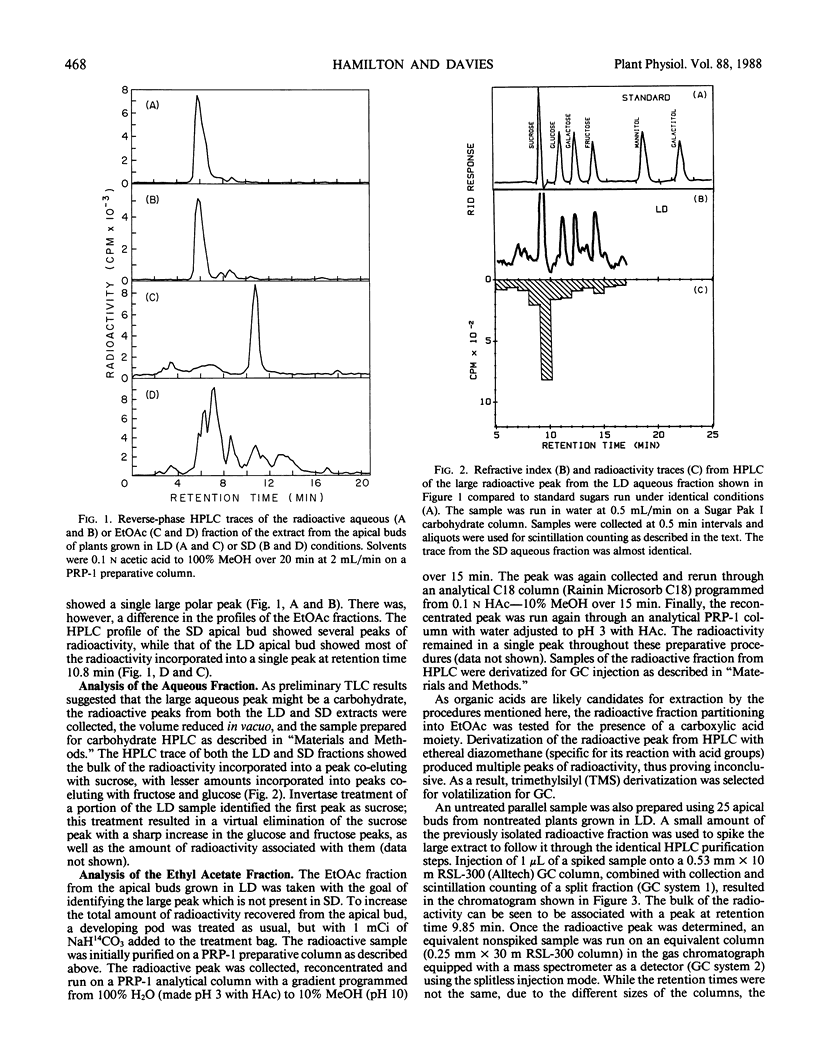
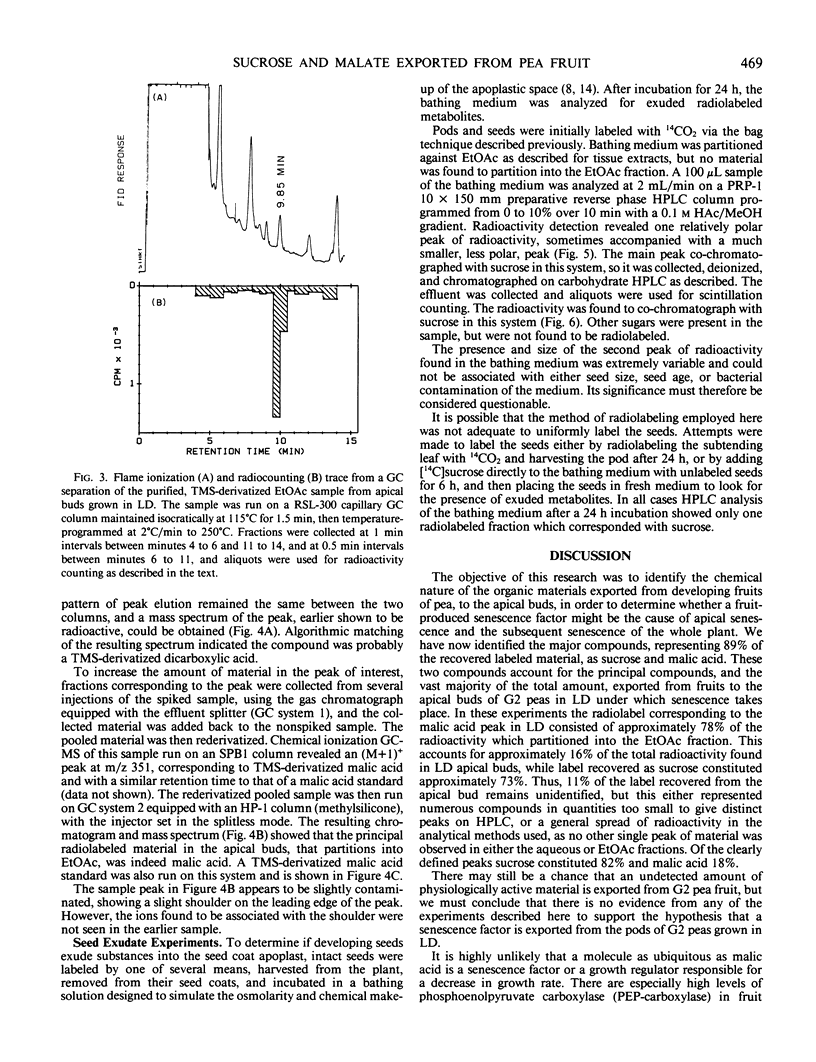
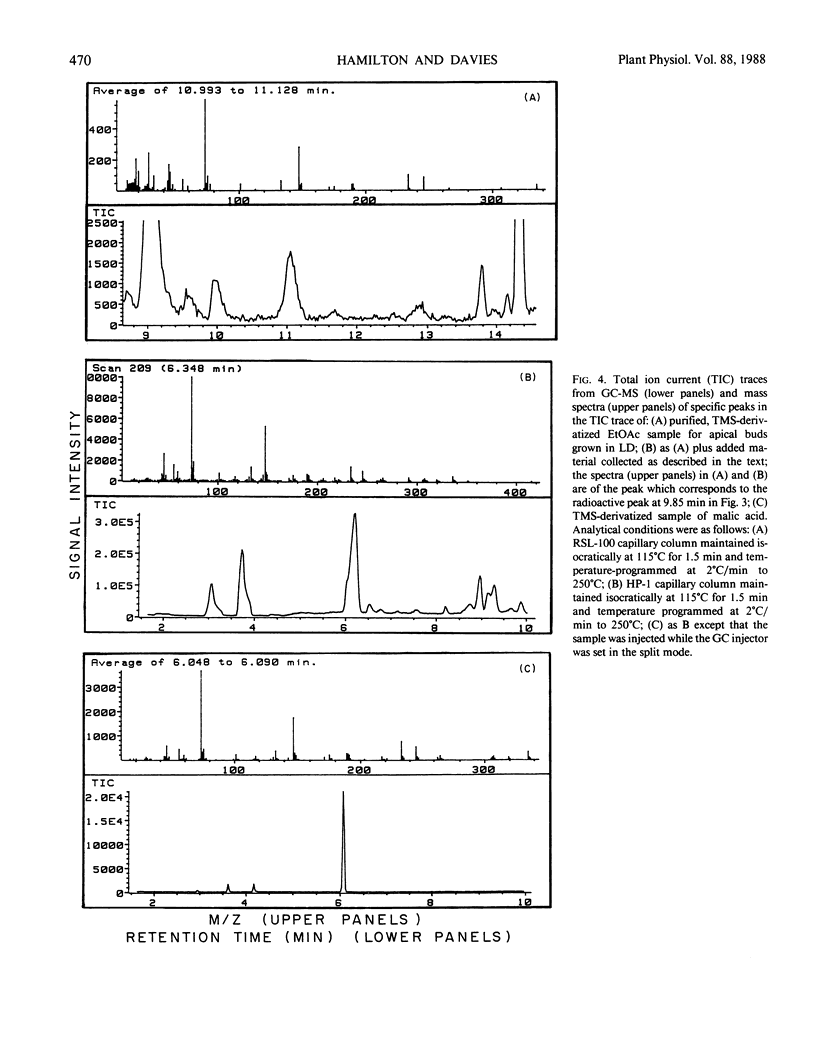
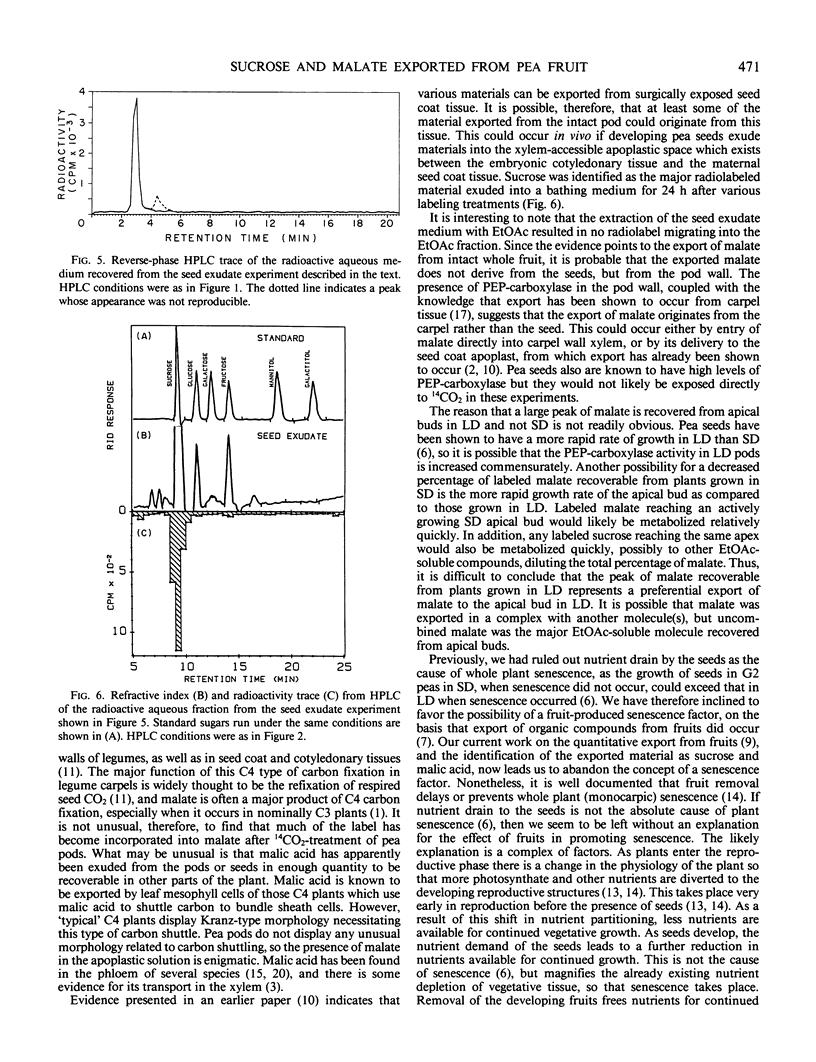
Abstract
The G2 line of peas (Pisum sativum L.) displays senescence and death of the apical bud only in long days and in the presence of fruit. As the removal of fruit prevents senescence, one possible mechanism by which fruits induce senescence is that the fruits produce some `senescence factor' under long day conditions, which is then transported to the apical bud. Allowing developing fruits to photosynthesize in the presence of 14CO2 results in the recovery of label in the apical bud. In order to determine the chemical nature of this radiolabeled material, fruits of G2 peas, growing under long days, were exposed to 14CO2 at the time when the first senescence symptoms start to appear. The radiolabeled material from apical buds was then extracted, purified, and identified. Using HPLC and GC-MS the major labeled compound found in the apical bud following exposure of pea fruits to 14CO2 was identified as sucrose, while malic acid was identified as the major ethyl acetate-soluble compound. These compounds accounted for about 73 and 16%, respectively, of the radioactivity in the apical bud. No other compounds were present in significant amounts. As neither of these chemicals is likely to have any kind of senescence effect, we report no evidence for a senescence factor.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett A. B., Sweger B. L., Spanswick R. M. Sink to source translocation in soybean. Plant Physiol. 1984 Feb;74(2):434–436. doi: 10.1104/pp.74.2.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford R. M., Thorne J. H. Sucrose Concentration at the Apoplastic Interface between Seed Coat and Cotyledons of Developing Soybean Seeds. Plant Physiol. 1985 Apr;77(4):863–868. doi: 10.1104/pp.77.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton D. A., Davies P. J. Mechanism of export of organic material from the developing fruits of pea. Plant Physiol. 1988 Mar;86(3):956–959. doi: 10.1104/pp.86.3.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly M. O., Davies P. J. Photoperiodic and genetic control of carbon partitioning in peas and its relationship to apical senescence. Plant Physiol. 1988 Mar;86(3):978–982. doi: 10.1104/pp.86.3.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtner F. T., Spanswick R. M. Electrogenic sucrose transport in developing soybean cotyledons. Plant Physiol. 1981 Apr;67(4):869–874. doi: 10.1104/pp.67.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate J. S., Peoples M. B., van Bel A. J., Kuo J., Atkins C. A. Diurnal water balance of the cowpea fruit. Plant Physiol. 1985 Jan;77(1):148–156. doi: 10.1104/pp.77.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proebsting W. M., Davies P. J., Marx G. A. Photoperiodic control of apical senescence in a genetic line of peas. Plant Physiol. 1976 Dec;58(6):800–802. doi: 10.1104/pp.58.6.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamas I. A., Engels C. J. Role of indoleacetic Acid and abscisic Acid in the correlative control by fruits of axillary bud development and leaf senescence. Plant Physiol. 1981 Aug;68(2):476–481. doi: 10.1104/pp.68.2.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieman T. J., Max M. H., Voyles C. R., Barrows G. H. Diversion of duodenal contents: its effect on the production of experimental gastric cancer. Arch Surg. 1980 Aug;115(8):959–961. doi: 10.1001/archsurg.1980.01380080053010. [DOI] [PubMed] [Google Scholar]


