Abstract
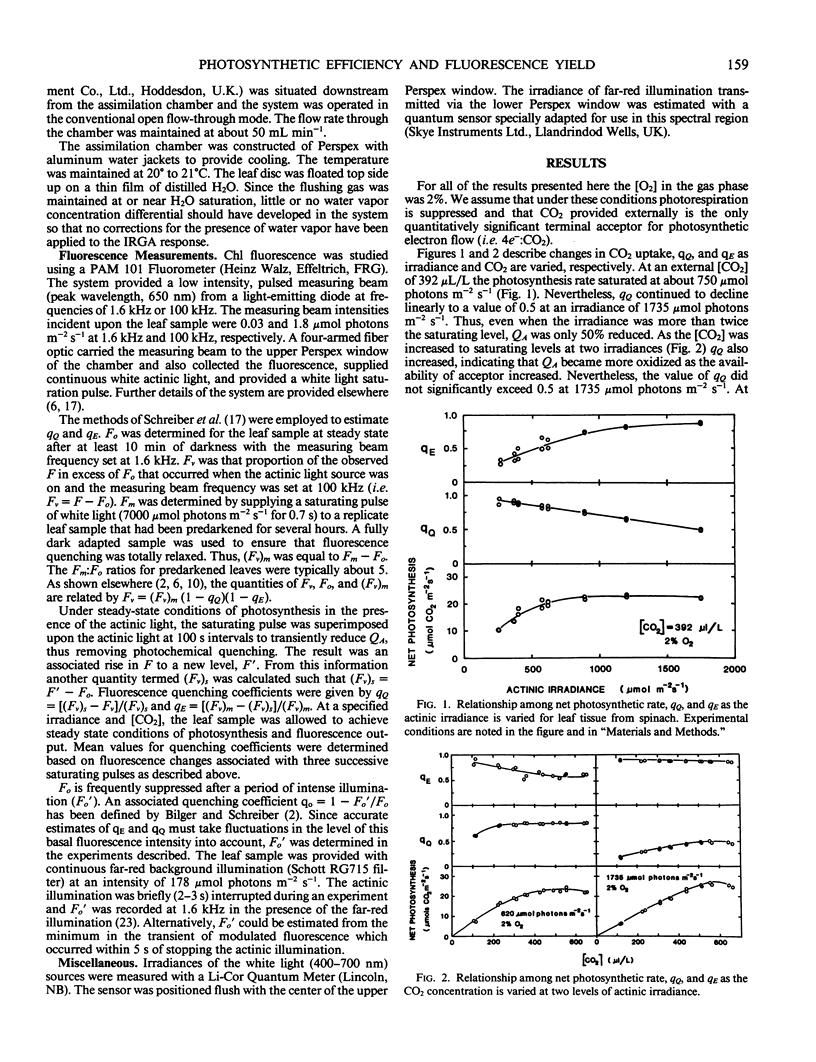
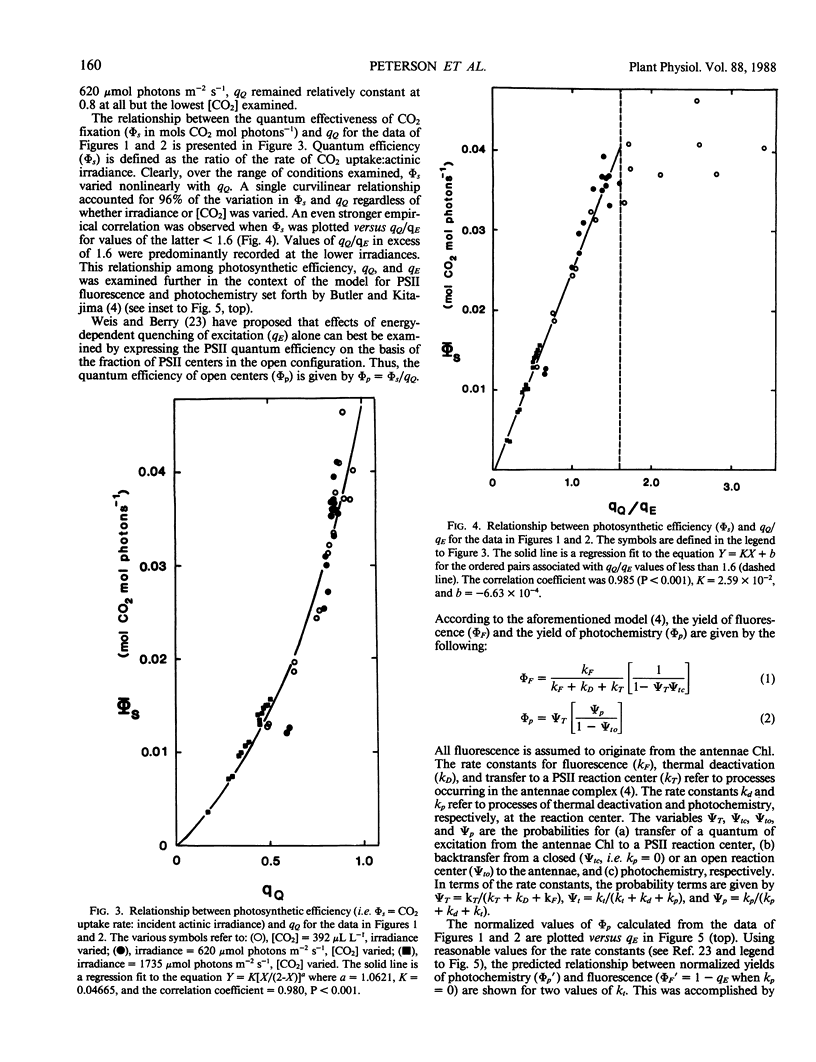
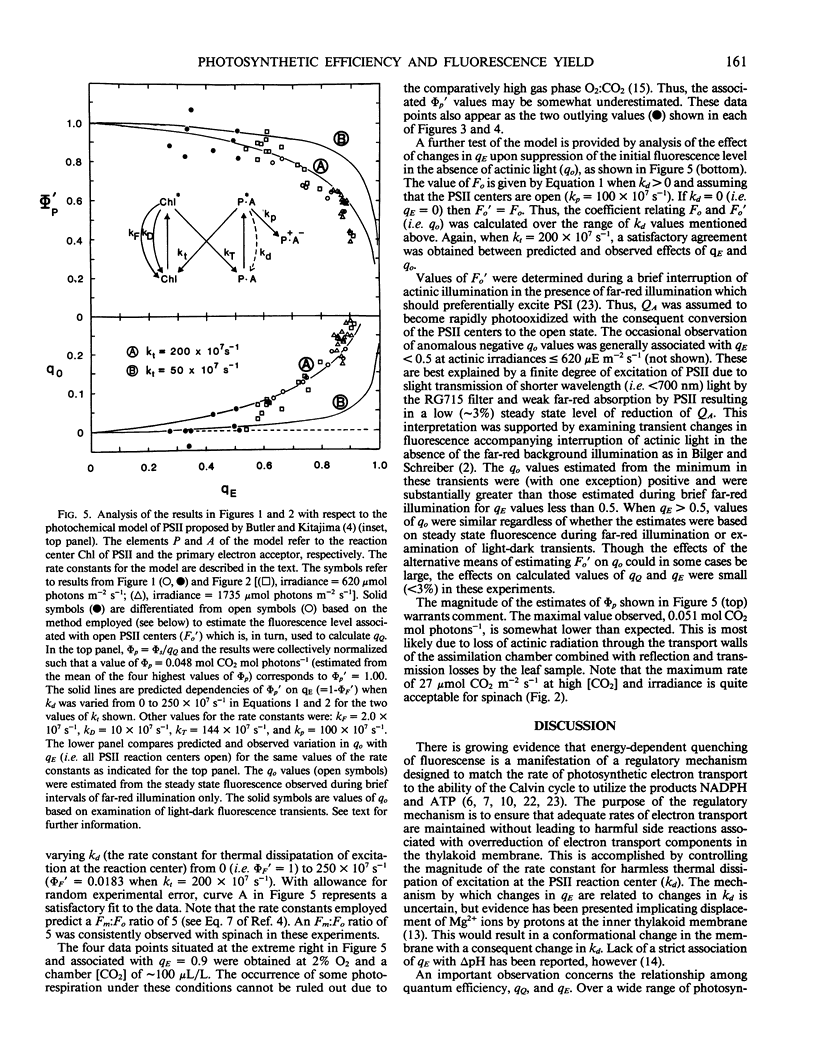
The relationship between steady-state photosynthetic efficiency, as moles CO2 per mole of incident visible photons under 2% O2, and chlorophyll fluorescence quenching has been investigated in intact leaf tissue of Spinacia oleracia. Fluorescence yield was measured using a pulse amplitude modulation technique that permitted rapid and sensitive resolution and quantitation of photochemical and nonphotochemical quenching coefficients. A highly linear relationship was observed between photosynthetic efficiency and the ratio of photochemical:nonphotochemical quenching coefficients for values of the latter less than 1.6. This relationship applied whether irradiance or CO2 concentration was varied. The observed relationships between photochemical yield and fluorescence yield were compatible with the photosystem II model proposed by Butler and Kitajima (1975 Biochim Biophys Acta 376: 116-125). The results are discussed with respect to the proposed role of nonphotochemical quenching in regulating radiant energy utilization and also the applicability of fluorescence measurements as a means of estimation of the rate of photosynthetic electron transport.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradbury M., Baker N. R. Analysis of the slow phases of the in vivo chlorophyll fluorescence induction curve. Changes in the redox state of photosystem II electron acceptors and fluorescence emission from photosystems I and II. Biochim Biophys Acta. 1981 May 13;635(3):542–551. doi: 10.1016/0005-2728(81)90113-4. [DOI] [PubMed] [Google Scholar]
- Butler W. L., Kitajima M. Fluorescence quenching in photosystem II of chloroplasts. Biochim Biophys Acta. 1975 Jan 31;376(1):116–125. doi: 10.1016/0005-2728(75)90210-8. [DOI] [PubMed] [Google Scholar]
- Demmig B., Winter K., Krüger A., Czygan F. C. Photoinhibition and zeaxanthin formation in intact leaves : a possible role of the xanthophyll cycle in the dissipation of excess light energy. Plant Physiol. 1987 Jun;84(2):218–224. doi: 10.1104/pp.84.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata N. Control of excitation transfer in photosynthesis. II. Magnesium ion-dependent distribution of excitation energy between two pigment systems in spinach chloroplasts. Biochim Biophys Acta. 1969 Oct 21;189(2):171–181. doi: 10.1016/0005-2728(69)90045-0. [DOI] [PubMed] [Google Scholar]
- Peterson R. B. Quantitation of the O(2)-Dependent, CO(2)-Reversible Component of the Postillumination CO(2) Exchange Transient in Tobacco and Maize Leaves. Plant Physiol. 1987 Jul;84(3):862–867. doi: 10.1104/pp.84.3.862. [DOI] [PMC free article] [PubMed] [Google Scholar]


