DAS and GPC analysed the data from studies for inclusion in the review and provided critical revisions. All authors contributed to manuscript revision, and have read and approved the final version of the manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had no personal involvement in the reviewing process, or any influence in terms of adjudicating on the final decision, for this article. The other authors declare that they have no competing interests.
References
- 1.Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5:374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- 2.Agorastos A, Chrousos GP. The neuroendocrinology of stress: The stress-related continuum of chronic disease development. Mol Psychiatry. 2022;27:502–513. doi: 10.1038/s41380-021-01224-9. [DOI] [PubMed] [Google Scholar]
- 3.Tsigos C, Kyrou I, Kassi E, Chrousos GP. Stress: Endocrine Physiology and Pathophysiology. In: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, et al., editors. Endotext. MDTextcom, Inc; South Dartmouth, MA: 2000. [PubMed] [Google Scholar]
- 4.Chrousos GP. The glucocorticoid receptor gene, longevity, and the complex disorders of Western societies. Am J Med. 2004;117:204–207. doi: 10.1016/j.amjmed.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Yan J, Chen Y, Luo M, Hu X, Li H, Liu Q, Zou Z. Chronic stress in solid tumor development: From mechanisms to interventions. J Biomed Sci. 2023;30:8. doi: 10.1186/s12929-023-00903-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lempesis IG, Karlafti E, Papalexis P, Fotakopoulos G, Tarantinos K, Lekakis V, Papadakos SP, Cholongitas E, Georgakopoulou VE. COVID-19 and liver injury in individuals with obesity. World J Gastroenterol. 2023;29:908–916. doi: 10.3748/wjg.v29.i6.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Georgakopoulou VE, Lembessis P, Skarlis C, Gkoufa A, Sipsas NV, Mavragani CP. Hematological abnormalities in COVID-19 disease: Association with type I interferon pathway activation and disease outcomes. Front Med (Lausanne) 2022;9:850472. doi: 10.3389/fmed.2022.850472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsamakis K, Gavriatopoulou M, Schizas D, Stravodimou A, Mougkou A, Tsiptsios D, Sioulas V, Spartalis E, Sioulas AD, Tsamakis C, et al. Oncology during the COVID-19 pandemic: Challenges, dilemmas and the psychosocial impact on cancer patients. Oncol Lett. 2020;20:441–447. doi: 10.3892/ol.2020.11599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Georgakopoulou VE, Makrodimitri S, Triantafyllou M, Samara S, Voutsinas PM, Anastasopoulou A, Papageorgiou CV, Spandidos DA, Gkoufa A, Papalexis P, et al. Immature granulocytes: Innovative biomarker for SARS-CoV-2 infection. Mol Med Rep. 2022;26:217. doi: 10.3892/mmr.2022.12733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lempesis IG, Georgakopoulou VE. Implications of obesity and adiposopathy on respiratory infections; focus on emerging challenges. World J Clin Cases. 2023;11:2925. doi: 10.12998/wjcc.v11.i13.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckerling A, Ricon-Becker I, Sorski L, Sandbank E, Ben-Eliyahu S. Stress and cancer: Mechanisms, significance and future directions. Nat Rev Cancer. 2021;21:767–785. doi: 10.1038/s41568-021-00395-5. [DOI] [PubMed] [Google Scholar]
- 12.Mravec B, Tibensky M, Horvathova L. Stress and cancer. Part I: Mechanisms mediating the effect of stressors on cancer. J Neuroimmunol. 2020;346:577311. doi: 10.1016/j.jneuroim.2020.577311. [DOI] [PubMed] [Google Scholar]
- 13.Deshaies-Gendron M. Enquiries Into the Nature, Knowledge, and Cure of Cancers. Gendron Deshaies. Done out of French. 2018 April 22; Gale Ecco, Print Editions. [Google Scholar]
- 14.Selye H. A syndrome produced by diverse nocuous agents. Nature. 1936;138:32–32. doi: 10.1176/jnp.10.2.230a. [DOI] [PubMed] [Google Scholar]
- 15.Antoni MH, Dhabhar FS. The impact of psychosocial stress and stress management on immune responses in patients with cancer. Cancer. 2019;125:1417–1431. doi: 10.1002/cncr.31943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hassan S, Karpova Y, Baiz D, Yancey D, Pullikuth A, Flores A, Register T, Cline JM, D'Agostino R, Jr, Danial N, et al. Behavioral stress accelerates prostate cancer development in mice. J Clin Invest. 2013;123:874–886. doi: 10.1172/JCI63324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inbar S, Neeman E, Avraham R, Benish M, Rosenne E, Ben-Eliyahu S. Do stress responses promote leukemia progression? An animal study suggesting a role for epinephrine and prostaglandin-E2 through reduced NK activity. PLoS One. 2011;6:e19246. doi: 10.1371/journal.pone.0019246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, Jennings NB, Armaiz-Pena G, Bankson JA, Ravoori M, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12:939–944. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- 19.Wu Y, Zhou L, Zhang X, Yang X, Niedermann G, Xue J. Psychological distress and eustress in cancer and cancer treatment: Advances and perspectives. Sci Adv. 2022;8:eabq7982. doi: 10.1126/sciadv.abq7982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heikkilä K, Nyberg ST, Theorell T, Fransson EI, Alfredsson L, Bjorner JB, Bonenfant S, Borritz M, Bouillon K, Burr H, et al. Work stress and risk of cancer: Meta-analysis of 5700 incident cancer events in 116 000 European men and women. BMJ. 2013;346:f165. doi: 10.1136/bmj.f165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang T, Qiao Y, Xiang S, Li W, Gan Y, Chen Y. Work stress and the risk of cancer: A meta-analysis of observational studies. Int J Cancer. 2019;144:2390–2400. doi: 10.1002/ijc.31955. [DOI] [PubMed] [Google Scholar]
- 22.Chida Y, Hamer M, Wardle J, Steptoe A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat Clin Pract Oncol. 2008;5:466–475. doi: 10.1038/ncponc1134. [DOI] [PubMed] [Google Scholar]
- 23.Coyne JC, Ranchor AV, Palmer SC. Meta-analysis of stress-related factors in cancer. Nat Rev Clin Oncol. 2010:7. doi: 10.1038/ncponc1134-c1. [DOI] [PubMed] [Google Scholar]
- 24.Mravec B, Tibensky M. Increased cancer incidence in 'cold' countries: An (un) sympathetic connection? J Therm Biol. 2020;89:102538. doi: 10.1016/j.jtherbio.2020.102538. [DOI] [PubMed] [Google Scholar]
- 25.Keinan-Boker L, Vin-Raviv N, Liphshitz I, Linn S, Barchana M. Cancer incidence in Israeli jewish survivors of World War II. J Natl Cancer Inst. 2009;101:1489–1500. doi: 10.1093/jnci/djp327. [DOI] [PubMed] [Google Scholar]
- 26.Huang T, Poole EM, Okereke OI, Kubzansky LD, Eliassen AH, Sood AK, Wang M, Tworoger SS. Depression and risk of epithelial ovarian cancer: Results from two large prospective cohort studies. Gynecol Oncol. 2015;139:481–486. doi: 10.1016/j.ygyno.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fang F, Fall K, Sparén P, Adami HO, Valdimarsdóttir HB, Lambe M, Valdimarsdóttir U. Risk of infection-related cancers after the loss of a child: A follow-up study in Sweden. Cancer Res. 2011;71:116–122. doi: 10.1158/0008-5472.CAN-10-0470. [DOI] [PubMed] [Google Scholar]
- 28.Schoemaker MJ, Jones ME, Wright LB, Griffin J, McFadden E, Ashworth A, Swerdlow AJ. Psychological stress, adverse life events and breast cancer incidence: A cohort investigation in 106,000 Women in the United Kingdom. Breast Cancer Res. 2016;18:72. doi: 10.1186/s13058-016-0733-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trudel-Fitzgerald C, Poole EM, Idahl A, Lundin E, Sood AK, Kawachi I, Kubzansky LD, Tworoger SS. The association of work characteristics with ovarian cancer risk and mortality. Psychosom Med. 2017;79:1059–1067. doi: 10.1097/PSY.0000000000000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carlson L, Angen M, Cullum J, Goodey E, Koopmans J, Lamont L, MacRae JH, Martin M, Pelletier G, Robinson J, et al. High levels of untreated distress and fatigue in cancer patients. Br J Cancer. 2004;90:2297–2304. doi: 10.1038/sj.bjc.6601887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitchell AJ, Chan M, Bhatti H, Halton M, Grassi L, Johansen C, Meader N. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: A meta-analysis of 94 interview-based studies. Lancet Oncol. 2011;12:160–174. doi: 10.1016/S1470-2045(11)70002-X. [DOI] [PubMed] [Google Scholar]
- 32.Lu D, Andersson TM, Fall K, Hultman CM, Czene K, Valdimarsdóttir U, Fang F. Clinical diagnosis of mental disorders immediately before and after cancer diagnosis: A nationwide matched cohort study in Sweden. JAMA Oncol. 2016;2:1188–1196. doi: 10.1001/jamaoncol.2016.0483. [DOI] [PubMed] [Google Scholar]
- 33.Batty GD, Russ TC, Stamatakis E, Kivimäki M. Psychological distress in relation to site specific cancer mortality: Pooling of unpublished data from 16 prospective cohort studies. BMJ. 2017;356:108. doi: 10.1136/bmj.j108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang X, Wang N, Zhong L, Wang S, Zheng Y, Yang B, Zhang J, Lin Y, Wang Z. Prognostic value of depression and anxiety on breast cancer recurrence and mortality: A systematic review and meta-analysis of 282,203 patients. Mol Psychiatry. 2020;25:3186–3197. doi: 10.1038/s41380-020-00865-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pinquart M, Duberstein P. Depression and cancer mortality: A meta-analysis. Psychol Med. 2010;40:1797–1810. doi: 10.1017/S0033291709992285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pinquart M, Duberstein PR. Associations of social networks with cancer mortality: A meta-analysis. Crit Rev Oncol Hematol. 2010;75:122–137. doi: 10.1016/j.critrevonc.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manjili MH. Tumor dormancy and relapse: From a natural byproduct of evolution to a disease state. Cancer Res. 2017;77:2564–2569. doi: 10.1158/0008-5472.CAN-17-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cannon WB. The wisdom of the body. 2nd. Norton and Co; New York, NY: 1939. [Google Scholar]
- 39.Cannon WB. Bodily changes in pain, hunger, fear, and rage An account of recent researches into the function of emotional excitement. D. Appleton and Company; 1915. [Google Scholar]
- 40.Selye H. The stress of life. McGraw-Hill Education; New York, NY: 1956. [Google Scholar]
- 41.Chrousos GP. Hans Selye memorial lecture: Stressors, stress and neuroendocrine integration of the adaptive response. Ann NY Acad Sci. 1997;851:311–335. doi: 10.1111/j.1749-6632.1998.tb09006.x. [DOI] [PubMed] [Google Scholar]
- 42.Lim CT, Khoo B. Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, et al. Endotext. MDTextcom, Inc; South Dartmouth, MA: 2000. Normal Physiology of ACTH and GH Release in the Hypothalamus and Anterior Pituitary in Man. [PubMed] [Google Scholar]
- 43.Gassen NC, Chrousos GP, Binder EB, Zannas AS. Life stress, glucocorticoid signaling, and the aging epigenome: Implications for aging-related diseases. Neurosci Biobehav Rev. 2017;74:356–365. doi: 10.1016/j.neubiorev.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 44.Nicolaides NC, Chrousos G, Kino T. Glucocorticoid Receptor. In: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, et al., editors. Endotext. MDText.com, Inc.; South Dartmouth MA: 2000. [Google Scholar]
- 45.Chrousos GP, McCarty R, Pacak K, Cizza G, Sternberg G, Gold P, Květňanský R. Stress: Basic mechanisms and clinical implications. 1995 [Google Scholar]
- 46.Chrousos GP, Gold PW. The concepts of stress and stress system disorders: Overview of physical and behavioral homeostasis. JAMA. 1992;267:1244–1252. [PubMed] [Google Scholar]
- 47.Chrousos GP. Organization and integration of the endocrine system: The arousal and sleep perspective. Sleep Med Clin. 2007;2:125–145. doi: 10.1016/j.jsmc.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Charmandari E, Tsigos C, Chrousos G. Neuroendocrinology of stress. Ann Rev Physiol. 2005;67:259–284. doi: 10.1146/annurev.physiol.67.040403.120816. [DOI] [PubMed] [Google Scholar]
- 49.Gold PW, Goodwin FK, Chrousos GP. Clinical and biochemical manifestations of depression. N Engl J Med. 1988;319:413–420. doi: 10.1056/NEJM198808183190706. [DOI] [PubMed] [Google Scholar]
- 50.Chrousos GP, Kino T. Glucocorticoid action networks and complex psychiatric and/or somatic disorders. Stress. 2007;10:213–219. doi: 10.1080/10253890701292119. [DOI] [PubMed] [Google Scholar]
- 51.Franchimont D, Kino T, Galon J, Meduri GU, Chrousos G. Glucocorticoids and Inflammation Revisited: The State of the ArtNIH clinical staff conference. Neuroimmunomodulation. 2003;10:247–260. doi: 10.1159/000069969. [DOI] [PubMed] [Google Scholar]
- 52.Chrousos GP. The stress response and immune function: Clinical implications. Ann NY Acad Sci. 2000;917:38–67. doi: 10.1111/j.1749-6632.2000.tb05371.x. [DOI] [PubMed] [Google Scholar]
- 53.Karalis K, Sano H, Redwine J, Listwak S, Wilder RL, Chrousos GP. Autocrine or paracrine inflammatory actions of corticotropin-releasing hormone in vivo. Science. 1991;254:421–423. doi: 10.1126/science.1925600. [DOI] [PubMed] [Google Scholar]
- 54.Sapolsky RM. Stress and the brain: Individual variability and the inverted-U. Nat Neurosci. 2015;18:1344–1346. doi: 10.1038/nn.4109. [DOI] [PubMed] [Google Scholar]
- 55.McEwen BS. Neurobiological and systemic effects of chronic stress. Chronic Stress (Thousand Oaks) 2017;1:2470547017692328. doi: 10.1177/2470547017692328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: Links to socioeconomic status, health, and disease. Ann N Y Acad Sci. 2010;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McEwen BS, Stellar E. Stress and the individual: Mechanisms leading to disease. Arch Intern Med. 1993;153:2093–2101. [PubMed] [Google Scholar]
- 58.Stefanaki C, Pervanidou P, Boschiero D, Chrousos GP. Chronic stress and body composition disorders: Implications for health and disease. Hormones (Athens) 2018;17:33–43. doi: 10.1007/s42000-018-0023-7. [DOI] [PubMed] [Google Scholar]
- 59.Chrousos GP, Tsigos C. Stress, obesity, and metabolic syndrome. Blackwell Pub on behalf of the New York Academy of Sciences; 2006. [DOI] [PubMed] [Google Scholar]
- 60.Chrousos G. The role of stress and the hypothalamic-pituitary-adrenal axis in the pathogenesis of the metabolic syndrome: Neuro-endocrine and target tissue-related causes. Int J Obes Relat Metab Disord. 2000;24(Suppl 2):S50–S55. doi: 10.1038/sj.ijo.0801278. [DOI] [PubMed] [Google Scholar]
- 61.Lempesis IG, Hoebers N, Essers Y, Jocken JWE, Dineen R, Blaak EE, Manolopoulos KN, Goossens GH. Distinct inflammatory signatures of upper and lower body adipose tissue and adipocytes in women with normal weight or obesity. Front Endocrinol (Lausanne) 2023;14:1600. doi: 10.3389/fendo.2023.1205799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lempesis IG, Tsilingiris D, Liu J, Dalamaga M. Of mice and men: Considerations on adipose tissue physiology in animal models of obesity and human studies. Metabol Open. 2022;15:100208. doi: 10.1016/j.metop.2022.100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lempesis IG, Georgakopoulou VE. Physiopathological mechanisms related to inflammation in obesity and type 2 diabetes mellitus. World J Exp Med. 2023;13:7–16. doi: 10.5493/wjem.v13.i3.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lempesis IG, Varrias D, Sagris M, Attaran RR, Altin ES, Bakoyiannis C, Palaiodimos L, Dalamaga M, Kokkinidis DG. Obesity and peripheral artery disease: Current evidence and controversies. Curr Obes Rep. 2023 May 27; doi: 10.1007/s13679-023-00510-7. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lempesis IG, van Meijel RLJ, Manolopoulos KN, Goossens GH. Oxygenation of adipose tissue: A human perspective. Acta Physiol (Oxf) 2020;228:e13298. doi: 10.1111/apha.13298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Spandidos DA. A unified theory for the development of cancer. Biosci Rep. 1986;6:691–708. doi: 10.1007/BF01116536. [DOI] [PubMed] [Google Scholar]
- 67.Spandidos DA, Anderson ML. Oncogenes and onco-suppressor genes: Their involvement in cancer. J Pathol. 1989;157:1–10. doi: 10.1002/path.1711570102. [DOI] [PubMed] [Google Scholar]
- 68.Simatou A, Simatos G, Goulielmaki M, Spandidos DA, Baliou S, Zoumpourlis V. Historical retrospective of the SRC oncogene and new perspectives (Review) Mol Clin Oncol. 2020;13:21. doi: 10.3892/mco.2020.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nitulescu GM, Van De Venter M, Nitulescu G, Ungurianu A, Juzenas P, Peng Q, Olaru OT, Grădinaru D, Tsatsakis A, Tsoukalas D, et al. The Akt pathway in oncology therapy and beyond (Review) Int J Oncol. 2018;53:2319–2331. doi: 10.3892/ijo.2018.4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fotakopoulos G, Georgakopoulou VE, Spandidos DA, Papalexis P, Angelopoulou E, Aravantinou-Fatorou A, Trakas N, Trakas I, Brotis AG. Role of miR-200 family in brain metastases: A systematic review. Mol Clin Oncol. 2023;18:15. doi: 10.3892/mco.2023.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Damaskos C, Garmpis N, Dimitroulis D, Garmpi A, Diamantis E, Sarantis P, Georgakopoulou VE, Patsouras A, Despotidis M, Prevezanos D, et al. The Role of SNHG15 in the pathogenesis of hepatocellular carcinoma. J Pers Med. 2022;12:753. doi: 10.3390/jpm12050753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Garmpis N, Damaskos C, Garmpi A, Nonni A, Georgakopoulou VE, Antoniou E, Schizas D, Sarantis P, Patsouras A, Syllaios A, et al. Histone deacetylases and their inhibitors in colorectal cancer therapy: Current evidence and future considerations. Curr Med Chem. 2022;29:2979–2994. doi: 10.2174/0929867328666210915105929. [DOI] [PubMed] [Google Scholar]
- 73.Garmpis N, Damaskos C, Angelou A, Garmpi A, Georgakopoulou VE, Valsami S, Schizas D, Voutyritsa E, Syllaios A, Diamantis E, et al. Animal models for the calculation of circulating tumor cells for experimental demonstration. Anticancer Res. 2020;40:6599–6607. doi: 10.21873/anticanres.14684. [DOI] [PubMed] [Google Scholar]
- 74.Tsatsakis A, Oikonomopoulou T, Nikolouzakis TK, Vakonaki E, Tzatzarakis M, Flamourakis M, Renieri E, Fragkiadaki P, Iliaki E, Bachlitzanaki M, et al. Role of telomere length in human carcinogenesis (Review) Int J Oncol. 2023;63:78. doi: 10.3892/ijo.2023.5526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Anderson ML, Spandidos DA. Onco-suppressor genes and their involvement in cancer (review) Anticancer Res. 1988;8:873–879. [PubMed] [Google Scholar]
- 76.Lang JC, Spandidos DA. The structure and function of eukaryotic enhancer elements and their role in oncogenesis. Anticancer Res. 1986;6:437–449. [PubMed] [Google Scholar]
- 77.Field JK, Spandidos DA. Expression of oncogenes in human tumours with special reference to the head and neck region. J Oral Pathol. 1987;16:97–107. doi: 10.1111/j.1600-0714.1987.tb01474.x. [DOI] [PubMed] [Google Scholar]
- 78.Spandidos DA, Anderson ML. A study of mechanisms of carcinogenesis by gene transfer of oncogenes into mammalian cells. Mutat Res. 1987;185:271–291. doi: 10.1016/0165-1110(87)90020-0. [DOI] [PubMed] [Google Scholar]
- 79.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 80.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 81.Fouad YA, Aanei C. Revisiting the hallmarks of cancer. Am J Cancer Res. 2017;7:1016. [PMC free article] [PubMed] [Google Scholar]
- 82.Spandidos DA, Anderson ML. A role of ras oncogenes in carcinogenesis and differentiation. Adv Exp Med Biol. 1990;265:127–131. doi: 10.1007/978-1-4757-5876-4_11. [DOI] [PubMed] [Google Scholar]
- 83.Gonos ES, Spandidos DA. Oncogenes in cellular immortalisation and differentiation (review) Anticancer Res. 1993;13:1117–1122. [PubMed] [Google Scholar]
- 84.Neagu M, Constantin C, Popescu ID, Zipeto D, Tzanakakis G, Nikitovic D, Fenga C, Stratakis CA, Spandidos DA, Tsatsakis AM. Inflammation and metabolism in cancer cell-mitochondria key player. Front Oncol. 2019;9:348. doi: 10.3389/fonc.2019.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pierouli K, Papakonstantinou E, Papageorgiou L, Diakou I, Mitsis T, Dragoumani K, Spandidos DA, Bacopoulou F, Chrousos GP, Goulielmos GN, et al. Long non-coding RNAs and microRNAs as regulators of stress in cancer (Review) Mol Med Rep. 2022;26:361. doi: 10.3892/mmr.2022.12878. [DOI] [PubMed] [Google Scholar]
- 86.Connerty P, Lock RB, De Bock CE. Long non-coding RNAs: Major regulators of cell stress in cancer. Front Oncol. 2020;10:285. doi: 10.3389/fonc.2020.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 88.Patidar A, Selvaraj S, Sarode A, Chauhan P, Chattopadhyay D, Saha B. DAMP-TLR-cytokine axis dictates the fate of tumor. Cytokine. 2018;104:114–123. doi: 10.1016/j.cyto.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 89.Melamed R, Rosenne E, Shakhar K, Schwartz Y, Abudarham N, Ben-Eliyahu S. Marginating pulmonary-NK activity and resistance to experimental tumor metastasis: Suppression by surgery and the prophylactic use of a beta-adrenergic antagonist and a prostaglandin synthesis inhibitor. Brain Behav Immun. 2005;19:114–126. doi: 10.1016/j.bbi.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 90.Melamed R, Rosenne E, Benish M, Goldfarb Y, Levi B, Ben-Eliyahu S. The marginating-pulmonary immune compartment in rats: Characteristics of continuous inflammation and activated NK cells. J Immunother. 2010;33:16–29. doi: 10.1097/CJI.0b013e3181b0b146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sorski L, Melamed R, Levi B, Matzner P, Lavon H, Rosenne E, Shaashua L, Ricon I, Sandbank E, Benbenishty A, Ben-Eliyahu S. Prevention of liver metastases through perioperative acute CpG-C immune stimulation. Cancer Immunol Immunother. 2020;69:2021–2031. doi: 10.1007/s00262-020-02596-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Strilic B, Offermanns S. Intravascular survival and extravasation of tumor cells. Cancer Cell. 2017;32:282–293. doi: 10.1016/j.ccell.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 93.Gonzalez H, Hagerling C, Werb Z. Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genes Dev. 2018;32:1267–1284. doi: 10.1101/gad.314617.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Neeman E, Ben-Eliyahu S. Surgery and stress promote cancer metastasis: New outlooks on perioperative mediating mechanisms and immune involvement. Brain Behav Immun. 2013;30(Suppl):S32–S40. doi: 10.1016/j.bbi.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Agorastos A, Nicolaides NC, Bozikas VP, Chrousos GP, Pervanidou P. Multilevel interactions of stress and circadian system: Implications for traumatic stress. Front Psychiatry. 2020;10:1003. doi: 10.3389/fpsyt.2019.01003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995;332:1351–1363. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- 97.Rosenne E, Shakhar G, Melamed R, Schwartz Y, Erdreich-Epstein A, Ben-Eliyahu S. Inducing a mode of NK-resistance to suppression by stress and surgery: A potential approach based on low dose of poly I-C to reduce postoperative cancer metastasis. Brain Behav Immun. 2007;21:395–408. doi: 10.1016/j.bbi.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ben-Eliyahu S, Shakhar G, Page GG, Stefanski V, Shakhar K. Suppression of NK cell activity and of resistance to metastasis by stress: A role for adrenal catecholamines and β-adrenoceptors. Neuroimmunomodulation. 2000;8:154–164. doi: 10.1159/000054276. [DOI] [PubMed] [Google Scholar]
- 99.Sloan EK, Priceman SJ, Cox BF, Yu S, Pimentel MA, Tangkanangnukul V, Arevalo JM, Morizono K, Karanikolas BD, Wu L, et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010;70:7042–7052. doi: 10.1158/0008-5472.CAN-10-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Du P, Zeng H, Xiao Y, Zhao Y, Zheng B, Deng Y, Liu J, Huang B, Zhang X, Yang K, et al. Chronic stress promotes EMT-mediated metastasis through activation of STAT3 signaling pathway by miR-337-3p in breast cancer. Cell Death Dis. 2020;11:761. doi: 10.1038/s41419-020-02981-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Madden KS, Szpunar MJ, Brown EB. Early impact of social isolation and breast tumor progression in mice. Brain Behav Immun. 2013;30(Suppl):S135–S141. doi: 10.1016/j.bbi.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cole SW, Sood AK. Molecular pathways: Beta-adrenergic signaling in cancer. Clin Cancer Res. 2012;18:1201–1206. doi: 10.1158/1078-0432.CCR-11-0641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Eng JWL, Kokolus KM, Reed CB, Hylander BL, Ma WW, Repasky EA. A nervous tumor microenvironment: The impact of adrenergic stress on cancer cells, immunosuppression, and immunotherapeutic response. Cancer Immunol Immunother. 2014;63:1115–1128. doi: 10.1007/s00262-014-1617-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cole SW, Nagaraja AS, Lutgendorf SK, Green PA, Sood AK. Sympathetic nervous system regulation of the tumour microenvironment. Nat Rev Cancer. 2015;15:563–572. doi: 10.1038/nrc3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Volden PA, Conzen SD. The influence of glucocorticoid signaling on tumor progression. Brain Behav Immun. 2013;30(Suppl):S26–S31. doi: 10.1016/j.bbi.2012.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Slominski RM, Raman C, Chen JY, Slominski AT. How cancer hijacks the Body's homeostasis through the neuroendocrine system. Trends Neurosci. 2023;46:263–275. doi: 10.1016/j.tins.2023.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rosch PJ. Stress and cancer: Disorders of communication, control, and civilization. In: Cooper CL, editor. Handbook of stress, medicine, and health. CRC Press/Routledge/Taylor and Francis Group; Oxfordshire: 1996. pp. 27–60. [Google Scholar]
- 108.Slawikowski GJ. Tumor development in adrenalectomized rats given inoculations of aged tumor cells after surgical stress. Cancer Res. 1960;20:316–320. [PubMed] [Google Scholar]
- 109.Helgesson Ö, Cabrera C, Lapidus L, Bengtsson C, Lissner L. Self-reported stress levels predict subsequent breast cancer in a cohort of Swedish women. Eur J Cancer Prev. 2003:377–381. doi: 10.1097/00008469-200310000-00006. [DOI] [PubMed] [Google Scholar]
- 110.Nielsen NR, Zhang ZF, Kristensen TS, Netterstr B, Schnohr P, Gr M. Self reported stress and risk of breast cancer: Prospective cohort study. BMJ. 2005;331:548. doi: 10.1136/bmj.38547.638183.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cui B, Peng F, Lu J, He B, Su Q, Luo H, Deng Z, Jiang T, Su K, Huang Y, et al. Cancer and stress: NextGen strategies. Brain Behav Immun. 2021;93:368–383. doi: 10.1016/j.bbi.2020.11.005. [DOI] [PubMed] [Google Scholar]
- 112.Yasuda MT, Sakakibara H, Shimoi K. Estrogen-and stress-induced DNA damage in breast cancer and chemoprevention with dietary flavonoid. Genes Environ. 2017;39:10. doi: 10.1186/s41021-016-0071-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jenkins FJ, Van Houten B, Bovbjerg DH. Effects on DNA damage and/or repair processes as biological mechanisms linking psychological stress to cancer risk. J Appl Biobehav Res. 2014;19:3–23. doi: 10.1111/jabr.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tomasetti C, Vogelstein B. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science. 2015;347:78–81. doi: 10.1126/science.1260825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chalkia AK, Spandidos DA, Detorakis ET. Viral involvement in the pathogenesis and clinical features of ophthalmic pterygium. Int J Mol Med. 2013;32:539–543. doi: 10.3892/ijmm.2013.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Filippakis H, Spandidos DA, Sourvinos G. Herpesviruses: Hijacking the Ras signaling pathway. Biochim Biophys Acta. 2010;1803:777–785. doi: 10.1016/j.bbamcr.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 117.Plummer M, de Martel C, Vignat J, Ferlay J, Bray F, Franceschi S. Global burden of cancers attributable to infections in 2012: A synthetic analysis. Lancet Glob Health. 2016;4:e609–e616. doi: 10.1016/S2214-109X(16)30143-7. [DOI] [PubMed] [Google Scholar]
- 118.de Martel C, Georges D, Bray F, Ferlay J, Clifford GM. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob Health. 2020;8:e180–e190. doi: 10.1016/S2214-109X(19)30488-7. [DOI] [PubMed] [Google Scholar]
- 119.Cacioppo JT, Kiecolt-Glaser JK, Malarkey WB, Laskowski BF, Rozlog LA, Poehlmann KM, Burleson MH, Glaser R. Autonomic and glucocorticoid associations with the steady-state expression of latent Epstein-Barr virus. Horm Behav. 2002;42:32–41. doi: 10.1006/hbeh.2002.1801. [DOI] [PubMed] [Google Scholar]
- 120.Glaser R, Friedman SB, Smyth J, Ader R, Bijur P, Brunell P, Cohen N, Krilov LR, Lifrak ST, Stone A, Toffler P. The differential impact of training stress and final examination stress on herpesvirus latency at the United States Military Academy at West Point. Brain Behav Immun. 1999;13:240–251. doi: 10.1006/brbi.1999.0566. [DOI] [PubMed] [Google Scholar]
- 121.Stamatiou DP, Derdas SP, Zoras OL, Spandidos DA. Herpes and polyoma family viruses in thyroid cancer. Oncol Lett. 2016;11:1635–1644. doi: 10.3892/ol.2016.4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mammas IN, Sourvinos G, Giannoudis A, Spandidos DA. Human papilloma virus (HPV) and host cellular interactions. Pathol Oncol Res. 2008;14:345–354. doi: 10.1007/s12253-008-9056-6. [DOI] [PubMed] [Google Scholar]
- 123.Antoni MH, Lutgendorf SK, Cole SW, Dhabhar FS, Sephton SE, McDonald PG, Stefanek M, Sood AK. The influence of bio-behavioural factors on tumour biology: Pathways and mechanisms. Nat Rev Cancer. 2006;6:240–248. doi: 10.1038/nrc1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nat Rev Immunol. 2011;11:625–632. doi: 10.1038/nri3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Collado-Hidalgo A, Sung C, Cole S. Adrenergic inhibition of innate anti-viral response: PKA blockade of Type I interferon gene transcription mediates catecholamine support for HIV-1 replication. Brain Behav Immun. 2006;20:552–563. doi: 10.1016/j.bbi.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 126.Fang CY, Miller SM, Bovbjerg DH, Bergman C, Edelson MI, Rosenblum NG, Bove BA, Godwin AK, Campbell DE, Douglas SD. Perceived stress is associated with impaired T-cell response to HPV16 in women with cervical dysplasia. Ann Behav Med. 2008;35:87–96. doi: 10.1007/s12160-007-9007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fischman HK, Pero RW, Kelly DD. Psychogenic stress induces chromosomal and DNA damage. Int J Neuroscience. 1996;84:219–227. doi: 10.3109/00207459608987267. [DOI] [PubMed] [Google Scholar]
- 128.Irie M, Asami S, Nagata S, Ikeda M, Miyata M, Kasai H. Psychosocial factors as a potential trigger of oxidative DNA damage in human leukocytes. Japanese J Cancer Res. 2001;92:367–376. doi: 10.1111/j.1349-7006.2001.tb01104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kiecolt-Glaser JK, Stephens RE, Lipetz PD, Speicher CE, Glaser R. Distress and DNA repair in human lymphocytes. J Behavioral Med. 1985;8:311–320. doi: 10.1007/BF00848366. [DOI] [PubMed] [Google Scholar]
- 130.Dimitroglou E, Zafiropoulou M, Messini-Nikolaki N, Doudounakis S, Tsilimigaki S, Piperakis SM. DNA damage in a human population affected by chronic psychogenic stress. Int J Hyg Environ Health. 2003;206:39–44. doi: 10.1078/1438-4639-00187. [DOI] [PubMed] [Google Scholar]
- 131.Flint MS, Baum A, Chambers WH, Jenkins FJ. Induction of DNA damage, alteration of DNA repair and transcriptional activation by stress hormones. Psychoneuroendocrinology. 2007;32:470–479. doi: 10.1016/j.psyneuen.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 132.Hara MR, Kovacs JJ, Whalen EJ, Rajagopal S, Strachan RT, Grant W, Towers AJ, Williams B, Lam CM, Xiao K, et al. A stress response pathway regulates DNA damage through β2-adrenoreceptors and β-arrestin-1. Nature. 2011;477:349–353. doi: 10.1038/nature10368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hara MR, Sachs BD, Caron MG, Lefkowitz RJ. Pharmacological blockade of a β2AR-β-arrestin-1 signaling cascade prevents the accumulation of DNA damage in a behavioral stress model. Cell Cycle. 2013;12:219–224. doi: 10.4161/cc.23368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Feng Z, Liu L, Zhang C, Zheng T, Wang J, Lin M, Zhao Y, Wang X, Levine AJ, Hu W. Chronic restraint stress attenuates p53 function and promotes tumorigenesis. Proc Natl Acad Sci USA. 2012;109:7013–7018. doi: 10.1073/pnas.1203930109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Magnon C, Hall SJ, Lin J, Xue X, Gerber L, Freedland SJ, Frenette PS. Autonomic nerve development contributes to prostate cancer progression. Science. 2013;341:1236361. doi: 10.1126/science.1236361. [DOI] [PubMed] [Google Scholar]
- 136.Zachos G, Varras M, Koffa M, Ergazaki M, Spandidos DA. The association of the H-ras oncogene and steroid hormone receptors in gynecological cancer. J Exp Ther Oncol. 1996;1:335–341. [PubMed] [Google Scholar]
- 137.Wrobel LJ, Le Gal FA. Inhibition of human melanoma growth by a non-cardioselective β-blocker. J Investigative Dermatol. 2015;135:525–531. doi: 10.1038/jid.2014.373. [DOI] [PubMed] [Google Scholar]
- 138.Armaiz-Pena GN, Allen JK, Cruz A, Stone RL, Nick AM, Lin YG, Han LY, Mangala LS, Villares GJ, Vivas-Mejia P, et al. Src activation by β-adrenoreceptors is a key switch for tumour metastasis. Nat Commun. 2013;4:1403. doi: 10.1038/ncomms2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shi M, Liu D, Duan H, Qian L, Wang L, Niu L, Zhang H, Yong Z, Gong Z, Song L, et al. The β2-adrenergic receptor and Her2 comprise a positive feedback loop in human breast cancer cells. Br Cancer Res Treat. 2011;125:351–362. doi: 10.1007/s10549-010-0822-2. [DOI] [PubMed] [Google Scholar]
- 140.Saul AN, Oberyszyn TM, Daugherty C, Kusewitt D, Jones S, Jewell S, Malarkey WB, Lehman A, Lemeshow S, Dhabhar FS. Chronic stress and susceptibility to skin cancer. J Natl Cancer Inst. 2005;97:1760–1767. doi: 10.1093/jnci/dji401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sumis A, Cook KL, Andrade FO, Hu R, Kidney E, Zhang X, Kim D, Carney E, Nguyen N, Yu W, et al. Social isolation induces autophagy in the mouse mammary gland: Link to increased mammary cancer risk. Endocr Relat Cancer. 2016;23:839–856. doi: 10.1530/ERC-16-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kokolus KM, Capitano ML, Lee CT, Eng JW, Waight JD, Hylander BL, Sexton S, Hong CC, Gordon CJ, Abrams SI, Repasky EA. Baseline tumor growth and immune control in laboratory mice are significantly influenced by subthermoneutral housing temperature. Proc Natl Acad Sci USA. 2013;110:20176–20181. doi: 10.1073/pnas.1304291110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Renz BW, Takahashi R, Tanaka T, Macchini M, Hayakawa Y, Dantes Z, Maurer HC, Chen X, Jiang Z, Westphalen CB, et al. β2 adrenergic-neurotrophin feedforward loop promotes pancreatic cancer. Cancer Cell. 2018;33:75–90.e7. doi: 10.1016/j.ccell.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hermes GL, Delgado B, Tretiakova M, Cavigelli SA, Krausz T, Conzen SD, McClintock MK. Social isolation dysregulates endocrine and behavioral stress while increasing malignant burden of spontaneous mammary tumors. Proc Natl Acad Sci USA. 2009;106:22393–22398. doi: 10.1073/pnas.0910753106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hasen NS, O'Leary KA, Auger AP, Schuler LA. Social isolation reduces mammary development, tumor incidence, and expression of epigenetic regulators in wild-type and p53-heterozygotic mice. Cancer Prev Res. 2010;3:620–629. doi: 10.1158/1940-6207.CAPR-09-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Riley V. Psychoneuroendocrine influences on immunocompetence and neoplasia. Science. 1981;212:1100–1109. doi: 10.1126/science.7233204. [DOI] [PubMed] [Google Scholar]
- 147.Leonardi GC, Candido S, Cervello M, Nicolosi D, Raiti F, Travali S, Spandidos DA, Libra M. The tumor microenvironment in hepatocellular carcinoma (review) Int J Oncol. 2012;40:1733–1747. doi: 10.3892/ijo.2012.1408. [DOI] [PubMed] [Google Scholar]
- 148.Wu T, Dai Y. Tumor microenvironment and therapeutic response. Cancer Lett. 2017;387:61–68. doi: 10.1016/j.canlet.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 149.Gysler SM, Drapkin R. Tumor innervation: Peripheral nerves take control of the tumor microenvironment. J Clin Invest. 2021;131:e147276. doi: 10.1172/JCI147276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Monje M, Borniger JC, D'Silva NJ, Deneen B, Dirks PB, Fattahi F, Frenette PS, Garzia L, Gutmann DH, Hanahan D, et al. Roadmap for the emerging field of cancer neuroscience. Cell. 2020;181:219–222. doi: 10.1016/j.cell.2020.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yang EV, Kim SJ, Donovan EL, Chen M, Gross AC, Webster Marketon JI, Barsky SH, Glaser R. Norepinephrine upregulates VEGF, IL-8, and IL-6 expression in human melanoma tumor cell lines: Implications for stress-related enhancement of tumor progression. Brain Behav Immun. 2009;23:267–275. doi: 10.1016/j.bbi.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Moretti S, Massi D, Farini V, Baroni G, Parri M, Innocenti S, Cecchi R, Chiarugi P. β-adrenoceptors are upregulated in human melanoma and their activation releases pro-tumorigenic cytokines and metalloproteases in melanoma cell lines. Lab Invest. 2013;93:279–290. doi: 10.1038/labinvest.2012.175. [DOI] [PubMed] [Google Scholar]
- 153.Madden KS, Szpunar MJ, Brown EB. β-Adrenergic receptors (β-AR) regulate VEGF and IL-6 production by divergent pathways in high β-AR-expressing breast cancer cell lines. Br Cancer Res Treat. 2011;130:747–758. doi: 10.1007/s10549-011-1348-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lutgendorf SK, Cole S, Costanzo E, Bradley S, Coffin J, Jabbari S, Rainwater K, Ritchie JM, Yang M, Sood AK. Stress-related mediators stimulate vascular endothelial growth factor secretion by two ovarian cancer cell lines. Clin Cancer Res. 2003;9:4514–4521. [PubMed] [Google Scholar]
- 155.Chen H, Liu D, Yang Z, Sun L, Deng Q, Yang S, Qian L, Guo L, Yu M, Hu M, et al. Adrenergic signaling promotes angiogenesis through endothelial cell-tumor cell crosstalk. Endocr Relat Cancer. 2014;21:783–795. doi: 10.1530/ERC-14-0236. [DOI] [PubMed] [Google Scholar]
- 156.Medeiros PJ, Jackson DN. Neuropeptide Y Y5-receptor activation on breast cancer cells acts as a paracrine system that stimulates VEGF expression and secretion to promote angiogenesis. Peptides. 2013;48:106–113. doi: 10.1016/j.peptides.2013.07.029. [DOI] [PubMed] [Google Scholar]
- 157.Shan T, Ma J, Ma Q, Guo K, Guo J, Li X, Li W, Liu J, Huang C, Wang F, Wu E. β2-AR-HIF-1α: A novel regulatory axis for stress-induced pancreatic tumor growth and angiogenesis. Curr Mol Med. 2013;13:1023–1034. doi: 10.2174/15665240113139990055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Xu P, He H, Gu Y, Wang Y, Sun Z, Yang L, Miao C. Surgical trauma contributes to progression of colon cancer by downregulating CXCL4 and recruiting MDSCs. Exp Cell Res. 2018;370:692–698. doi: 10.1016/j.yexcr.2018.07.035. [DOI] [PubMed] [Google Scholar]
- 159.Budiu RA, Vlad AM, Nazario L, Bathula C, Cooper KL, Edmed J, Thaker PH, Urban J, Kalinski P, Lee AV, et al. Restraint and social isolation stressors differentially regulate adaptive immunity and tumor angiogenesis in a breast cancer mouse model. Cancer Clin Oncol. 2017;6:12–24. doi: 10.5539/cco.v6n1p12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hulsurkar M, Li Z, Zhang Y, Li X, Zheng D, Li W. Beta-adrenergic signaling promotes tumor angiogenesis and prostate cancer progression through HDAC2-mediated suppression of thrombospondin-1. Oncogene. 2017;36:1525–1536. doi: 10.1038/onc.2016.319. [DOI] [PubMed] [Google Scholar]
- 161.Le CP, Nowell CJ, Kim-Fuchs C, Botteri E, Hiller JG, Ismail H, Pimentel MA, Chai MG, Karnezis T, Rotmensz N, et al. Chronic stress in mice remodels lymph vasculature to promote tumour cell dissemination. Nat Commun. 2016;7:10634. doi: 10.1038/ncomms10634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Colon-Echevarria CB, Lamboy-Caraballo R, Aquino-Acevedo AN, Armaiz-Pena GN. Neuroendocrine regulation of tumor-associated immune cells. Front Oncol. 2019;9:1077. doi: 10.3389/fonc.2019.01077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Qiao G, Chen M, Bucsek MJ, Repasky EA, Hylander BL. Adrenergic signaling: A targetable checkpoint limiting development of the antitumor immune response. Front Immunol. 2018;9:164. doi: 10.3389/fimmu.2018.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Huan HB, Wen XD, Chen XJ, Wu L, Wu LL, Zhang L, Yang DP, Zhang X, Bie P, Qian C, Xia F. Sympathetic nervous system promotes hepatocarcinogenesis by modulating inflammation through activation of alpha1-adrenergic receptors of Kupffer cells. Brain Behav Immun. 2017;59:118–134. doi: 10.1016/j.bbi.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 166.Nagaraja AS, Dorniak PL, Sadaoui NC, Kang Y, Lin T, Armaiz-Pena G, Wu SY, Rupaimoole R, Allen JK, Gharpure KM, et al. Sustained adrenergic signaling leads to increased metastasis in ovarian cancer via increased PGE2 synthesis. Oncogene. 2016;35:2390–2397. doi: 10.1038/onc.2015.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Qin JF, Jin FJ, Li N, Guan HT, Lan L, Ni H, Wang Y. Adrenergic receptor β2 activation by stress promotes breast cancer progression through macrophages M2 polarization in tumor microenvironment. BMB Rep. 2015;48:295. doi: 10.5483/BMBRep.2015.48.5.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Armaiz-Pena GN, Gonzalez-Villasana V, Nagaraja AS, Rodriguez-Aguayo C, Sadaoui NC, Stone RL, Matsuo K, Dalton HJ, Previs RA, Jennings NB, et al. Adrenergic regulation of monocyte chemotactic protein 1 leads to enhanced macrophage recruitment and ovarian carcinoma growth. Oncotarget. 2015;6:4266–4273. doi: 10.18632/oncotarget.2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lamkin DM, Ho H-Y, Ong TH, Kawanishi CK, Stoffers VL, Ahlawat N, Ma JCY, Arevalo JMG, Cole SW, Sloan EK. β-Adrenergic-stimulated macrophages: Comprehensive localization in the M1-M2 spectrum. Brain Behav Immun. 2016;57:338–346. doi: 10.1016/j.bbi.2016.07.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bower JE, Shiao SL, Sullivan P, Lamkin DM, Atienza R, Mercado F, Arevalo J, Asher A, Ganz PA, Cole SW. Prometastatic molecular profiles in breast tumors from socially isolated women. JNCI Cancer Spectr. 2018;2:pky029. doi: 10.1093/jncics/pky029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Shakhar G, Ben-Eliyahu S. In vivo β-adrenergic stimulation suppresses natural killer activity and compromises resistance to tumor metastasis in rats. J Immunol. 1998;160:3251–3258. [PubMed] [Google Scholar]
- 172.Rosenne E, Sorski L, Shaashua L, Neeman E, Matzner P, Levi B, Ben-Eliyahu S. In vivo suppression of NK cell cytotoxicity by stress and surgery: Glucocorticoids have a minor role compared to catecholamines and prostaglandins. Brain Behav Immun. 2014;37:207–219. doi: 10.1016/j.bbi.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lutgendorf SK, Sood AK, Anderson B, McGinn S, Maiseri H, Dao M, Sorosky JI, De Geest K, Ritchie J, Lubaroff DM. Social support, psychological distress, and natural killer cell activity in ovarian cancer. J Clin Oncol. 2005;23:7105–7113. doi: 10.1200/JCO.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 174.Hou N, Zhang X, Zhao L, Zhao X, Li Z, Song T, Huang C. A novel chronic stress-induced shift in the Th1 to Th2 response promotes colon cancer growth. Biochem Biophys Res Commun. 2013;439:471–476. doi: 10.1016/j.bbrc.2013.08.101. [DOI] [PubMed] [Google Scholar]
- 175.Bucsek MJ, Qiao G, MacDonald CR, Giridharan T, Evans L, Niedzwecki B, Liu H, Kokolus KM, Eng JW, Messmer MN, et al. β-Adrenergic signaling in mice housed at standard temperatures suppresses an effector phenotype in CD8+ T cells and undermines checkpoint inhibitor therapy. Cancer Res. 2017;77:5639–5651. doi: 10.1158/0008-5472.CAN-17-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lutgendorf SK, Lamkin DM, DeGeest K, Anderson B, Dao M, McGinn S, Zimmerman B, Maiseri H, Sood AK, Lubaroff DM. Depressed and anxious mood and T-cell cytokine expressing populations in ovarian cancer patients. Brain Behav Immun. 2008;22:890–900. doi: 10.1016/j.bbi.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mohammadpour H, MacDonald CR, Qiao G, Chen M, Dong B, Hylander BL, McCarthy PL, Abrams SI, Repasky EA. β2 adrenergic receptor-mediated signaling regulates the immunosuppressive potential of myeloid-derived suppressor cells. J Clin Invest. 2019;129:5537–5552. doi: 10.1172/JCI129502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mundy-Bosse BL, Thornton LM, Yang HC, Andersen BL, Carson WE. Psychological stress is associated with altered levels of myeloid-derived suppressor cells in breast cancer patients. Cell Immunol. 2011;270:80–87. doi: 10.1016/j.cellimm.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Nguyen KD, Qiu Y, Cui X, Goh YP, Mwangi J, David T, Mukundan L, Brombacher F, Locksley RM, Chawla A. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–108. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Flierl MA, Rittirsch D, Nadeau BA, Chen AJ, Sarma JV, Zetoune FS, McGuire SR, List RP, Day DE, Hoesel LM, et al. Phagocyte-derived catecholamines enhance acute inflammatory injury. Nature. 2007;449:721–725. doi: 10.1038/nature06185. [DOI] [PubMed] [Google Scholar]
- 181.Wong HPS, Yu L, Lam EKY, Tai EKK, Wu WKK, Cho CH. Nicotine promotes cell proliferation via α7-nicotinic acetylcholine receptor and catecholamine-synthesizing enzymes-mediated pathway in human colon adenocarcinoma HT-29 cells. Toxicol Appl Pharmacol. 2007;221:261–267. doi: 10.1016/j.taap.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 182.Amaro F, Silva D, Reguengo H, Oliveira JC, Quintas C, Vale N, Gonçalves J, Fresco P. β-adrenoceptor activation in breast MCF-10A cells induces a pattern of catecholamine production similar to that of tumorigenic MCF-7 cells. Int J Mol Sci. 2020;21:7968. doi: 10.3390/ijms21217968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zhang X, Zhang Y, He Z, Yin K, Li B, Zhang L, Xu Z. Chronic stress promotes gastric cancer progression and metastasis: An essential role for ADRB2. Cell Death Dis. 2019;10:788. doi: 10.1038/s41419-019-2030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhi X, Li B, Li Z, Zhang J, Yu J, Zhang L, Xu Z. Adrenergic modulation of AMPK-dependent autophagy by chronic stress enhances cell proliferation and survival in gastric cancer. Int J Oncol. 2019;54:1625–1638. doi: 10.3892/ijo.2019.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wong HP, Ho JW, Koo MW, Yu L, Wu WK, Lam EK, Tai EK, Ko JK, Shin VY, Chu KM, Cho CH. Effects of adrenaline in human colon adenocarcinoma HT-29 cells. Life Sci. 2011;88:1108–1112. doi: 10.1016/j.lfs.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 186.Sood AK, Armaiz-Pena GN, Halder J, Nick AM, Stone RL, Hu W, Carroll AR, Spannuth WA, Deavers MT, Allen JK, et al. Adrenergic modulation of focal adhesion kinase protects human ovarian cancer cells from anoikis. J Clin Invest. 2010;120:1515–1523. doi: 10.1172/JCI40802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Liu H, Wang C, Xie N, Zhuang Z, Liu X, Hou J, Huang H. Activation of adrenergic receptor β2 promotes tumor progression and epithelial mesenchymal transition in tongue squamous cell carcinoma. Int J Mol Med. 2018;41:147–154. doi: 10.3892/ijmm.2017.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kim-Fuchs C, Le CP, Pimentel MA, Shackleford D, Ferrari D, Angst E, Hollande F, Sloan EK. Chronic stress accelerates pancreatic cancer growth and invasion: A critical role for beta-adrenergic signaling in the pancreatic microenvironment. Brain Behav Immun. 2014;40:40–47. doi: 10.1016/j.bbi.2014.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Pu J, Zhang X, Luo H, Xu L, Lu X, Lu J. Adrenaline promotes epithelial-to-mesenchymal transition via HuR-TGFβ regulatory axis in pancreatic cancer cells and the implication in cancer prognosis. Biochem Biophys Res Commun. 2017;493:1273–1279. doi: 10.1016/j.bbrc.2017.09.146. [DOI] [PubMed] [Google Scholar]
- 190.Liu J, Qu L, Wan C, Xiao M, Ni W, Jiang F, Fan Y, Lu C, Ni R. A novel β2-AR/YB-1/β-catenin axis mediates chronic stress-associated metastasis in hepatocellular carcinoma. Oncogenesis. 2020;9:84. doi: 10.1038/s41389-020-00268-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Chen H, Liu D, Guo L, Cheng X, Guo N, Shi M. Chronic psychological stress promotes lung metastatic colonization of circulating breast cancer cells by decorating a pre-metastatic niche through activating β-adrenergic signaling. J Pathol. 2018;244:49–60. doi: 10.1002/path.4988. [DOI] [PubMed] [Google Scholar]
- 192.Lamkin DM, Sloan EK, Patel AJ, Chiang BS, Pimentel MA, Ma JC, Arevalo JM, Morizono K, Cole SW. Chronic stress enhances progression of acute lymphoblastic leukemia via β-adrenergic signaling. Brain Behav Immun. 2012;26:635–641. doi: 10.1016/j.bbi.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Chang A, Le CP, Walker AK, Creed SJ, Pon CK, Albold S, Carroll D, Halls ML, Lane JR, Riedel B, et al. β2-Adrenoceptors on tumor cells play a critical role in stress-enhanced metastasis in a mouse model of breast cancer. Brain Behav Immun. 2016;57:106–115. doi: 10.1016/j.bbi.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zahalka AH, Frenette PS. Nerves in cancer. Nat Rev Cancer. 2020;20:143–157. doi: 10.1038/s41568-019-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lutgendorf SK, DeGeest K, Dahmoush L, Farley D, Penedo F, Bender D, Goodheart M, Buekers TE, Mendez L, Krueger G, et al. Social isolation is associated with elevated tumor norepinephrine in ovarian carcinoma patients. Brain Behav Immun. 2011;25:250–255. doi: 10.1016/j.bbi.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Schuller HM, Cole B. Regulation of cell proliferation by β-adrenergjc receptors in a human lung adenocarcinoma cell line. Carcinogenesis. 1989;10:1753–1755. doi: 10.1093/carcin/10.9.1753. [DOI] [PubMed] [Google Scholar]
- 197.Huang XY, Wang HC, Yuan Z, Huang J, Zheng Q. Norepinephrine stimulates pancreatic cancer cell proliferation, migration and invasion via β-adrenergic receptor-dependent activation of P38/MAPK pathway. Hepatogastroenterology. 2012;59:889–893. doi: 10.5754/hge11476. [DOI] [PubMed] [Google Scholar]
- 198.Lackovicova L, Banovska L, Bundzikova J, Janega P, Bizik J, Kiss A, Mravec B. Chemical sympathectomy suppresses fibrosarcoma development and improves survival of tumor-bearing rats. Neoplasma. 2011;58:424–429. doi: 10.4149/neo_2011_05_424. [DOI] [PubMed] [Google Scholar]
- 199.Simon WE, Albrecht M, Trams G, Dietel M, Hölzel F. In vitro growth promotion of human mammary carcinoma cells by steroid hormones, tamoxifen, and prolactin. J Natl Cancer Inst. 1984;73:313–321. doi: 10.1093/jnci/73.2.313. [DOI] [PubMed] [Google Scholar]
- 200.Zhao XY, Malloy PJ, Krishnan AV, Swami S, Navone NM, Peehl DM, Feldman D. Glucocorticoids can promote androgen-independent growth of prostate cancer cells through a mutated androgen receptor. Nat Med. 2000;6:703–706. doi: 10.1038/76287. [DOI] [PubMed] [Google Scholar]
- 201.Horvathova L, Padova A, Tillinger A, Osacka J, Bizik J, Mravec B. Sympathectomy reduces tumor weight and affects expression of tumor-related genes in melanoma tissue in the mouse. Stress. 2016;19:528–534. doi: 10.1080/10253890.2016.1213808. [DOI] [PubMed] [Google Scholar]
- 202.Chin CC, Li JM, Lee KF, Huang YC, Wang KC, Lai HC, Cheng CC, Kuo YH, Shi CS. Selective β2-AR blockage suppresses colorectal cancer growth through regulation of EGFR-Akt/ERK1/2 signaling, G1-phase arrest, and apoptosis. J Cell Physiol. 2016;231:459–472. doi: 10.1002/jcp.25092. [DOI] [PubMed] [Google Scholar]
- 203.Masur K, Niggemann B, Zanker KS, Entschladen F. Norepinephrine-induced migration of SW 480 colon carcinoma cells is inhibited by β-blockers. Cancer Res. 2001;61:2866–2869. [PubMed] [Google Scholar]
- 204.Yang EV, Sood AK, Chen M, Li Y, Eubank TD, Marsh CB, Jewell S, Flavahan NA, Morrison C, Yeh PE, et al. Norepinephrine up-regulates the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, and MMP-9 in nasopharyngeal carcinoma tumor cells. Cancer Res. 2006;66:10357–10364. doi: 10.1158/0008-5472.CAN-06-2496. [DOI] [PubMed] [Google Scholar]
- 205.Sood AK, Bhatty R, Kamat AA, Landen CN, Han L, Thaker PH, Li Y, Gershenson DM, Lutgendorf S, Cole SW. Stress hormone-mediated invasion of ovarian cancer cells. Clin Cancer Res. 2006;12:369–375. doi: 10.1158/1078-0432.CCR-05-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lutgendorf SK, Lamkin DM, Jennings NB, Arevalo JM, Penedo F, DeGeest K, Langley RR, Lucci JA, III, Cole SW, Lubaroff DM, Sood AK. Biobehavioral influences on matrix metalloproteinase expression in ovarian carcinoma. Clin Cancer Res. 2008;14:6839–6846. doi: 10.1158/1078-0432.CCR-08-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Campbell JP, Karolak MR, Ma Y, Per rien DS, Masood-Campbell SK, Penner NL, Munoz SA, Zijlstra A, Yang X, Sterling JA, Elefteriou F. Stimulation of host bone marrow stromal cells by sympathetic nerves promotes breast cancer bone metastasis in mice. PLoS Biol. 2012;10:e1001363. doi: 10.1371/journal.pbio.1001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Simpson CD, Anyiwe K, Schimmer AD. Anoikis resistance and tumor metastasis. Cancer Lett. 2008;272:177–185. doi: 10.1016/j.canlet.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 209.Benish M, Bartal I, Goldfarb Y, Levi B, Avraham R, Raz A, Ben-Eliyahu S. Perioperative use of beta-blockers and COX-2 inhibitors may improve immune competence and reduce the risk of tumor metastasis. Ann Surg Oncol. 2008;15:2042–2052. doi: 10.1245/s10434-008-9890-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kaira K, Kamiyoshihara M, Kawashima O, Endoh H, Imaizumi K, Sugano M, Tanaka S, Fujita A, Kogure Y, Shimizu A, et al. Prognostic impact of β2 adrenergic receptor expression in surgically resected pulmonary pleomorphic carcinoma. Anticancer Res. 2019;39:395–403. doi: 10.21873/anticanres.13125. [DOI] [PubMed] [Google Scholar]
- 211.Choy C, Raytis JL, Smith DD, Duenas M, Neman J, Jandial R, Lew MW. Inhibition of β2-adrenergic receptor reduces triple-negative breast cancer brain metastases: The potential benefit of perioperative β-blockade. Oncol Rep. 2016;35:3135–3142. doi: 10.3892/or.2016.4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Al-Niaimi A, Dickson EL, Albertin C, Karnowski J, Niemi C, Spencer R, Shahzad MM, Uppal S, Saha S, Rice L, Nally AM. The impact of perioperative β blocker use on patient outcomes after primary cytoreductive surgery in high-grade epithelial ovarian carcinoma. Gynecol Oncol. 2016;143:521–525. doi: 10.1016/j.ygyno.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 213.Barron TI, Connolly RM, Sharp L, Bennett K, Visvanathan K. Beta blockers and breast cancer mortality: A population-based study. J Clin Oncol. 2011;29:2635–2644. doi: 10.1200/JCO.2010.33.5422. [DOI] [PubMed] [Google Scholar]
- 214.Lemeshow S, Sørensen HT, Phillips G, Yang EV, Antonsen S, Riis AH, Lesinski GB, Jackson R, Glaser R. β-Blockers and survival among Danish patients with malignant melanoma: A population-based cohort study. Cancer Epidemiol Biomarkers Prev. 2011;20:2273–2279. doi: 10.1158/1055-9965.EPI-11-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Cata JP, Villarreal J, Keerty D, Thakar DR, Liu DD, Sood AK, Gottumukkala V. Perioperative beta-blocker use and survival in lung cancer patients. J Clin Anesth. 2014;26:106–117. doi: 10.1016/j.jclinane.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 216.Heitz F, Hengsbach A, Harter P, Traut A, Ataseven B, Schneider S, Prader S, Kurzeder C, Sporkmann M, du Bois A. Intake of selective beta blockers has no impact on survival in patients with epithelial ovarian cancer. Gynecol Oncol. 2017;144:181–186. doi: 10.1016/j.ygyno.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 217.Kim TH, Gill NK, Nyberg KD, Nguyen AV, Hohlbauch SV, Geisse NA, Nowell CJ, Sloan EK, Rowat AC. Cancer cells become less deformable and more invasive with activation of β-adrenergic signaling. J Cell Sci. 2016;129:4563–4575. doi: 10.1242/jcs.194803. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.