Graphical abstract
Keywords: Microplastic, Avian, Gut vascular barrier, Gut–liver axis, Multi-omics analysis
Highlights
-
•
Molecular toxicity of microplastic to the avian digestive system was characterized.
-
•
Unlike other species, microplastic caused intestinal necrosis and pyroptosis in avian.
-
•
The avian liver toxicity caused by microplastic is lipid metabolism disorder and apoptosis.
-
•
The gut microbiota participates in microplastic induced liver injury by the gut-liver axis.
-
•
Caffeine and melanin may be potential natural resistances to microplastic toxicity.
Abstract
Introduction
Microplastic pollution seriously threatens the health and safety of humans and wildlife. Avian is one of the main species endangered by microplastics. However, the damage mechanism of microplastics to the digestive system of avian is not clear.
Objectives
The gut-liver axis is a bidirectional channel that regulates the exchange of information between the gut and the liver and is also a key target for tissue damage caused by pollutants. This study aimed to elucidate the digestive toxicity of microplastics in avian and the key role of the gut-liver axis in it.
Methods
We constructed an exposure model for microplastics in environmental concentrations and toxicological concentrations in chickens and reveal the digestive toxicity of polystyrene microplastics (PS-MPs) in avian by 16S rRNA, transcriptomics and metabolomics.
Results
PS-MPs changed the death mode from apoptosis to necrosis and pyroptosis by upregulating Caspase 8, disrupting the intestinal vascular barrier, disturbing the intestinal flora and promoting the accumulation of lipopolysaccharide. Harmful flora and metabolites were translocated to the liver through the liver-gut axis, eliciting hepatic immune responses and promoting hepatic lipid metabolism disorders and apoptosis. Liver injury involves multiple molecular effects of mitochondrial dynamics disturbance, oxidative stress, endoplasmic reticulum stress, and cell cycle disturbance. Furthermore, metabolomics suggested that caffeine and melanin metabolites may be potential natural resistance substances for microplastics.
Conclusion
Taken together, our data demonstrate the digestive damage of PS-MPs in avian, revealing a critical role of the liver-gut axis in it. This will provide a reference for protecting the safety of avian populations.
Introduction
Microplastic (MPs) pollution is widespread in the natural environment [1], [2], [3]. Due to their small size, MPs are ingested by animals for bioaccumulation, ultimately harming the ecosystem [4]. MPs are found in almost all trophic-grade organisms [5], [6]. Vertebrates are at the top of the food chain, so they are more severely damaged by MPs toxicity [7]. Most of these studies focused on fish (79.8 %), while birds were only 4.3 % [8]. Birds are usually apex predators and are more likely to accumulate MPs into their bodies and cause damage. More than half of seabird species ingest marine plastic [9]. MPs were detected in kingfishers along the Ticino River in northern Italy [10]. MPs have seriously threatened the health and safety of bird populations. Although the toxic damage of MPs to fish has been extensively studied, the results cannot correctly infer the response of birds, so the related research on birds is seriously lacking.
Intestinal damage is the main target organ of microplastic toxicity. Microplastic exposure affects the intestinal flora and carbohydrate metabolism and amino acid metabolism activities of zebrafish [11]. Due to the unique structure of the digestive system (crop, gizzard, glandular stomach), the digestion process of microplastics after being ingested by avian species is completely different from that of fish and mammals. Digestive damage caused by microplastics in avian species has not been reported. The microbial community in the intestine participates in the regulation of host metabolism and physiological functions [12]. The intestinal barrier is a physical and immune barrier against environmental threats and resident microbiota. After the intestinal barrier is damaged, microorganisms and their products such as lipopolysaccharide (LPS) enter the portal vein and invade the liver tissue through the intestinal-liver axis [13]. The intestinal source of LPS has been considered to be one of the main factors of liver damage [14]. The intestinal barrier is composed of three parts: mucus barrier, gut vascular barrier (GVB), intestinal epithelial barrier. GVB is the deepest protective layer of the intestine, and it is the last obstacle for microorganisms and their metabolites to reach the systemic circulation and organs far away from the intestine [15]. The Wnt/β-catenin pathway is related to several pathological conditions related to GVB destruction, and GVB destruction is often accompanied by an increase in the plasma membrane vesicle protein PV-1 [16]. Whether microplastics can destroy GVB and cause the translocation of microbes and harmful metabolites has not been reported yet.
The gut-liver axis relates to a bidirectional manner through the portal circulation and bile ducts, and its functions involve neuroendocrine and immune crosstalk. The gut microbiota participates in the pathophysiological processes of hepatic metabolic diseases through the gut-liver axis [17]. At present, studies have reported that microplastics cause hepatitis and lipid metabolism disorders [18]. However, the role of the liver-gut axis in microplastic-induced digestive damage remains unclear.
Although there has been good research on the digestive toxicity of microplastics, most have focused on the fish species. Avian has a higher level of food chain and a wider range of biological activities, and its toxicity from microplastics may be higher than that of fish. Therefore, it is urgent to understand the digestive toxicity of microplastics to avian species. In this study, through multi-omics analysis of gut microbiome, liver transcriptome and metabolome, the toxicity mechanism of microplastics on avian digestive system was comprehensively dissected, which provided a reference for protecting the population safety of avian species.
Materials and methods
Ethics statement
All experiments involving animals were conducted according to the ethical policies and procedures approved by the ethics committee of the Northeast Forestry University, numbered UT-31.
Construction of animal models
MPs (5 μm) used in this study were purchased from Tesulang Chemical Company. The aquatic environment is the main source of bird feeding, so this study used the water pollution concentration of MPs as one of the study concentrations.[8]. In this study, the low-exposure group of PS-MPs used environmental concentrations (1 mg/L) [19], [20]. 120 One-day-old chickens were randomly divided into four groups. Control group (C group, 0 mg/L), low concentration of polystyrene microplastic (L-PS group, 1 mg/L), medium concentration of polystyrene microplastic (M−PS group, 10 mg/L), high concentration of polystyrene microplastic (H-PS group, 100 mg/L). The water containing MPs was well shaken for 20 min and changed daily. Chickens were exposed to 6 week continuously, and their body weight was recorded every week. After 6 W, each group of chickens was killed, the liver and gut were taken out. Part of the tissue was fixed with tissue fixative, and the remaining tissue was frozen and stored in a −80 °C freezer.
H&E staining
The tissue was prepared into a wax block and cut into 5 μm sections. Sections were first dehydrated with different concentrations of alcohol, and then replaced with xylene. After H&E staining, the sections were sealed in paraffin and observed using an optical microscope.
Transmission electron microscopy
The fixed tissue was dehydrated in a gradient of ethanol at low temperature. The tissue was then dehydrated with 100 % acetone, embedded and coagulated. Tissue blocks were cut into 90 nm slices using a microtome, and the slices were stained with lead citrate and uranyl acetate. The samples were observed using TEM (JEM-1200ES, Japan).
Oil red O staining
The fixed liver tissue was made into frozen sections and then treated with 60 % isopropanol for 3 min. The sections were immersed in 0.3 % oil red O for 25 min. Then washed with 60 % isopropanol once, and washed three times with PBS. Nuclei were counterstained with hematoxylin and observed under a microscope after mounting.
Biochemical indicators
Alanine transaminase (ALT) (wanleibio, china), aspartate transaminase (AST) (wanleibio, china), triglycerides (TG), catalase (CAT) (wanleibio, china), total superoxide dismutase (T-SOD) (wanleibio, china), malondialdehyde (MDA) (wanleibio, china), glutathione (GSH) (wanleibio, china) and total antioxidant capacity (T-AOC), Diamine oxidase (DAO) were tested by the kits.
16S rRNA sequencing
Fresh cecal contents were stored in a −80 °C freezer. The cecal contents were sent to Majorbio company for testing, and the data were analyzed online (https://www.majorbio.com).
Transcriptomics
Transcriptome detection was performed on liver tissues of the control group, L-PS and H-PS groups, with three replicates in each group. The data were analyzed online (https://www.majorbio.com). A total of 20,214 expressed genes were detected in this analysis, including 19,947 known genes and 267 new genes; 65,118 expressed transcripts, including 55,930 known transcripts and 9188 new transcripts.
LC-MS non-targeted metabolomics
Liver tissue samples were analyzed for metabolomics by Majorbio company. The treatment method is detailed in the team's previous research [21].
Cell culture and cell viability analysis
Chicken LMH hepatocytes were cultured in DMEM high glucose medium (HyClone, Sigma-Aldrich). Add fetal bovine serum (concentration to 10 %) and penicillin–streptomycin (100 units/mL) to the medium. The cells were cultured at 38.5 °C (5 % carbon dioxide). The cells were passaged once every 2–3 days.
The cell suspension was added to a 96-well plate and cultured in a 38.5 °C, 5 % CO2 incubator for more than 48 h. After the cells adhered, the culture medium containing PS-MPs at concentrations of 0 μg/ml, 100 μg/ml, 300 μg/ml, 500 μg/ml, 800 μg/ml, 1000 μg/ml, 1500 μg/ml, 2000 μg/ml and cultured for 24 h. Cell viability was tested by CCK8 kit (Beyotime, China).
Reactive oxygen species (ROS) detection
One-third (L-PS), one-half (M−PS), and LC50 (H-PS) of PS-MPs were selected for exposure to hepatocytes for 24H. Then remove the medium and MPs, wash three times with PBS, add DCFH-DA (10 μM) (Beyotime, China), and incubate at 37 °C for 30 min. Fluorescence microscopy and flow cytometry were used to detect the fluorescence intensity of ROS in cells.
Apoptosis detection
Annexin V-FITC/PI kit (Wanlei Biotechnology, China) was used to detect apoptosis. After the cells were treated with PS-MPs for 24 h, cells were washed with phosphate buffer saline (PBS) to remove PS-MPs. Each group of cells was digested with trypsin, and then centrifuged at 1500 r/min. Resuspend the cells in 200 μl buffer, add 4 μl PI and 2 μl FITC, incubate for 15 min. Then cells were detection by flow cytometer and fluorescence microscope.
Cell cycle detection
Cell cycle detection kit (Wanlei Biotechnology, China) was used to detect cell cycle. After the in vitro model was constructed, the cells were collected. The cells were fixed with 70 % alcohol fixative for 24 h, then the fixative was removed by PBS. Add 100 μl of RNaseA to the cell pellet, water bath at 37 °C for 30 min, add 400 μl of PI and incubate for 30 min. The cell cycle is detected by flow cytometry.
qRT-PCR
Tissue and cells were fixed using trizol. RNA was reverse transcribed into cDNA using the kit. The mRNA expression level was detected by LightCycler 480 (Roche, Switzerland). The target gene sequence was searched on NCBI (https://www.ncbi.nlm.nih.gov/), and the CDS region sequence was copied, and then the primer sequence was designed online using (https://www.sangon.com/login), and the primer sequence was verified by Blast analysis. The primer sequences used in this study are shown in Table 1. The relative expression of mRNA was calculated according to 2-ΔΔCt, and β-actin was used as an internal reference gene.
Table 1.
Gene special primers used in the real-time quantitative reverse transcription PCR.
| Gene | Primer Sequence (5′ – 3′) |
|---|---|
| Fasn | Forward:TCTCTGCCATCTCCCGAACTTCC |
| Reverse:TCTCAATTAGCCACTGTGCCAACTC | |
| SREBP1C | Forward:CAGCAACAGCAGCAGTGACTCC |
| Reverse:GGCAGAGGAAGACAAAGGCACAG | |
| ACACA | Forward:TCCATCGCATCTTCCATTATGTCCTG |
| Reverse:TCTCAGTGTCTTCATTAGTCGCTCAAC | |
| SCD | Forward:CACATGGCTTGGCTGCTGGTAC |
| Reverse:CTTGTAGTATCTCCGCTGGAACATCAC | |
| APOB | Forward:AGAGGTAGAGGCAGGACGCATATC |
| Reverse:TCTGAAGGTGGCATGTGAATTGTGAG | |
| MTTP | Forward:TTCAGGCATTCCGTGACCAAGTATG |
| Reverse:TTCCAACATTTCTGCTTTCCCTCTCC | |
| FABP4 | Forward:AGTTTGATGAGACCACAGCAGATGAC |
| Reverse:CATTCCACCAGCAGGTTCCCATC | |
| FATP1 | Forward:CGTCGCAGTGTATGGAGTGGAAG |
| Reverse:GGGCGTAGGAAGGCAGAACTTTC | |
| CD36 | Forward:GCGATTTGGTTAATGGCACTGATGG |
| Reverse:TCCCTTCACGGTCTTACTGGTCTG | |
| PPAR-α | Forward:TTTCACCAGCATCCAGTCCTTCATC |
| Reverse:ACCTTCACAAGCATGTACTCCGTAATG | |
| CPT1 | Forward:CGAGTCAGACACCACAGCAACAC |
| Reverse:CACCGTAACCATCATCAGCCACAG | |
| CPT2 | Forward:CCGTCCAAGCATAGCACAGAAGAG |
| Reverse:ACCCAAACAGATGTCGGTCAAATCC | |
| PDK4 | Forward:GTCACCCGAGGCACATTGGAAG |
| Reverse:CTGGTCACATAGCATCTTGGAACTTTG | |
| PLIN1 | Forward:GCATCTCCAGCGTGAAGAAGGTC |
| Reverse:AGCATCCACCGTCTCCATAGCC | |
| ABCG5 | Forward:TGCTCTTCAGAAGTGCTCCAACAAC |
| Reverse:GACGCCTCTCACCTCCAGAAATTC | |
| ABCG8 | Forward:TCTCAGCTTTCCTTGGCAATGTCC |
| Reverse:GTCCAGAGTTGTTCCAGGCTTATCAC | |
| HMGCR | Forward:TGTAGGCGTAGCAGGACCACTATAC |
| Reverse:ATACGGCTCCTTGCTCCTCCAC | |
| HMGCS1 | Forward:CTGGAGGCAGGAGTTGAAGTTGTC |
| Reverse:AGGGATTCTTGGCACTTTCTTAGCAG | |
| C/EBPα | Forward:CCATCGACATCAGCGCCTACATC |
| Reverse:CTGCTTGCTGTGCTGGAAGAGG | |
| COX2 | Forward:TGTCCTTTCACTGCTTTCCAT |
| Reverse:TTCCATTGCTGTGTTTGAGGT | |
| TNF-α | Forward:CTTCCTGCTGGGGTGCATAG |
| Reverse:AAGAACCAACGTGGGCATTG | |
| NF-kB | Forward:TCAACGCAGGACCTAAAGACAT |
| Reverse:GCAGATAGCCAAGTTCAGGAT | |
| IL1β | Forward:GAGGAGGTTTTTGAGCCCGT |
| Reverse:GCACGAAGCACTTCTGGTTG | |
| TLR-2 | Forward:TCCTCATCCTGGTGGTCGTTGG |
| Reverse:AAGCGTCGTAGCAGATGTCTTTCG | |
| TLR-4 | Forward:CATCCCAACCCAACCACAGTAGC |
| Reverse:CTGAGCAGCACCAATGAGTAGTATAGC | |
| MFF | Forward:TGGGAAGGCTGAAGAGAGAA |
| Reverse:GGTGTTCCCTCAAGTGTGGT | |
| MFN2 | Forward:TCTTGGATGGACAATGCTGGTGAAC |
| Reverse:AGACTGGGATTTGCTGGTGTTAAAGG | |
| MFN1 | Forward:TGAGCATGTAGCAACGGAAG |
| Reverse:AGCAAGCTGATTGACGGTCT | |
| OPA1 | Forward:GCTACGGACCAGGGTTATGA |
| Reverse:GCTCAAGCATCCGTTGGTAT | |
| DRP1 | Forward:GGCAGTCACAGCAGCTAACA |
| Reverse:GCATCCATGAGATCCAGCTT | |
| P53 | Forward:GCGGAGGAGATGGAACCATTGC |
| Reverse:GCTCCTGCCAGTTGCTGTGATC | |
| BAX | Forward:TATGGGACACCAGGAGGGTA |
| Reverse:CGTAGACCTTGCGGATAAAGC | |
| BAK | Forward:ACCCGGAGATCATGGAGA |
| Reverse:GATGCCTTGCTGGTAGACG | |
| Caspase 8 | Forward:CCTCTTGGGCATGGCTA |
| Reverse:TGCTGCTCACCTCTTGATT | |
| Caspase 9 | Forward:CCGAAGGAGCAAGCACG |
| Reverse:AGGTTGGACTGGGATGGAC |
Western blotting
Proteins from cells and tissues were extracted using RIPA lysate containing PMSF. Protein samples were separated by SDS-PAGE gel electrophoresis. Proteins were then transferred to PVDF membranes and blocked with 5 % skim milk for two hours. Then use the primary antibody to incubate for 12 h. SREBP1c, PPAR-α, PGC1, β-actin, IκB, TNF-α, Pro-IL1β, Mature IL1β, NF-κB, MFN1, MFN2, DRP1, P53, Cladin-3, BCL-2, ZO-1, BAX, Caspase 3, Occludin, Caspase 9, Wnt3a, β-catenin, PV-1, Caspase 8, Cyt-c, RIPK1, MLKL, GSDMD, ASC, NLRP3, Caspase 1, COX2, IL-18 (Wanleibio, china). PVDF membranes were incubated with HRP-conjugated secondary antibody for two hours. Results were imaged using an ImageQuant LAS 4000.
Statistical analysis
The data results in this study are at least three replicates, expressed as mean ± SD. The data was analyzed by GraphPad Prism 8. Differences between groups were analyzed using one way anova analysis of variance. P < 0.05 means that the data is significantly different.
Result
PS-MPs mediate the destruction of the gut vascular barrier through the Wnt/β-catenin pathway in chicken
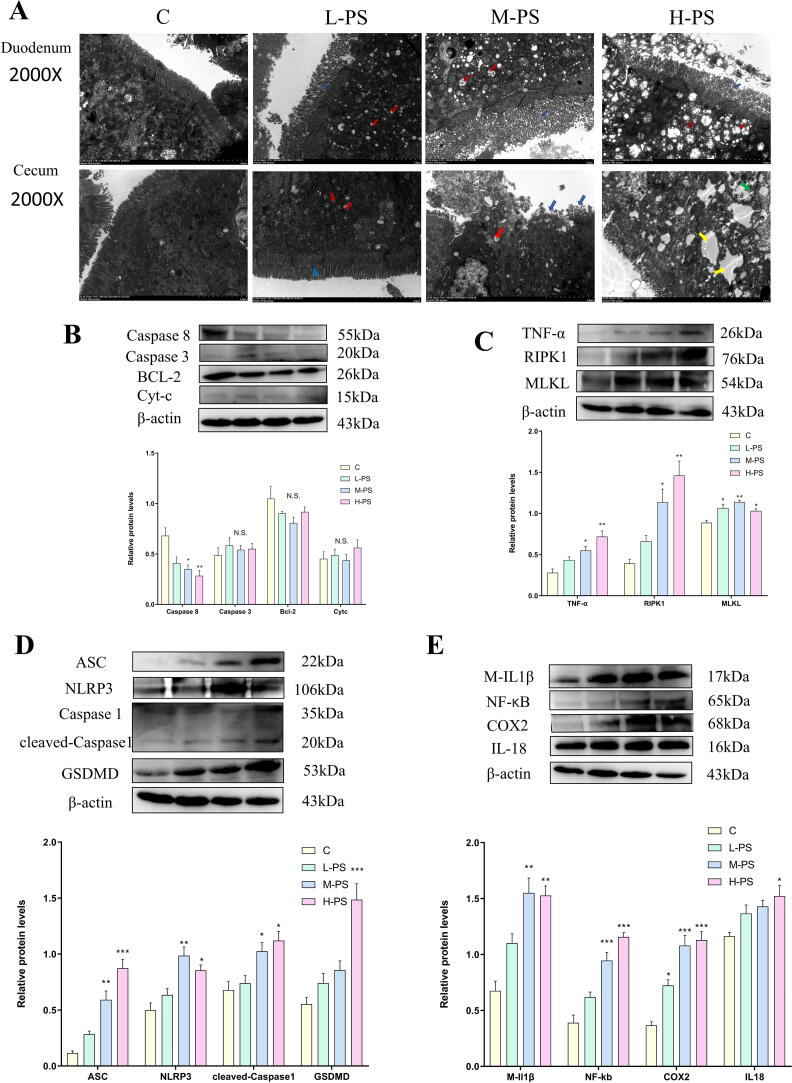
After chickens were exposed to PS-MPs, their body weight was recorded every week. At six weeks, there was no significant difference in body weight between the groups (Fig. 1A). But when PS-MPs were exposed to 2 weeks and 5 weeks, the body weight of chickens increased to different degrees. Anatomical observation of the intestine revealed that obvious blood spots (black arrow) appeared after PS-MPs treatment, the intestinal wall was smooth, and the intestinal folds were reduced (Fig. 1B). HE staining observed the pathological damage of the duodenum and cecum (Fig. 1C). The results showed that PS-MPs caused strong inflammation (red Arrow) of the intestinal tissue, and the duodenum and the cecum of the L-PS group and the M−PS group had the strongest inflammatory reaction. The length of the intestinal crypts (yellow arrow) became shorter and the width increased, and the number of cecal intestinal crypts in the H-PS group decreased. In the H-PS group, the duodenal intestinal villi gap was widened, and the intestinal villi of the cecum were severely missing. The length of the intestinal crypts became shorter and the width increased, and the number of cecal intestinal crypts in the H-PS group decreased. In the H-PS group, the duodenal intestinal villi gap was widened, and the intestinal villi of the cecum were severely missing. After PS-MPs treatment, the submucosa of the cecum is looser than the submucosa of the duodenum, and there are obvious gaps (blue arrows).
Fig 1.
PS-MPs mediate the destruction of the gut vascular barrier through the Wnt/β-catenin pathway. A: The weekly weight of chickens after PS-MPs exposure. B: Observation of duodenum anatomy, the black arrow is the bleeding point. C: Representative figure of HE staining of duodenum and cecum, red arrow: inflammatory infiltration, yellow arrow: intestinal crypt, blue arrow: submucosa. D: The content of diamine oxidase in serum. E-G: The expression levels of tight junction proteins and Wnt/β-catenin signaling pathway proteins. *P < 0.05, **P < 0.01, ***P < 0.001 (one-way analysis of variance (ANOVA) followed by post hoc Tukey’s test). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The content of diamine oxidase (DAO) in serum increased with the increase of PS-MPs concentration, indicating that intestinal permeability increased (Fig. 1D). The expression of Claudin-3, Occudin and ZO-1 in the duodenum and cecum was reduced by PS-MPs (Fig. 1E). The gut vascular barrier marker PV-1 was up-regulated by PS-MPs, indicating that the gut vascular barrier was destroyed and its regulatory pathway Wnt/β-catenin was inhibited (Fig. 1E).
PS-MPs change the mode of death from apoptosis to pyroptosis and necroptosis by inhibiting the expression of Caspase 8 in chicken intestine
For explore which cell death model PS-MPs used to cause intestinal tissue damage. The organelle damage in the duodenum and cecum was observed using transmission electron microscopy (Fig. 2A). The results showed that, consistent with the HE results, the intestinal villi (blue arrow) of the duodenum and cecum in the L-PS group were sparsely arranged, the cecal villi of the M−PS group were partially shedding, and the intestinal villi of the H-PS group were significantly shedding. Mitochondrial (red arrow) swelling and vacuolization occurred in each group, which was dose-dependent. The most serious intestinal injury was observed in the H-PS group of cecum. The euchromatin in the nucleus disappeared (yellow arrow), and the cytoplasm was edged (green arrow), showing local necrosis.
Fig 2.
PS-MPs exposure caused intestinal tissue pyrolysis and necrosis instead of apoptosis. A: The cecum and duodenum were observed under a transmission electron microscope. Red arrow: mitochondria, blue arrow: intestinal villi, yellow arrow: nucleus, green arrow: cytoplasmic edge aggregation. B: Apoptosis-related protein expression level. C: Necroptosis-related protein expression level. D: Pyrolysis-related protein expression level. E: Inflammation-related protein expression level. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Then we tested the expression levels of key proteins of apoptosis, pyrolysis and necroptosis in the cecum tissue. There was no significant difference in the expression of apoptosis-related proteins among the treatment groups (Fig. 2B). And the expression of Caspase 8 also decreased with the increase of PS-MPs. PS-MPs activate TNF-α/RIPK1/MLKL signaling pathway and cause necroptosis (Fig. 2C). Similarly, PS-MPs treatment activates ASC and NLRP3, and then cleaves Caspase 1 to induce the release of GSDMD, causing pyrolysis (Fig. 2D). While pyrolysis and necroptosis are usually accompanied by inflammation, the expression of NF-kB, COX2, M-IL1β, and IL18 increase, which is consistent with the results of HE staining (Fig. 2E).
PS-MPs disrupt the intestinal flora leading to an increase in gram-negative bacteria and bacterial biofilms form in chicken
We studied the effects of MPs exposure on gut microbes. MPs has little effect on the alpha diversity of intestinal microbes (Fig. 3A), the Chao index did not change, only the M−PS group increased the simpson index and decreased the Shannon index, and the simpson index and shannon index of the H-PS group had a decreasing trend but no significant difference. Then the Venn diagram was used to analyze the similarity of the composition of each treatment group (Fig. 3B). As the concentration of MPs changed, the microbial composition also changed. PS-MPs changed the β diversity of microorganisms (Fig. 3C). The results of PCoA and PLS-DA analysis showed that the microbiota of the L-PS group and the M−PS group and the blank group still had overlapping parts, but the H-PS group was completely separated from the blank group. MPs treatment significantly changed the bacterial abundance at the phylum and family level (Fig. 3D). The phylum of Proteobacteria in the H-PS group increased to 6 times of that of the C group, and the phylum of Actinomycetes increased to 2.2 times of that of the C group. Lacetospirillum, Lactobacillus, and Rumenococcus species hydrolyze sugar and starch to produce short-chain fatty acids like butyrate [22]. PS-MPs reduced the abundance of Lachnospiraceae and Lactobacillaceae but did not affect Ruminococcaceae. The abundance of Lactobacillaceae in M−PS group was increased significantly. The abundance of Oscillospiraceae increased in the L-PS group and the M−PS group, but decreased in the H-PS group. The abundance of Butyricicoccaceae and Erysipelatoclostridiaceae in each treatment group decreased, and the abundance of Peptostreptococcaceae increased. Interestingly, the abundance of Enterobacteriaceae, Eggerthellaceae, and Erysipelotrichaceae in the M−PS group decreased, but the abundance of the H-PS group increased.
Fig 3.
The intestinal flora changes after PS-MPs exposure. A: The α diversity index of intestinal flora includes Shannon index, Simpson index and Chao index. B: Venn diagram analysis of species between groups. C: Beta diversity analysis, PCoA and PLS-DA analysis. D: Relative abundance of intestinal bacteria at the phylum and family level. E: BugBase phenotype prediction, including seven categories: Gram Positive, Gram Negative, Biofilm Forming, Pathogenic, Mobile Element Containing, Oxygen Utilizing and Oxidative Stress Tolerant.
BugBase was used to predict the phenotype (Fig. 3E), and the results showed that only Gram Negative, Biofilm Forming and Oxidative Stress Tolerant had a significant increase, and there were no significant differences in other types of phenotypes.
PS-MPs exposure does not affect liver coefficient but still causes liver damage in chicken
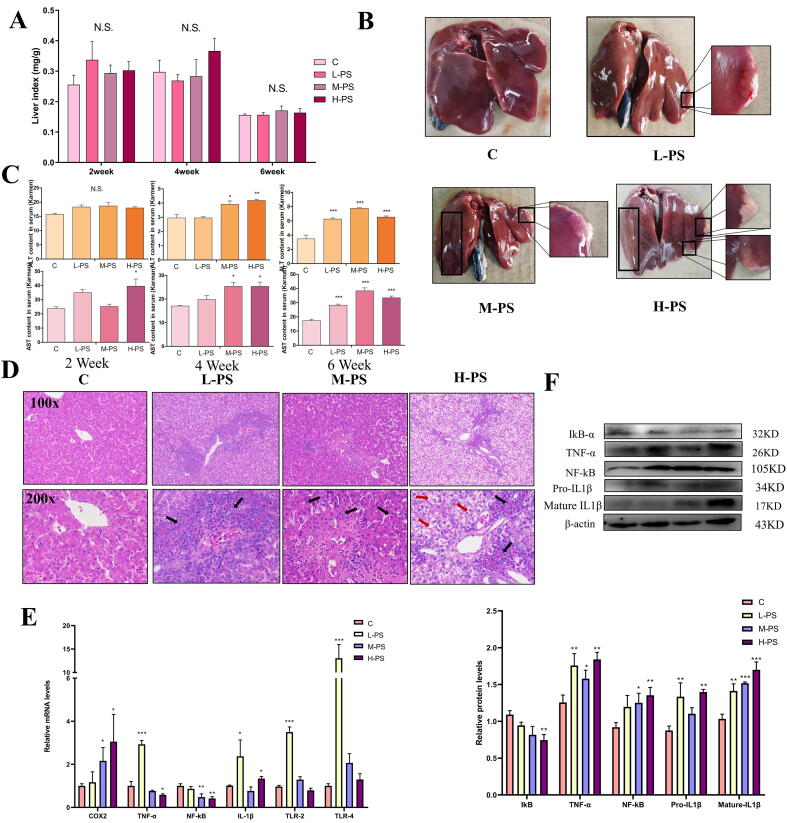
Next, we investigated whether PS-MPs caused the destruction of the gut vascular barrier and the disturbance of the intestinal flora to cause liver damage through the intestinal liver axis. Although the liver coefficient of the PS-MPs exposure group had an increasing trend, there was no significant difference from the C group (Fig. 4A). After that, we performed anatomical observation of the liver. The liver colors after PS-MPs exposure was yellow, and white lesions appeared in the liver lobules (Fig. 4B). Liver damaged was concentration-dependent. The liver injury indexes ALT and AST increased with time and concentration (Fig. 4C).
Fig 4.
Changes of physiological and biochemical indexes of chickens exposed to PS-MPs for 6 weeks. A: The ratio of liver weight to body weight (mg/g) (n = 6). Data are mean ± SEM. B: Anatomical observation of liver tissue. C: Serum levels of ALT and AST in each treatment group after exposure to PS-MPs for 2, 4, and 6 weeks (n = 6). Data are mean ± SEM. D: HE staining of the liver (100X and 200X microscope), the black arrow points to inflammatory cell infiltration, and the red arrow points to the ballooning degeneration of hepatocytes. E-F: The expression level of inflammation-related genes and proteins. *P < 0.05, **P < 0.01, ***P < 0.001 (one-way analysis of variance (ANOVA) followed by post hoc Tukey’s test). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
As a unique chemical component in the outer wall of gram-negative bacteria, LPS can strongly cause tissue inflammation. HE staining of liver showed that PS-MPs exposure caused strong inflammatory cell infiltration (Fig. 4D). The hepatocytes in the H-PS group showed ballooning degeneration. At the mRNA level, PS-MPs exposure significantly up-regulated the expression of inflammatory markers (COX2, TNF-α, NF-κB, IL1β, TLR2, TLR4), and the expression levels of some genes in the H-PS group were lower than those in the L-PS group (Fig. 4E). Similar results were obtained at the protein level (Fig. 4F). Although the expression levels of TNF-α and NF-κB proteins in the H-PS group were increased, while mRNA expression was declined. This may be due to the body's self-regulation in order to relieve the strong inflammatory response and reduce the mRNA expression. Our results show that even lower concentrations of PS-MPs caused inflammation after exposure, and high concentrations of PS-MPs cause ballooning degeneration of hepatocytes.
Changes in chicken liver transcriptomics caused by PS-MPs exposure
In order to clarify the specific mechanism of liver damaged caused by PS-MPs exposure, RNA-seq was used to screen differentially expressed genes (DEGs) between the C group and the PS-MPs exposed group. Between the C group and the L-PS group, 451 DEGs, 228 up-regulated genes and 223 down-regulated genes were identified. Between the control group and the H-PS group, 432 DEGs, 248 up-regulated genes and 184 down-regulated genes were identified (Fig. S1A). The distribution of DEGs is shown in the volcano map (Fig. S1B). Interestingly, the gene expression pattern of PS-MPs (L-PS group) at environmental concentration is different from that of H-PS group (Fig. S1C).
The genes with the most and least fold difference between L-PS group, H-PS group and C group are shown in Fig. S1DE. Then, DEGs were analyzed by GO enrichment. Exposure to the L-PS group mainly affected the synthesis and metabolism of sterols, cholesterol and steroids (Fig. S1F). The molecular functions of DEGs were enriched in oxygen binding, growth factor binding, oxygen carrier activity and transmembrane receptor protrin tyrosine kinase activity, suggesting that the oxidation functions of mitochondria may be damaged. H-PS group exposure mainly affects the immune response to bacteria, organic acid synthesis, lipid synthesis and ion transport (Fig. S1G). This is consistent with the results of intestinal injury, the H-PS group had the most serious “intestinal leakage”.The molecular functions of DEGs were enriched in transferase activity and transferring amino-acyl groups.
The most enriched pathway of DEGs in the L-PS group was steroid biosynthesis, followed by transcription disorders in cancer and PI3K-AKT signaling pathway (Fig. S1H). The pathway of DEGs enrichment in the H-PS group mainly involved immune function, diabetes, bacterial infection, cancer and lipid anabolism (Fig. S1I).
Changes in chicken liver metabolomics caused by PS-MPs exposure
Metabolomics analysis explored the changes in liver metabolism profile after PS-MPs exposure. The results of principal component analysis showed that the metabolites of each treatment group were similar, but as the exposure concentration increased, the confidence ellipses gradually converged and shrank (Fig. S2A). Then a Venn diagram was constructed for the metabolic set, and the H-PS group produced more differential metabolites (Fig. S2B). The H-PS group produced more differential metabolites, and only seven metabolites overlapped in the two metabolic sets. The differential metabolites were further classified by HMDB (Fig. S2C). Lipids and lipid-like molec in the L-PS group and H-PS group were the most abundant in the differential metabolites, accounting for 44.68 % and 35.71 %, respectively. This is consistent with the transcriptome results. The remaining differential metabolites in the H-PS group are organic acids and derivatives (25.53 %), phenylpropanoids and polyketides (10.64 %), organic oxygen compounds (8.51 %), organoheterocyclic compounds (5.32 %), organic nitrogen compounds (3.19 %), benzenoids (1.06 %), nucleosides, nucleotides, and analogues (1.06 %). The remaining differential metabolites in the L-PS group are phenylpropanoids and polyketides (28.57 %), Nucleosides, Nucleotides, and analogues (7.14 %), Organic acids and derivatives (7.14 %), Organic nitrogen compounds (7.14 %), Organic oxygen compounds (7.14 %), organoheterocyclic compounds (7.14 %).Through clustering heat maps and VIP bar graphs, the expression patterns of metabolites in each sample in each difference group and the P value of metabolites in the VIP and single-dimensional statistics of multivariate statistical analysis are displayed (Fig. S2D and E). It is worth noting that Brevetoxin B2, which is the second highest expressed in the differential metabolites of the H-PS group, may be derived from disordered intestinal microbial products or secondary metabolites. It is a biogenic toxin that can destroy thioredoxin reductase and cause toxic damage to the gastrointestinal tract and nerves [23].
Finally, KEGG enrichment analysis was performed on differential metabolites. The most enriched pathway in the H-PS group was apoptosis, followed by melanogenesis, necroptosis (Fig. S2F). The enriched pathways in the L-PS group are caffeine metabolism and purine metabolism.
PS-MPs exposure disrupts lipid metabolism and synthesis balance by inhibiting mitochondrial dynamics
According to the analysis of transcriptome data and metabolome data, the lipid synthesis and metabolism of the liver was affected by PS-MPs. The level of lipid deposition in liver tissues was observed by oil red o staining. PS-MPs promoted the accumulation of lipids in the liver and showed a concentration-dependent manner (Fig. 5A). The level of triglycerides (TG) in the liver also increased, while the level of TG in the serum decreased (Fig. 5B). The content of total cholesterol in serum and liver increased after PS-MPs exposure, and the L-PS group was higher than the H-PS group (Fig. 5B). Although previous studies have found that lipid deposition is one of MPs-induced hepatotoxicity, the detailed mechanism has not been explored. Next, we explored how PS-MPs caused liver lipid deposition, and examined the expression of proteins and genes related to fatty acid synthesis, transport, metabolism. The expression of fatty acid synthesis genes was up-regulated by PS-MPs at ambient concentrations. However, the expression of some fatty acid synthesis genes (FASN, SREBP1c and ACACA) was down-regulated by medium and high concentrations of PS-MPs (Fig. 5C). Fatty acids need to be transported to the cytoplasm for metabolic consumption with the assistance of APOB and MTTP. The mRNA expression of APOB and MTTP in the L-PS group increased while the expression of the M−PS and the H-PS group decreased (Fig. 5D). The mRNA expression of other fatty acid transporters (FABP4, FATP1 and CD36) were all up-regulated by PS-MPs (Fig. 5D). Next, we tested genes related to fatty acid metabolism. The expression of key genes for fatty acid β oxidation was down-regulated by PS-MPs, and only peroxisome proliferator activated receptor alpha (PPAR-α) at environmental concentrations was up-regulated (Fig. 5E). The up-regulated PDK4 of PS-MPs enhances the conversion of fatty acids into triglycerides; the up-regulated PLIN1 prevents the contact of lipase with triglycerides (Fig. 5E). In addition, the cholesterol transports genes ABCG5 and ABCG8 were only up-regulated by L-PS. The cholesterol synthesis gene HMGCS1 was only down-regulated in the H-PS group and up-regulated in the L-PS and M−PS groups (Fig. 5F). The protein level showed that PS-MPs increased lipid synthesis (SREBP1c) and decreased lipid metabolism (PPAR-α and PGC1) (Fig. 5G). PS-MPs exposure activates LXR (Fig. 5H) and inhibits FXR (Fig. 5I) in a dose-dependent manner. Our data show that PS-MPs disrupt the balance of liver lipid synthesis and metabolism, and the degree of lipid deposition is concentration-dependent.
Fig 5.
PS-MPs exposure disrupts mitochondrial dynamics and disrupts lipid metabolism homeostasis. A: Liver Oil Red O staining (100X and 200X microscope). B: The content of triglycerides and cholesterol in the liver and plasma. Data are mean ± SEM. C-E: The mRNA expression levels of fatty acid synthesis, transport and metabolism-related genes in the liver of each treatment group. Data are mean ± SEM. F: The mRNA expression levels of cholesterol transport and metabolism-related genes in the liver of each treatment group. Data are mean ± SEM. G: The expression levels of liver SREBP1, PPAR-α, PGC1 protein in each treatment group. Data are mean ± SEM. H, I:The expression levels of liver LXR and FXR mRNA in each treatment group. J: Electron microscope observation of liver cell damage. Mitochondria (yellow arrow), nucleus (blue arrow), lipid droplet (red arrow). K: The protein expression level of genes related to mitochondrial dynamics. Data are mean ± SEM. L: The mRNA expression level of genes related to mitochondrial dynamics. Data are mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 (one-way analysis of variance (ANOVA) followed by post hoc Tukey’s test). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Lipid metabolism is mainly carried out in mitochondria, and then observe whether organelles were damaged. As shown in Fig. 5J, exposure to PS-MPs caused nuclear shrinkage of hepatocytes (blue arrow), suggesting that apoptosis may have occurred. In addition, the electron microscope results showed abnormal morphology of mitochondria, manifested as mitochondrial swelling, vacuum degeneration, and loss of mitochondrial cristae structure (yellow arrow). Lipid droplets (red arrows) were also observed in the M−PS and H-PS groups.
Mitochondria are a highly dynamic organelle that maintains mitochondrial homeostasis through division and fusion, so genes and proteins related to mitochondrial dynamics were tested (Fig. 5k and L). Mitochondrial outer membrane fusion (MFN1, MFN2) was inhibited by medium and high concentrations of PS-MPs, but PS-MPs at ambient concentrations up-regulated the expression of MFN1 and MFN2. While the mitochondrial inner membrane fuses, the formation of mitochondrial cristae (OPA1) was inhibited by PS-MPs. The expression of mitochondrial division-related genes (MFF, DRP1) was down-regulated by PS-MPs. At the protein level, we got similar results. In short, PS-MPs disturbed the balance of mitochondrial dynamics, leading to increased lipid synthesis and inhibiting the β-oxidation of fatty acids, and finally causing lipid deposition.
PS-MPs exposure increased the ER stress level, disrupt the antioxidant system balance and trigger apoptosis
The ER is a workshop for lipid synthesis, and lipid synthesis enzymes are located in the smooth ER. ER stress in the liver was caused by PS-MPs in a concentration-dependent manner. Since the previous results showed damage to the mitochondria, the steady state of the antioxidant system was tested. PS-MPs significantly inhibited SOD enzyme activity, increased MDA content, and up-regulated the enzyme activities of T-AOC, CAT, GSH (Fig. 6A).
Fig 6.
PS-MPs exposure caused liver antioxidant system disorders, endoplasmic reticulum stress and apoptosis. A: Detection of CAT, SOD, GSH, MDA and T-AOC content in liver tissue. Data are mean ± SEM. B: Detection of mRNA expression levels of apoptosis-related genes. Data are mean ± SEM. C: Detection of protein expression levels of apoptosis-related genes. Data are mean ± SEM. D: Detection of protein expression levels of ER stress-related genes. Data are mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 (one-way analysis of variance (ANOVA) followed by post hoc Tukey’s test).
According to the results of electron microscopy and metabonomics analysis, apoptosis may be the main way of liver cell death. In the electron microscope results, it was observed that hepatocytes had nuclear shrinkage, mitochondrial damage, ER stress and oxidative stress all led to the occurrence of cell apoptosis. Next, we tested the relevant indicators of hepatocytes apoptosis. After PS-MPs exposures, the expression of apoptotic proteins increased, and the expression of anti-apoptotic protein was decreased (Fig. 6B and C). The decreased of P53 protein expression may be related to the potential carcinogenic effects of PS-MPs. The expression trends of Caspase 9 gene was opposite to that of protein. The body reduces the possibility of cell canceration through apoptosis mechanism, while PS-MPs hinder the expression of related genes mRNA to enhance canceration. PS-MPs treatment increased the phosphorylation level of protein kinase R like ER kinase (PERK) and up-regulated the genes and proteins levels of inositol-requiring enzyme 1 (IRE1), glucose-regulated protein 78 (GRP78) and activating transcription factor 6 (ATF6) (Fig. 6D). The above results indicated that PS-MPs induced oxidative stress and ER stress and lead to apoptosis.
PS-MPs caused oxidative stress and cell cycle arrest, leading to apoptosis of chicken LMH liver cells
Then, we evaluated the effect of PS-MPs exposure to liver cells. CCK8 was used to detect cell viability (Fig. 7A). The cells morphology was observed under a microscope (Fig. 7B), LMH cells showed ballooning degeneration (green arrow), and PS-MPs (purple arrow) gathered around normal cells (yellow arrow). ROS levels were significantly induced by PS-MPs (Fig. 7C and D). The apoptosis of LMH cells induced by PS-MPs is mainly early apoptosis, and the proportion of late apoptosis and necrosis are small (Fig. 7E). Then, hoechst staining showed that the cells exposed to M−PS group and H-PS group had obvious nuclear condensation (Fig. 7F). The expression levels of the protein also showed the occurrence of apoptosis (Fig. 7G).
Fig 7.
PS-MPs exposure caused damage to chicken LMH liver cells. A: Cell viability was tested by CCK8. Data are mean ± SEM. B: Observe the cell morphology with light microscope, normal cells (yellow arrow), microplastics (purple arrow), swollen cells (green arrow). C: ROS fluorescent probe staining of LMH cells. D: Flow cytometric detection of ROS fluorescence intensity of LMH cells. E: Flow cytometric detection of the level of apoptosis of LMH cells. F: Hoechst (yellow box) of LMH cells. Condensation of cell nuclei appears in Hoechst staining (orange arrow). G: Protein expression levels of apoptosis-related genes. H: Flow cytometry detects the cell cycle of LMH cell. I: Protein expression levels of cell cycle-related genes. *P < 0.05, **P < 0.01, ***P < 0.001 (one-way analysis of variance (ANOVA) followed by post hoc Tukey’s test). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Next we explored whether the cell cycles was blocked by PS-MPs. The results of flow cytometry showed that the cell cycles was blocked in S phase and G2/M phase (Fig. 7H), and similar results were obtained fo protein expression (Fig. 7I). Our results indicated that PS-MPs induced oxidative stress, cell cycle arrest and apoptosis in LMH cells in vitro.
Discussion
In the past few years, the ecological threat of MPs to wild animals and humans has drawn international attention [24], [25]. In this study, we report the toxic effects of environmental and toxicological concentrations on the digestive system of chicken. PS-MPs exposure disrupted GVB in chickens and resulted in intestinal tissue damage and intestinal flora disturbance. Harmful flora and metabolites are translocated to the liver through the liver-gut axis, eliciting hepatic immune responses and promoting hepatic lipid metabolism disorders and apoptosis.
The destruction of the intestinal barrier caused by MPs has been initially observed in multiple studies [26]. However, whether MPs exposure affects the integrity of GVB has not been reported yet. GVB is the last barrier for microorganisms and their metabolites to enter the systemic circulation, and is regulated by the typical Wnt/β-catenin signaling pathway. Salmonella typhimurium causes GVB damaged, accompanied by a decrease in β-catenin expression and an increase in PV-1 [16]. Our results indicated that PS-MPs exposure reduced the expression of intestinal tight junction proteins and inhibited the Wnt/β-catenin signaling pathway, leading to the destruction of the gut vascular barrier. Compared to the duodenum, this damage is more serious about the cecum, manifested by loss of intestinal villi and widening of the submucosal space. Caspase 8 has rich biological functions and participates in regulating a variety of death modes such as apoptosis, pyrolysis, and necroptosis [27]. Caspase 8 forms a signaling complex (DISC) with proteins such as FADD and cleaves the effector protein to cause apoptosis, and cleaves RIPK1 and RIPK3 in apoptotic bodies, thereby blocking necrosis [28]. In addition, caspase-8 can be used as a scaffold to induce pyrolysis in intestinal epithelial cells, independent of its activity [29]. In this study, PS-MPs exposure inhibited the expression of Caspase 8 and changed the mode of death from apoptosis to pyrolysis and necroptosis. Both pyrolysis and necroptosis are inflammatory death ways, and the exposure to PS-MPs caused a strong inflammatory response to the intestinal tissue. The aminoacyl-tRNA biosynthesis pathway is associated with lesions and diseases of the digestive tract, so the high enrichment of aminoacyl-tRNA biosynthesis in metabolites is consistent with chicken intestinal lesions [30]. There are currently very limited reports on the way microplastics lead to cell death in intestinal tissue. Exposure to microplastics induces apoptosis in human colonic epithelial cells and intestinal tissues of freshwater benthic clams, mice, and planarians [31], [32], [33]. This difference may be due to the ability of the bird's intestine to move food back and forth in the intestine through reverse peristalsis, a unique way of digestion that may lead to microplastics rubbing against the intestinal wall for a long time [34].
The gut microbiota participates in the pathophysiological process of various metabolic diseases through the gut-liver axis [17]. Proteobacteria is the main source of intestinal endotoxin LPS, and its abundance is related to the content of LPS [35]. Rumenococcus, Lactobacteriaceae and Lacetospirillaceae families produce butyrate and other short-chain fatty acids by hydrolyzing starch and sugars [36]. Butyrate is used as an energy source by the intestinal epithelium, and SCFA enhance mucosal barrier function by increasing intestinal mucosal mucin and IgA production [37]. N-butyrate reduces intestinal inflammation by promoting the differentiation of regulatory T cells [38]. Acetate negatively regulates the insulin signal in fat cells to inhibit fat deposition [39]. Therefore, PS-MPs exposure may reduced the production of butyrate and short-chain fatty acids by reducing the abundance of Laevis and Lactobacillus, further destroying the intestinal barrier, causing intestinal inflammation and promoting liver lipid deposition. Escherichia coli and Enterobacteriaceae, Peptococcus, Coriobacteriale Eggerthella, and various Clostridia, such as Oscillospiraceae, Anaerotruncus, and Lachnoclostridium, are significantly increased and may be involved in the degradation of the protein backbone of mucin. The affinity with Escherichia coli and Enterobacteriaceae for mucin may help to enter the intestinal mucosa, promote intestinal barrier damaged and trigger systemic inflammation [40]. The increase in peptostreptococcaceae is related to intestinal bleeding, which may affect the repair ability or proliferation rated of epithelial cells [41]. 60–80 % of body infections are related to biofilms [42]. The destruction of mucosal epithelium is conducive to the growth of microorganisms across the epithelial barrier and pathogenic biofilms. Therefore, our research showed that the imbalance of the gut microbiota associated with PS-MPs leads to the overproduction of LPS, destruction of the intestinal barrier, inflammation, and subsequent lipid accumulation [43].
Omics analysis is suitable for analyzing the state of tissue organ function and metabolism [44], [45]. Transcriptomics and metabolomics were used to comprehensively evaluate PS-MPs induced liver injury in chickens. The liver receives blood from the portal vein exported from the intestine, and it is the first organ to come into contact with intestinal microbes and their components and metabolites [46]. Consistently, the transcriptome was enriched for bacterial immune responses and bacterial infections. LPS is the central mediator of liver inflammation [47]. LPS or free fatty acids (FFA) mediated activation of TLR4 signaling enhanced inflammatory responses and ROS production [48], NLRP3 inflammasome-related IL-1β production, and NF-κB mediated TNF-α production [49]. HE stained, protein and mRNA expression of inflammatory cytokines indicated that PS-MPs induced inflammatory response in chicken liver.
Lipid deposition is one of the main ways that microplastics exert hepatotoxicity. Saturate fatty acids (SFAs) induce hepatocyte apoptosis by activating the mitochondrial death pathway and increasing ER stress [50]. In this study, the most differential metabolites in both the L-PS group and the H-PS group are lipids and lipid molecules. The transcriptome analysis pathway is enriched in steroid biosynthesis and lipid synthesis. Lipid accumulation is regulated by fatty acid synthesis, transport, metabolism, and various signaling pathways [51]. Mitochondria and endoplasmic reticulum are key organelles involved in lipid metabolism. In this study, mitochondrial damage occurred in the duodenum, cecum and liver, and the liver transcriptome DEGs were enriched to oxygen binding and oxygen carrier activity, indicating that mitochondria may be the target organs of PS-MPs toxicity. Mitochondria have a variety of biological functions and participate in the regulation of ATP production, apoptosis, lipid metabolism, calcium signaling and other biological processes [52]. They continue to carry out a coordinated cycle of division and fusion to maintain metabolic homeostasis [53]. The endoplasmic reticulum (ER) and mitochondria interact physically and functionally. ER stress increases ER-mitochondrial coupling, thereby improving mitochondrial respiration and bioenergy [54]. The ER is the main site of lipid synthesis of hepatocytes. The homeostasis of the liver ER is the key to maintaining the composition of membrane lipids and controlling the lipid homeostasis in the liver and plasma. Under high fructose feeding, the inhibition of PERK-eIF2α-ATF4 can protect mice from steatosis by reducing neonatal adipogenesis [55]. ATF6 interacts with PPARα to enhance its transcription activity on liver fatty acid oxidation [56]. ER stress is also involved in regulating the inflammatory. ATF6 activates NF-κB through the phosphorylation of AKT. IRE1α and PERK are essential for the activation of NF-κB induced by endogenous stress [57]. Inflammatory factors are also involved in the process of regulating lipid metabolism. Both TNF-α and IL-1β directly inhibit the activation of PPARα [58]. TNF-α inhibits β-oxidation by inhibiting peroxisome fatty acyl-CoA oxidase (ACOX2) [59]. In short, PS-MPs exposure disrupted mitochondrial dynamics and leads to impaired mitochondrial function, trigger ER stress, resulting in increased lipid synthesis, interference with fatty acid transport, and inhibition of β-oxidation to promote lipid deposition.
The occurrence of oxidative stress is the main ways for MPs to exert toxic damage [60]. During ER stress, Ca2+ leaked from the ER is absorbed by mitochondria, leading to the opening of transition pores and the release of cytochrome c, which leads to an increase in ROS [61]. Transcriptome data analysis showed that the most enriched DEGs in the L-PS group was steroid biosynthesis. Steroids include cholesterol, bile acids, steroid hormones and many other substances[62]. Cholesterol induces mitochondrial dysfunction and increases the production of ROS [63]. The increase in oxidative stress leads to the auto-oxidation of oxysterols produced by excessive cholesterol, and promotes the occurrence of inflammation and apoptosis [64]. Glutamate drives the synthesis of glutathione for antioxidant function [65]. After PS-MPs treatmented, glutamine and glutamate synthesis in metabolites were significantly enriched, and the activity of antioxidant enzymes in the liver was reduced, indicating that chicken livers suffered from oxidative stress.
In fact, mitochondrial damage, ER stress and oxidative stress, they all lead to cell death, especially cell apoptosis[66]. In this study, the most enriched KEGG pathway in liver metabolomics analysis is apoptosis. KCs in the liver also swallow apoptotic bodies, increase TNF and TRAIL, and further promote hepatocytes apoptosis to cause liver inflammation and fibrosis [67]. Interestingly, although P53 promoted the occurrence of apoptosis, PS-MPs reduced the expression of P53. As a transcription factor, p53 regulates a variety of biological processes, but the most important thing is to hinder the occurrence of cancer [68]. Inactivation of P53 is the most common mutation in human cancer. The inheritance of mutant P53 alleles makes patients susceptible to early cancer [69]. The transcriptome results showed that DEGs in the liver after exposure to PS-MPs were enriched in cancer. This suggests that avian exposed to PS-MPs are at risk of developing cancer.
In this study, we also found increased synthesis of some metabolites, namely caffeine and melanin. Caffeine reduces intrahepatic lipid content by stimulating β-oxidation and autophagy-lysosomal pathways [70]. And in a mouse model of disseminated salmonellosis, caffeine also has anti-inflammatory and anti-infective effects [71]. Melanin has a strong ROS scavenging ability and can promote the polarization of macrophages for cardiac repair [72]. Similar melanin also has good anti-inflammatory properties and can prevent and treat fatty liver disease [73]. These two metabolites may be resistant substances produced by the body in response to the toxic damage to PS-MPs exposure, and may serve as potential antagonists of microplastics. We compared metabolomic results from species such as oysters [74], zebrafish [75], earthworms, microbes [76], microcystis [77], and maize [78], where fatty acid metabolism and energy metabolism were commonly enriched metabolic pathways. Notably, the same differential metabolic pathways as in this study emerged only from earthworms, which were aminoacyl-tRNA biosynthesis and glutamine and glutamate metabolism [79]. This may be due to structural differences between terrestrial and aquatic animals, and we still need more research to confirm this.
Conclusion
This study showed that even exposure to higher concentrations of PS-MPs did not cause significant mortality in avian, but caused significant damage to the digestive system. Possibly due to differences in physiology, PS-MPs exposure induced pyroptosis and necrosis of intestinal tissue instead of apoptosis, and gut GVB structure was disrupted and permeability increased. 16sRNA and liver transcriptomic results indicated that the gut microbiota was perturbed by PS-MPs, and that harmful bacteria and the resulting metabolites were involved in liver injury through translocation of the gut-liver axis. Metabolomic and transcriptomic data showed that PS-MPs induced liver injury was mainly caused by lipid metabolism disorder and apoptosis. Metabolomics suggested that melanin and caffeine may be natural microplastics resistant to toxicity. The results of this study fill a gap in the research on microplastic toxicity to avian and reveal the important role of the gut-liver axis in microplastic damaged.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
This review was funded by Heilongjiang Provincial Natural Science Foundation (ZD2020C005) and the Fundamental Research Funds for the Central Universities (2572021AW04).
Compliance with Ethics Requirements
All Institutional and National Guidelines for the care and use of animals (fisheries) were followed.
Footnotes
All authors have read the manuscript and agreed to submit it in its current form for consideration for publication in the Journal.
Peer review under responsibility of Cairo University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2022.10.015.
Contributor Information
Yu Wang, Email: wangyu2013@nefu.edu.cn.
Mingwei Xing, Email: xingmingwei@nefu.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Lindeque P.K., Cole M., Coppock R.L., Lewis C.N., Miller R.Z., Watts A.J.R., et al. Are we underestimating microplastic abundance in the marine environment? A comparison of microplastic capture with nets of different mesh-size. Environ Pollut. 2020;265:12. doi: 10.1016/j.envpol.2020.114721. [DOI] [PubMed] [Google Scholar]
- 2.Grbic J., Helm P., Athey S., Rochman C.M. Microplastics entering northwestern Lake Ontario are diverse and linked to urban sources. Water Res. 2020;174:10. doi: 10.1016/j.watres.2020.115623. [DOI] [PubMed] [Google Scholar]
- 3.Wright S.L., Ulke J., Font A., Chan K.L.A., Kelly F.J. Atmospheric microplastic deposition in an urban environment and an evaluation of transport. Environ Int. 2020;136:7. doi: 10.1016/j.envint.2019.105411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yao L.M., Hui L., Yang Z., Chen X.D., Xiao A.R. Freshwater microplastics pollution: Detecting and visualizing emerging trends based on Citespace II. Chemosphere. 2020;245:8. doi: 10.1016/j.chemosphere.2019.125627. [DOI] [PubMed] [Google Scholar]
- 5.Horton A.A., Walton A., Spurgeon D.J., Lahive E., Svendsen C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci Total Environ. 2017;586:127–141. doi: 10.1016/j.scitotenv.2017.01.190. [DOI] [PubMed] [Google Scholar]
- 6.Brookson C.B., De Solla S.R., Fernie K.J., Cepeda M., Rochman C.M. Microplastics in the diet of nestling double-crested cormorants (Phalacrocorax auritus), an obligate piscivore in a freshwater ecosystem. Can J Fish Aquat Sci. 2019;76(11):2156–2163. doi: 10.1139/cjfas-2018-0388. [DOI] [Google Scholar]
- 7.Bhagat J., Zang L.Q., Nishimura N., Shimada Y. Zebrafish: An emerging model to study microplastic and nanoplastic toxicity. Sci Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138707. [DOI] [PubMed] [Google Scholar]
- 8.Prokic M.D., Gavrilovic B.R., Radovanovic T.B., Gavric J.P., Petrovic T.G., Despotovic S.G., et al. Studying microplastics: Lessons from evaluated literature on animal model organisms and experimental approaches. J Hazard Mater. 2021;414 doi: 10.1016/j.jhazmat.2021.125476. [DOI] [PubMed] [Google Scholar]
- 9.Wilcox C., Van Sebille E., Hardesty B.D. Threat of plastic pollution to seabirds is global, pervasive, and increasing. Proc Natl Acad Sci U S A. 2015;112(38):11899–11904. doi: 10.1073/pnas.1502108112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winkler A., Nessi A., Antonioli D., Laus M., Tremolada P. Occurrence of microplastics in pellets from the common kingfisher (Alcedo atthis) along the Ticino River, North Italy. Environ Sci Pollut Res. 2020;1–2 doi: 10.1007/s11356-020-10163-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marana M.H., Poulsen R., Thormar E.A., Clausen C.G., Thit A., Mathiessen H., et al. Plastic nanoparticles cause mild inflammation, disrupt metabolic pathways, change the gut microbiota and affect reproduction in zebrafish: A full generation multi-omics study. J Hazard Mater. 2022;424 doi: 10.1016/j.jhazmat.2021.127705. [DOI] [PubMed] [Google Scholar]
- 12.Duan Y., Llorente C., Lang S., Brandl K., Chu H.K., Jiang L., et al. Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature. 2019;575(7783):505. doi: 10.1038/s41586-019-1742-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albillos A., de Gottardi A., Rescigno M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J Hepatol. 2020;72(3):558–577. doi: 10.1016/j.jhep.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Han Y.H., Onufer E.J., Huang L.H., Sprung R.W., Davidson W.S., Czepielewski R.S., et al. Enterically derived high-density lipoprotein restrains liver injury through the portal vein. Science. 2021;373(6553):410. doi: 10.1126/science.abe6729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brescia P., Rescigno M. The gut vascular barrier: a new player in the gut–liver–brain axis. Trends Mol Med. 2021;27(9):844–855. doi: 10.1016/j.molmed.2021.06.007. [DOI] [PubMed] [Google Scholar]
- 16.Odenwald M.A., Turner J.R. The intestinal epithelial barrier: a therapeutic target? Nat Rev Gastroenterol Hepatol. 2017;14(1):9–21. doi: 10.1038/nrgastro.2016.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aron-Wisnewsky J., Warmbrunn M.V., Nieuwdorp M., Clément K. Nonalcoholic Fatty Liver Disease: Modulating Gut Microbiota to Improve Severity? Gastroenterology. 2020;158(7):1881–1898. doi: 10.1053/j.gastro.2020.01.049. [DOI] [PubMed] [Google Scholar]
- 18.Lu L., Wan Z.Q., Luo T., Fu Z.W., Jin Y.X. Polystyrene microplastics induce gut microbiota dysbiosis and hepatic lipid metabolism disorder in mice. Sci Total Environ. 2018;631–632:449–458. doi: 10.1016/j.scitotenv.2018.03.051. [DOI] [PubMed] [Google Scholar]
- 19.Sun T., Zhan J.F., Li F., Ji C.L., Wu H.F. Environmentally relevant concentrations of microplastics influence the locomotor activity of aquatic biota. J Hazard Mater. 2021;414 doi: 10.1016/j.jhazmat.2021.125581. [DOI] [PubMed] [Google Scholar]
- 20.Sun T., Zhan J., Li F., Ji C., Wu H. Effect of microplastics on aquatic biota: A hormetic perspective. Environmental pollution (Barking. Essex. 1987;2021(285) doi: 10.1016/j.envpol.2021.117206. [DOI] [PubMed] [Google Scholar]
- 21.Yin K., Wang D., Zhao H., Wang Y., Zhang Y., Liu Y., et al. Polystyrene microplastics up-regulates liver glutamine and glutamate synthesis and promotes autophagy-dependent ferroptosis and apoptosis in the cerebellum through the liver-brain axis. Environmental pollution (Barking, Essex. 1987;2022(307) doi: 10.1016/j.envpol.2022.119449. [DOI] [PubMed] [Google Scholar]
- 22.Vacca M., Celano G., Calabrese F.M., Portincasa P., Gobbetti M., De Angelis M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms. 2020;8(4) doi: 10.3390/microorganisms8040573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Annunziato M., Eeza M.N.H., Bashirova N., Lawson A., Matysik J., Benetti D., et al. An integrated systems-level model of the toxicity of brevetoxin based on high-resolution magic-angle spinning nuclear magnetic resonance (HRMAS NMR) metabolic profiling of zebrafish embryos. Sci Total Environ. 2022;803 doi: 10.1016/j.scitotenv.2021.149858. [DOI] [PubMed] [Google Scholar]
- 24.Yin K., Wang Y., Zhao H.J., Wang D.X., Guo M.H., Mu M.Y., et al. A comparative review of microplastics and nanoplastics: Toxicity hazards on digestive, reproductive and nervous system. Sci Total Environ. 2021;774 doi: 10.1016/j.scitotenv.2021.145758. [DOI] [Google Scholar]
- 25.Yin K., Wang D., Zhao H., Wang Y., Guo M., Liu Y., et al. Microplastics pollution and risk assessment in water bodies of two nature reserves in Jilin Province: Correlation analysis with the degree of human activity. The Science of the total environment. 2021;799 doi: 10.1016/j.scitotenv.2021.149390. [DOI] [PubMed] [Google Scholar]
- 26.Li Z.L., Feng C.H., Pang W., Tian C.H., Zhao Y. Nanoplastic-Induced Genotoxicity and Intestinal Damage in Freshwater Benthic Clams (Corbicula fluminea): Comparison with Microplastics. ACS Nano. 2021;15(6):9469–9481. doi: 10.1021/acsnano.1c02407. [DOI] [PubMed] [Google Scholar]
- 27.Schwarzer R., Laurien L., Pasparakis M. New insights into the regulation of apoptosis, necroptosis, and pyroptosis by receptor interacting protein kinase 1 and caspase-8. Curr Opin Cell Biol. 2020;63:186–193. doi: 10.1016/j.ceb.2020.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Tummers B., Green D.R. Caspase-8: regulating life and death. Immunol Rev. 2017;277(1):76–89. doi: 10.1111/imr.12541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fritsch M., Gunther S.D., Schwarzer R., Albert M.C., Schorn F., Werthenbach J.P., et al. Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature. 2019;575(7784):683-+. doi: 10.1038/s41586-019-1770-6. [DOI] [PubMed] [Google Scholar]
- 30.Gao X.L., Guo R., Li Y.H., Kang G.L., Wu Y., Cheng J., et al. Contribution of upregulated aminoacyl-tRNA biosynthesis to metabolic dysregulation in gastric cancer. J Gastroenterol Hepatol. 2021;36(11):3113–3126. doi: 10.1111/jgh.15592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Z, Feng C, Pang W, Tian C, Zhao YJAn. Corbicula flumineaNanoplastic-Induced Genotoxicity and Intestinal Damage in Freshwater Benthic Clams (Corbicula fluminea): Comparison with Microplastics. 2021;15(6):9469-81. 10.1021/acsnano.1c02407. [DOI] [PubMed]
- 32.Xu D.H., Ma Y.H., Han X.D., Chen Y.B. Systematic toxicity evaluation of polystyrene nanoplastics on mice and molecular mechanism investigation about their internalization into Caco-2 cells. J Hazard Mater. 2021:417. doi: 10.1016/j.jhazmat.2021.126092. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y., Wang S., Olga V., Xue Y., Lv S., Diao X., et al. The potential effects of microplastic pollution on human digestive tract cells. Chemosphere. 2022;291 doi: 10.1016/j.chemosphere.2021.132714. [DOI] [PubMed] [Google Scholar]
- 34.Hu J.H., Fuller L., McDougald L.R. Infection of turkeys with Histomonas meleagridis by the cloacal drop le method. Avian Dis. 2004;48(4):746–750. doi: 10.1637/7152. [DOI] [PubMed] [Google Scholar]
- 35.Moya-Pérez A., Neef A., Sanz Y. Bifidobacterium pseudocatenulatum CECT 7765 Reduces Obesity-Associated Inflammation by Restoring the Lymphocyte-Macrophage Balance and Gut Microbiota Structure in High-Fat Diet-Fed Mice. PLoS ONE. 2015;10(7):e0126976. doi: 10.1371/journal.pone.0126976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Biddle A., Stewart L., Blanchard J., Leschine S.J.D. Untangling the Genetic Basis of Fibrolytic Specialization by Lachnospiraceae and Ruminococcaceae in Diverse Gut Communities. 2013;5(3):627–640. [Google Scholar]
- 37.Kimura I., Ozawa K., Inoue D., Imamura T., Kimura K., Maeda T., et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun. 2013:4. doi: 10.1038/ncomms2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Donohoe D.R., Collins L.B., Wali A., Bigler R., Sun W., Bultman S.J. The Warburg Effect Dictates the Mechanism of Butyrate-Mediated Histone Acetylation and Cell Proliferation. Mol Cell. 2012;48(4):612–626. doi: 10.1016/j.molcel.2012.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aoki R., Onuki M., Hattori K., Ito M., Yamada T., Kamikado K., et al. Commensal microbe-derived acetate suppresses NAFLD/NASH development via hepatic FFAR2 signalling in mice. Microbiome. 2021;9(1) doi: 10.1186/s40168-021-01125-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raimondi S., Musmeci E., Candeliere F., Amaretti A., Rossi M. Identification of mucin degraders of the human gut microbiota. Sci Rep. 2021;11(1) doi: 10.1038/s41598-021-90553-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu L., Chen C., Liu X., Chen B., Ding C., Liang J. Altered Gut Microbiota Associated With Hemorrhage in Chronic Radiation Proctitis. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.637265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tytgat H.L.P., Nobrega F.L., van der Oost J., de Vos W.M. Bowel Biofilms: Tipping Points between a Healthy and Compromised Gut? Trends Microbiol. 2019;27(1):17–25. doi: 10.1016/j.tim.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 43.Hoarau G., Mukherjee P.K., Gower-Rousseau C., Hager C., Chandra J., Retuerto M.A., et al. Bacteriome and Mycobiome Interactions Underscore Microbial Dysbiosis in Familial Crohn's Disease. Mbio. 2016;7(5) doi: 10.1128/mBio.01250-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xue Z., Ansari A.R., Zhao X., Zang K., Liu H. Rna-seq-based gene expression pattern and morphological alterations in chick thymus during postnatal development. International Journal of Genomics. 2019;5:1–11. doi: 10.1155/2019/6905194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haibo, Huang, Liu, Hui, Wu, & Rahman, A. , et al. Transcriptome analysis indicated that salmonella lipopolysaccharide-induced thymocyte death and thymic atrophy were related to tlr4-fos/jun pathway in chicks. BMC genomics, 2016, 322. 10.1186/s12864-016-2674-6. [DOI] [PMC free article] [PubMed]
- 46.Mazagova M., Wang L.R., Anfora A.T., Wissmueller M., Lesley S.A., Miyamoto Y., et al. Commensal microbiota is hepatoprotective and prevents liver fibrosis in mice. Faseb J. 2015;29(3):1043–1055. doi: 10.1096/fj.14-259515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soares J.B., Pimentel-Nunes P., Roncon-Albuquerque R., Leite-Moreira A. The role of lipopolysaccharide/toll-like receptor 4 signaling in chronic liver diseases. Hepatol Int. 2010;4(4):659–672. doi: 10.1007/s12072-010-9219-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferro D., Baratta F., Pastori D., Cocomello N., Colantoni A., Angelico F., et al. New Insights into the Pathogenesis of Non-Alcoholic Fatty Liver Disease: Gut-Derived Lipopolysaccharides and Oxidative Stress. Nutrients. 2020;12(9):14. doi: 10.3390/nu12092762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Csak T., Ganz M., Pespisa J., Kodys K., Dolganiuc A., Szabo G. Fatty Acid and Endotoxin Activate Inflammasomes in Mouse Hepatocytes that Release Danger Signals to Stimulate Immune Cells. Hepatology. 2011;54(1):133–144. doi: 10.1002/hep.24341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leamy A.K., Egnatchik R.A., Young J.D. Molecular mechanisms and the role of saturated fatty acids in the progression of non-alcoholic fatty liver disease. Prog Lipid Res. 2013;52(1):165–174. doi: 10.1016/j.plipres.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scorletti E, Byrne CD. Omega-3 Fatty Acids, Hepatic Lipid Metabolism, and Nonalcoholic Fatty Liver Disease. In: Cousins RJ, editor. Annual Review of Nutrition, Vol 33. Annual Review of Nutrition. 33. Palo Alto: Annual Reviews; 2013. p. 231-48. [DOI] [PubMed]
- 52.Zemirli N., Morel E., Molino D. Mitochondrial Dynamics in Basal and Stressful Conditions. Int J Mol Sci. 2018;19(2):19. doi: 10.3390/ijms19020564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liesa M., Shirihai O.S. Mitochondrial Dynamics in the Regulation of Nutrient Utilization and Energy Expenditure. Cell Metab. 2013;17(4):491–506. doi: 10.1016/j.cmet.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bravo R., Vicencio J.M., Parra V., Troncoso R., Munoz J.P., Bui M., et al. Increased ER-mitochondrial coupling promotes mitochondrial respiration and bioenergetics during early phases of ER stress. 2011;124(14):2511- doi: 10.1242/jcs.080762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xiao G.Z., Zhang T., Yu S.B., Lee S.J., Calabuig-Navarro V., Yamauchi J., et al. ATF4 Protein Deficiency Protects against High Fructose-induced Hypertriglyceridemia in Mice. J Biol Chem. 2013;288(35):25350–25361. doi: 10.1074/jbc.M113.470526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen X.Q., Zhang F.F., Gong Q., Cui A.Y., Zhuo S., Hu Z.M., et al. Hepatic ATF6 Increases Fatty Acid Oxidation to Attenuate Hepatic Steatosis in Mice Through Peroxisome Proliferator-Activated Receptor alpha. Diabetes. 2016;65(7):1904–1915. doi: 10.2337/db15-1637. [DOI] [PubMed] [Google Scholar]
- 57.Yamazaki H., Hiramatsu N., Hayakawa K., Tagawa Y., Okamura M., Ogata R., et al. Activation of the Akt-NF-kappa B Pathway by Subtilase Cytotoxin through the ATF6 Branch of the Unfolded Protein Response. J Immunol. 2009;183(2):1480–1487. doi: 10.4049/jimmunol.0900017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beier K., Völkl A., Fahimi H.D. TNF-α downregulates the peroxisome proliferator activated receptor-α and the mRNAs encoding peroxisomal proteins in rat liver. FEBS Lett. 1997;412(2):385–387. doi: 10.1016/S0014-5793(97)00805-3. [DOI] [PubMed] [Google Scholar]
- 59.Beier K., Völkl A., Fahimi H.D. Suppression of peroxisomal lipid β-oxidation enzymes by TNF-α. FEBS Lett. 1992;310(3):273–276. doi: 10.1016/0014-5793(92)81347-O. [DOI] [PubMed] [Google Scholar]
- 60.Shengchen W., Jing L., Yujie Y., Yue W., Shiwen X. Polystyrene microplastics-induced ROS overproduction disrupts the skeletal muscle regeneration by converting myoblasts into adipocytes. J Hazard Mater. 2021;417 doi: 10.1016/j.jhazmat.2021.125962. [DOI] [PubMed] [Google Scholar]
- 61.Magee N., Zou A., Zhang Y.X. Pathogenesis of Nonalcoholic Steatohepatitis: Interactions between Liver Parenchymal and Nonparenchymal Cells. Biomed Research. International. 2016; 2016. doi: 10.1155/2016/5170402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang S.S., Sheng F.Y., Zou L., Xiao J.B., Li P. Hyperoside attenuates non-alcoholic fatty liver disease in rats via cholesterol metabolism and bile acid metabolism. J Adv Res. 2021;34:109–122. doi: 10.1016/j.jare.2021.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Min H.K., Kapoor A., Fuchs M., Mirshahi F., Zhou H.P., Maher J., et al. Increased Hepatic Synthesis and Dysregulation of Cholesterol Metabolism Is Associated with the Severity of Nonalcoholic Fatty Liver Disease. Cell Metab. 2012;15(5):665–674. doi: 10.1016/j.cmet.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Choi S., Diehl A.M. Role of inflammation in nonalcoholic steatohepatitis. Current Opinion in Gastroenterology. 2005;21(6):702–707. doi: 10.1097/01.mog.0000182863.96421.47. [DOI] [PubMed] [Google Scholar]
- 65.Sappington D.R., Siegel E.R., Hiatt G., Desai A., Penney R.B., Jamshidi-Parsian A., et al. Glutamine drives glutathione synthesis and contributes to radiation sensitivity of A549 and H460 lung cancer cell lines. Biochimica Et Biophysica Acta-General Subjects. 2016;1860(4):836–843. doi: 10.1016/j.bbagen.2016.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lian C.-Y., Chu B.-X., Xia W.-H., Wang Z.-Y., Fan R.-F., Wang L. Persistent activation of Nrf2 in a p62-dependent non-canonical manner aggravates lead-induced kidney injury by promoting apoptosis and inhibiting autophagy. J Adv Res. 2022 doi: 10.1016/j.jare.2022.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Canbay A., Feldstein A.E., Higuchi H., Werneburg N., Grambihler A., Bronk S.F., et al. Kupffer cell engulfment of apoptotic bodies stimulates death ligand and cytokine expression. Hepatology. 2003;38(5):1188–1198. doi: 10.1053/jhep.2003.50472. [DOI] [PubMed] [Google Scholar]
- 68.Boutelle A.M., Attardi L.D. p53 and Tumor Suppression: It Takes a Network. Trends Cell Biol. 2021;31(4):298–310. doi: 10.1016/j.tcb.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kandoth C., McLellan M.D., Vandin F., Ye K., Niu B.F., Lu C., et al. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502(7471):333. doi: 10.1038/nature12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sinha R.A., Farah B.L., Singh B.K., Siddique M.M., Li Y., Wu Y.J., et al. Caffeine Stimulates Hepatic Lipid Metabolism by the Autophagy-Lysosomal Pathway in Mice. Hepatology. 2014;59(4):1366–1380. doi: 10.1002/hep.26667. [DOI] [PubMed] [Google Scholar]
- 71.Dorvigny B., Tavares L., de Almeida I., Santana L., de Souza S.E., de Souza J., et al. Antiinflammatory and antiinfective effect of caffeine in a mouse model of disseminated salmonellosis. 2021 doi: 10.1002/ptr.7349. [DOI] [PubMed] [Google Scholar]
- 72.Zhou J., Liu W., Zhao X.Y., Xian Y.F., Wu W., Zhang X., et al. Natural Melanin/Alginate Hydrogels Achieve Cardiac Repair through ROS Scavenging and Macrophage Polarization. Advanced. Science. 2021;8(20) doi: 10.1002/advs.202100505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Page S., Chandhoke V., Baranova A. Melanin and melanogenesis in adipose tissue: possible mechanisms for abating oxidative stress and inflammation? Obes Rev. 2011;12(501):e21–e31. doi: 10.1111/j.1467-789X.2010.00773.x. [DOI] [PubMed] [Google Scholar]
- 74.Huang W., Wang X.H., Chen D.Y., Xu E.G., Luo X., Zeng J.N., et al. Toxicity mechanisms of polystyrene microplastics in marine mussels revealed by high-coverage quantitative metabolomics using chemical isotope labeling liquid chromatography mass spectrometry. J Hazard Mater. 2021:417. doi: 10.1016/j.jhazmat.2021.126003. [DOI] [PubMed] [Google Scholar]
- 75.Marana M.H., Poulsen R., Thormar E.A., Clausen C.G., Thit A., Mathiessen H., et al. Plastic nanoparticles cause mild inflammation, disrupt metabolic pathways, change the gut microbiota and affect reproduction in zebrafish: A full generation multi-omics study. J Hazard Mater. 2022:424. doi: 10.1016/j.jhazmat.2021.127705. [DOI] [PubMed] [Google Scholar]
- 76.Wu C.C., Ma Y.J., Wang D., Shan Y.P., Song X.P., Hu H.Y., et al. Integrated microbiology and metabolomics analysis reveal plastic mulch film residue affects soil microorganisms and their metabolic functions. J Hazard Mater. 2022:423. doi: 10.1016/j.jhazmat.2021.127258. [DOI] [PubMed] [Google Scholar]
- 77.Cao J., Liao Y.C., Yang W.S., Jiang X.F., Li M. Enhanced microalgal toxicity due to polystyrene nanoplastics and cadmium co-exposure: From the perspective of physiological and metabolomic profiles. J Hazard Mater. 2022:427. doi: 10.1016/j.jhazmat.2021.127937. [DOI] [PubMed] [Google Scholar]
- 78.Zhang Y., Yang X., Luo Z.X., Lai J.L., Li C., Luo X.G. Effects of polystyrene nanoplastics (PSNPs) on the physiology and molecular metabolism of corn (Zea mays L.) seedlings. Sci Total Environ. 2022:806. doi: 10.1016/j.scitotenv.2021.150895. [DOI] [PubMed] [Google Scholar]
- 79.Chen K., Tang R., Luo Y., Chen Y., Ei-Naggar A., Du J., et al. Transcriptomic and metabolic responses of earthworms to contaminated soil with polypropylene and polyethylene microplastics at environmentally relevant concentrations. J Hazard Mater. 2022;427 doi: 10.1016/j.jhazmat.2021.128176. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.