Abstract
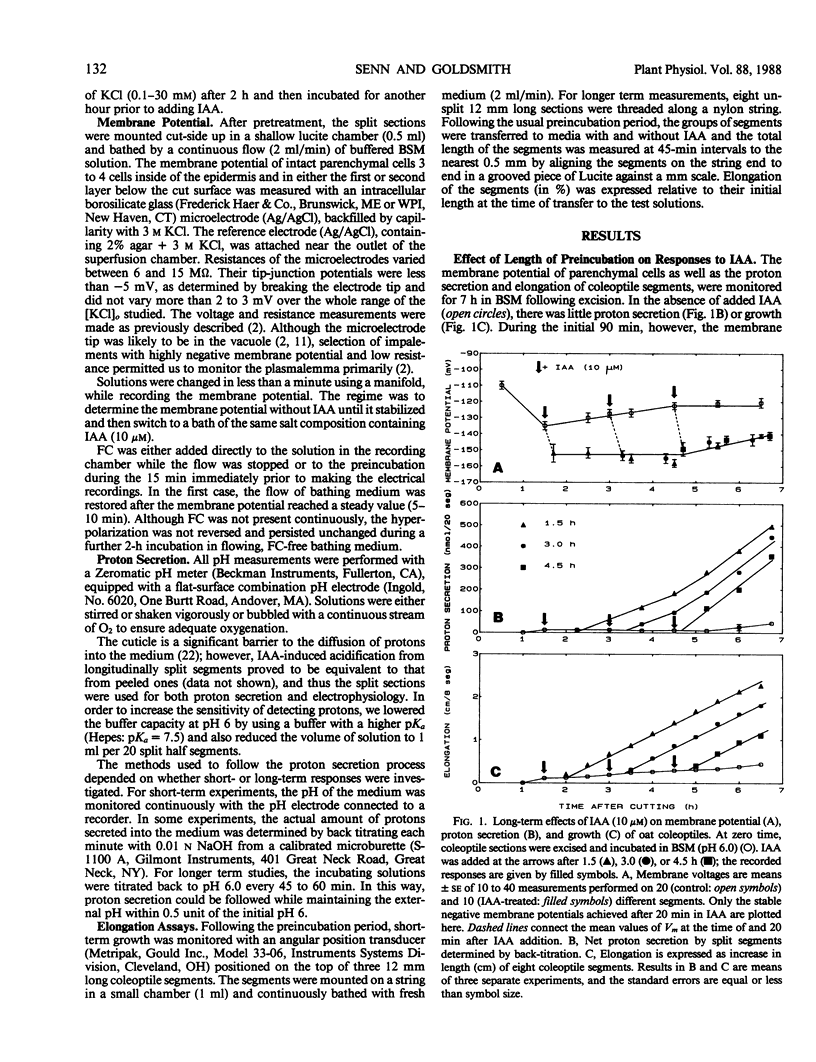
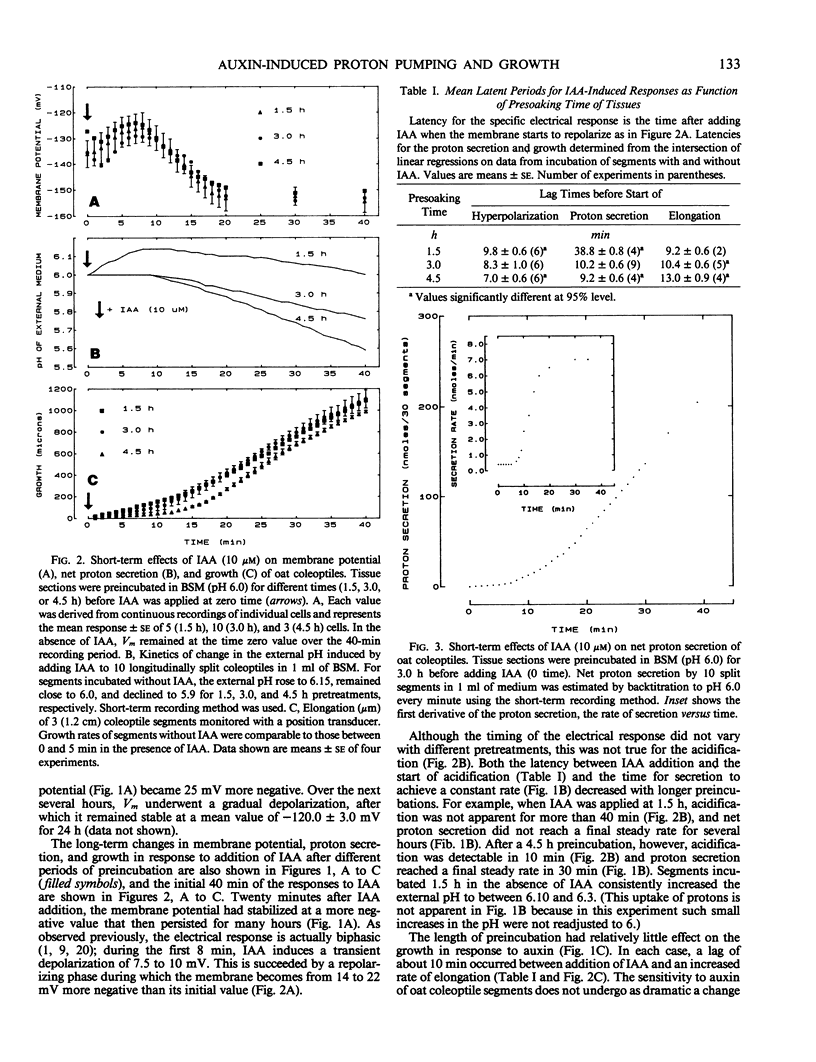
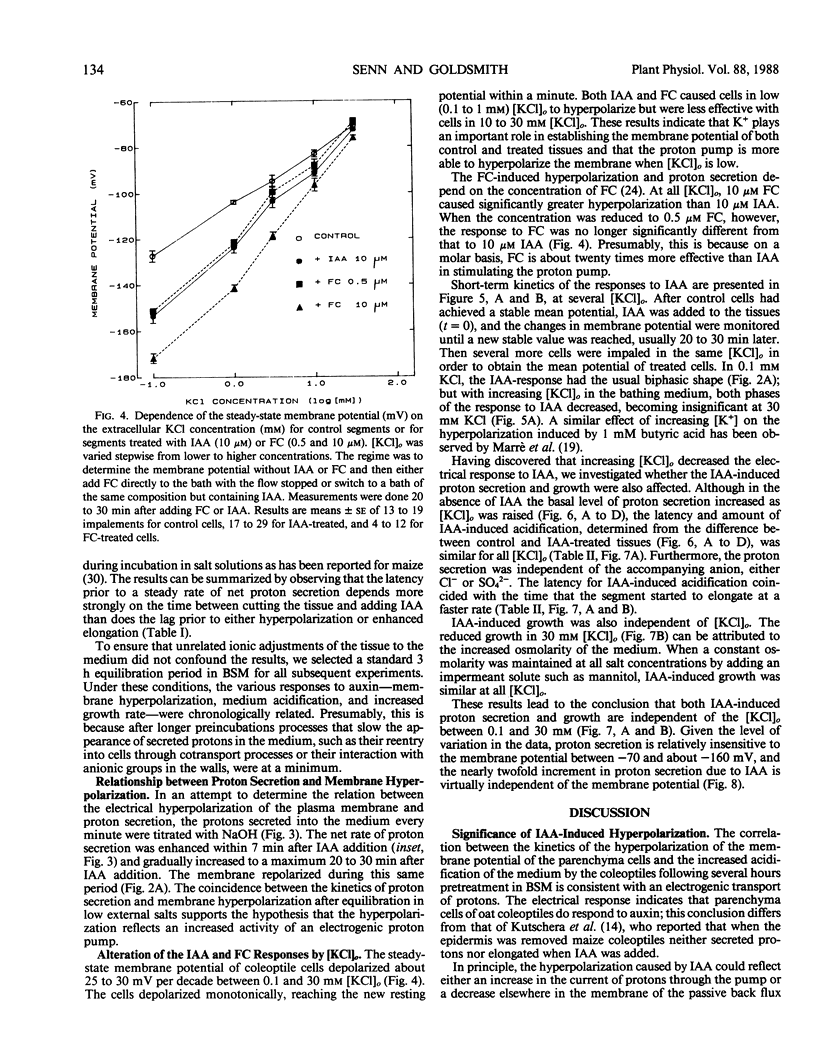
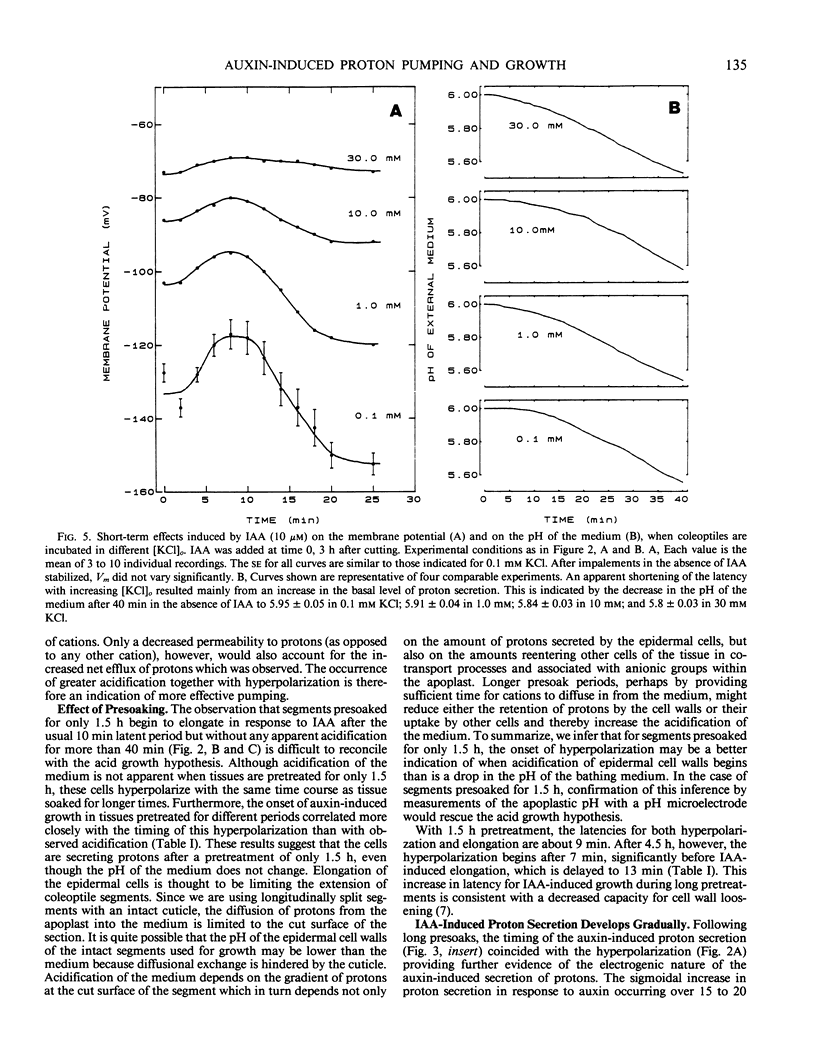
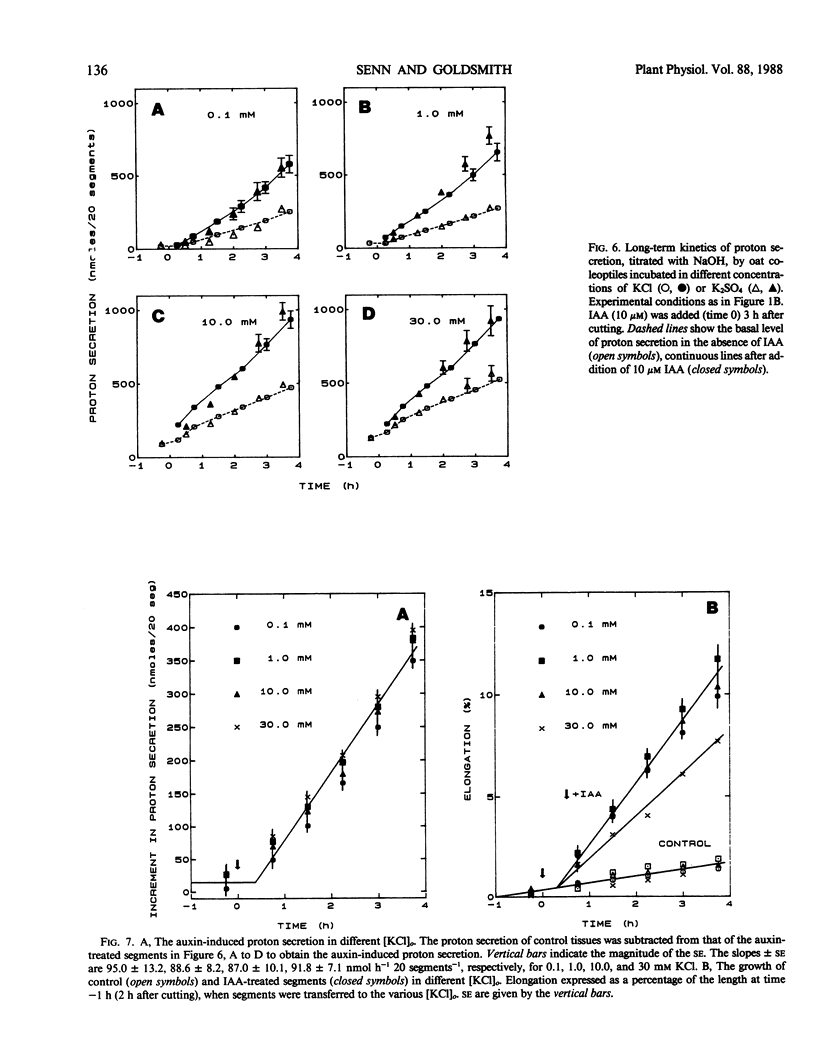
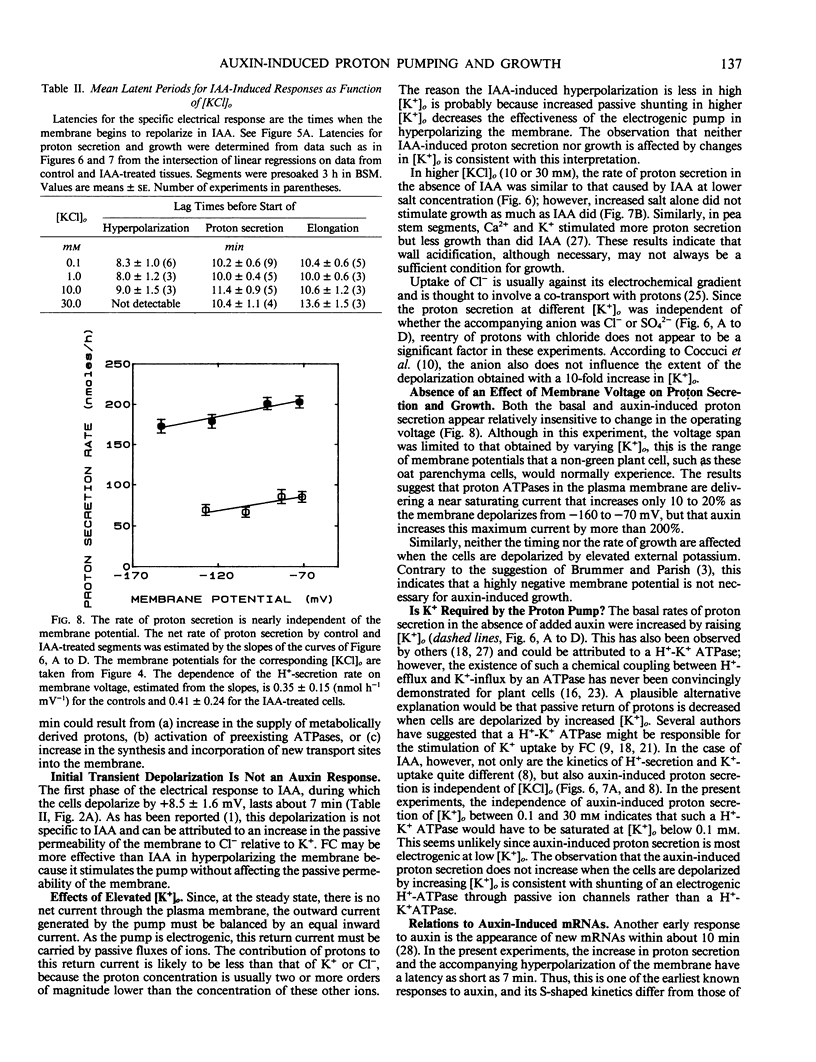
The temporal relations between early responses to indoleacetic acid (IAA), proton secretion, hyperpolarization of the membrane potential, and growth change during the incubation of segments of oat (Avena sativa L.) coleoptiles in a low salt medium. When IAA is added after pretreatment of several hours, proton secretion increases after a latency of 7 minutes and reaches its maximum 10 to 15 minutes later. This timing coincides with both the increase in growth of the segments and the hyperpolarization of the membrane potential of parenchyma cells, consistent with the hypothesis that the change in membrane voltage reflects the activity of an electrogenic proton pump. The extent of IAA-induced hyperpolarization is substantially reduced by elevating [KCl]0, most likely because this increases the passive conductance of the membrane. Neither growth nor proton secretion is affected by high [KCl]0 (30 millimolar), indicating that neither process is limited by the magnitude of the membrane potential. These results are consistent with the acid growth hypothesis. Following short incubation times, however, IAA-induced hyperpolarization and growth are detected within 10 minutes, while acidification of the medium is delayed for more than 40 minutes. This result is seemingly in conflict with the acid growth hypothesis, but in freshly cut tissue, the pH of the external medium may not reflect the pH of the epidermal cell walls. The temporal coincidence of auxin-induced growth and hyperpolarization suggests that in freshly isolated segments the hyperpolarization is a more sensitive indication of proton secretion than is acidification of the external aqueous environment.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cleland R. E. Kinetics of Hormone-induced H Excretion. Plant Physiol. 1976 Aug;58(2):210–213. doi: 10.1104/pp.58.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland R. E., Prins H. B., Harper J. R., Higinbotham N. Rapid Hormone-induced Hyperpolarization of the Oat Coleoptile Transmembrane Potential. Plant Physiol. 1977 Mar;59(3):395–397. doi: 10.1104/pp.59.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs M., Ray P. M. Rapid Auxin-induced Decrease in Free Space pH and Its Relationship to Auxin-induced Growth in Maize and Pea. Plant Physiol. 1976 Aug;58(2):203–209. doi: 10.1104/pp.58.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayle D. L., Cleland R. Control of plant cell enlargement by hydrogen ions. Curr Top Dev Biol. 1977;11:187–214. doi: 10.1016/s0070-2153(08)60746-2. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Navarro A., Blatt M. R., Slayman C. L. A potassium-proton symport in Neurospora crassa. J Gen Physiol. 1986 May;87(5):649–674. doi: 10.1085/jgp.87.5.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein B. Responses of Avena coleoptiles to suboptimal fusicoccin: kinetics and comparisons with indoleacetic Acid. Plant Physiol. 1981 Sep;68(3):543–547. doi: 10.1104/pp.68.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry M. E., Jones R. L. Effect of salt on auxin-induced acidification and growth by pea internode sections. Plant Physiol. 1981 Jul;68(1):59–64. doi: 10.1104/pp.68.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesper M. J., Evans M. L. Time-dependent Changes in the Auxin Sensitivity of Coleoptile Segments: Apparent Sensory Adaptation. Plant Physiol. 1978 Feb;61(2):204–208. doi: 10.1104/pp.61.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]


