Abstract
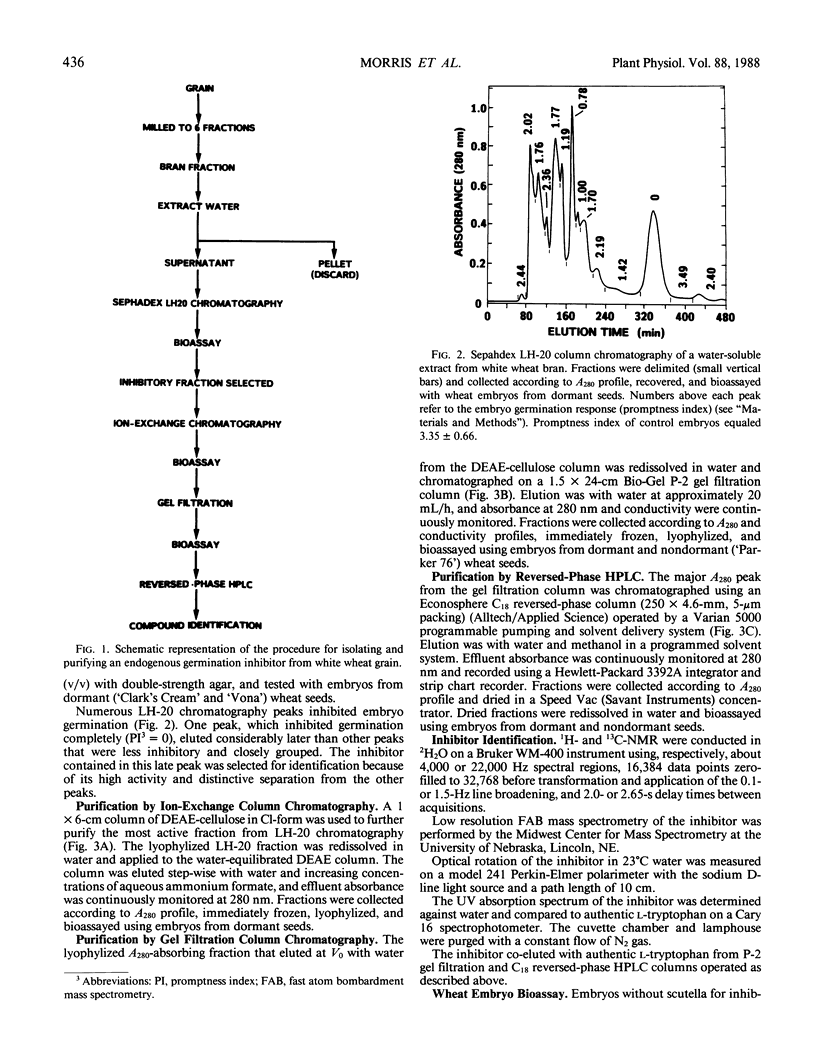
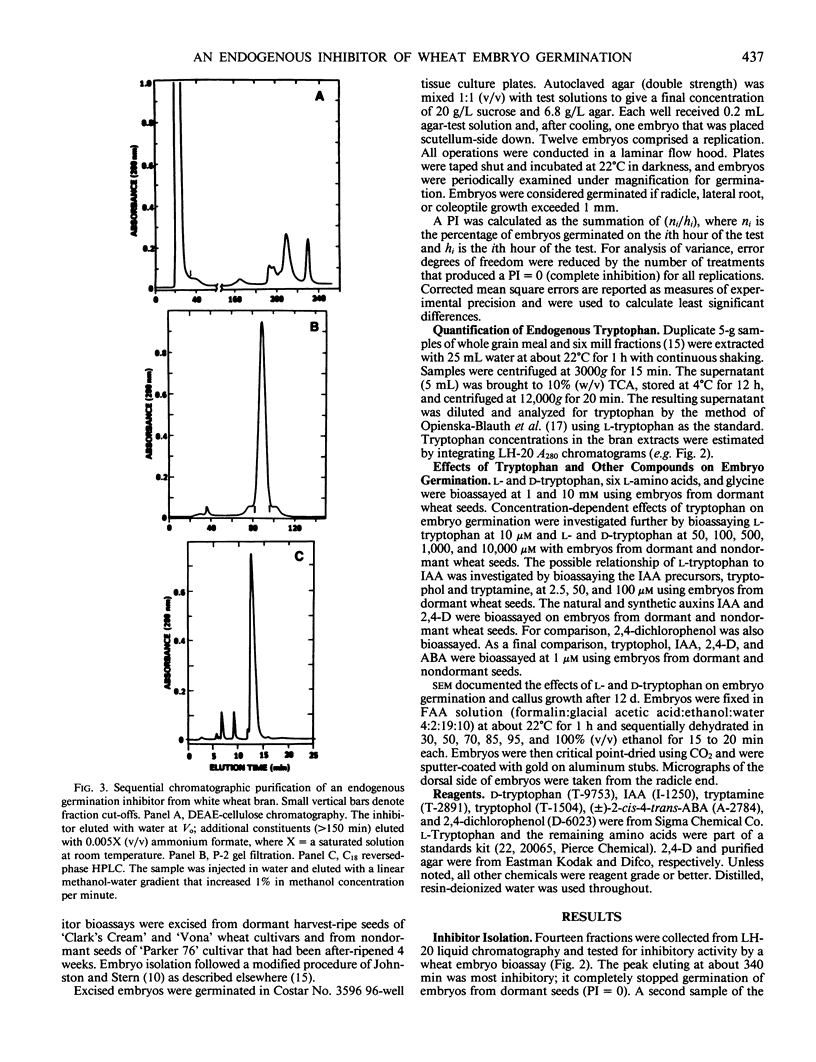
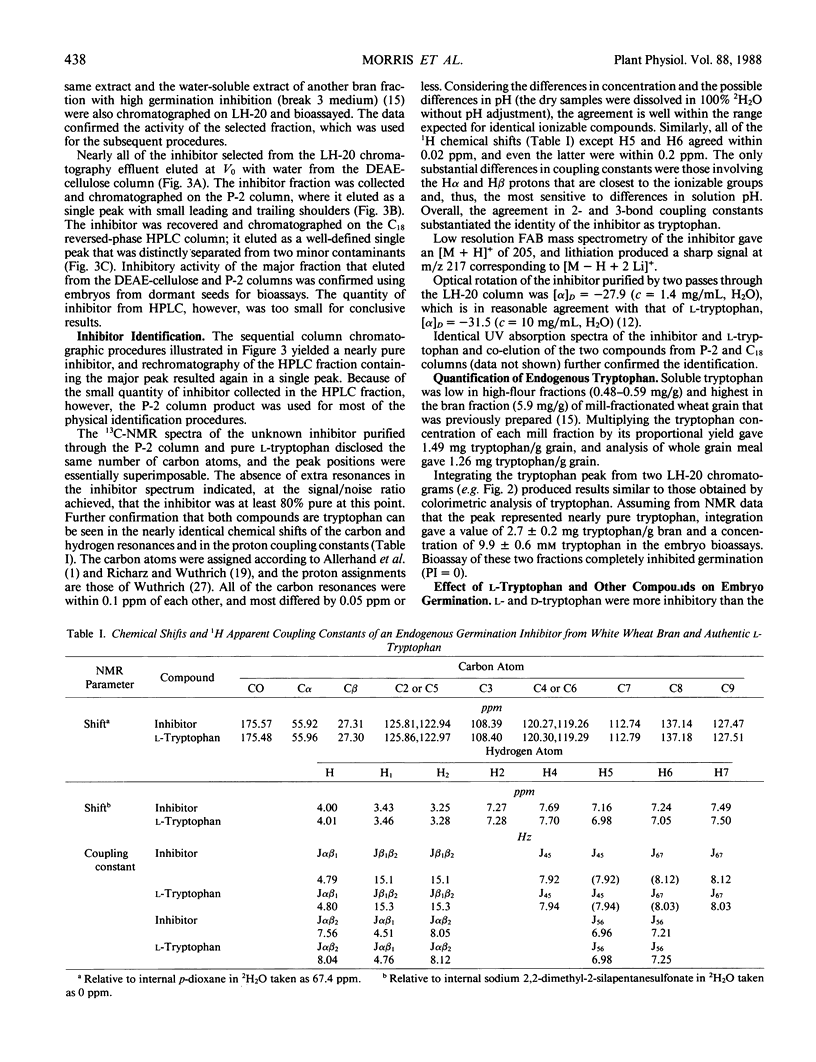
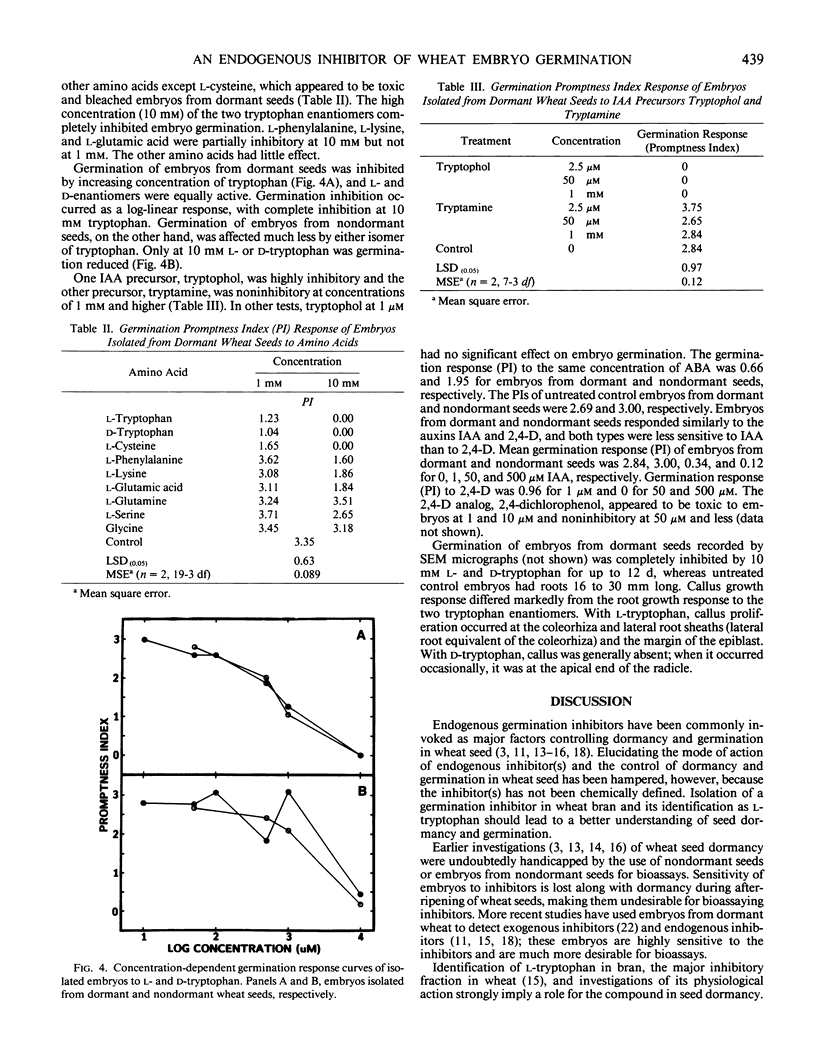
An endogenous germination inhibitor(s) in wheat (Triticum aestivum L.) grain has been implicated in seed dormancy and germination, but its identity and mode of action have not been elucidated. We isolated and identified an endogenous germination inhibitor in white wheat grain and compared its activity with that of known compounds. A water extract of wheat bran chromatographed on Sephadex LH-20 yielded an inhibitory fraction that was detected by bioassay on embryos excised from dormant wheat seeds. The inhibitor was sequentially purified by DEAE-cellulose, P-2 gel filtration, and C18 reversed-phase HPLC and identified as l-tryptophan by carbon and proton nuclear magnetic resonance spectroscopy, mass spectrometry, optical rotation, UV absorption spectrum, and chromatographic co-elution with authentic tryptophan. Extractable tryptophan occurred in wheat grain and bran at about 1.3 to 1.5 and 2.7 to 5.9 milligrams per gram, respectively. Low millimolar concentrations of l-tryptophan similar to physiological levels and d-tryptophan were more inhibitory to embryos from dormant seeds than to embryos from nondormant seeds. Tryptophol and tryptamine, putative intermediates between tryptophan and IAA, were highly inhibitory and noninhibitory of embryo germination, respectively. IAA and 2,4-D also inhibited germination, but embryos from dormant and nondormant seeds responded similarly. l-Tryptophan may be an important germination inhibitor of excised embryos, but its role in situ remains to be determined.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allerhand A., Childers R. F., Oldfield E. Natrual-abundance carbon-13 nuclear magnetic resonance studies in 20-mm sample tubes. Observation of numerous single-carbon resonances of hen egg-white lysozyme. Biochemistry. 1973 Mar 27;12(7):1335–1341. doi: 10.1021/bi00731a013. [DOI] [PubMed] [Google Scholar]
- JOHNSTON F. B., STERN H. Mass isolation of viable wheat embryos. Nature. 1957 Jan 19;179(4551):160–161. doi: 10.1038/179160b0. [DOI] [PubMed] [Google Scholar]
- Miyamoto T., Tolbert N. E., Everson E. H. Germination inhibitors related to dormancy in wheat seeds. Plant Physiol. 1961 Nov;36(6):739–746. doi: 10.1104/pp.36.6.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OPIENSKA-BLAUTH J., CHAREZINSKI M., BERBEC H. A new, rapid method of determining tryptophan. Anal Biochem. 1963 Jul;6:69–76. doi: 10.1016/0003-2697(63)90009-5. [DOI] [PubMed] [Google Scholar]
- Walker-Simmons M. ABA Levels and Sensitivity in Developing Wheat Embryos of Sprouting Resistant and Susceptible Cultivars. Plant Physiol. 1987 May;84(1):61–66. doi: 10.1104/pp.84.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]


