Abstract
BACKGROUND:
Chronic subdural hematoma (CSDH) is an increasingly prevalent disease in the aging population. Patients with CSDH frequently suffer from concurrent vascular disease or develop secondary thrombotic complications requiring antithrombotic treatment.
OBJECTIVE:
To determine the safety and impact of early reinitiation of antithrombotics after middle meningeal artery embolization for chronic subdural hematoma.
METHODS:
This is a single-institution, retrospective study of patients who underwent middle meningeal artery (MMA) embolizations for CSDH. Patient with or without antithrombotic initiation within 5 days postembolization were compared. Primary outcome was the rate of recurrence within 60 days. Secondary outcomes included rate of reoperation, reduction in CSDH thickness, and midline shift.
RESULTS:
Fifty-seven patients met inclusion criteria. The median age was 66 years (IQR 58-76) with 21.1% females. Sixty-six embolizations were performed. The median length to follow-up was 20 days (IQR 14-44). Nineteen patients (33.3%) had rapid reinitiation of antithrombotics (5 antiplatelet, 11 anticoagulation, and 3 both). Baseline characteristics between the no antithrombotic (no-AT) and the AT groups were similar. The recurrence rate was higher in the AT group (no-AT vs AT, 9.3 vs 30.4%, P = .03). Mean absolute reduction in CSDH thickness and midline shift was similar between groups. Rate of reoperation did not differ (4.7 vs 8.7%, P = .61).
CONCLUSION:
Rapid reinitiation of AT after MMA embolization for CSDH leads to higher rates of recurrence with similar rates of reoperation. Care must be taken when initiating antithrombotics after treatment of CSDH with MMA embolization.
KEY WORDS: Subdural hemorrhage, Anticoagulation, Embolization, Endovascular therapy
ABBREVIATIONS:
- AC
anticoagulant
- AP
antiplatelet
- AT
antithrombotic
- CSDH
chronic subdural hematoma
- DOAC
direct oral anticoagulant
- INR
international normalized ratio
- MMA
middle meningeal artery
- PTT
partial thromboplastin time
- SDH
subdural hematoma.
Chronic subdural hematomas (CSDHs) are prevalent, with a rising frequency in an aging population.1 In addition, concurrent increase in vascular disease within an elderly population will augment the rates of coexisting antithrombotic use. Moreover, in this neurologically ill population, the rate of venous thromboembolism requiring early anticoagulation is ∼7%.2 However, given the evidence of increased risk of recurrence with antithrombotic use, cessation or abstention remains a mainstay in the treatment of CSDH.3,4 Middle meningeal artery (MMA) embolization has emerged as a promising alternative or adjunct to surgical evacuation, with an absolute risk reduction for recurrence of approximately 16.7%.5 Although MMA embolization is frequently quoted as a potentially effective and preferred treatment in patients requiring rapid initiation or continuation of antithrombotics, data to support this conjecture are lacking. Thus, we sought to understand the relative efficacy of MMA embolization in patients who require initiation of antithrombotics in close proximity to MMA embolization.
METHODS
Study Design
This is a single-center, retrospective cohort study performed at a Level 1 Academic Trauma Center from January 2018 to December 2021. The study was approved by the local Institutional Review Board (IRB approval #HSC-MS-21-0792), which determined that informed consent was not required because of the retrospective study design and lack of patient interaction or additional visits or imaging. Inclusion criterion was defined as all consecutive patients who underwent MMA embolization for CSDH at our institution during the above specified period. Decisions for embolization as either definitive treatment or adjunctive treatment (ie, after surgical evacuation) was at the discretion of the treating physician at the time of embolization. Liquid embolic agents were used in an off-label but well-supported fashion. Rapid antithrombotic (AT) initiation was defined as the start of any antiplatelet (AP) or anticoagulant (AC) agent (at therapeutic doses, not prophylactic) within 5 days after the embolization procedure.
Outcome Measures
Primary outcome was defined as the incidence of radiographic hematoma recurrence within 60 days after embolization. The 60-day time frame was determined given that our local practice is to follow up CSDH closely within the 2 month time frame and given the lack of consensus on appropriate follow-up as can be seen in the ongoing MMA embolization trials. Recurrence was defined as any increase in CSDH thickness after hematoma evacuation and embolization or progression of CSDH thickness after embolization alone within 60 days. Secondary outcomes included incidence of reoperation, reduction in CSDH size, and reduction in midline shift at follow-up. Sensitivity analysis was performed to uncover the influence of AP or AC use individually.
Imaging Analysis
CSDH was diagnosed based on admission computed tomography (CT) scan demonstrating mixed density and/or hypodense subdural collections and verified by radiology reporting. CSDH thickness was measured as the longest distance between the central part of the crescent-shaped subdural and the perpendicular inner table of the skull on axial CT imaging using scale bars. Midline shift was measured as lateral displacement of the septum pellucidum, in millimeters, at the level of the foramen of Monro on axial CT imaging in a similar fashion. Presence of septations was defined as any CSDH with hyperdense “streaking” within the lesion. Radiographic measurement was performed by neuroendovascular physicians.
Statistical Analysis
Baseline characteristics and clinical outcomes were compared between AT and no-AT groups using the Student t test for continuous variables and the Fisher exact test for categorical variables. Baseline characteristics were performed on a per-patient basis. All outcome data analysis was performed on a per-subdural basis. Survival curves for recurrence of radiographic hematoma were plotted using the Kaplan-Meier approach stratified by treatment groups, and differences in curves were assessed using the log-rank test. No imputation was performed for missing data points. Significance levels were set at P < .05 for 2-tailed tests, and all analyses were performed using STATA 16.0 (StataCorp).
RESULTS
After screening, 57 patients who underwent 66 embolizations (9 bilateral) met inclusion criteria. The median age was 66 years (IQR 58-76) and 21.1% were females. The mean subdural hematoma (SDH) thickness was 13.2 mm, and the mean midline shift was 3.2 mm. Forty-one subdurals of the 66 (62%) in 34 patients underwent pre-embolization evacuation (30 burr holes and 11 craniotomies). Embolizations were performed with coils (73%), particles (70%) and liquid embolics (27%). The median time to last follow-up CT was 20 days (IQR 14-44).
Of the cohort, 19/57 patients (33.33%) had rapid reinitiation of antithrombotics (5 AP, 11 AC, and 3 both). Baseline characteristics between the groups were similar except for higher premorbid use of warfarin and heparin in the AC group (Table 1). There was a total of 23 SDHs in the 19 patients with rapid reinitiation (5 in AP, 14 in AC, and 4 in group of both). Among the AC cohort (18/23), the agents used postembolization were heparin (52.2%), warfarin (34.8%), and direct oral anticoagulants (DOACs) (4.4%). The mean time to initiation was 1 day postembolization. Most common indications for rapid AC initiation after embolization included mechanical valves (35.7%) and atrial fibrillation (28.6%; Supplemental Table 1, http://links.lww.com/NEU/D446). Among the AP group (9/23), aspirin was the only antiplatelet agent started within a prespecified time frame of 5 days (39.1%). The mean time to initiation was 2 days postembolization. A single patient was started on aspirin and then on clopidogrel in a delayed time frame of 21 days. Most common indications for AP initiation after embolization included history of stroke/TIA (50%) and CAD/stents (37.5%; Supplemental Table 2, http://links.lww.com/NEU/D446). There were 3 patients with overlapping aspirin and anticoagulant use. Collectively, the mean antithrombotic initiation was 1 day (IQR 0-2).
TABLE 1.
Baseline Characteristics by Treatment Group
| Variable | Total (n = 57) | No-AT (n = 38) | AT (n = 19) | P value |
|---|---|---|---|---|
| Age, y, median (IQR) | 66 (58-76) | 66 (58-76) | 70 (58-76) | .89 |
| Female sex, n (%) | 12 (21.1) | 6 (15.8) | 6 (31.6) | .19 |
| Bilateral lesions, n (%) | 9 (15.8) | 5 (13.2) | 4 (21.1) | .46 |
| Evacuation, n (%) | ||||
| Burr holes | 23 (40.4) | 18 (47.4) | 5 (26.3) | .16 |
| Craniotomy | 11 (19.3) | 6 (15.8) | 5 (26.3) | .48 |
| Embolization material, n (%) | ||||
| Particles | 42 (73.7) | 29 (76.3) | 13 (68.4) | .54 |
| Coils | 44 (77.2) | 31 (81.6) | 13 (68.4) | .32 |
| Onyx | 13 (22.8) | 7 (18.4) | 6 (31.6) | .32 |
| Pre-antiplatelet agents, n (%) | 15 (26.3) | 7 (18.4) | 8 (42.1) | .11 |
| Aspirin | 15 (26.3) | 7 (18.4) | 8 (42.1) | .11 |
| Clopidogrel | 2 (3.5) | 1 (2.6) | 1 (5.3) | 1 |
| Pre-anticoagulants, n (%) | 13 (22.8) | 2 (5.3) | 11 (57.9) | <.001 |
| Heparin | 3 (5.3) | 0 (0.0) | 3 (15.8) | .033 |
| Warfarin | 9 (15.8) | 1 (2.6) | 8 (42.1) | <.001 |
| DOAC | 3 (5.3) | 1 (2.6) | 2 (10.5) | .26 |
| Post-antiplatelet agents, n (%) | 8 (14.0) | 8 (42.1) | ||
| Aspirin | 8 (14.0) | 8 (42.1) | ||
| Clopidogrel | 1 (1.8) | 1 (5.3) | ||
| Postoperative start, d, median (IQR) | 2 (.5-2.5) | 2 (.5-2.5) | ||
| Post-anticoagulant, n (%) | 14 (24.6) | 14 (73.7) | ||
| Heparina | 10 (17.5) | 10 (52.6) | ||
| PTT, s, median (range) | 59.6 (24-175) | |||
| Warfarina | 6 (10.5) | 6 (31.6) | ||
| INR, median (range) | 2.0 (1.5-2.8) | |||
| DOAC | 1 (1.8) | 1 (5.3) | ||
| Postoperative start, d, median (IQR) | 1 (0-2) | 1 (0-2) |
ATs, antithrombotics; DOAC, direct oral anticoagulant; INR, international normalized ratio; PTT, partial thromboplastin time.
Four cases were on heparin bridge to warfarin. Sample size refers to per patient and not per subdural hematoma.
Data were analyzed by the Student t test for continuous variables or the Fisher exact test for categorical variables.
Incidence of recurrence in the complete cohort was 16.7%. Patients who were rapidly started on AT had significantly higher rates of recurrence (9.3% vs 30.4%, P = .04; Table 2). Kaplan-Meier analysis with an end point of CSDH recurrence demonstrates significant difference between survival curves of groups (P = .023, log-rank test; Figure). However, the rate of reoperation did not differ between groups (no-AT vs AT, 4.7% vs 8.7%, P = .61). Subgroup analysis for recurrence rate individually in AP (9.3% vs 44.4%, P = .02; Supplemental Table 3, http://links.lww.com/NEU/D446) and AC (9.3% vs 33.3%, P = .05; Supplemental Table 4, http://links.lww.com/NEU/D446) populations continued to demonstrate a higher rate of recurrence with rapid initiation of either medication class. Reoperation rate was not different in either groups (Supplemental Tables 3 and 4, http://links.lww.com/NEU/D446). Indication for reoperation was symptomatic recurrence in all cases.
TABLE 2.
Lesion Characteristics and Outcomes by Treatment Group
| Variable | Total (n = 66) | No-AT (n = 43) | AT (n = 23) | P value |
|---|---|---|---|---|
| Septate, n (%) | 45 (68.2) | 31 (72.1) | 14 (60.9) | .41 |
| SDH thickness, mm, mean (SD) | 13.2 (5.3) | 13.9 (5.5) | 11.9 (5.0) | .15 |
| Follow-up SDH thickness, mm, mean (SD) | 8.6 (5.5) | 8.4 (5.1) | 8.9 (6.2) | .74 |
| Reduction in SDH thickness, mm, mean (SD)a | −4.6 (5.8) | −5.5 (5.4) | −3.0 (6.4) | .10 |
| Recurrence, n (%) | 11 (16.7) | 4 (9.3) | 7 (30.4) | .04 |
| Reoperation, n (%) | 4 (6.1) | 2 (4.7) | 2 (8.7) | .61 |
| Midline shift, mm, mean (SD) | 3.2 (3.1) | 3.2 (3.2) | 3.1 (2.8) | .82 |
| Follow-up midline shift, mm, mean (SD) | 1.4 (2.8) | 1.2 (2.4) | 1.8 (3.4) | .43 |
| Reduction in midline shift, mm, mean (SD)a | −1.8 (3.0) | −2.0 (3.2) | −1.3 (2.6) | .34 |
AT, antithrombotics; SDH, subdural hematoma.
Only in nonrecurrent lesions.
FIGURE 1.
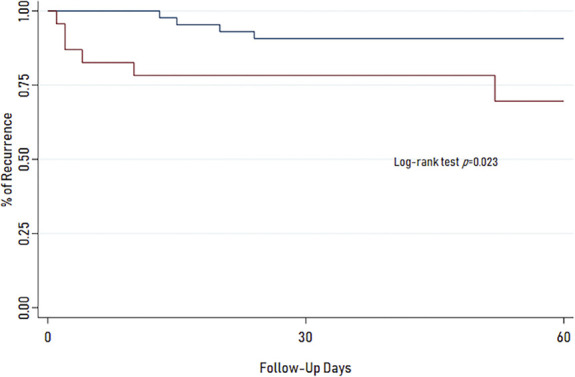
Kaplan-Meier curves for no-AT and AT groups. Curves show time to end point of recurrence within 60 days. Survival curve for AT is significantly different from those in the no-AT group (P = .023, log-rank test). Blue line = no AT use and red line = AT use. AT, antithrombotic.
The radiographic response measured by mean reduction in CSDH thickness (no-AT vs AT, −5.5 vs −3.0 mm, P = .10) and midline shift (−2 vs −1.3 mm, P = .34) was similar between groups. No difference was observed in either of these secondary outcomes when investigating either AP or AC as subgroups (Supplemental Tables 1 and 2, http://links.lww.com/NEU/D446). The effect of AT on recurrence was not driven solely by surgically treated patients or nonsurgically treated patients given that both had similarly higher rates of recurrence with AT (Supplemental Table 5, http://links.lww.com/NEU/D446).
DISCUSSION
The reported recurrence rate of CSDH after initiation of antithrombotics varies widely, from no significant difference to up to 40 times higher compared with controls.6,7 MMA embolization has revolutionized the treatment of CSDH, with a significant impact on reduction of delayed recurrences.8 Given this, it has become a common adjuvant to surgical evacuation, especially in cases in whom a patient requires AT use. The efficacy of this procedure in patients requiring rapid reinitiation of AT is not clear. We aimed to understand the recurrence rate of CSDH after MMA embolization after rapid initiation of AT. In our cohort, the mean initiation of AT was 1 day. After this, the incidence of CSDH recurrence was significantly different (no-AT vs AT; 9.3% vs 30.4%, P = .04) with no difference in the reoperation rate (4.7% vs 8.7%, P = .61). Taken together, our data suggest that rapid AT use confers a higher risk of recurrence after MMA embolization but was not associated with higher reoperation rates.
A recent case-control study comparing CSDH MMA embolization in a postembolization AT group (23/56) vs controls (33/56) observed no significant difference in the response to MMA embolization as measured by rate of >50% reabsorption of CSDH at follow-up.9 In our larger cohort, we observed a dissimilar result. A few possible explanations for this discordance exist. In their patient population, only 38% of patients underwent surgical evacuation before embolization, with only a single recurrence; in our cohort, these rates were 62% evacuations and 16.7% recurrence. Our higher 62% surgical evacuation was on the other hand similar to other MMA embolization studies like 62.5% evacuations in the study by Ban al.8 Mir et al9 also started antithrombotics at a mean of 2.4 days after embolization, compared with a mean of 1 day in our cohort. In addition, our trauma center protocol is to provide an early MMA embolization as sole treatment or adjunct within the acute phase of symptomatic CSDH encounters, which may select for a particular subset of patients. Given the cohort median length of stay of 1 day in the study by Mir et al,9 it is evident that our patient populations may differ. Moreover, our recurrence rate of 9.3% for no-AT patients more closely approximates that of previously published studies and is thus potentially more representative of clinical practice at large trauma centers.5,10
Limitations
Our study has a few limitations, including its retrospective nature and a relatively small sample size. Given that our outcomes were heavily dependent on repeat imaging, we only included patients who returned to follow-up with imaging which may have biased the sample to patients who have better social or medical support or toward a greater representation of patients with continued symptoms after treatment. In addition, patients on AT could potentially be more closely monitored with imaging, yielding a higher screening rate as well. To this point, there were 4/20 (20%) excluded patients who were on AT compared with 23/66 (35%) in included patients, thus likely not a significant source of bias. To note, our imaging follow-ups were within the 60-day time frame and not at the 60-day time point given the retrospective design of our study, with the median follow-up being 20 days (IQR 14-44). This led to inclusion of very early imaging follow-up patients. Nevertheless, looking at the time to recurrence in our Kaplan-Meier analysis, most recurrences were seen within the first 2 to 4 weeks in both groups.
CONCLUSION
MMA embolization has been repeatedly shown to be an effective sole or adjuvant treatment for CSDH. In patients undergoing MMA embolization for CSDH, rapid (within 5 day) initiation of antithrombotics is associated with a higher rate of recurrence. Care must be taken when considering postembolization antithrombotic initiation. Our results conflict with other recently published research. This may be reflective of the differences in patient populations treated. Further research is needed to define the optimal timing of restarting antithrombotic agents after MMA embolization.
Footnotes
Supplemental digital content is available for this article at neurosurgery-online.com.
Contributor Information
Juan Carlos Martinez-Gutierrez, Email: juan.c.martinezgutierrez@uth.tmc.edu.
Michael I. Nahhas, Email: michael.i.nahhas@uth.tmc.edu.
Matthew J. Kole, Email: Mkole1@geisinger.edu.
Youngran Kim, Email: Youngran.Kim@uth.tmc.edu.
Hyun Woo Kim, Email: hyun.woo.kim@uth.tmc.edu.
Peng Roc Chen, Email: peng.r.chen@uth.tmc.edu.
Spiros L. Blackburn, Email: spiros.blackburn@uth.tmc.edu.
Gary Spiegel, Email: DrSpiegel@NIR-ETX.com.
Sunil A. Sheth, Email: sunil.a.sheth@uth.tmc.edu.
Ryan S. Kitagawa, Email: ryan.s.kitagawa@uth.tmc.edu.
Mark J. Dannenbaum, Email: mark.dannenbaum@uth.tmc.edu.
Funding
This study did not receive any funding or financial support. Dr Sheth reports NIH funding.
Disclosures
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Supplemental Digital Content
Supplemental Tables. Supplemental Table 1. Indications for anticoagulant medications pre-MMA and post-MMA embolization (MMAE). Antiphospholipid antibody syndrome (APLAS). Overlap of indications exist; n listed correspond to total patients on anticoagulants, and percentages are based on this total.
Supplemental Table 2. Indications for antiplatelet medications pre-MMA and post-MMA embolization (MMAE). Overlap of indications exist; n listed correspond to total patients on antiplatelet medications, and percentages are based on this total.
Supplemental Table 3. Lesion Characteristics and Outcomes by Treatment Group. AC = anticoagulants, *Only in nonrecurrent lesions.
Supplemental Table 4. Lesion Characteristics and Outcomes by Treatment Group. AP = antiplatelet agents, *Only in nonrecurrent lesions.
Supplemental Table 5. Subgroup analysis of the effect of AT on surgical and nonsurgical patients.
COMMENTS
The authors should be commended for tackling this important question in neurosurgery. The study offers a single-center, retrospective review of patients treated with MMA embolization for subdural hematoma to compare rates of recurrence and reoperation in those requiring re-initiation of anticoagulation or antiplatelet therapy. The conclusions of the study generally match intuition that there would be higher recurrence rate in those needing rapid re-introduction of anticoagulation or antiplatelet therapy; interestingly, the mean reduction in SDH thickness did not significantly differ between the groups at follow-up, and notably, there was no difference in re-operation rates. However, the study carries several limitations and should be interpreted with caution until larger, multicenter, prospective studies can confirm the findings. An important limitation and potential confounder is the presence of a large cohort of patients who underwent surgical evacuation preceding the MMA embolization, although the authors point out this similar limitation in prior studies also and did perform a subgroup analysis. Also, the follow-up timing varied widely among patients with a median of 20 days, despite the 60-day follow-up goal within the primary outcome, which further impacts the interpretation of the findings of this study.
Laura Stone McGuire
Ali Alaraj
Chicago, Illinois, USA
REFERENCES
- 1.Balser D, Farooq S, Mehmood T, Reyes M, Samadani U. Actual and projected incidence rates for chronic subdural hematomas in United States Veterans Administration and civilian populations. J Neurosurg. 2015;123(5):1209-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldstein JN, Fazen LE, Wendell L, et al. Risk of thromboembolism following acute intracerebral hemorrhage. Neurocrit Care. 2009;10(1):28-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Motiei-Langroudi R, Stippler M, Shi S, et al. Factors predicting reoperation of chronic subdural hematoma following primary surgical evacuation. J Neurosurg. 2018;129(5):1143-1150. [DOI] [PubMed] [Google Scholar]
- 4.De Bonis P, Trevisi G, de Waure C, et al. Antiplatelet/anticoagulant agents and chronic subdural hematoma in the elderly. PLoS One. 2013;8(7):e68732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ironside N, Nguyen C, Do Q, et al. Middle meningeal artery embolization for chronic subdural hematoma: a systematic review and meta-analysis. J Neurointerv Surg. 2021;13(10):951-957. [DOI] [PubMed] [Google Scholar]
- 6.Rust T, Kiemer N, Erasmus A. Chronic subdural haematomas and anticoagulation or anti-thrombotic therapy. J Clin Neurosci. 2006;13(8):823-827. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Zhou J, Fan C, et al. Influence of antithrombotic agents on the recurrence of chronic subdural hematomas and the quest about the recommencement of antithrombotic agents: a meta-analysis. J Clin Neurosci. 2017;38:79-83. [DOI] [PubMed] [Google Scholar]
- 8.Ban SP, Hwang G, Byoun HS, et al. Middle meningeal artery embolization for chronic subdural hematoma. Radiology. 2018;286(3):992-999. [DOI] [PubMed] [Google Scholar]
- 9.Mir O, Yaghi S, Pujara D, et al. Safety of antithrombotic resumption in chronic subdural hematoma patients with middle meningeal artery embolization: a case control study. J Stroke Cerebrovasc Dis. 2022;31(4):106318. [DOI] [PubMed] [Google Scholar]
- 10.Kan P, Maragkos GA, Srivatsan A, et al. Middle meningeal artery embolization for chronic subdural hematoma: a multi-center experience of 154 consecutive embolizations. Neurosurgery. 2021;88(2):268-277. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Tables. Supplemental Table 1. Indications for anticoagulant medications pre-MMA and post-MMA embolization (MMAE). Antiphospholipid antibody syndrome (APLAS). Overlap of indications exist; n listed correspond to total patients on anticoagulants, and percentages are based on this total.
Supplemental Table 2. Indications for antiplatelet medications pre-MMA and post-MMA embolization (MMAE). Overlap of indications exist; n listed correspond to total patients on antiplatelet medications, and percentages are based on this total.
Supplemental Table 3. Lesion Characteristics and Outcomes by Treatment Group. AC = anticoagulants, *Only in nonrecurrent lesions.
Supplemental Table 4. Lesion Characteristics and Outcomes by Treatment Group. AP = antiplatelet agents, *Only in nonrecurrent lesions.
Supplemental Table 5. Subgroup analysis of the effect of AT on surgical and nonsurgical patients.


